The Reign of Follistatin in Tumors and Their Microenvironment: Implications for Drug Resistance
Abstract
Simple Summary
Abstract
1. Introduction
2. Follistatin and Its Intimate Tango with TGF-β
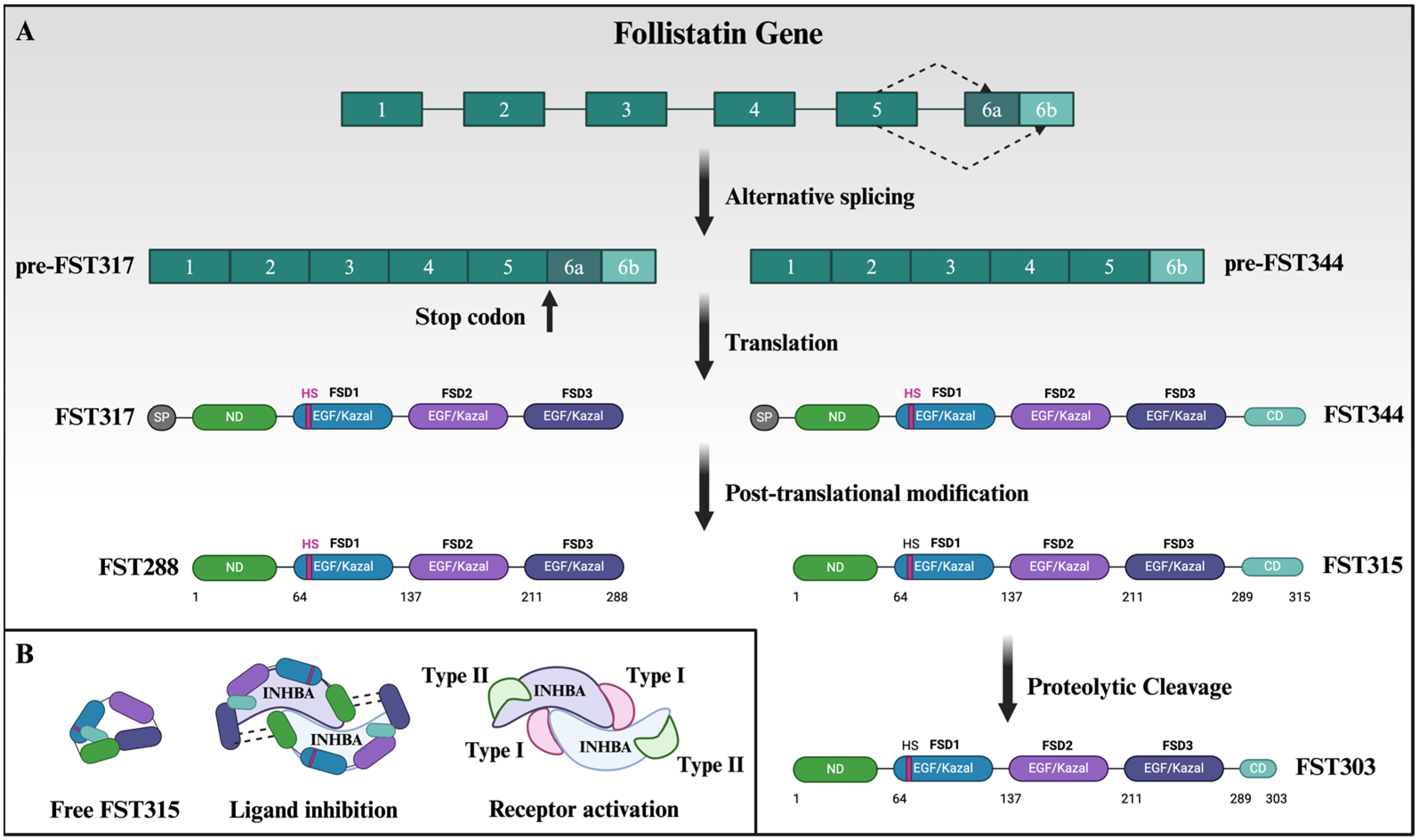
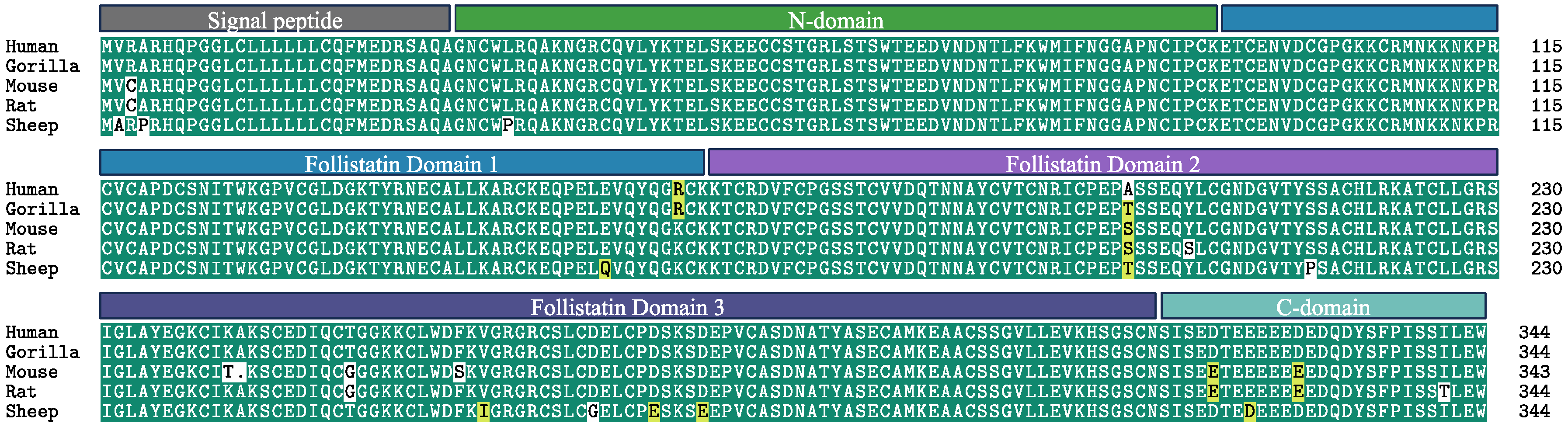
3. Complexity of Follistatin Isoforms and Structure Can Be Exploited for Targeted Therapy
4. Post-Translational Modification and Intracellular Localization of Follistatin
5. Expression of Follistatin in Tissues and Organs
6. The TGF-β Paradox and the Possible Role of Follistatin
7. Follistatin in the Ecology of Cancer: Cancer Hallmarks
7.1. Maintenance of a Progenitor State in Epithelial-Rich Tissues
7.2. Sustaining the Growth Machinery
7.3. Cancer Metastasis
7.4. Angiogenesis
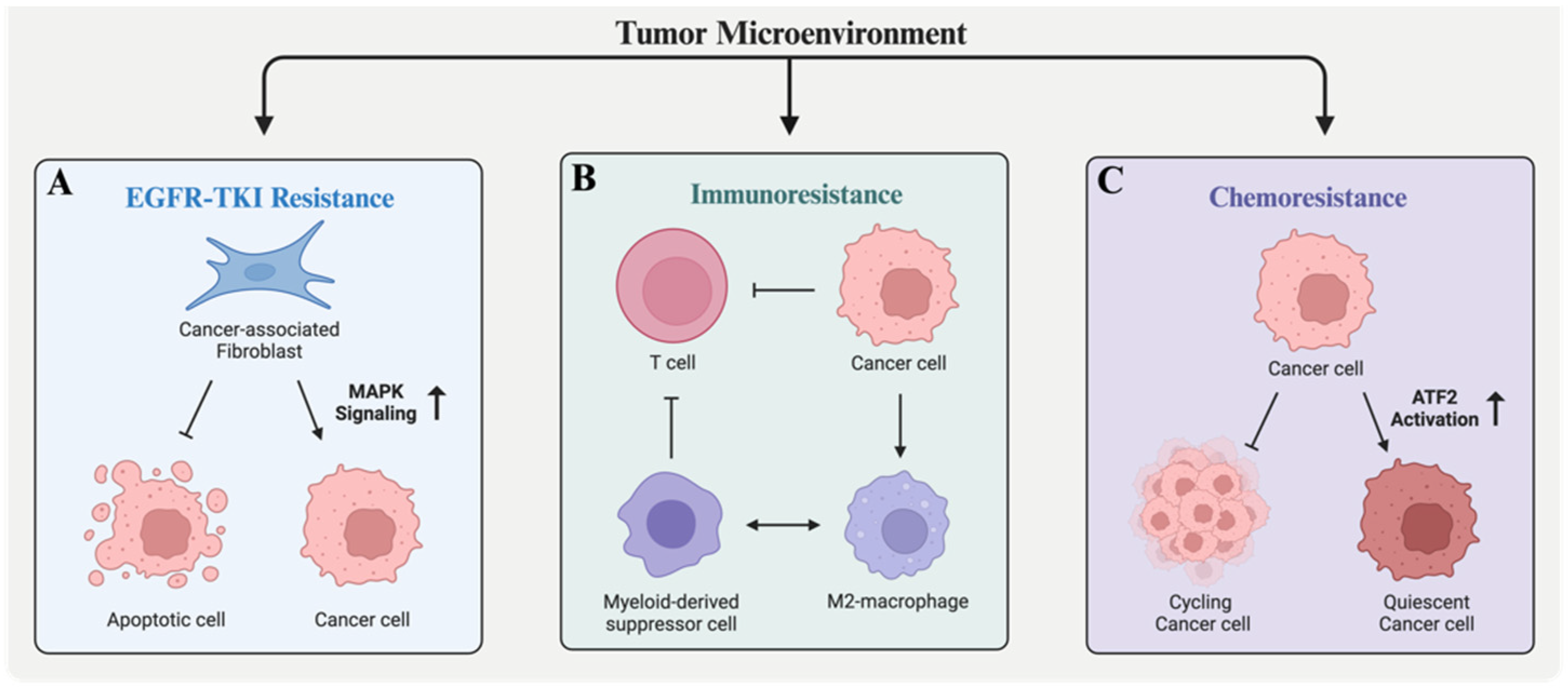
8. Follistatin in Drug Resistance: Vignettes from Three Major Cancer Types
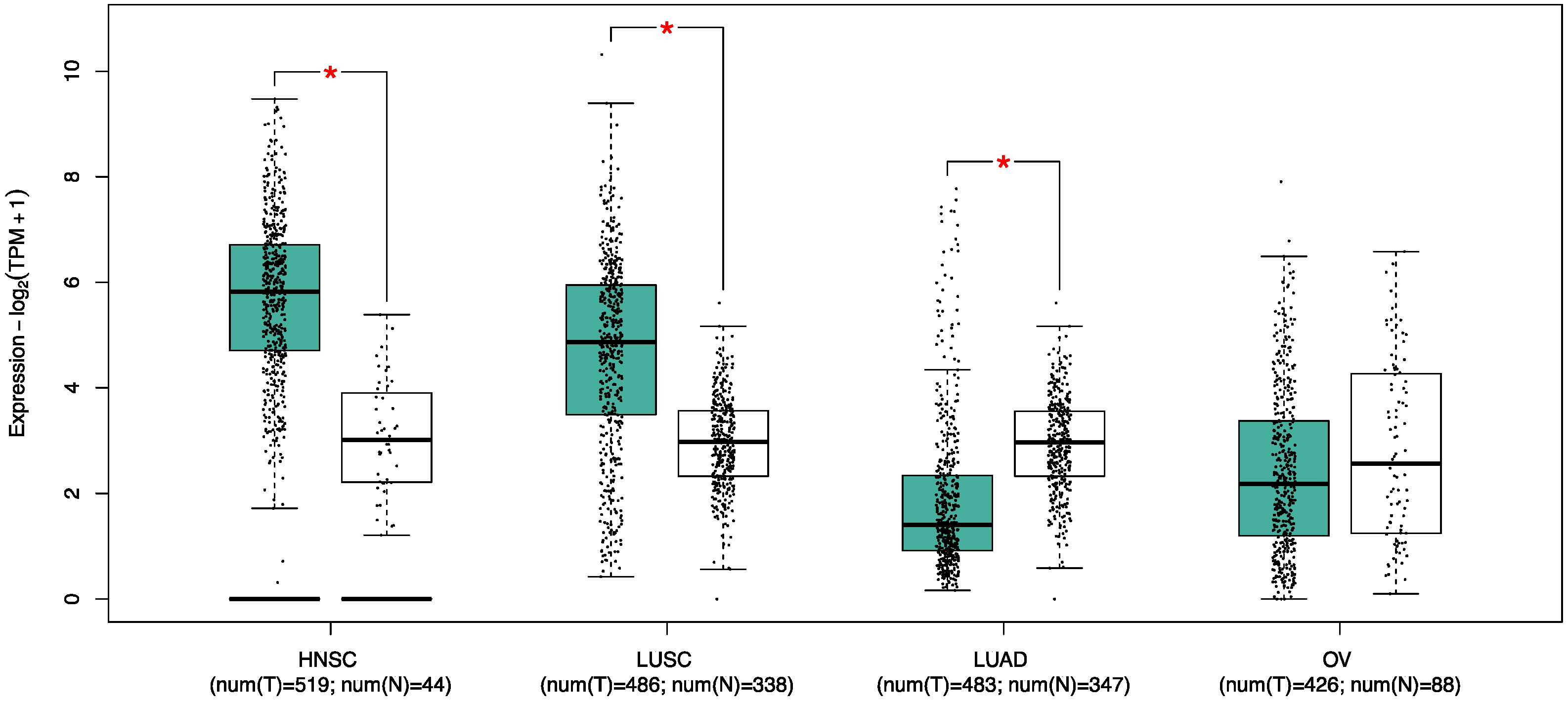
8.1. Lung Cancer
8.2. Ovarian Cancer
8.3. Head and Neck Squamous Cell Carcinoma
9. Mechanisms Driving Follistatin Expression in Cancer
10. Concluding Remarks and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sidis, Y.; Mukherjee, A.; Keutmann, H.; Delbaere, A.; Sadatsuki, M.; Schneyer, A. Biological activity of follistatin isoforms and follistatin-like-3 is dependent on differential cell surface binding and specificity for activin, myostatin, and bone morphogenetic proteins. Endocrinology 2006, 147, 3586–3597. [Google Scholar] [CrossRef]
- Shi, Y.; Massagué, J. Mechanisms of TGF-β Signaling from Cell Membrane to the Nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Heldin, C.H.; Miyazono, K.; ten Dijke, P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 1997, 390, 465–471. [Google Scholar] [CrossRef]
- Neumann, E. Activin can activate p38 MAPK. Breast Cancer Res. 2001, 3, 68468. [Google Scholar] [CrossRef]
- Hu, J.; Wang, X.; Wei, S.-M.; Tang, Y.-H.; Zhou, Q.; Huang, C.-X. Activin A stimulates the proliferation and differentiation of cardiac fibroblasts via the ERK1/2 and p38-MAPK pathways. Eur. J. Pharmacol. 2016, 789, 319–327. [Google Scholar] [CrossRef]
- Zhang, Y.E. Non-Smad pathways in TGF-β signaling. Cell Res. 2009, 19, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.E. Non-Smad Signaling Pathways of the TGF-β Family. Cold Spring Harb. Perspect. Biol. 2017, 9, a022129. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.B.; Lerch, T.F.; Cook, R.W.; Woodruff, T.K.; Jardetzky, T.S. The Structure of the Follistatin:Activin Complex Reveals Antagonism of Both Type I and Type II Receptor Binding. Dev. Cell 2005, 9, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Harrington, A.E.; Morris-Triggs, S.A.; Ruotolo, B.T.; Robinson, C.V.; Ohnuma, S.-I.; Hyvönen, M. Structural basis for the inhibition of activin signalling by follistatin. EMBO J. 2006, 25, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Winbanks, C.E.; Weeks, K.L.; Thomson, R.E.; Sepulveda, P.V.; Beyer, C.; Qian, H.; Chen, J.L.; Allen, J.M.; Lancaster, G.I.; Febbraio, M.A.; et al. Follistatin-mediated skeletal muscle hypertrophy is regulated by Smad3 and mTOR independently of myostatin. J. Cell Biol. 2012, 197, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Braga, M.; Reddy, S.T.; Lee, S.J.; Parveen, M.; Grijalva, V.; Vergnes, L.; Pervin, S. Follistatin Targets Distinct Pathways to Promote Brown Adipocyte Characteristics in Brown and White Adipose Tissues. Endocrinology 2017, 158, 1217–1230. [Google Scholar] [CrossRef] [PubMed]
- He, B.L.; Yang, N.; Man, C.H.; Ng, N.K.; Cher, C.Y.; Leung, H.C.; Kan, L.L.; Cheng, B.Y.; Lam, S.S.; Wang, M.L.; et al. Follistatin is a novel therapeutic target and biomarker in FLT3/ITD acute myeloid leukemia. EMBO Mol. Med. 2020, 12, e10895. [Google Scholar] [CrossRef]
- Klumpe, H.E.; Langley, M.A.; Linton, J.M.; Su, C.J.; Antebi, Y.E.; Elowitz, M.B. The context-dependent, combinatorial logic of BMP signaling. Cell Syst. 2022, 13, 388–407.e310. [Google Scholar] [CrossRef]
- Nickel, J.; ten Dijke, P.; Mueller, T.D. TGF-β family co-receptor function and signaling. Acta Biochim. Biophys. Sin. 2017, 50, 12–36. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, X.-F. Signaling cross-talk between TGF-β/BMP and other pathways. Cell Res. 2009, 19, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Turley, S.J.; Akhurst, R.J. TGFβ biology in cancer progression and immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 9–34. [Google Scholar] [CrossRef]
- Shimasaki, S.; Koga, M.; Esch, F.; Mercado, M.; Cooksey, K.; Koba, A.; Ling, N. Porcine follistatin gene structure supports two forms of mature follistatin produced by alternative splicing. Biochem. Biophys. Res. Commun. 1988, 152, 717–723. [Google Scholar] [CrossRef]
- Shimasaki, S.; Koga, M.; Esch, F.; Cooksey, K.; Mercado, M.; Koba, A.; Ueno, N.; Ying, S.Y.; Ling, N.; Guillemin, R. Primary structure of the human follistatin precursor and its genomic organization. Proc. Natl. Acad. Sci. USA 1988, 85, 4218–4222. [Google Scholar] [CrossRef]
- Sugino, K.; Kurosawa, N.; Nakamura, T.; Takio, K.; Shimasaki, S.; Ling, N.; Titani, K.; Sugino, H. Molecular heterogeneity of follistatin, an activin-binding protein. Higher affinity of the carboxyl-terminal truncated forms for heparan sulfate proteoglycans on the ovarian granulosa cell. J. Biol. Chem. 1993, 268, 15579–15587. [Google Scholar] [CrossRef]
