Levodopa Impairs Lysosomal Function in Sensory Neurons In Vitro
Simple Summary
Abstract
1. Introduction
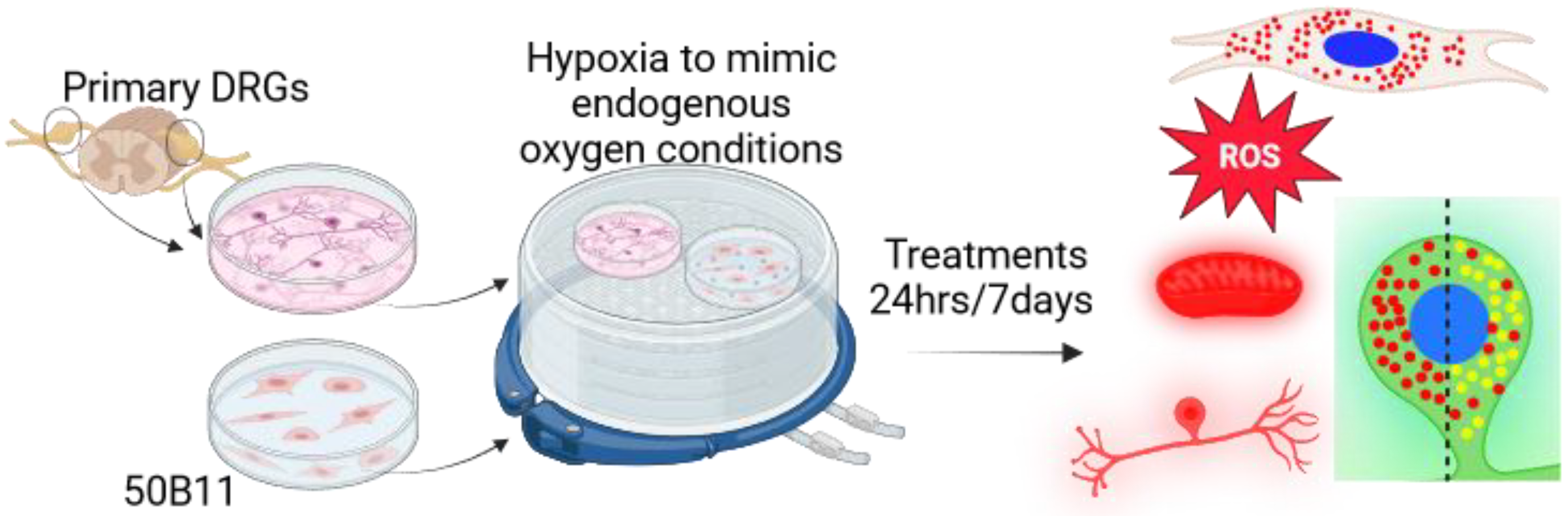
2. Materials and Methods
2.1. Dorsal Root Ganglia
2.1.1. Dorsal Root Ganglia Preparation
2.1.2. Dorsal Root Ganglia Treatments
2.1.3. Measurement of Mitochondrial Membrane Potential
2.1.4. Oxidative Stress Assay
2.1.5. Immunostaining
2.1.6. Lysosome Content and Acidity Measurements
2.2. 50B11 Cells
2.2.1. Culturing
2.2.2. 50B11 Treatments
2.2.3. Lysosome Analysis
2.3. Statistics
3. Results
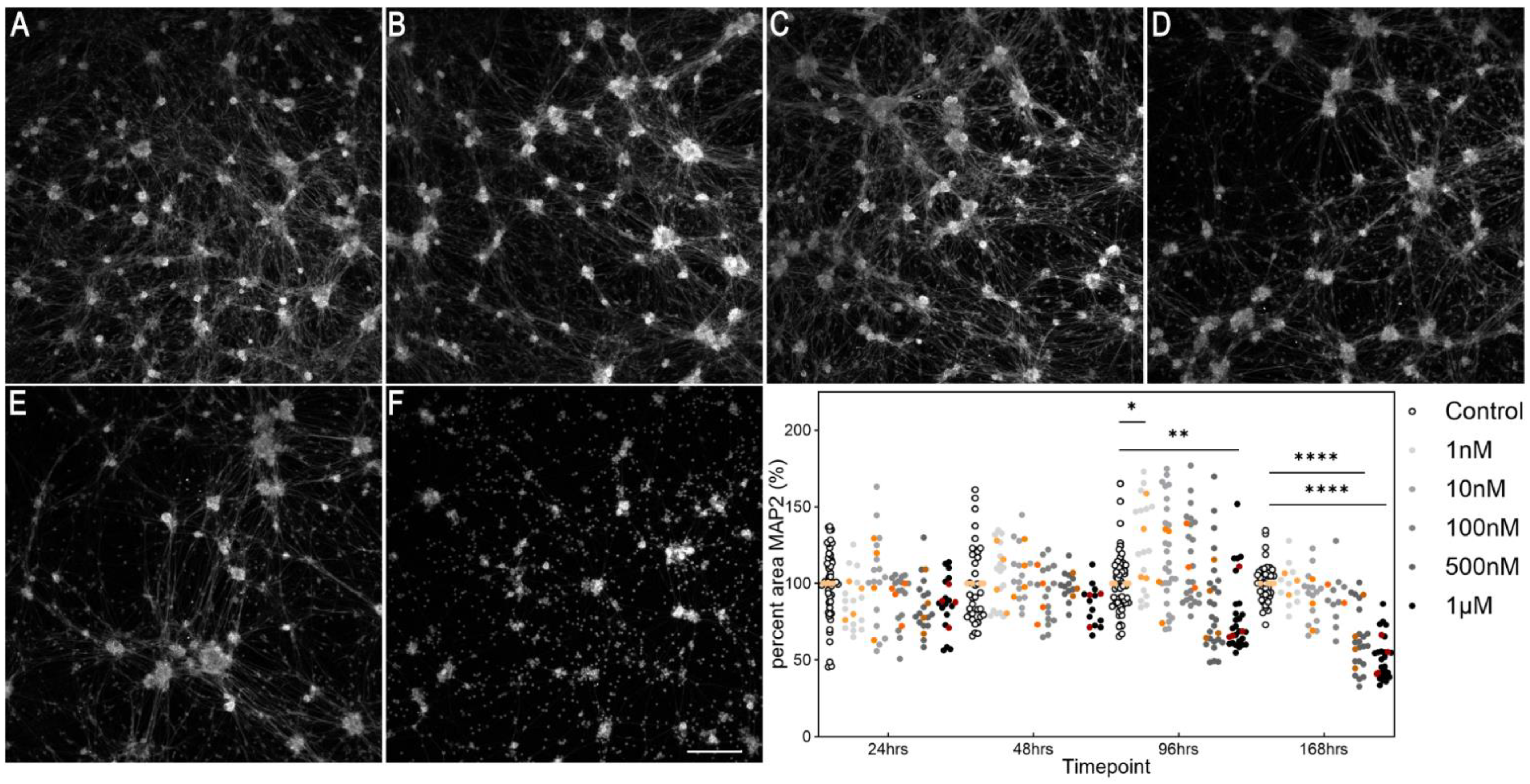
3.1. Establishing a Parkinsonian Primary Sensory Neuronal Cell Model
3.2. Effect of Levodopa on Sensory Neurons Alone and in the Context of Parkinsonism
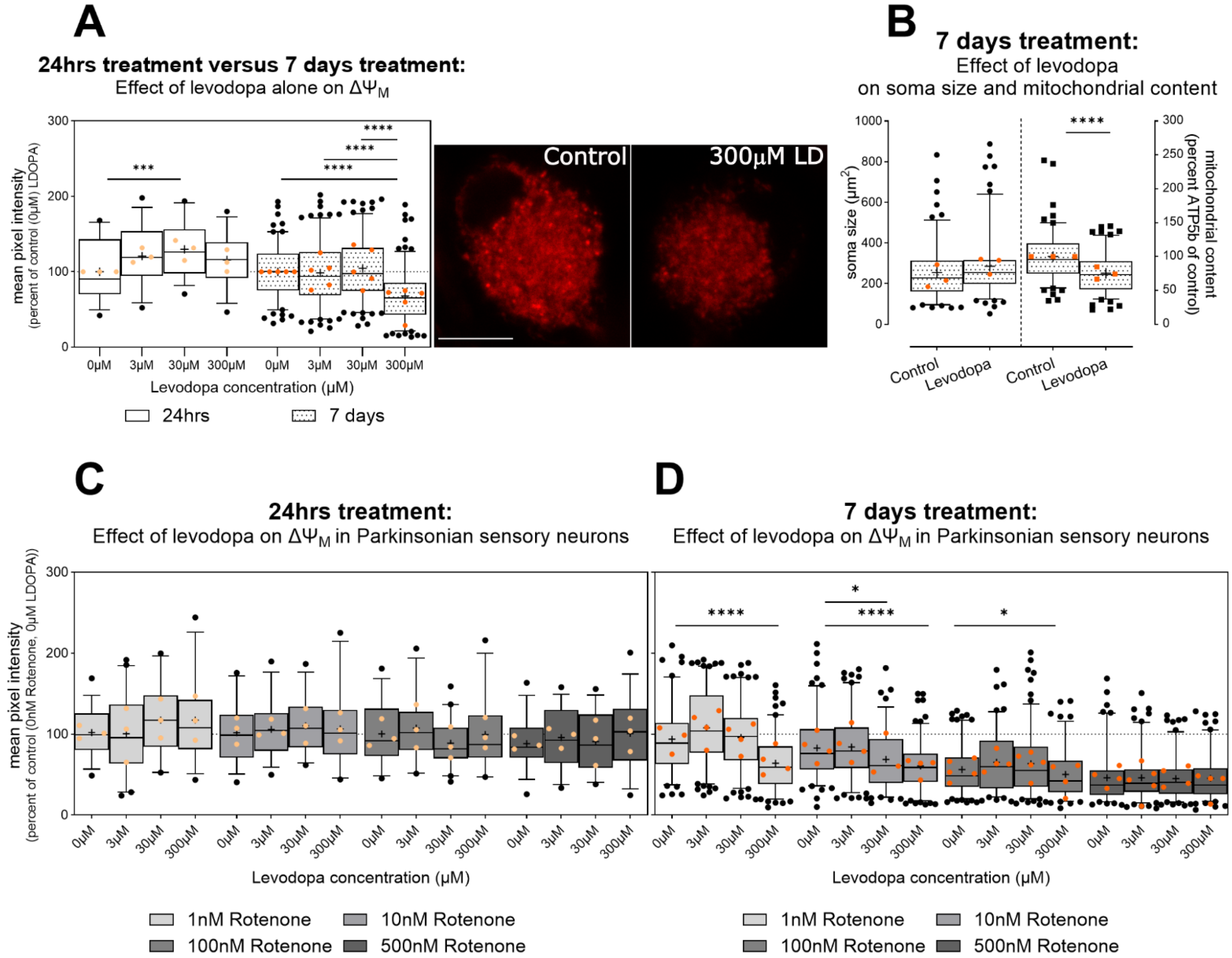
3.2.1. Levodopa Exacerbates Mitochondrial Impairment in Parkinsonism
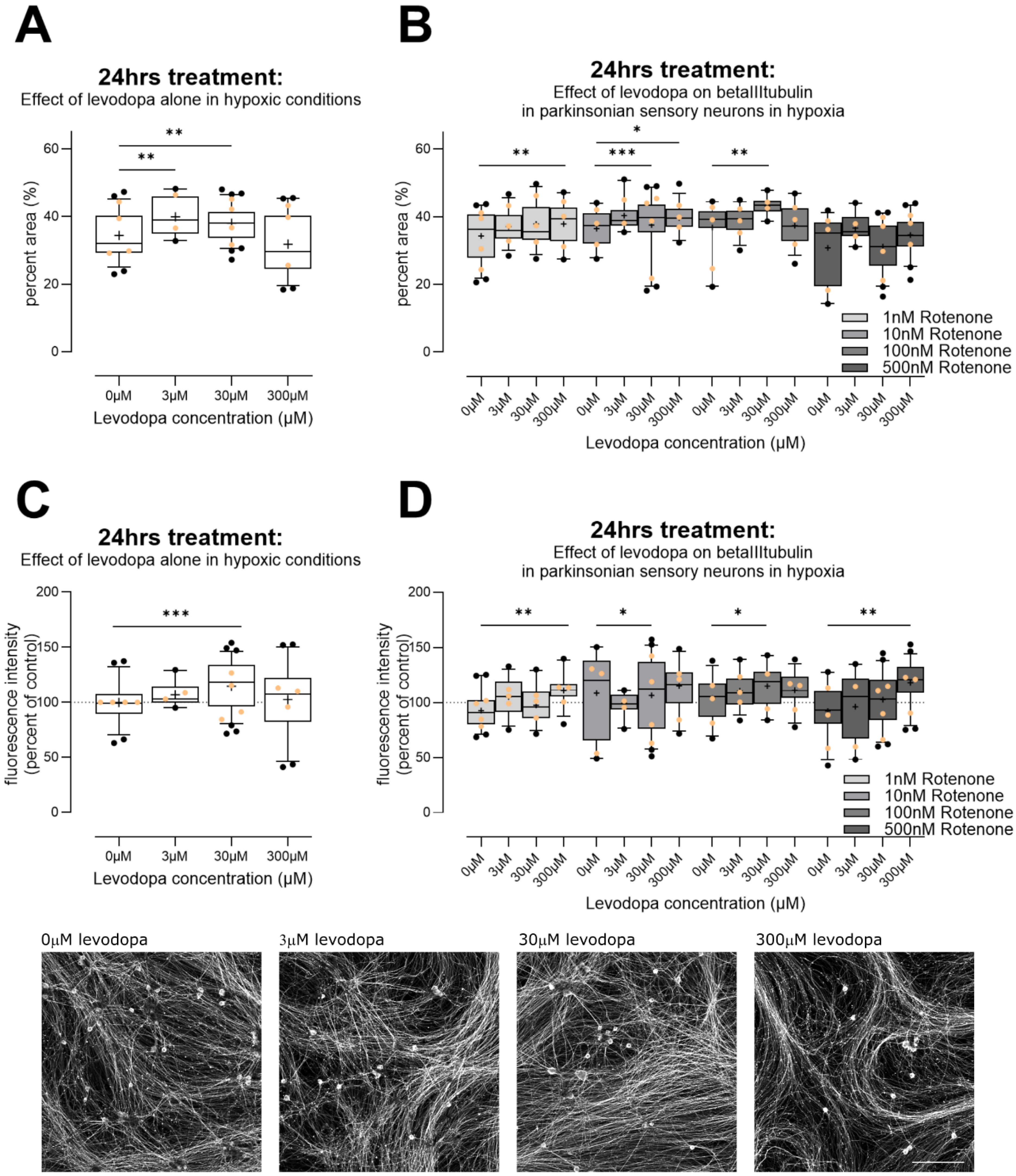
3.2.2. Chronic Levodopa Initially Increases and Then Ameliorates Oxidative Stress, at Concentrations Observed In Vivo
3.2.3. Levodopa Stabilizes Tubulin at Concentrations Observed In Vivo
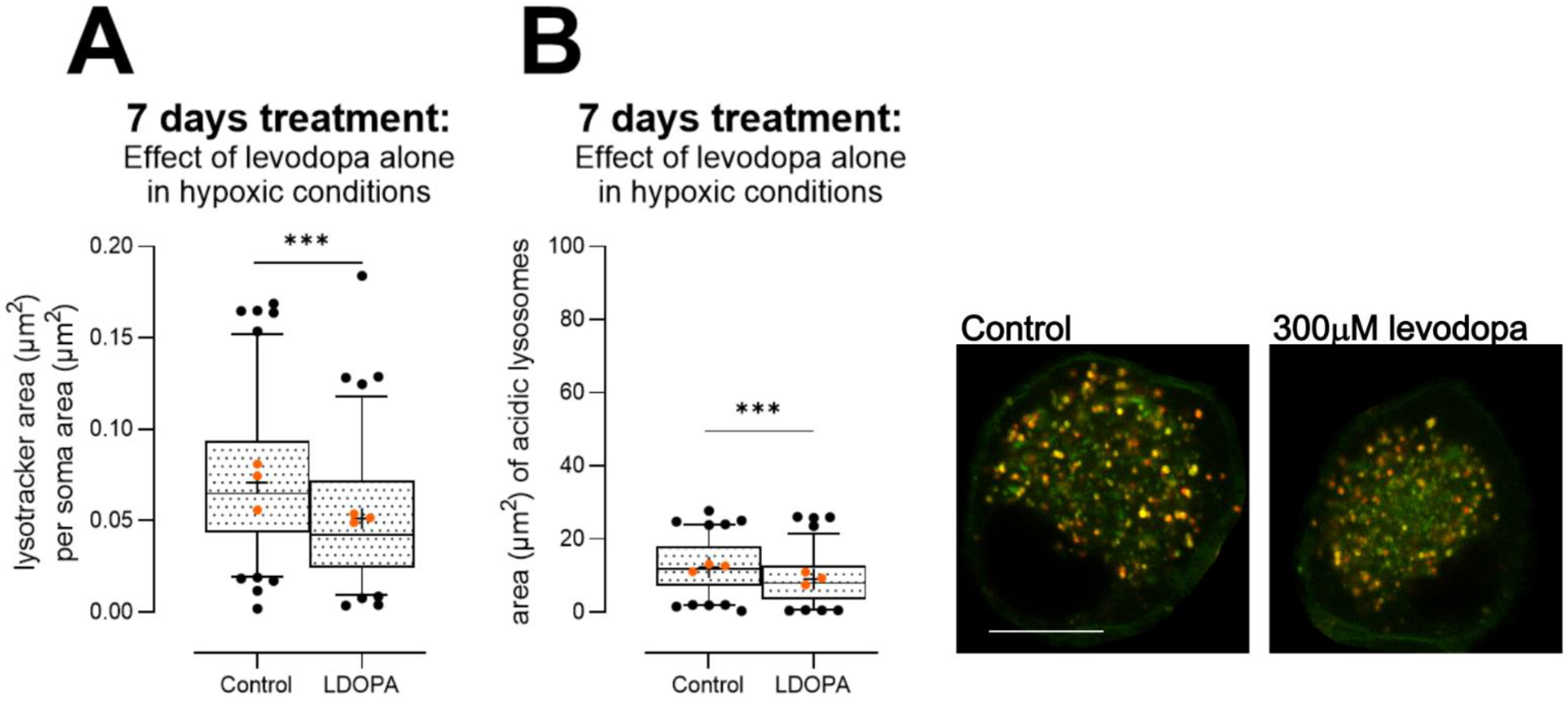
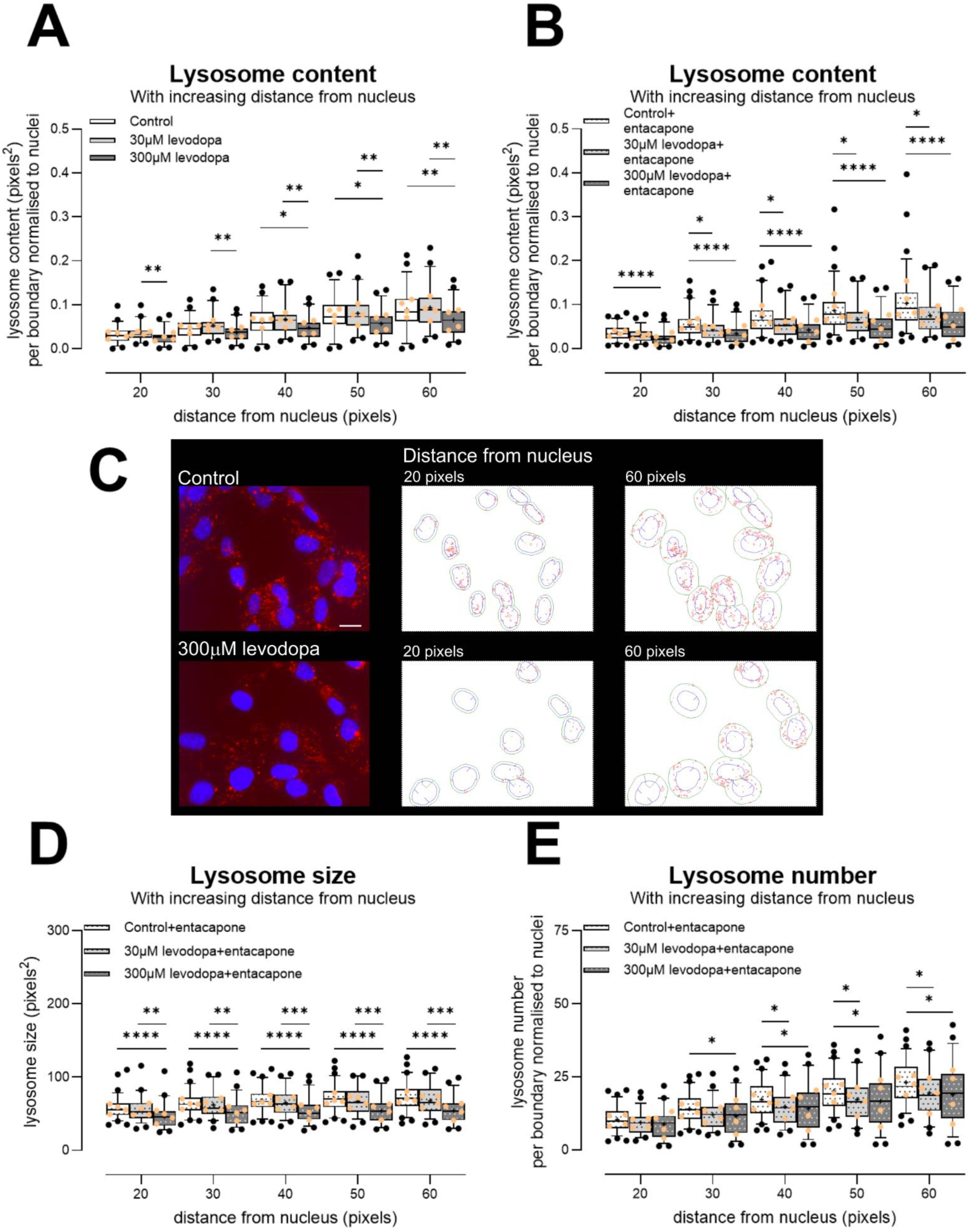
3.2.4. Levodopa Reduces Lysosome Content at Concentrations Observed In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steinmetz, J.D.; Seeher, K.M.; Schiess, N.; Nichols, E.; Cao, B.; Servili, C.; Cavallera, V.; Cousin, E.; Hagins, H.; Moberg, M.E.; et al. Global, Regional, and National Burden of Disorders Affecting the Nervous System, 1990–2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 344–381. [Google Scholar] [CrossRef] [PubMed]
- Ehringer, H.; Hornykiewicz, O. Verteilung Von Noradrenalin Und Dopamin (3-Hydroxytyramin) Im Gehirn Des Menschen Und Ihr Verhalten Bei Erkrankungen Des Extrapyramidalen Systems. Klin. Wochenschr. 1960, 38, 1236–1239. [Google Scholar] [CrossRef] [PubMed]
