Effects of Light Color on the Growth, Feeding, Digestion, and Antioxidant Enzymes of Tripneustes gratilla (Linnaeus, 1758)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sea Urchins
2.2. Experimental Design
2.3. Sample Collection
2.4. Measurement and Analysis of Samples
2.4.1. Growth Performance Measurement
2.4.2. Feeding Performance Measurement
2.4.3. Enzyme Activity Measurement
2.5. Data Analysis
3. Results
3.1. Growth Performance of Sea Urchins under Different Light Colors
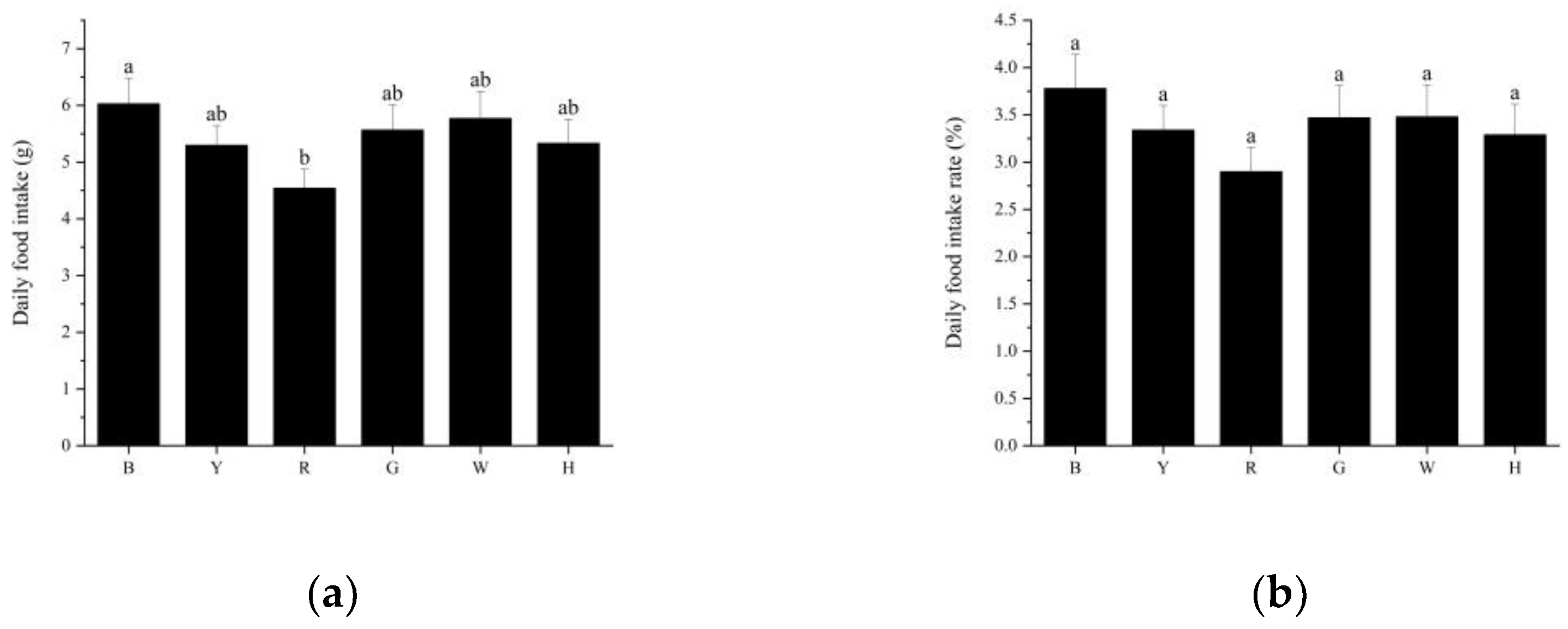
3.2. Feeding Performance of Sea Urchins under Different Light Colors
3.3. Changes in the Digestive Enzyme Activities of Sea Urchins under Different Light Colors
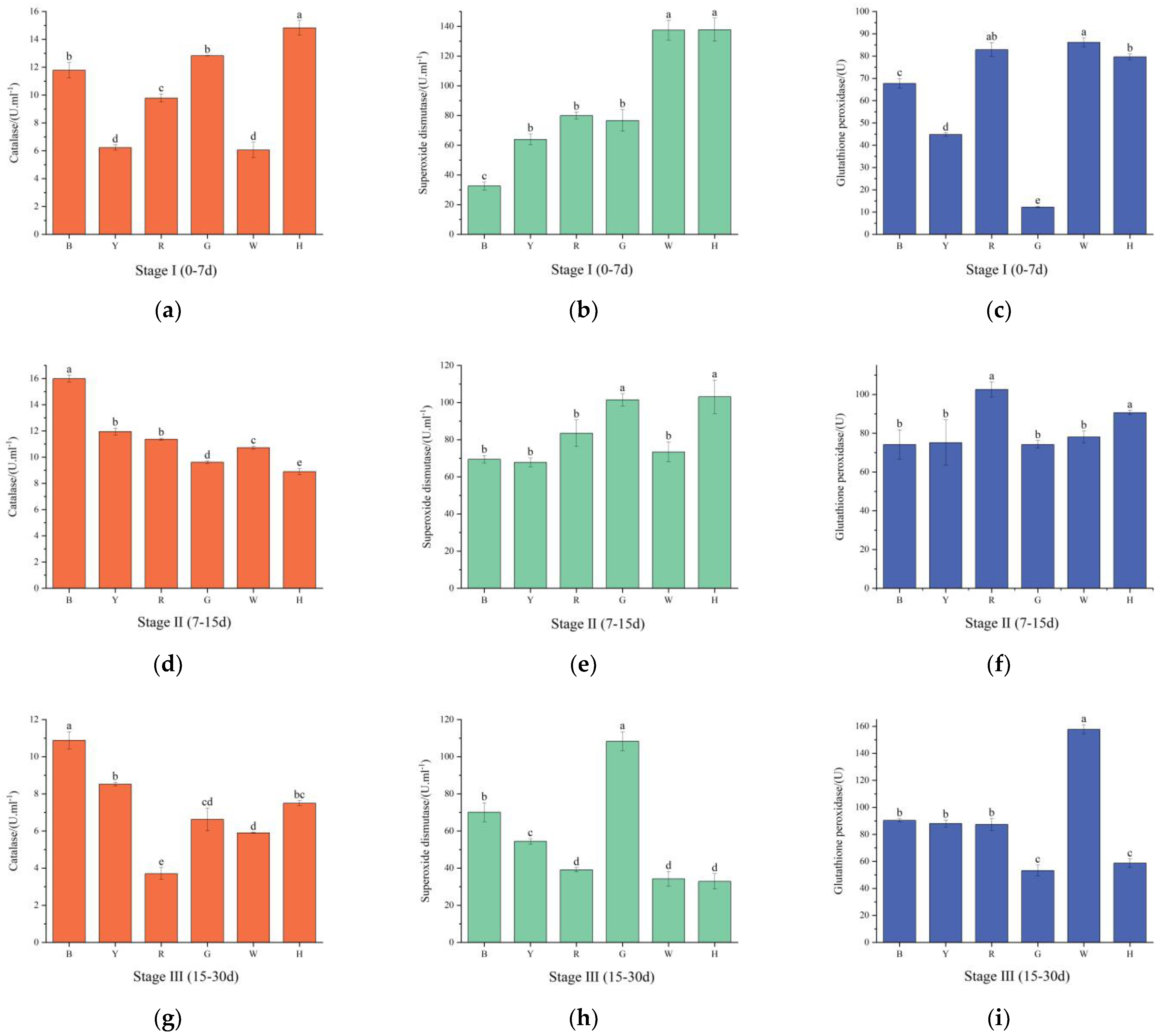
3.4. Changes in the Antioxidant Enzyme Activities of Sea Urchins under Different Light Colors
4. Discussion
4.1. The Effect of Light Color on the Feeding and Growth of T. gratilla
4.2. The Effect of Light Color on the Digestive Enzyme Activities of T. gratilla
4.3. The Effect of Light Color on the Antioxidant Enzyme Activities of T. gratilla
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Villamizar, N.; Blanco-Vives, B.; Migaud, H.; Davie, A.; Carboni, S.; Sánchez-Vázquez, F.J. Effects of light during early larval development of some aquacultured teleosts: A review. Aquaculture 2011, 315, 86–94. [Google Scholar] [CrossRef]
- Liu, S.; Cai, H.; Liu, Y.; Zhang, Y.; Fang, Y.; Sun, F.; Wu, Y.; Li, X.; Lv, L.; Zhang, Q.; et al. Effects of LED spectra on the growth and physiological mechanism of juvenile Sebastes schlegelii. Part I: Growth, feeding and digestion and metabolism. Aquaculture 2023, 580, 740295. [Google Scholar] [CrossRef]
- Yang, M.; Chen, Z.; Hu, F.; Sun, J.; Ding, J.; Chang, Y.; Zhao, C. Light spectra regulated foraging and feeding behaviors shed light on stock enhancement of the sea urchin Strongylocentrotus intermedius. Aquac. Rep. 2020, 18, 100480. [Google Scholar] [CrossRef]
- Wang, M.-H.; Hsieh, Y.-J.; Chang, H.-H.; Wang, Y.-S. Effects of different visible light spectrums on phototaxis and bottom preference behavior of sea cucumber. Aquaculture 2024, 578, 740112. [Google Scholar] [CrossRef]
- Villamizar, N.; García-Alcazar, A.; Sánchez-Vázquez, F.J. Effect of light spectrum and photoperiod on the growth, development and survival of European sea bass (Dicentrarchus labrax) larvae. Aquaculture 2009, 292, 80–86. [Google Scholar] [CrossRef]
- Karakatsouli, N.; Papoutsoglou, E.S.; Sotiropoulos, N.; Mourtikas, D.; Stigen-Martinsen, T.; Papoutsoglou, S.E. Effects of light spectrum, rearing density and light intensity on growth performance of scaled and mirror common carp Cyprinus carpio reared under recirculating system conditions. Aquac. Eng. 2010, 42, 121–127. [Google Scholar] [CrossRef]