- Lerch, T.F.; Shimasaki, S.; Woodruff, T.K.; Jardetzky, T.S. Structural and biophysical coupling of heparin and activin binding to follistatin isoform functions. J. Biol. Chem. 2007, 282, 15930–15939. [Google Scholar] [CrossRef]
- Schneyer, A.L.; Wang, Q.; Sidis, Y.; Sluss, P.M. Differential distribution of follistatin isoforms: Application of a new FS315-specific immunoassay. J. Clin. Endocrinol. Metab. 2004, 89, 5067–5075. [Google Scholar] [CrossRef]
- Zhang, F.; Beaudet, J.M.; Luedeke, D.M.; Walker, R.G.; Thompson, T.B.; Linhardt, R.J. Analysis of the interaction between heparin and follistatin and heparin and follistatin-ligand complexes using surface plasmon resonance. Biochemistry 2012, 51, 6797–6803. [Google Scholar] [CrossRef]
- Cash, J.N.; Rejon, C.A.; McPherron, A.C.; Bernard, D.J.; Thompson, T.B. The structure of myostatin:follistatin 288: Insights into receptor utilization and heparin binding. EMBO J. 2009, 28, 2662–2676. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.; Guo, Y.; DePaolo, L.; Shimonaka, M.; Ling, N.; Shimasaki, S. Recombinant expression of human follistatin with 315 and 288 amino acids: Chemical and biological comparison with native porcine follistatin. Endocrinology 1991, 129, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Innis, C.A.; Hyvönen, M. Crystal Structures of the Heparan Sulfate-binding Domain of Follistatin: Insights into ligand binding. J. Biol. Chem. 2003, 278, 39969–39977. [Google Scholar] [CrossRef] [PubMed]
- Sidis, Y.; Schneyer, A.L.; Sluss, P.M.; Johnson, L.N.; Keutmann, H.T. Follistatin: Essential Role for the N-terminal Domain in Activin Binding and Neutralization. J. Biol. Chem. 2001, 276, 17718–17726. [Google Scholar] [CrossRef] [PubMed]
- Keutmann, H.T.; Schneyer, A.L.; Sidis, Y. The Role of Follistatin Domains in Follistatin Biological Action. Mol. Endocrinol. 2004, 18, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Cash, J.N.; Angerman, E.B.; Keutmann, H.T.; Thompson, T.B. Characterization of follistatin-type domains and their contribution to myostatin and activin A antagonism. Mol. Endocrinol. 2012, 26, 1167–1178. [Google Scholar] [CrossRef]
- Pearsall, R.S.; Davies, M.V.; Cannell, M.; Li, J.; Widrick, J.; Mulivor, A.W.; Wallner, S.; Troy, M.E.; Spaits, M.; Liharska, K.; et al. Follistatin-based ligand trap ACE-083 induces localized hypertrophy of skeletal muscle with functional improvement in models of neuromuscular disease. Sci. Rep. 2019, 9, 11392. [Google Scholar] [CrossRef]
- Castonguay, R.; Lachey, J.; Wallner, S.; Strand, J.; Liharska, K.; Watanabe, A.E.; Cannell, M.; Davies, M.V.; Sako, D.; Troy, M.E.; et al. Follistatin-288-Fc Fusion Protein Promotes Localized Growth of Skeletal Muscle. J. Pharmacol. Exp. Ther. 2019, 368, 435–445. [Google Scholar] [CrossRef]
- Gao, X.; Hu, H.; Zhu, J.; Xu, Z. Identification and characterization of follistatin as a novel angiogenin-binding protein. FEBS Lett. 2007, 581, 5505–5510. [Google Scholar] [CrossRef]
- Maguer-Satta, V.; Forissier, S.; Bartholin, L.; Martel, S.; Jeanpierre, S.; Bachelard, E.; Rimokh, R. A novel role for fibronectin type I domain in the regulation of human hematopoietic cell adhesiveness through binding to follistatin domains of FLRG and follistatin. Exp. Cell Res. 2006, 312, 434–442. [Google Scholar] [CrossRef]
- Gao, X.; Wei, S.; Lai, K.; Sheng, J.; Su, J.; Zhu, J.; Dong, H.; Hu, H.; Xu, Z. Nucleolar follistatin promotes cancer cell survival under glucose-deprived conditions through inhibiting cellular rRNA synthesis. J. Biol. Chem. 2010, 285, 36857–36864. [Google Scholar] [CrossRef]
- Saito, S.; Sidis, Y.; Mukherjee, A.; Xia, Y.; Schneyer, A. Differential biosynthesis and intracellular transport of follistatin isoforms and follistatin-like-3. Endocrinology 2005, 146, 5052–5062. [Google Scholar] [CrossRef][Green Version]
- Hyuga, M.; Itoh, S.; Kawasaki, N.; Ohta, M.; Ishii, A.; Hyuga, S.; Hayakawa, T. Analysis of site-specific glycosylation in recombinant human follistatin expressed in Chinese hamster ovary cells. Biologicals 2004, 32, 70–77. [Google Scholar] [CrossRef]
- Datta-Mannan, A.; Huang, L.; Pereira, J.; Yaden, B.; Korytko, A.; Croy, J.E. Insights into the Impact of Heterogeneous Glycosylation on the Pharmacokinetic Behavior of Follistatin-Fc–Based Biotherapeutics. Drug Metab. Dispos. 2015, 43, 1882. [Google Scholar] [CrossRef] [PubMed]
- Payano, V.J.H.; Lopes, L.V.A.; Peixoto, L.R.; Silva, K.A.D.; Ortiga-Carvalho, T.M.; Tafuri, A.; Vago, A.R.; Bloise, E. Immunostaining of βA-Activin and Follistatin Is Decreased in HPV(+) Cervical Pre-Neoplastic and Neoplastic Lesions. Viruses 2023, 15, 1031. [Google Scholar] [CrossRef] [PubMed]