- Holtz, P. Dopadecarboxylase. Naturwissenschaften 1939, 27, 724–725. [Google Scholar] [CrossRef]
- Birkmayer, W.; Hornykiewicz, O. The L-3,4-dioxyphenylalanine (DOPA)-effect in Parkinson-akinesia. Wien. Klin. Wochenschr. 1961, 73, 787–788. [Google Scholar]
- Birkmayer, W.; Hornykiewicz, O. Der L-Dioxyphenylalanin (=L-DOPA)-Effekt beim Parkinson-Syndrom des Menschen: Zur Pathogenese und Behandlung der Parkinson-Akinese. Arch. Psychiatr. Z. Ges. Neurol. 1962, 203, 560–574. [Google Scholar] [CrossRef] [PubMed]
- Cotzias, G.C.; Van Woert, M.H.; Schiffer, L.M. Aromatic Amino Acids and Modification of Parkinsonism. N. Engl. J. Med. 1967, 276, 374–379. [Google Scholar] [CrossRef]
- PD Med Collaborative Group. Long-Term Effectiveness of Dopamine Agonists and Monoamine Oxidase B Inhibitors Compared with Levodopa as Initial Treatment for Parkinson’s Disease (PD MED): A Large, Open-Label, Pragmatic Randomised Trial. Lancet 2014, 384, 1196–1205. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s Disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Antonini, A.; Moro, E.; Godeiro, C.; Reichmann, H. Medical and Surgical Management of Advanced Parkinson’s Disease. Mov. Disord. 2018, 33, 900–908. [Google Scholar] [CrossRef]
- Foltynie, T.; Bruno, V.; Fox, S.; Kühn, A.A.; Lindop, F.; Lees, A.J. Medical, Surgical, and Physical Treatments for Parkinson’s Disease. Lancet 2024, 403, 305–324. [Google Scholar] [CrossRef]
- Faisal, M.; Rusetskaya, A.; Väli, L.; Taba, P.; Minajeva, A.; Hickey, M.A. No Evidence of Sensory Neuropathy in a Traditional Mouse Model of Idiopathic Parkinson’s Disease. Cells 2024, 13, 799. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.M.; Ekhator, O.R.; Ghisays, V. Assessment of Sensorimotor Function in Mouse Models of Parkinson’s Disease. J. Vis. Exp. JoVE 2013, 76, e50303. [Google Scholar] [CrossRef]