- Takahashi, A.; Kasagi, S.; Murakami, N.; Furufuji, S.; Kikuchi, S.; Mizusawa, K.; Andoh, T. Chronic effects of light irradiated from LED on the growth performance and endocrine properties of barfin flounder Verasper moseri. Gen. Comp. Endocrinol. 2016, 232, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Xiaolong, G.; Mo, Z.; Xian, L.; Ce, S.; Changbin, S.; Ying, L. Effects of LED light quality on the growth, metabolism, and energy budgets of Haliotis discus discus. Aquaculture 2016, 453, 31–39. [Google Scholar] [CrossRef]
- Yang, M.; Hu, F.; Leng, X.; Chi, X.; Yin, D.; Ding, J.; Li, X.; Zuo, R.; Chang, Y.; Zhao, C. Long-term effects of light spectra on fitness related behaviors and growth of the sea urchin Strongylocentrotus intermedius. Aquaculture 2021, 537, 736518. [Google Scholar] [CrossRef]
- Zhao, W.; Wen, W.; Tan, C.; Huang, X.; Yang, R.; Chen, M.; Yang, Q.; Chen, X. Effects of starvation stress on the activities of immune enzymes in differenttissues of Harpiosquilla harpax. Acta Sci. Nat. Univ. Sunyatseni 2021, 60, 26–33. [Google Scholar] [CrossRef]
- Wang, M.; Lv, C.; Yang, D.; Zhao, J. Effects of Lactobacillus plantarum(LP HMX-3) on growth, digestion, immunityand intestinal flora of Apostichopus japonicus. J. Fish. China 2023, 47, 129111. [Google Scholar]
- Yi, M.; Zhai, W.L.; Wang, M.; Wang, H.; Liu, Z.; Gao, F.-Y.; Ke, X.-L.; Song, C.; Cao, J.; Lu, M.-X. The Welfare of Nile Tilapia (Oreochromis niloticus, GIFT Strain) Juveniles Cultured in Different Light Spectra. Front. Mar. Sci. 2022, 9, 924110. [Google Scholar] [CrossRef]
- Hou, Z.-S.; Wen, H.-S.; Li, J.-F.; He, F.; Li, Y.; Qi, X.; Zhao, J.; Zhang, K.-Q.; Tao, Y.-X. Effects of photoperiod and light Spectrum on growth performance, digestive enzymes, hepatic biochemistry and peripheral hormones in spotted sea bass (Lateolabrax maculatus). Aquaculture 2019, 507, 419–427. [Google Scholar] [CrossRef]
- Yang, S.; Yan, T.; Wu, H.; Xiao, Q.; Fu, H.M.; Luo, J.; Zhou, J.; Zhao, L.L.; Wang, Y.; Yang, S.Y.; et al. Acute hypoxic stress: Effect on blood parameters, antioxidant enzymes, and expression of HIF-1alpha and GLUT-1 genes in largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2017, 67, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Trenzado, C.E.; Hidalgo, F.; Villanueva, D.; Furné, M.; Díaz-Casado, M.E.; Merino, R.; Sanz, A. Study of the enzymatic digestive profile in three species of Mediterranean sea urchins. Aquaculture 2012, 344–349, 174–180. [Google Scholar] [CrossRef]
- Xi, S.; Qin, C.; Ma, Z.; Yu, G.; Sun, J.; Pan, W.; Zuo, T.; Ma, H.; Zhu, W. Effects of dietary microalgae on growth and survival of larval development of sea urchin (Anthocidaris crassispina). South China Fish. Sci. 2020, 16, 115–120. [Google Scholar]