- Janik, S.; Bekos, C.; Hacker, P.; Raunegger, T.; Schiefer, A.I.; Mullauer, L.; Veraar, C.; Dome, B.; Klepetko, W.; Ankersmit, H.J.; et al. Follistatin impacts Tumor Angiogenesis and Outcome in Thymic Epithelial Tumors. Sci. Rep. 2019, 9, 17359. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, C.; Liu, J.; Xie, W.; Xu, W.; Liang, F.; Huang, K.; He, X. Intraperitoneal administration of follistatin promotes adipocyte browning in high-fat diet-induced obese mice. PLoS ONE 2019, 14, e0220310. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.; Reddy, S.T.; Vergnes, L.; Pervin, S.; Grijalva, V.; Stout, D.; David, J.; Li, X.; Tomasian, V.; Reid, C.B.; et al. Follistatin promotes adipocyte differentiation, browning, and energy metabolism. J. Lipid Res. 2014, 55, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.S.; Rutti, S.; Arous, C.; Clemmesen, J.O.; Secher, N.H.; Drescher, A.; Gonelle-Gispert, C.; Halban, P.A.; Pedersen, B.K.; Weigert, C.; et al. Circulating Follistatin Is Liver-Derived and Regulated by the Glucagon-to-Insulin Ratio. J. Clin. Endocrinol. Metab. 2016, 101, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Borné, Y.; Gao, R.; López Rodriguez, M.; Roell, W.C.; Wilson, J.M.; Regmi, A.; Luan, C.; Aly, D.M.; Peter, A.; et al. Elevated circulating follistatin associates with an increased risk of type 2 diabetes. Nat. Commun. 2021, 12, 6486. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Miao, Y.; Gur-Cohen, S.; Gomez, N.; Yang, H.; Nikolova, M.; Polak, L.; Hu, Y.; Verma, A.; Elemento, O.; et al. The aging skin microenvironment dictates stem cell behavior. Proc. Natl. Acad. Sci. USA 2020, 117, 5339–5350. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Jin, S.; Chen, J.; Li, Z.; Lin, Z.; Tang, L.; Nie, Q.; Andersen, B. Murine interfollicular epidermal differentiation is gradualistic with GRHL3 controlling progression from stem to transition cell states. Nat. Commun. 2020, 11, 5434. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Yin, Y.; Wang, H.; Zhou, Z.; Sheng, X.; Fu, H.; Guo, R.; Wang, H.; Yang, J.; Gong, P.; et al. Telomere dysfunction impairs epidermal stem cell specification and differentiation by disrupting BMP/pSmad/P63 signaling. PLoS Genet. 2019, 15, e1008368. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Oyelakin, A.; Gluck, C.; Bard, J.E.; Song, E.C.; Smalley, K.; Che, M.; Flores, E.; Sinha, S.; Romano, R.A. p63 and Its Target Follistatin Maintain Salivary Gland Stem/Progenitor Cell Function through TGF-beta/Activin Signaling. iScience 2020, 23, 101524. [Google Scholar] [CrossRef]
- Abnaof, K.; Mallela, N.; Walenda, G.; Meurer, S.K.; Seré, K.; Lin, Q.; Smeets, B.; Hoffmann, K.; Wagner, W.; Zenke, M.; et al. TGF-β stimulation in human and murine cells reveals commonly affected biological processes and pathways at transcription level. BMC Syst. Biol. 2014, 8, 55. [Google Scholar] [CrossRef]
- Gangopadhyay, S.S. Systemic administration of follistatin288 increases muscle mass and reduces fat accumulation in mice. Sci. Rep. 2013, 3, 2441. [Google Scholar] [CrossRef]
- Haidet, A.M.; Rizo, L.; Handy, C.; Umapathi, P.; Eagle, A.; Shilling, C.; Boue, D.; Martin, P.T.; Sahenk, Z.; Mendell, J.R.; et al. Long-term enhancement of skeletal muscle mass and strength by single gene administration of myostatin inhibitors. Proc. Natl. Acad. Sci. USA 2008, 105, 4318–4322. [Google Scholar] [CrossRef]
- Iyer, C.C.; Chugh, D.; Bobbili, P.J.; Iii, A.J.B.; Crum, A.E.; Yi, A.F.; Kaspar, B.K.; Meyer, K.C.; Burghes, A.H.M.; Arnold, W.D. Follistatin-induced muscle hypertrophy in aged mice improves neuromuscular junction innervation and function. Neurobiol. Aging 2021, 104, 32–41. [Google Scholar] [CrossRef]
- Rose, F.F., Jr.; Mattis, V.B.; Rindt, H.; Lorson, C.L. Delivery of recombinant follistatin lessens disease severity in a mouse model of spinal muscular atrophy. Hum. Mol. Genet. 2009, 18, 997–1005. [Google Scholar] [CrossRef]
- Kawakami, S.; Fujii, Y.; Winters, S.J. Follistatin production by skin fibroblasts and its regulation by dexamethasone. Mol. Cell. Endocrinol. 2001, 172, 157–167. [Google Scholar] [CrossRef]
- Schneyer, A.L.; Hall, H.A.; Lambert-Messerlian, G.; Wang, Q.F.; Sluss, P.; Crowley, W.F., Jr. Follistatin-activin complexes in human serum and follicular fluid differ immunologically and biochemically. Endocrinology 1996, 137, 240–247. [Google Scholar] [CrossRef]
- Massagué, J.; Sheppard, D. TGF-β signaling in health and disease. Cell 2023, 186, 4007–4037. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J. TGFbeta in Cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Anumanthan, G.; Halder, S.K.; Osada, H.; Takahashi, T.; Massion, P.P.; Carbone, D.P.; Datta, P.K. Restoration of TGF-β signalling reduces tumorigenicity in human lung cancer cells. Br. J. Cancer 2005, 93, 1157–1167. [Google Scholar] [CrossRef]
- Ye, Z.; Kilic, G.; Dabelsteen, S.; Marinova, I.N.; Thøfner, J.F.B.; Song, M.; Rudjord-Levann, A.M.; Bagdonaite, I.; Vakhrushev, S.Y.; Brakebusch, C.H.; et al. Characterization of TGF-β signaling in a human organotypic skin model reveals that loss of TGF-βRII induces invasive tissue growth. Sci. Signal. 2022, 15, eabo2206. [Google Scholar] [CrossRef]
- Tan, X.; Tong, L.; Li, L.; Xu, J.; Xie, S.; Ji, L.; Fu, J.; Liu, Q.; Shen, S.; Liu, Y.; et al. Loss of Smad4 promotes aggressive lung cancer metastasis by de-repression of PAK3 via miRNA regulation. Nat. Commun. 2021, 12, 4853. [Google Scholar] [CrossRef]
- Ries, A.; Schelch, K.; Falch, D.; Pany, L.; Hoda, M.A.; Grusch, M. Activin A: An emerging target for improving cancer treatment? Expert Opin. Ther. Targets 2020, 24, 985–996. [Google Scholar] [CrossRef]
- Hulmi, J.J.; Nissinen, T.A.; Penna, F.; Bonetto, A. Targeting the Activin Receptor Signaling to Counteract the Multi-Systemic Complications of Cancer and Its Treatments. Cells 2021, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.J.; Cangemi, N.A.; Makker, V.; Cadoo, K.A.; Liu, J.F.; Rasco, D.W.; Navarro, W.H.; Haqq, C.M.; Hyman, D.M. First-in-Human Phase I Study of the Activin a Inhibitor, STM 434, in Patients with Granulosa Cell Ovarian Cancer and Other Advanced Solid Tumors. Clin. Cancer Res. 2019, 25, 5458–5465. [Google Scholar] [CrossRef]