- Ogawa, M.; Zhou, Y.; Tsuji, R.; Goto, S.; Kasahara, J. Video-Based Assessments of the Hind Limb Stepping in a Mouse Model of Hemi-Parkinsonism. Neurosci. Res. 2020, 154, 56–59. [Google Scholar] [CrossRef]
- Schneider-Thoma, J.; Chalkou, K.; Dörries, C.; Bighelli, I.; Ceraso, A.; Huhn, M.; Siafis, S.; Davis, J.M.; Cipriani, A.; Furukawa, T.A.; et al. Comparative Efficacy and Tolerability of 32 Oral and Long-Acting Injectable Antipsychotics for the Maintenance Treatment of Adults with Schizophrenia: A Systematic Review and Network Meta-Analysis. Lancet 2022, 399, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Schrag, A.; Bohlken, J.; Dammertz, L.; Teipel, S.; Hermann, W.; Akmatov, M.K.; Bätzing, J.; Holstiege, J. Widening the Spectrum of Risk Factors, Comorbidities, and Prodromal Features of Parkinson Disease. JAMA Neurol. 2023, 80, 161. [Google Scholar] [CrossRef] [PubMed]
- Tanner, C.M.; Kamel, F.; Ross, G.W.; Hoppin, J.A.; Goldman, S.M.; Korell, M.; Marras, C.; Bhudhikanok, G.S.; Kasten, M.; Chade, A.R.; et al. Rotenone, Paraquat, and Parkinson’s Disease. Environ. Health Perspect. 2011, 119, 866–872. [Google Scholar] [CrossRef]
- Innos, J.; Hickey, M.A. Using Rotenone to Model Parkinson’s Disease in Mice: A Review of the Role of Pharmacokinetics. Chem. Res. Toxicol. 2021, 34, 1223–1239. [Google Scholar] [CrossRef]
- Morris, H.R.; Spillantini, M.G.; Sue, C.M.; Williams-Gray, C.H. The Pathogenesis of Parkinson’s Disease. Lancet 2024, 403, 293–304. [Google Scholar] [CrossRef]
- Del Tredici, K.; Braak, H. Review: Sporadic Parkinson’s Disease: Development and Distribution of α -Synuclein Pathology. Neuropathol. Appl. Neurobiol. 2016, 42, 33–50. [Google Scholar] [CrossRef]
- Sulzer, D.; Edwards, R.H. The Physiological Role of A-synuclein and Its Relationship to Parkinson’s Disease. J. Neurochem. 2019, 150, 475–486. [Google Scholar] [CrossRef]