- Wang, W.; Han, L.; Zhang, X.; Liu, P.; Yang, X.; Wang, L.; Zhang, W.; Chang, Y.; Ding, J. Effects of Thermal Stress on the Activities of Antioxidant Enzymes and Mitochondrial Structure and Function in Strongylocentrotus intermedius. J. Guangdong Ocean Univ. 2022, 42, 42–48. [Google Scholar]
- Ackleson, S.G. Light in shallow waters: A brief research review. Limnol. Oceanogr. 2003, 48, 323–328. [Google Scholar] [CrossRef]
- Lawrence, J.M. Chapter 2—Sea Urchin Life History Strategies. In Developments in Aquaculture and Fisheries Science; Lawrence, J.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 38, pp. 15–23. [Google Scholar]
- Brink, M.; Kuys, R.D.; Rhode, C.; Macey, B.M.; Christison, K.W.; Roodt-Wilding, R. Genetic diversity and population connectivity of the sea urchin Tripneustes gratilla along the South African coast. Afr. J. Mar. Sci. 2018, 40, 149–156. [Google Scholar] [CrossRef]
- Cyrus, M.; Bolton, J.; de Wet, L.; Macey, B. The development of a formulated feed containing Ulva (Chlorophyta) to promote rapid growth and enhanced production of high quality roe in the sea urchin Tripneustes gratilla (Linnaeus). Aquac. Res. 2012, 45, 159–176. [Google Scholar] [CrossRef]
- Scholtz, R.; Bolton, J.; Macey, B. Effects of different microalgal feeds and their influence on larval development in the white-spined sea urchin Tripneustes gratilla. Afr. J. Mar. Sci. 2013, 35, 25–34. [Google Scholar] [CrossRef]
- Zhang, T.; Li, X.; Cao, R.; Zhang, Q.; Qu, Y.; Wang, Q.; Dong, Z.; Zhao, J. Interactive effects of ocean acidification, ocean warming, and diurnal temperature cycling on antioxidant responses and energy budgets in two sea urchins Strongylocentrotus intermedius and Tripneustes gratilla from different latitudes. Sci. Total Environ. 2022, 824, 153780. [Google Scholar] [CrossRef] [PubMed]
- Seymour, S.; Paul, N.A.; Dworjanyn, S.A.; de Nys, R. Feeding preference and performance in the tropical sea urchin Tripneustes gratilla. Aquaculture 2013, 400–401, 6–13. [Google Scholar] [CrossRef]
- Dworjanyn, S.A.; Pirozzi, I. Induction of settlement in the sea urchin Tripneustes gratilla by macroalgae, biofilms and conspecifics: A role for bacteria? Aquaculture 2008, 274, 268–274. [Google Scholar] [CrossRef]
- Dworjanyn, S.A.; Pirozzi, I.; Liu, W. The effect of the addition of algae feeding stimulants to artificial diets for the sea urchin Tripneustes gratilla. Aquaculture 2007, 273, 624–633. [Google Scholar] [CrossRef]
- Brink-Hull, M.; Cyrus, M.D.; Macey, B.M.; Rhode, C.; Hull, K.L.; Roodt-Wilding, R. Dietary effects on the reproductive performance of the sea urchin Tripneustes gratilla ll: Implications for offspring performance. Aquaculture 2022, 553, 738034. [Google Scholar] [CrossRef]