- Tumminello, F.M.; Badalamenti, G.; Fulfaro, F.; Incorvaia, L.; Crescimanno, M.; Flandina, C.; Sepporta, M.V.; Leto, G. Serum follistatin in patients with prostate cancer metastatic to the bone. Clin. Exp. Metastasis 2010, 27, 549–555. [Google Scholar] [CrossRef]
- Tomoda, T.; Nouso, K.; Miyahara, K.; Kobayashi, S.; Kinugasa, H.; Toyosawa, J.; Hagihara, H.; Kuwaki, K.; Onishi, H.; Nakamura, S.; et al. Prognotic impact of serum follistatin in patients with hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2013, 28, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ruan, Y.; Xiao, J.; Chen, F.; Zhang, X. Association of serum follistatin levels with histological types and progression of tumor in human lung cancer. Cancer Cell Int. 2018, 18, 162. [Google Scholar] [CrossRef] [PubMed]
- Refaat, B.; Zekri, J.; Aslam, A.; Ahmad, J.; Baghdadi, M.A.; Meliti, A.; Idris, S.; Sultan, S.; Alardati, H.; Saimeh, H.A.; et al. Profiling Activins and Follistatin in Colorectal Cancer According to Clinical Stage, Tumour Sidedness and Smad4 Status. Pathol. Oncol. Res. 2021, 27, 1610032. [Google Scholar] [CrossRef] [PubMed]
- Stove, C.; Vanrobaeys, F.; Devreese, B.; Van Beeumen, J.; Mareel, M.; Bracke, M. Melanoma cells secrete follistatin, an antagonist of activin-mediated growth inhibition. Oncogene 2004, 23, 5330–5339. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Resaul, J.; Owen, S.; Ye, L.; Jiang, W.G. Clinical and Therapeutic Implications of Follistatin in Solid Tumours. Cancer Genom. Proteom. 2016, 13, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ren, P.; Feng, Y.; Liu, H.; Sun, Y.; Liu, Z.; Ge, J.; Cui, X. Follistatin is a novel biomarker for lung adenocarcinoma in humans. PLoS ONE 2014, 9, e111398. [Google Scholar] [CrossRef]
- Huang, J.J.; Blobe, G.C. Dichotomous roles of TGF-β in human cancer. Biochem. Soc. Trans. 2016, 44, 1441–1454. [Google Scholar] [CrossRef]
- Kahata, K.; Dadras, M.S.; Moustakas, A. TGF-β Family Signaling in Epithelial Differentiation and Epithelial-Mesenchymal Transition. Cold Spring Harb. Perspect. Biol. 2018, 10, a022194. [Google Scholar] [CrossRef]
- Jiang, M.; Ku, W.Y.; Zhou, Z.; Dellon, E.S.; Falk, G.W.; Nakagawa, H.; Wang, M.L.; Liu, K.; Wang, J.; Katzka, D.A.; et al. BMP-driven NRF2 activation in esophageal basal cell differentiation and eosinophilic esophagitis. J. Clin. Investig. 2015, 125, 1557–1568. [Google Scholar] [CrossRef]
- Cheng, J.B.; Sedgewick, A.J.; Finnegan, A.I.; Harirchian, P.; Lee, J.; Kwon, S.; Fassett, M.S.; Golovato, J.; Gray, M.; Ghadially, R.; et al. Transcriptional Programming of Normal and Inflamed Human Epidermis at Single-Cell Resolution. Cell Rep. 2018, 25, 871–883. [Google Scholar] [CrossRef]
- Finnegan, A.; Cho, R.J.; Luu, A.; Harirchian, P.; Lee, J.; Cheng, J.B.; Song, J.S. Single-Cell Transcriptomics Reveals Spatial and Temporal Turnover of Keratinocyte Differentiation Regulators. Front. Genet. 2019, 10, 775. [Google Scholar] [CrossRef]
- Beites, C.L.; Hollenbeck, P.L.; Kim, J.; Lovell-Badge, R.; Lander, A.D.; Calof, A.L. Follistatin modulates a BMP autoregulatory loop to control the size and patterning of sensory domains in the developing tongue. Development 2009, 136, 2187–2197. [Google Scholar] [CrossRef]
- Herrera, S.C.; Sainz de la Maza, D.; Grmai, L.; Margolis, S.; Plessel, R.; Burel, M.; O’Connor, M.; Amoyel, M.; Bach, E.A. Proliferative stem cells maintain quiescence of their niche by secreting the Activin inhibitor Follistatin. Dev. Cell 2021, 56, 2284–2294.e2286. [Google Scholar] [CrossRef] [PubMed]
- Gaviño, M.A.; Wenemoser, D.; Wang, I.E.; Reddien, P.W. Tissue absence initiates regeneration through Follistatin-mediated inhibition of Activin signaling. eLife 2013, 2, e00247. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Morgan, C.; Goff, L.A.; Doetzlhofer, A. Follistatin promotes LIN28B-mediated supporting cell reprogramming and hair cell regeneration in the murine cochlea. Sci. Adv. 2022, 8, eabj7651. [Google Scholar] [CrossRef] [PubMed]
- Lepletier, A.; Hun, M.L.; Hammett, M.V.; Wong, K.; Naeem, H.; Hedger, M.; Loveland, K.; Chidgey, A.P. Interplay between Follistatin, Activin A, and BMP4 Signaling Regulates Postnatal Thymic Epithelial Progenitor Cell Differentiation during Aging. Cell Rep. 2019, 27, 3887–3901.e3884. [Google Scholar] [CrossRef] [PubMed]
- Forrester, H.B.; Ivashkevich, A.; McKay, M.J.; Leong, T.; de Kretser, D.M.; Sprung, C.N. Follistatin is induced by ionizing radiation and potentially predictive of radiosensitivity in radiation-induced fibrosis patient derived fibroblasts. PLoS ONE 2013, 8, e77119. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ran, P.; Wang, J. mRNA expression of Follistatin and follistatin-like 3, bone morphogenetic protein-4 antagonists in lung tissues of hypoxic mice. Chin. J. Tuberc. Respir. Dis. 2015, 38, 119–121. [Google Scholar]