- Stocchi, F.; Vacca, L.; Ruggieri, S.; Olanow, C.W. Intermittent vs. Continuous Levodopa Administration in Patients With Advanced Parkinson Disease: A Clinical and Pharmacokinetic Study. Arch. Neurol. 2005, 62, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.A.; Espay, A.J.; Ellenbogen, A.L.; Fernandez, H.H.; Isaacson, S.H.; LeWitt, P.A.; Ondo, W.G.; Pahwa, R.; Schwarz, J.; Stocchi, F.; et al. IPX203 vs Immediate-Release Carbidopa-Levodopa for the Treatment of Motor Fluctuations in Parkinson Disease: The RISE-PD Randomized Clinical Trial. JAMA Neurol. 2023, 80, 1062. [Google Scholar] [CrossRef]
- Romagnolo, A.; Merola, A.; Artusi, C.A.; Rizzone, M.G.; Zibetti, M.; Lopiano, L. Levodopa-Induced Neuropathy: A Systematic Review. Mov. Disord. Clin. Pract. 2019, 6, 96–103. [Google Scholar] [CrossRef]
- Jeziorska, M.; Atkinson, A.; Kass-Iliyya, L.; Kobylecki, C.; Gosal, D.; Marshall, A.; Malik, R.A.; Silverdale, M. Small Fibre Neuropathy in Parkinson’s Disease: Comparison of Skin Biopsies from the More Affected and Less Affected Sides. J. Park. Dis. 2019, 9, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Vacchi, E.; Senese, C.; Chiaro, G.; Disanto, G.; Pinton, S.; Morandi, S.; Bertaina, I.; Bianco, G.; Staedler, C.; Galati, S.; et al. Alpha-Synuclein Oligomers and Small Nerve Fiber Pathology in Skin Are Potential Biomarkers of Parkinson’s Disease. npj Park. Dis. 2021, 7, 119. [Google Scholar] [CrossRef] [PubMed]
- Kühn, E.; Averdunk, P.; Huckemann, S.; Müller, K.; Biesalski, A.; Hof Zum Berge, F.; Motte, J.; Fisse, A.L.; Schneider-Gold, C.; Gold, R.; et al. Correlates of Polyneuropathy in Parkinson’s Disease. Ann. Clin. Transl. Neurol. 2020, 7, 1898–1907. [Google Scholar] [CrossRef]
- Lee, J.J.; Baik, J.S. Peripheral Neuropathy in de Novo Patients with Parkinson’s Disease. Yonsei Med. J. 2020, 61, 1050–1053. [Google Scholar] [CrossRef]