- Cyrus, M.D.; Bolton, J.J.; Macey, B.M. The role of the green seaweed Ulva as a dietary supplement for full life-cycle grow-out of Tripneustes gratilla. Aquaculture 2015, 446, 187–197. [Google Scholar] [CrossRef]
- Shpigel, M.; Erez, J. Effect of diets and light regimes on calcification and somatic growth of the sea urchin Tripneustes gratilla elatensis. Aquaculture 2020, 529, 735547. [Google Scholar] [CrossRef]
- Hernández-Almaraz, P.; Rivera, M.J.; Mazariegos-Villarreal, A.; Méndez-Rodríguez, L.C.; Serviere-Zaragoza, E. Macroalgae contribution to the diet of two sea urchins in Sargassum Beds: Tripneustes depressus (Camarodonta: Toxopneustidae) and Eucidaris thouarsii (Cidaroide: Cidaridae). Reg. Stud. Mar. Sci. 2022, 53, 102456. [Google Scholar] [CrossRef]
- Casilagan, I.L.N.; Juinio-Meñez, M.A.; Crandall, E.D. Genetic diversity, population structure, and demographic history of exploited sea urchin populations (Tripneustes gratilla) in the Philippines. J. Exp. Mar. Biol. Ecol. 2013, 449, 284–293. [Google Scholar] [CrossRef][Green Version]
- Toha, A.H.A.; Sumitro, S.B.; Widodo; Hakim, L. Color diversity and distribution of sea urchin Tripneustes gratilla in Cenderawasih Bay ecoregion of Papua, Indonesia. Egypt. J. Aquat. Res. 2015, 41, 273–278. [Google Scholar] [CrossRef][Green Version]
- Loew, E.R.; McFarland, W.N. The underwater visual environment. In The Visual System of Fish; Douglas, R., Djamgoz, M., Eds.; Springer: Dordrecht, The Netherlands, 1990; pp. 1–43. [Google Scholar]
- Migaud, H.; Taylor, J.F.; Taranger, G.L.; Davie, A.; Cerdá-Reverter, J.M.; Carrillo, M.; Hansen, T.; Bromage, N.R. A comparative ex vivo and in vivo study of day and night perception in teleosts species using the melatonin rhythm. J Pineal Res 2006, 41, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zheng, S.; Zeng, C. Comparative study of the absorption spectra of several species of green, brown and red algae. J. Integr. Plant Biol. 1974, 16, 146–155. [Google Scholar]
- de Mooij, T.; de Vries, G.; Latsos, C.; Wijffels, R.H.; Janssen, M. Impact of light color on photobioreactor productivity. Algal Res. 2016, 15, 32–42. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Hanelt, D.; Wiencke, C. Growth and DNA damage in young Laminaria sporophytes exposed to ultraviolet radiation: Implication for depth zonation of kelps on Helgoland (North Sea). Mar. Biol. 2006, 148, 1201–1211. [Google Scholar] [CrossRef][Green Version]
- Cheng, X. Response of Photosynthetic Activity of Macroalgae to Different Temperatures and Light Conditions. Master’s Thesis, Shanghai Ocean University., Shanghai, China, 2019. [Google Scholar]