- Mehta, N.; Gava, A.L.; Zhang, D.; Gao, B.; Krepinsky, J.C. Follistatin Protects Against Glomerular Mesangial Cell Apoptosis and Oxidative Stress to Ameliorate Chronic Kidney Disease. Antioxid. Redox Signal. 2019, 31, 551–571. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, K.; Zhao, C.; Zhou, L.; Cheng, J.; Wang, D.W.; Zhao, C. Follistatin Attenuates Myocardial Fibrosis in Diabetic Cardiomyopathy via the TGF-β–Smad3 Pathway. Front. Pharmacol. 2021, 12, 683335. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Liu, S.F. Lentivirus-Mediated Short Hairpin RNA for Follistatin Downregulation Suppresses Tumor Progression in Hypopharyngeal Carcinoma. Curr. Med. Sci. 2022, 42, 832–840. [Google Scholar] [CrossRef]
- Iyer, S.; Zhang, S.; Yucel, S.; Horn, H.; Smith, S.G.; Reinhardt, F.; Hoefsmit, E.; Assatova, B.; Casado, J.; Meinsohn, M.C.; et al. Genetically Defined Syngeneic Mouse Models of Ovarian Cancer as Tools for the Discovery of Combination Immunotherapy. Cancer Discov. 2021, 11, 384–407. [Google Scholar] [CrossRef]
- Karve, T.M.; Preet, A.; Sneed, R.; Salamanca, C.; Li, X.; Xu, J.; Kumar, D.; Rosen, E.M.; Saha, T. BRCA1 regulates follistatin function in ovarian cancer and human ovarian surface epithelial cells. PLoS ONE 2012, 7, e37697. [Google Scholar] [CrossRef]
- Yu, M.; Xiao, L.; Chen, Y.; Wang, H.; Gao, Y.; Wang, A. Identification of a potential target for treatment of squamous cell carcinoma of the tongue: Follistatin. Br. J. Oral. Maxillofac. Surg. 2020, 58, 437–442. [Google Scholar] [CrossRef]
- Krneta, J.; Kroll, J.; Alves, F.; Prahst, C.; Sananbenesi, F.; Dullin, C.; Kimmina, S.; Phillips, D.J.; Augustin, H.G. Dissociation of angiogenesis and tumorigenesis in follistatin- and activin-expressing tumors. Cancer Res. 2006, 66, 5686–5695. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.J.; Mellor, S.L.; Wang, H.; Evans, L.W.; Groome, N.P.; Risbridger, G.P. Expression of activin A and follistatin core proteins by human prostate tumor cell lines. Endocrinology 1999, 140, 5303–5309. [Google Scholar] [CrossRef]
- Mange, A.; Dimitrakopoulos, L.; Soosaipillai, A.; Coopman, P.; Diamandis, E.P.; Solassol, J. An integrated cell line-based discovery strategy identified follistatin and kallikrein 6 as serum biomarker candidates of breast carcinoma. J. Proteom. 2016, 142, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Seachrist, D.D.; Sizemore, S.T.; Johnson, E.; Abdul-Karim, F.W.; Bonk, K.L.W.; Keri, R.A. Follistatin is a metastasis suppressor in a mouse model of HER2-positive breast cancer. Breast Cancer Res. 2017, 19, 66. [Google Scholar] [CrossRef]
- Liu, S.; Liu, B.; Zhao, Q.; Shi, J.; Gu, Y.; Guo, Y.; Li, Y.; Liu, Y.; Cheng, Y.; Qiao, Y.; et al. Down-regulated FST expression is involved in the poor prognosis of triple-negative breast cancer. Cancer Cell Int. 2021, 21, 267. [Google Scholar] [CrossRef] [PubMed]
- Zabkiewicz, C.; Resaul, J.; Hargest, R.; Jiang, W.G.; Ye, L. Increased Expression of Follistatin in Breast Cancer Reduces Invasiveness and Clinically Correlates with Better Survival. Cancer Genom. Proteom. 2017, 14, 241–251. [Google Scholar] [CrossRef]
- Klingberg, F.; Hinz, B.; White, E.S. The myofibroblast matrix: Implications for tissue repair and fibrosis. J. Pathol. 2013, 229, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, B.; Webb, D.J. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem. Soc. Trans. 2017, 45, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Belhabib, I.; Zaghdoudi, S.; Lac, C.; Bousquet, C.; Jean, C. Extracellular Matrices and Cancer-Associated Fibroblasts: Targets for Cancer Diagnosis and Therapy? Cancers 2021, 13, 3466. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Xia, X.; Huang, L.-B.; An, H.; Cao, M.; Kim, G.D.; Chen, H.-N.; Zhang, W.-H.; Shu, Y.; Kong, X.; et al. Pan-cancer single-cell analysis reveals the heterogeneity and plasticity of cancer-associated fibroblasts in the tumor microenvironment. Nat. Commun. 2022, 13, 6619. [Google Scholar] [CrossRef] [PubMed]
- Cords, L.; Tietscher, S.; Anzeneder, T.; Langwieder, C.; Rees, M.; de Souza, N.; Bodenmiller, B. Cancer-associated fibroblast classification in single-cell and spatial proteomics data. Nat. Commun. 2023, 14, 4294. [Google Scholar] [CrossRef]
- Wu, Y.; Clark, K.C.; Niranjan, B.; Chüeh, A.C.; Horvath, L.G.; Taylor, R.A.; Daly, R.J. Integrative characterisation of secreted factors involved in intercellular communication between prostate epithelial or cancer cells and fibroblasts. Mol. Oncol. 2023, 17, 469–486. [Google Scholar] [CrossRef]
- Hu, H.; Piotrowska, Z.; Hare, P.J.; Chen, H.; Mulvey, H.E.; Mayfield, A.; Noeen, S.; Kattermann, K.; Greenberg, M.; Williams, A.; et al. Three subtypes of lung cancer fibroblasts define distinct therapeutic paradigms. Cancer Cell 2021, 39, 1531–1547.e1510. [Google Scholar] [CrossRef]
- Le Bras, G.F.; Loomans, H.A.; Taylor, C.J.; Revetta, F.L.; Andl, C.D. Activin A balance regulates epithelial invasiveness and tumorigenesis. Lab. Investig. 2014, 94, 1134–1146. [Google Scholar] [CrossRef]
- Tomita, M.; Matsuzaki, Y.; Edagawa, M.; Maeda, M.; Shimizu, T.; Hara, M.; Onitsuka, T. Correlation between tumor angiogenesis and invasiveness in thymic epithelial tumors. J. Thorac. Cardiovasc. Surg. 2002, 124, 493–498. [Google Scholar] [CrossRef]
- Maeshima, A.; Zhang, Y.-Q.; Furukawa, M.; Naruse, T.; Kojima, I. Hepatocyte growth factor induces branching tubulogenesis in MDCK cells by modulating the activin-follistatin system. Kidney Int. 2000, 58, 1511–1522. [Google Scholar] [CrossRef]