- Conradt, C.; Guo, D.; Miclea, A.; Nisslein, T.; Ismail, C.; Chatamra, K.; Andersohn, F. Increased Prevalence of Polyneuropathy in Parkinson’s Disease Patients: An Observational Study. J. Park. Dis. 2018, 8, 141–144. [Google Scholar] [CrossRef]
- Corrà, M.F.; Vila-Chã, N.; Sardoeira, A.; Hansen, C.; Sousa, A.P.; Reis, I.; Sambayeta, F.; Damásio, J.; Calejo, M.; Schicketmueller, A.; et al. Peripheral Neuropathy in Parkinson’s Disease: Prevalence and Functional Impact on Gait and Balance. Brain 2023, 146, 225–236. [Google Scholar] [CrossRef]
- Jeziorska, M.; Atkinson, A.; Kass-Iliyya, L.; Javed, S.; Kobylecki, C.; Gosal, D.; Marshall, A.; Silverdale, M.; Malik, R.A. Increased Intraepidermal Nerve Fiber Degeneration and Impaired Regeneration Relate to Symptoms and Deficits in Parkinson’s Disease. Front. Neurol. 2019, 10, 111. [Google Scholar] [CrossRef]
- Lauria, G.; Hsieh, S.T.; Johansson, O.; Kennedy, W.R.; Leger, J.M.; Mellgren, S.I.; Nolano, M.; Merkies, I.S.J.; Polydefkis, M.; Smith, A.G.; et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the Use of Skin Biopsy in the Diagnosis of Small Fiber Neuropathy. Report of a Joint Task Force of the European Fe-Deration of Neurological Societies and the Peripheral Ne. Eur. J. Neurol. 2010, 17, 903-e49. [Google Scholar] [CrossRef] [PubMed]
- Bove, F.; Luigetti, M.; Gallicchio, L.; Recchia, V.; Petruzzellis, A.; Di Iorio, R.; Tamma, F.; Fasano, A. Central Conduction Abnormalities in Patients Receiving Levodopa-Carbidopa Intestinal Gel Infusion. Neurol. Sci. 2017, 38, 1869–1872. [Google Scholar] [CrossRef] [PubMed]
- Hörmann, P.; Delcambre, S.; Hanke, J.; Geffers, R.; Leist, M.; Hiller, K. Impairment of Neuronal Mitochondrial Function by L-DOPA in the Absence of Oxygen-Dependent Auto-Oxidation and Oxidative Cell Damage. Cell Death Discov. 2021, 7, 151. [Google Scholar] [CrossRef]