- Sui, X.; Ren, W.; Yan, W.; Liu, S.; Yang, N. Effects of light wavelength on growth and reproduction of the gametophytes of Laminaria japonica Aresch. Mar. Sci. 2011, 35, 33–36. [Google Scholar]
- Mizuta, H.; Kai, T.; Tabuchi, K.; Yasui, H. Effects of light quality on the reproduction and morphology of sporophytes of Laminaria japonica (Phaeophyceae). Aquac. Res. 2007, 38, 1323–1329. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Y.; Zhang, H.; Fang, L.; Wang, Q.; Ruan, G. Effect of yeast hydrolysate on growth performance, digestion, antioxidant and immunity of crayfish (Litopenaeus vannamei). Acta Hydrobiol. Sin. 2024, 48, 393–404. [Google Scholar]
- Ren, J.; Wei, P.; Fei, F.; Dai, M.; Ma, H.; Gao, D.; Song, C.; Chen, T.; Liu, Y. Effects of LED spectroscopy on feeding, growth and energy partitioning in juvenile tongue-toothed perch (Lepomis macrocephalus). J. Fish. China 2019, 43, 1821–1829. [Google Scholar]
- Li, M.; Zhang, F.; Ding, J.; Zuo, R.; Chang, Y. Effects of lipid sources on the growth performance, gonad development, fatty acid profile and transcription of related genes in juvenile sea urchin (Strongylocentrotus intermedius). Aquac. Nutr. 2021, 27, 28–38. [Google Scholar] [CrossRef]
- Moyano, F.J.; Díaz, M.; Alarcón, F.J.; Sarasquete, M.C. Characterization of digestive enzyme activity during larval development of gilthead seabream (Sparus aurata). Fish Physiol. Biochem. 1996, 15, 121–130. [Google Scholar] [CrossRef]
- Ren, X. Influence of Environmental Factors and Nutritional Levels on Digestive Enzyme Activities in Half-Smooth Tongue Sole (Solea solea). Master’s Thesis, Ocean University of China, Qingdao, China, 2008. [Google Scholar]
- Chen, J.; Xi, S.; Qin, C.; Guo, Y.; Pan, W.; Shao, G. Effect of light intensity on the growth and digestive enzyme activities of purple sea urchin (Heliocidaris crassispina, Agassiz, 1864) planktonic larvae. Prog. Fish. Sci. 2021, 42, 125–131. [Google Scholar] [CrossRef]
- Shi, J.; Feng, Y.; Jiang, X.; Liu, X. Effects of different algae and polyculture ratio of sea cucumber Apostichopus japonicus and sea urchin Hemicentrotus pulcherrimus on growth, body composition and digestive enzyme activities in sea cucumber and sea urchin. J. Dalian Ocean Univ. 2020, 35, 509–515. [Google Scholar] [CrossRef]
- Zuo, R.; Hou, S.; Li, G.; Chang, Y. Study on the activities of digestive enzyme and anti-oxidative enzymes injuvenile sea urchin (Strongylocentrotus intermedius). Feed Ind. 2016, 37, 26–29. [Google Scholar] [CrossRef]
- Zhao, C.; Feng, W.; Wei, J.; Zhang, L.; Sun, P.; Chang, Y. Effects of temperature and feeding regime on food consumption, growth, gonad production and quality of the sea urchin Strongylocentrotus intermedius. J. Mar. Biol. Assoc. 2016, 96, 185–195. [Google Scholar] [CrossRef]