- Owusu, B.Y.; Galemmo, R.; Janetka, J.; Klampfer, L. Hepatocyte Growth Factor, a Key Tumor-Promoting Factor in the Tumor Microenvironment. Cancers 2017, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Kozian, D.H.; Ziche, M.; Augustin, H.G. The activin-binding protein follistatin regulates autocrine endothelial cell activity and induces angiogenesis. Lab. Investig. 1997, 76, 267–276. [Google Scholar] [PubMed]
- Klagsbrun, M.; Moses, M.A. Molecular angiogenesis. Chem. Biol. 1999, 6, R217–R224. [Google Scholar] [CrossRef] [PubMed]
- Fahmy-Garcia, S.; Farrell, E.; Witte-Bouma, J.; Robbesom-van den Berge, I.; Suarez, M.; Mumcuoglu, D.; Walles, H.; Kluijtmans, S.; van der Eerden, B.C.J.; van Osch, G.; et al. Follistatin Effects in Migration, Vascularization, and Osteogenesis in vitro and Bone Repair in vivo. Front. Bioeng. Biotechnol. 2019, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Ping, Q.; Yan, R.; Cheng, X.; Wang, W.; Zhong, Y.; Hou, Z.; Shi, Y.; Wang, C.; Li, R. Cancer-associated fibroblasts: Overview, progress, challenges, and directions. Cancer Gene Ther. 2021, 28, 984–999. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Zhou, X.; Yang, J.; Shi, H.; Li, H.; Zhao, X.; Ma, X. The Role of Tumor-Stroma Interactions in Drug Resistance Within Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 637675. [Google Scholar] [CrossRef] [PubMed]
- Dhungel, N.; Youngblood, R.; Chu, M.; Carroll, J.; Dragoi, A.M. Assessing the epithelial-to-mesenchymal plasticity in a small cell lung carcinoma (SCLC) and lung fibroblasts co-culture model. Front. Mol. Biosci. 2023, 10, 1096326. [Google Scholar] [CrossRef]
- Wu, L.; Ke, L.; Zhang, Z.; Yu, J.; Meng, X. Development of EGFR TKIs and Options to Manage Resistance of Third-Generation EGFR TKI Osimertinib: Conventional Ways and Immune Checkpoint Inhibitors. Front. Oncol. 2020, 10, 602762. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M.; Atagi, S.; Goto, K.; Hosomi, Y.; Seto, T.; Hida, T.; Nakagawa, K.; Yoshioka, H.; Nogami, N.; Maemondo, M.; et al. Biomarker analysis of the phase II JO25567 study comparing erlotinib with or without bevacizumab in first-line advanced EGFR(+)non-small-cell lung cancer. Transl. Lung Cancer Res. 2023, 12, 1167–1184. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Fan, J.; He, Y.; Xiong, A.; Yu, J.; Li, Y.; Zhang, Y.; Zhao, W.; Zhou, F.; Li, W.; et al. Single-cell profiling of tumor heterogeneity and the microenvironment in advanced non-small cell lung cancer. Nat. Commun. 2021, 12, 2540. [Google Scholar] [CrossRef]
- Marini, K.D.; Croucher, D.R.; McCloy, R.A.; Vaghjiani, V.; Gonzalez-Rajal, A.; Hastings, J.F.; Chin, V.; Szczepny, A.; Kostyrko, K.; Marquez, C.; et al. Inhibition of activin signaling in lung adenocarcinoma increases the therapeutic index of platinum chemotherapy. Sci. Transl. Med. 2018, 10, eaat3504. [Google Scholar] [CrossRef] [PubMed]
- Wankell, M.; Kaesler, S.; Zhang, Y.Q.; Florence, C.; Werner, S.; Duan, R. The activin binding proteins follistatin and follistatin-related protein are differentially regulated in vitro and during cutaneous wound repair. J. Endocrinol. 2001, 171, 385–395. [Google Scholar] [CrossRef]
- Hardy, C.L.; Nguyen, H.A.; Mohamud, R.; Yao, J.; Oh, D.Y.; Plebanski, M.; Loveland, K.L.; Harrison, C.A.; Rolland, J.M.; O’Hehir, R.E. The activin A antagonist follistatin inhibits asthmatic airway remodelling. Thorax 2013, 68, 9–18. [Google Scholar] [CrossRef]
- Hardy, C.L.; King, S.J.; Mifsud, N.A.; Hedger, M.P.; Phillips, D.J.; Mackay, F.; de Kretser, D.M.; Wilson, J.W.; Rolland, J.M.; O’Hehir, R.E. The activin A antagonist follistatin inhibits cystic fibrosis-like lung inflammation and pathology. Immunol. Cell Biol. 2015, 93, 567–574. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, S.; Guo, Z.; Bi, Y.; Zhou, M.; Li, P.; Seyedsadr, M.; Xu, X.; Li, J.L.; Markovic-Plese, S.; et al. The TGF-β superfamily cytokine Activin-A is induced during autoimmune neuroinflammation and drives pathogenic Th17 cell differentiation. Immunity 2021, 54, 308–323.e306. [Google Scholar] [CrossRef]
- Rautela, J.; Dagley, L.F.; de Oliveira, C.C.; Schuster, I.S.; Hediyeh-Zadeh, S.; Delconte, R.B.; Cursons, J.; Hennessy, R.; Hutchinson, D.S.; Harrison, C.; et al. Therapeutic blockade of activin-A improves NK cell function and antitumor immunity. Sci. Signal. 2019, 12, eaat7527. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.J.; Panesso-Gomez, S.; Shah, J.S.; Ebai, T.; Jiang, Q.; Gumusoglu-Acar, E.; Bello, M.G.; Vlad, A.; Modugno, F.; Edwards, R.P.; et al. Quiescent ovarian cancer cells secrete follistatin to induce chemotherapy resistance in surrounding cells in response to chemotherapy. Clin. Cancer Res. 2023, 29, 1969–1983. [Google Scholar] [CrossRef] [PubMed]
- Steg, A.D.; Bevis, K.S.; Katre, A.A.; Ziebarth, A.; Dobbin, Z.C.; Alvarez, R.D.; Zhang, K.; Conner, M.; Landen, C.N. Stem Cell Pathways Contribute to Clinical Chemoresistance in Ovarian Cancer. Clin. Cancer Res. 2012, 18, 869–881. [Google Scholar] [CrossRef]
- Orr, B.; Mahdi, H.; Fang, Y.; Strange, M.; Uygun, I.; Rana, M.; Zhang, L.; Suarez Mora, A.; Pusateri, A.; Elishaev, E.; et al. Phase I Trial Combining Chemokine-Targeting with Loco-Regional Chemoimmunotherapy for Recurrent, Platinum-Sensitive Ovarian Cancer Shows Induction of CXCR3 Ligands and Markers of Type 1 Immunity. Clin. Cancer Res. 2022, 28, 2038–2049. [Google Scholar] [CrossRef]