- Connolly, N.M.C.; Theurey, P.; Adam-Vizi, V.; Bazan, N.G.; Bernardi, P.; Bolaños, J.P.; Culmsee, C.; Dawson, V.L.; Deshmukh, M.; Duchen, M.R.; et al. Guidelines on Experimental Methods to Assess Mitochondrial Dysfunction in Cellular Models of Neurodegenerative Diseases. Cell Death Differ. 2018, 25, 542–572. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.W.; Norman, J.P.; Barbieri, J.; Brown, E.B.; Gelbard, H.A. Mitochondrial Membrane Potential Probes and the Proton Gradient: A Practical Usage Guide. BioTechniques 2011, 50, 98–115. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Stirling, D.R.; Swain-Bowden, M.J.; Lucas, A.M.; Carpenter, A.E.; Cimini, B.A.; Goodman, A. CellProfiler 4: Improvements in Speed, Utility and Usability. BMC Bioinform. 2021, 22, 433. [Google Scholar] [CrossRef] [PubMed]
- Corvol, J.; Bonnet, C.; Charbonnier-Beaupel, F.; Bonnet, A.; Fiévet, M.; Bellanger, A.; Roze, E.; Meliksetyan, G.; Ben Djebara, M.; Hartmann, A.; et al. The COMT Val158Met Polymorphism Affects the Response to Entacapone in Parkinson’s Disease: A Randomized Crossover Clinical Trial. Ann. Neurol. 2011, 69, 111–118. [Google Scholar] [CrossRef]
- Zoccolella, S.; Lamberti, P.; Armenise, E.; Mari, M.D.; Lamberti, S.V.; Mastronardi, R.; Fraddosio, A.; Iliceto, G.; Livrea, P. Plasma Homocysteine Levels in Parkinson’s Disease: Role of Antiparkinsonian Medications. Park. Relat. Disord. 2005, 11, 131–133. [Google Scholar] [CrossRef]
- Müller, T.; Kuhn, W. Homocysteine Levels after Acute Levodopa Intake in Patients with Parkinson’s Disease. Mov. Disord. 2009, 24, 1339–1343. [Google Scholar] [CrossRef]
- MATLAB Version: 24.1.0.2644111 (R2024a) Update 4; The MathWorks, Inc.: Natick, MA, USA; Available online: https://www.mathworks.com (accessed on 30 August 2024).