- Franco, R.; Sánchez-Olea, R.; Reyes-Reyes, E.M.; Panayiotidis, M.I. Environmental toxicity, oxidative stress and apoptosis: Ménage à Trois. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2009, 674, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Fattman, C.L.; Schaefer, L.M.; Oury, T.D. Extracellular superoxide dismutase in biology and medicine. Free Radic. Biol. Med. 2003, 35, 236–256. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, Y.; Li, S.; Guo, X.; Fu, Y.; He, N.; Ruan, G.; Wang, Q.; Gao, W.; Fang, L. Impact of nitrite exposure on oxidative stress and antioxidative-related genes responses in the gills of Procambarus clarkii. Fish Shellfish Immunol. 2022, 131, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Jiang, F.; Shi, Y.; Xu, J.; Liu, Y.; Deng, P. Effects of high temperature stress on antioxidative and non-specific immunity indices of one-year-old Alosa sapidissima. J. Zhejiang Univ. (Agric. Life Sci.) 2021, 47, 107–117. [Google Scholar]
- Wu, Z.; You, F.; Wang, Y.; Wen, A.; Ma, D.; Xu, Y.; Zhang, P. The effects of hypoxia and hyperoxia on nucleus anomaly, SOD, CAT activities and MDA content in juvenile turbot Scophthalmus maximus. J. Shanghai Ocean Univ. 2011, 20, 808–813. [Google Scholar]
- Zhai, S.; Fu, H.; Qiao, H.; Zhang, W.; Jin, S.; Jiang, S.; Xiong, Y.; Xu, L.; Wang, Y.; Hu, Y.; et al. Effects of high temperature on heat shock proteins, antioxidant enzymeactivity, and histology of oriental river prawn Macrobrachium nipponense. J. Fish. Sci. China 2022, 29, 684–695. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Guo, Y.; Li, J.; Ma, Z.; Yu, G.; Qin, C. Effects of Light Color on the Growth, Feeding, Digestion, and Antioxidant Enzymes of Tripneustes gratilla (Linnaeus, 1758). Biology 2024, 13, 65. https://doi.org/10.3390/biology13020065
Zhao X, Guo Y, Li J, Ma Z, Yu G, Qin C. Effects of Light Color on the Growth, Feeding, Digestion, and Antioxidant Enzymes of Tripneustes gratilla (Linnaeus, 1758). Biology. 2024; 13(2):65. https://doi.org/10.3390/biology13020065
Chicago/Turabian StyleZhao, Xinye, Yu Guo, Jiayang Li, Zhenhua Ma, Gang Yu, and Chuanxin Qin. 2024. "Effects of Light Color on the Growth, Feeding, Digestion, and Antioxidant Enzymes of Tripneustes gratilla (Linnaeus, 1758)" Biology 13, no. 2: 65. https://doi.org/10.3390/biology13020065
APA StyleZhao, X., Guo, Y., Li, J., Ma, Z., Yu, G., & Qin, C. (2024). Effects of Light Color on the Growth, Feeding, Digestion, and Antioxidant Enzymes of Tripneustes gratilla (Linnaeus, 1758). Biology, 13(2), 65. https://doi.org/10.3390/biology13020065