- Liu, J.; Lichtenberg, T.; Hoadley, K.A.; Poisson, L.M.; Lazar, A.J.; Cherniack, A.D.; Kovatich, A.J.; Benz, C.C.; Levine, D.A.; Lee, A.V.; et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 2018, 173, 400–416.e411. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, B.S.; Jang, J.Y.; Lee, Y.S.; Kim, H.J.; Roh, J.; Shin, Y.S.; Woo, H.G.; Kim, C.H. Single-cell transcriptome profiling of the stepwise progression of head and neck cancer. Nat. Commun. 2023, 14, 1055. [Google Scholar] [CrossRef]
- Oyelakin, A.; Sosa, J.; Nayak, K.B.; Glathar, A.; Gluck, C.; Sethi, I.; Tsompana, M.; Nowak, N.; Buck, M.; Romano, R.-A.; et al. An integrated genomic approach identifies follistatin as a target of the p63-epidermal growth factor receptor oncogenic network in head and neck squamous cell carcinoma. NAR Cancer 2023, 5, zcad038. [Google Scholar] [CrossRef]
- Arora, R.; Cao, C.; Kumar, M.; Sinha, S.; Chanda, A.; McNeil, R.; Samuel, D.; Arora, R.K.; Matthews, T.W.; Chandarana, S.; et al. Spatial transcriptomics reveals distinct and conserved tumor core and edge architectures that predict survival and targeted therapy response. Nat. Commun. 2023, 14, 5029. [Google Scholar] [CrossRef] [PubMed]
- Puram, S.V.; Tirosh, I.; Parikh, A.S.; Patel, A.P.; Yizhak, K.; Gillespie, S.; Rodman, C.; Luo, C.L.; Mroz, E.A.; Emerick, K.S.; et al. Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell 2017, 171, 1611–1624.e1624. [Google Scholar] [CrossRef]
- Lawson, D.A.; Bhakta, N.R.; Kessenbrock, K.; Prummel, K.D.; Yu, Y.; Takai, K.; Zhou, A.; Eyob, H.; Balakrishnan, S.; Wang, C.Y.; et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature 2015, 526, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Yerly, L.; Pich-Bavastro, C.; Di Domizio, J.; Wyss, T.; Tissot-Renaud, S.; Cangkrama, M.; Gilliet, M.; Werner, S.; Kuonen, F. Integrated multi-omics reveals cellular and molecular interactions governing the invasive niche of basal cell carcinoma. Nat. Commun. 2022, 13, 4897. [Google Scholar] [CrossRef] [PubMed]
- Ji, A.L.; Rubin, A.J.; Thrane, K.; Jiang, S.; Reynolds, D.L.; Meyers, R.M.; Guo, M.G.; George, B.M.; Mollbrink, A.; Bergenstråhle, J.; et al. Multimodal Analysis of Composition and Spatial Architecture in Human Squamous Cell Carcinoma. Cell 2020, 182, 497–514.e422. [Google Scholar] [CrossRef]
- Du, B.; Shim, J.S. Targeting Epithelial-Mesenchymal Transition (EMT) to Overcome Drug Resistance in Cancer. Molecules 2016, 21, 965. [Google Scholar] [CrossRef]
- Teixeira, A.F.; ten Dijke, P.; Zhu, H.-J. On-Target Anti-TGF-β Therapies Are Not Succeeding in Clinical Cancer Treatments: What Are Remaining Challenges? Front. Cell Dev. Biol. 2020, 8, 605. [Google Scholar] [CrossRef]
- Cook, D.P.; Vanderhyden, B.C. Context specificity of the EMT transcriptional response. Nat. Commun. 2020, 11, 2142. [Google Scholar] [CrossRef]
- McDermott, S.C.; Rodriguez-Ramirez, C.; McDermott, S.P.; Wicha, M.S.; Nör, J.E. FGFR signaling regulates resistance of head and neck cancer stem cells to cisplatin. Oncotarget 2018, 9, 25148–25165. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.P.; Waldron, L.; Perez-Ordonez, B.; Pintilie, M.; Galloni, N.N.; Xuan, Y.; Cervigne, N.K.; Warner, G.C.; Makitie, A.A.; Simpson, C.; et al. A gene signature in histologically normal surgical margins is predictive of oral carcinoma recurrence. BMC Cancer 2011, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Méndez, E.; Houck, J.; Fan, W.; Lohavanichbutr, P.; Doody, D.; Yueh, B.; Futran, N.D.; Upton, M.; Farwell, D.G.; et al. Gene expression profiling identifies genes predictive of oral squamous cell carcinoma. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2152–2162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, K.; Han, B.; Xu, Z.; Gao, X. The emerging role of follistatin under stresses and its implications in diseases. Gene 2018, 639, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Blount, A.L.; Schmidt, K.; Justice, N.J.; Vale, W.W.; Fischer, W.H.; Bilezikjian, L.M. FoxL2 and Smad3 coordinately regulate follistatin gene transcription. J. Biol. Chem. 2009, 284, 7631–7645. [Google Scholar] [CrossRef] [PubMed]
- Barany, N.; Rozsas, A.; Megyesfalvi, Z.; Grusch, M.; Hegedus, B.; Lang, C.; Boettiger, K.; Schwendenwein, A.; Tisza, A.; Renyi-Vamos, F.; et al. Clinical relevance of circulating activin A and follistatin in small cell lung cancer. Lung Cancer 2021, 161, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.P.; Kao, H.K.; Liang, Y.; Cheng, M.H.; Chang, Y.L.; Liu, S.C.; Lin, Y.C.; Ko, T.Y.; Lee, Y.S.; Tsai, C.L.; et al. Overexpression of activin A in oral squamous cell carcinoma: Association with poor prognosis and tumor progression. Ann. Surg. Oncol. 2010, 17, 1945–1956. [Google Scholar] [CrossRef]
- Memon, D.; Gill, M.B.; Papachristou, E.K.; Ochoa, D.; D’Santos, C.S.; Miller, M.L.; Beltrao, P. Copy number aberrations drive kinase rewiring, leading to genetic vulnerabilities in cancer. Cell Rep. 2021, 35, 109155. [Google Scholar] [CrossRef]
- Zhou, R.W.; Parsons, R.E. Etiology of super-enhancer reprogramming and activation in cancer. Epigenetics Chromatin 2023, 16, 29. [Google Scholar] [CrossRef]
- Cheng, Y.; He, C.; Wang, M.; Ma, X.; Mo, F.; Yang, S.; Han, J.; Wei, X. Targeting epigenetic regulators for cancer therapy: Mechanisms and advances in clinical trials. Signal Transduct. Target. Ther. 2019, 4, 62. [Google Scholar] [CrossRef]