- Heckman, M.G.; Davis, J.M.; Crowson, C.S. Post Hoc Power Calculations: An Inappropriate Method for Interpreting the Findings of a Research Study. J. Rheumatol. 2022, 49, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Dziak, J.J.; Dierker, L.C.; Abar, B. The Interpretation of Statistical Power after the Data Have Been Gathered. Curr. Psychol. 2020, 39, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Greenland, S.; Senn, S.J.; Rothman, K.J.; Carlin, J.B.; Poole, C.; Goodman, S.N.; Altman, D.G. Statistical Tests, P Values, Confidence Intervals, and Power: A Guide to Misinterpretations. Eur. J. Epidemiol. 2016, 31, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Carreau, A.; Hafny-Rahbi, B.E.; Matejuk, A.; Grillon, C.; Kieda, C. Why Is the Partial Oxygen Pressure of Human Tissues a Crucial Parameter? Small Molecules and Hypoxia. J. Cell. Mol. Med. 2011, 15, 1239–1253. [Google Scholar] [CrossRef] [PubMed]
- Adamiak-Giera, U.; Jawień, W.; Pierzchlińska, A.; Białecka, M.; Kobierski, J.D.; Janus, T.; Gawrońska-Szklarz, B. Pharmacokinetics of Levodopa and 3-O-Methyldopa in Parkinsonian Patients Treated with Levodopa and Ropinirole and in Patients with Motor Complications. Pharmaceutics 2021, 13, 1395. [Google Scholar] [CrossRef]
- Müller, T.; Thiede, H.M. Bound, Free, and Total l-Dopa Measurement in Plasma of Parkinson’s Disease Patients. J. Neural Transm. 2019, 126, 1417–1420. [Google Scholar] [CrossRef]
- Othman, A.A.; Dutta, S. Population Pharmacokinetics of Levodopa in Subjects with Advanced P Arkinson’s Disease: Levodopa-carbidopa Intestinal Gel Infusion vs. Oral Tablets. Brit J. Clin. Pharma 2014, 78, 94–105. [Google Scholar] [CrossRef]
- Sagar, K.A.; Smyth, M.R. Bioavailability Studies of Oral Dosage Forms Containing Levodopa and Carbidopa Using Column-Switching Chromatography Followed by Electrochemical Detection. Analyst 2000, 125, 439–445. [Google Scholar] [CrossRef]
- Shiraishi, T.; Nishikawa, N.; Mukai, Y.; Takahashi, Y. High Levodopa Plasma Concentration after Oral Administration Predicts Levodopa-Induced Dyskinesia in Parkinson’s Disease. Park. Relat. Disord. 2020, 75, 80–84. [Google Scholar] [CrossRef]
- Stocchi, F.; Vacca, L.; Grassini, P.; Pawsey, S.; Whale, H.; Marconi, S.; Torti, M. L-Dopa Pharmacokinetic Profile with Effervescent Melevodopa/Carbidopa versus Standard-Release Levodopa/Carbidopa Tablets in Parkinson’s Disease: A Randomised Study. Park. Dis. 2015, 2015, 369465. [Google Scholar] [CrossRef]
- Zorgniotti, A.; Ditamo, Y.; Arce, C.A.; Bisig, C.G. Irreversible Incorporation of L-Dopa into the C-Terminus of α-Tubulin Inhibits Binding of Molecular Motor KIF5B to Microtubules and Alters Mitochondrial Traffic along the Axon. Neurobiol. Dis. 2021, 147, 105164. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, K.J.; Hume, P.M.; Dunlop, R.A.; Dean, R.T. Biosynthesis and Turnover of DOPA-Containing Proteins by Human Cells. Free Radic. Biol. Med. 2004, 37, 1756–1764. [Google Scholar] [CrossRef]
- Lang, M.; Pramstaller, P.P.; Pichler, I. Crosstalk of Organelles in Parkinson’s Disease—MiT Family Transcription Factors as Central Players in Signaling Pathways Connecting Mitochondria and Lysosomes. Mol. Neurodegener. 2022, 17, 50. [Google Scholar] [CrossRef]
- Kendrick, A.A.; Christensen, J.R. Bidirectional Lysosome Transport: A Balancing Act between ARL8 Effectors. Nat. Commun. 2022, 13, 5261. [Google Scholar] [CrossRef] [PubMed]
- Wallings, R.L.; Humble, S.W.; Ward, M.E.; Wade-Martins, R. Lysosomal Dysfunction at the Centre of Parkinson’s Disease and Frontotemporal Dementia/Amyotrophic Lateral Sclerosis. Trends Neurosci. 2019, 42, 899–912. [Google Scholar] [CrossRef]
- Chen, W.; Mi, R.; Haughey, N.; Oz, M.; Höke, A. Immortalization and Characterization of a Nociceptive Dorsal Root Ganglion Sensory Neuronal Line. J. Peripher. Nerv. Syst. 2007, 12, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Dusan, M.; Jastrow, C.; Alyce, M.M.; Yingkai, W.; Shashikanth, M.; Andelain, E.; Christine, B.M.; Stuart, B.M.; Oliver, B.G.; Michael, M.Z.; et al. Differentiation of the 50B11 Dorsal Root Ganglion Cells into NGF and GDNF Responsive Nociceptor Subtypes. Mol. Pain 2020, 16, 174480692097036. [Google Scholar] [CrossRef]
- WHO. Web Annex A. World Health Organization Model List of Essential Medicines—23rd List, 2023; The selection and use of essential medicines 2023: Executive summary of the report of the 24th WHO Expert Committee on the Selection and Use of Essential Medicines, 24–28 April 2023; WHO/MHP/HPS/EML/2023.02; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Leta, V.; Klingelhoefer, L.; Longardner, K.; Campagnolo, M.; Levent, H.Ç.; Aureli, F.; Metta, V.; Bhidayasiri, R.; Chung-Faye, G.; Falup-Pecurariu, C.; et al. Gastrointestinal Barriers to Levodopa Transport and Absorption in Parkinson’s Disease. Euro J. Neurol. 2023, 30, 1465–1480. [Google Scholar] [CrossRef]