- Chen, N.; Golczer, G.; Ghose, S.; Lin, B.; Langenbucher, A.; Webb, J.; Bhanot, H.; Abt, N.B.; Lin, D.; Varvares, M.; et al. YAP1 maintains active chromatin state in head and neck squamous cell carcinomas that promotes tumorigenesis through cooperation with BRD4. Cell Rep. 2022, 39, 110970. [Google Scholar] [CrossRef]
- Ning, B.; Tilston-Lunel, A.M.; Simonetti, J.; Hicks-Berthet, J.; Matschulat, A.; Pfefferkorn, R.; Spira, A.; Edwards, M.; Mazzilli, S.; Lenburg, M.E.; et al. Convergence of YAP/TAZ, TEAD and TP63 activity is associated with bronchial premalignant severity and progression. J. Exp. Clin. Cancer Res. 2023, 42, 116. [Google Scholar] [CrossRef]
- Bartholin, L.; Maguer-Satta, V.; Hayette, S.; Martel, S.; Gadoux, M.; Corbo, L.; Magaud, J.P.; Rimokh, R. Transcription activation of FLRG and follistatin by activin A, through Smad proteins, participates in a negative feedback loop to modulate activin A function. Oncogene 2002, 21, 2227–2235. [Google Scholar] [CrossRef]
- Matzuk, M.M.; Lu, N.; Vogel, H.; Sellheyer, K.; Roop, D.R.; Bradley, A. Multiple defects and perinatal death in mice deficient in follistatin. Nature 1995, 374, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.C.; Lidral, A.C.; McCoy, J.C.; Liu, H.; Cox, L.L.; Zhu, Y.; Anderson, R.D.; Moreno Uribe, L.M.; Anand, D.; Deng, M.; et al. Mutations in GDF11 and the extracellular antagonist, Follistatin, as a likely cause of Mendelian forms of orofacial clefting in humans. Hum. Mutat. 2019, 40, 1813–1825. [Google Scholar] [CrossRef] [PubMed]
- Grusch, M.; Drucker, C.; Peter-Vörösmarty, B.; Erlach, N.; Loser, A.; Parzefall, W.; Berger, W.; Grasl-Kraupp, B.; Schulte-Hermann, R. Overexpression of follistatin and follistatin-like 3 may be involved in hepatocarcinogenesis. Cancer Res. 2006, 66, 718–719. [Google Scholar]
- Prajapati-DiNubila, M.; Benito-Gonzalez, A.; Golden, E.J.; Zhang, S.; Doetzlhofer, A. A counter gradient of Activin A and follistatin instructs the timing of hair cell differentiation in the murine cochlea. eLife 2019, 8, e47613. [Google Scholar] [CrossRef]
- Tao, R.; Wang, C.; Stöhr, O.; Qiu, W.; Hu, Y.; Miao, J.; Dong, X.C.; Leng, S.; Stefater, M.; Stylopoulos, N.; et al. Inactivating hepatic follistatin alleviates hyperglycemia. Nat. Med. 2018, 24, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Florio, P.; Reis, F.M.; Torres, P.B.; Calonaci, F.; Abrao, M.S.; Nascimento, L.L.; Franchini, M.; Cianferoni, L.; Petraglia, F. High serum follistatin levels in women with ovarian endometriosis. Hum. Reprod. 2009, 24, 2600–2606. [Google Scholar] [CrossRef] [PubMed]
- Panagiotou, G.; Ghaly, W.; Upadhyay, J.; Pazaitou-Panayiotou, K.; Mantzoros, C.S. Serum Follistatin Is Increased in Thyroid Cancer and Is Associated With Adverse Tumor Characteristics in Humans. J. Clin. Endocrinol. Metab. 2021, 106, e2137–e2150. [Google Scholar] [CrossRef] [PubMed]
- Colussi, C.; Gaetano, C.; Capogrossi, M.C. AAV-dependent targeting of myostatin function: Follistatin strikes back at muscular dystrophy. Gene Ther. 2008, 15, 1075–1076. [Google Scholar] [CrossRef]
- Mendell, J.R.; Sahenk, Z.; Malik, V.; Gomez, A.M.; Flanigan, K.M.; Lowes, L.P.; Alfano, L.N.; Berry, K.; Meadows, E.; Lewis, S.; et al. A phase 1/2a follistatin gene therapy trial for becker muscular dystrophy. Mol. Ther. 2015, 23, 192–201. [Google Scholar] [CrossRef]
- Ozawa, T.; Morikawa, M.; Morishita, Y.; Ogikubo, K.; Itoh, F.; Koinuma, D.; Nygren, P.-Å.; Miyazono, K. Systemic administration of monovalent follistatin-like 3-Fc-fusion protein increases muscle mass in mice. iScience 2021, 24, 102488. [Google Scholar] [CrossRef]
- Schumann, C.; Nguyen, D.X.; Norgard, M.; Bortnyak, Y.; Korzun, T.; Chan, S.; Lorenz, A.S.; Moses, A.S.; Albarqi, H.A.; Wong, L.; et al. Increasing lean muscle mass in mice via nanoparticle-mediated hepatic delivery of follistatin mRNA. Theranostics 2018, 8, 5276–5288. [Google Scholar] [CrossRef]
- Korzun, T.; Moses, A.S.; Kim, J.; Patel, S.; Schumann, C.; Levasseur, P.R.; Diba, P.; Olson, B.; Rebola, K.G.O.; Norgard, M.; et al. Nanoparticle-Based Follistatin Messenger RNA Therapy for Reprogramming Metastatic Ovarian Cancer and Ameliorating Cancer-Associated Cachexia. Small 2022, 18, e2204436. [Google Scholar] [CrossRef]
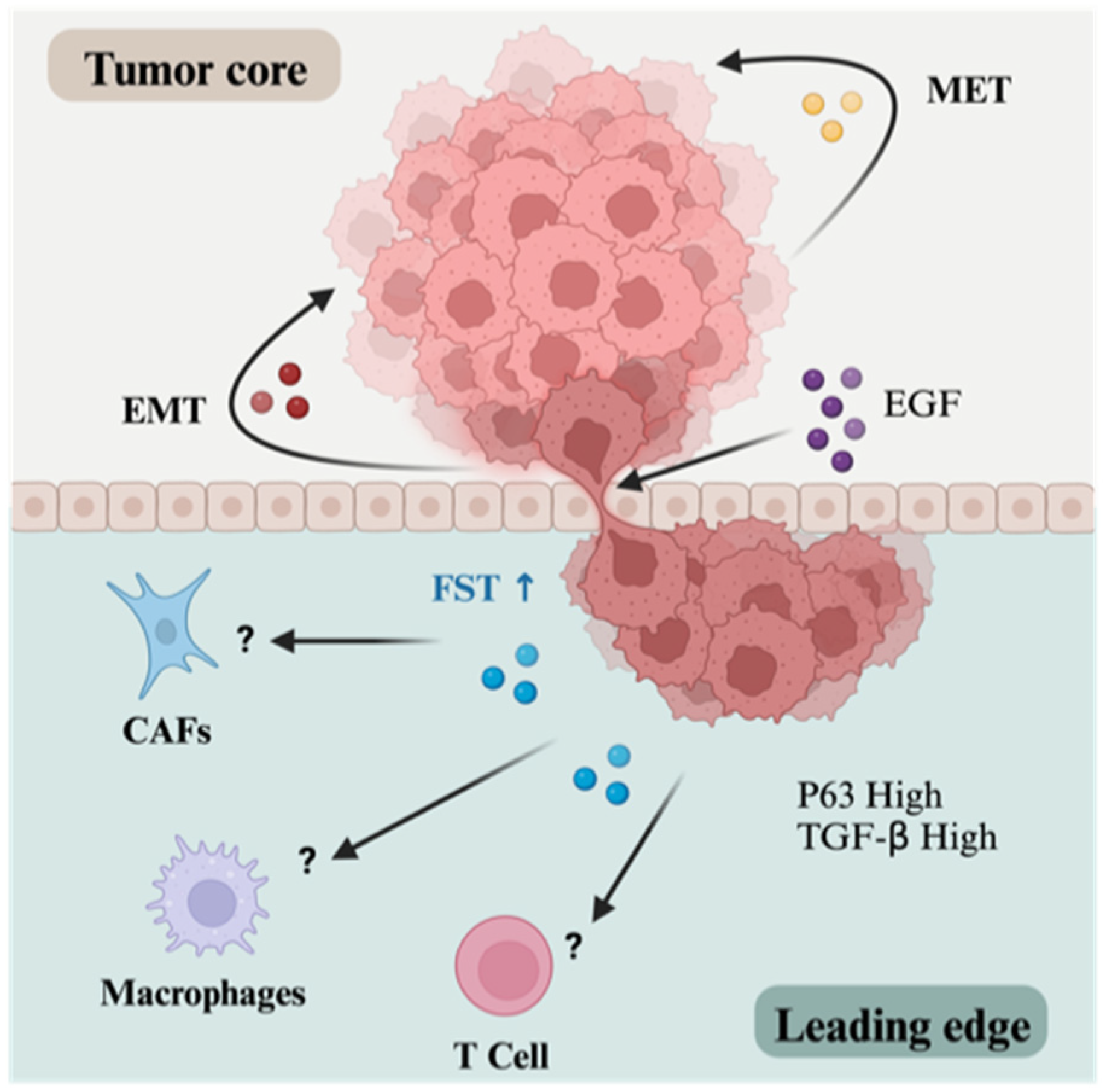
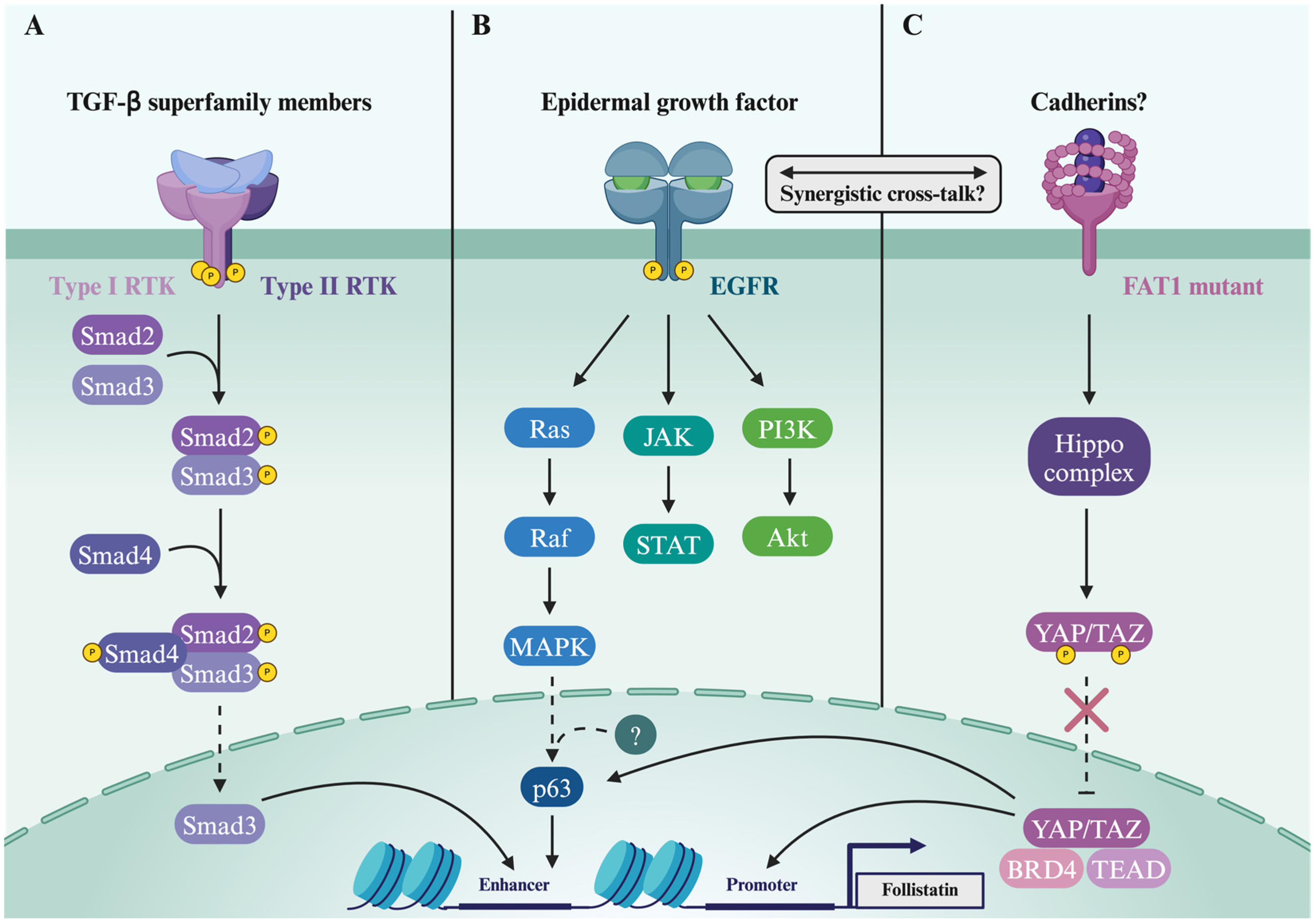
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sosa, J.; Oyelakin, A.; Sinha, S. The Reign of Follistatin in Tumors and Their Microenvironment: Implications for Drug Resistance. Biology 2024, 13, 130. https://doi.org/10.3390/biology13020130
Sosa J, Oyelakin A, Sinha S. The Reign of Follistatin in Tumors and Their Microenvironment: Implications for Drug Resistance. Biology. 2024; 13(2):130. https://doi.org/10.3390/biology13020130
Chicago/Turabian StyleSosa, Jennifer, Akinsola Oyelakin, and Satrajit Sinha. 2024. "The Reign of Follistatin in Tumors and Their Microenvironment: Implications for Drug Resistance" Biology 13, no. 2: 130. https://doi.org/10.3390/biology13020130
APA StyleSosa, J., Oyelakin, A., & Sinha, S. (2024). The Reign of Follistatin in Tumors and Their Microenvironment: Implications for Drug Resistance. Biology, 13(2), 130. https://doi.org/10.3390/biology13020130