- Lee, E.-S.Y.; Chen, H.; King, J.; Charlton, C. The Role of 3-O-Methyldopa in the Side Effects of l-Dopa. Neurochem. Res. 2008, 33, 401–411. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, D.; Hou, J.; Li, J.; Zhang, Y.; Tian, M.; Li, Z.; Tie, T.; Cheng, Y.; Su, X.; et al. High-Concentration Homocysteine Inhibits Mitochondrial Respiration Function and Production of Reactive Oxygen Species in Neuron Cells. J. Stroke Cerebrovasc. Dis. 2020, 29, 105109. [Google Scholar] [CrossRef]
- Deep, S.N.; Seelig, S.; Paul, S.; Poddar, R. Homocysteine-Induced Sustained GluN2A NMDA Receptor Stimulation Leads to Mitochondrial ROS Generation and Neurotoxicity. J. Biol. Chem. 2024, 300, 107253. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gou, M.; Guo, X. Features of Plasma Homocysteine, Vitamin B12, and Folate in Parkinson’s Disease: An Updated Meta-Analysis. J. Integr. Neurosci. 2023, 22, 115. [Google Scholar] [CrossRef]
- Periñán, M.T.; Macías-García, D.; Jesús, S.; Martín-Rodríguez, J.F.; Muñoz-Delgado, L.; Jimenez-Jaraba, M.V.; Buiza-Rueda, D.; Bonilla-Toribio, M.; Adarmes-Gómez, A.D.; Gómez-Garre, P.; et al. Homocysteine Levels, Genetic Background, and Cognitive Impairment in Parkinson’s Disease. J. Neurol. 2023, 270, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Wu, R. Plasma Homocysteine, Folate and Vitamin B12 Levels in Parkinson’s Disease in China: A Meta-Analysis. Clin. Neurol. Neurosurg. 2020, 188, 105587. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Feng, H.; Peng, S.; Xiao, J.; Zhang, J. Association of Plasma Homocysteine, Vitamin B12 and Folate Levels with Cognitive Function in Parkinson’s Disease: A Meta-Analysis. Neurosci. Lett. 2017, 636, 190–195. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; He, X.; Jia, X.; Zhang, X.; Lu, B.; Zhao, J.; Lu, J.; Chen, L.; Dong, Z.; et al. Proteomics Reveal the Inhibitory Mechanism of Levodopa Against Esophageal Squamous Cell Carcinoma. Front. Pharmacol. 2020, 11, 568459. [Google Scholar] [CrossRef]
- De Araujo, M.E.G.; Liebscher, G.; Hess, M.W.; Huber, L.A. Lysosomal Size Matters. Traffic 2020, 21, 60–75. [Google Scholar] [CrossRef]
- Xu, H.; Ren, D. Lysosomal Physiology. Annu. Rev. Physiol. 2015, 77, 57–80. [Google Scholar] [CrossRef]
- Bouhamdani, N.; Comeau, D.; Turcotte, S. A Compendium of Information on the Lysosome. Front. Cell Dev. Biol. 2021, 9, 798262. [Google Scholar] [CrossRef]
- Dentesano, Y.M.; Ditamo, Y.; Hansen, C.; Arce, C.A.; Bisig, C.G. Post-translational Incorporation of 3,4-dihydroxyphenylalanine into the C Terminus of A-tubulin in Living Cells. FEBS J. 2018, 285, 1064–1078. [Google Scholar] [CrossRef]
- Song, Q.; Meng, B.; Xu, H.; Mao, Z. The Emerging Roles of Vacuolar-Type ATPase-Dependent Lysosomal Acidification in Neurodegenerative Diseases. Transl. Neurodegener. 2020, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Yadavalli, N.; Ferguson, S.M. LRRK2 Suppresses Lysosome Degradative Activity in Macrophages and Microglia through MiT-TFE Transcription Factor Inhibition. Proc. Natl. Acad. Sci. USA 2023, 120, e2303789120. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Ruvkun, G. Lysosomal Activity Regulates Caenorhabditis Elegans Mitochondrial Dynamics through Vitamin B12 Metabolism. Proc. Natl. Acad. Sci. USA 2020, 117, 19970–19981. [Google Scholar] [CrossRef] [PubMed]
- Vanderschuren, H.; Boycheva, S.; Li, K.-T.; Szydlowski, N.; Gruissem, W.; Fitzpatrick, T.B. Strategies for Vitamin B6 Biofortification of Plants: A Dual Role as a Micronutrient and a Stress Protectant. Front. Plant Sci. 2013, 4, 143. [Google Scholar] [CrossRef]
- Ahlskog, J.E. Levodopa, Homocysteine and Parkinson’s Disease: What’s the Problem? Park. Relat. Disord. 2023, 109, 105357. [Google Scholar] [CrossRef]

| Condition | Median (95% CIs 2) | Dunn’s Multiple Comparisons Test (Versus Control) | Dunn’s Multiple Comparisons Test (Versus Control + Entacapone) | Mann–Whitney U Test Compared with Control Cells (No Entacapone) |
|---|---|---|---|---|
| Control | 35 (34–35) | - | ||
| 30 µM levodopa | 36 (35–36) | * | ||
| 300 µM levodopa | 31 (30–32) | **** | ||
| Control + 1 µM entacapone | 35 (35–36) | - | ||
| 30 µM levodopa + 1 µM entacapone | 33 (32–33) | **** | ||
| 300 µM levodopa + 1 µM entacapone | 30 (29–30) | **** | ||
| Homocysteine 20 µM | 38 (37–38) | **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olaoye, O.J.; Aksoy, A.E.; Hyytiäinen, S.V.; Narits, A.A.; Hickey, M.A. Levodopa Impairs Lysosomal Function in Sensory Neurons In Vitro. Biology 2024, 13, 893. https://doi.org/10.3390/biology13110893
Olaoye OJ, Aksoy AE, Hyytiäinen SV, Narits AA, Hickey MA. Levodopa Impairs Lysosomal Function in Sensory Neurons In Vitro. Biology. 2024; 13(11):893. https://doi.org/10.3390/biology13110893
Chicago/Turabian StyleOlaoye, Oyedele J., Asya Esin Aksoy, Santeri V. Hyytiäinen, Aia A. Narits, and Miriam A. Hickey. 2024. "Levodopa Impairs Lysosomal Function in Sensory Neurons In Vitro" Biology 13, no. 11: 893. https://doi.org/10.3390/biology13110893
APA StyleOlaoye, O. J., Aksoy, A. E., Hyytiäinen, S. V., Narits, A. A., & Hickey, M. A. (2024). Levodopa Impairs Lysosomal Function in Sensory Neurons In Vitro. Biology, 13(11), 893. https://doi.org/10.3390/biology13110893






