Estrogen Receptor Beta Agonist Influences Presynaptic NMDA Receptor Distribution in the Paraventricular Hypothalamic Nucleus Following Hypertension in a Mouse Model of Perimenopause
Abstract
Simple Summary
Abstract
1. Introduction
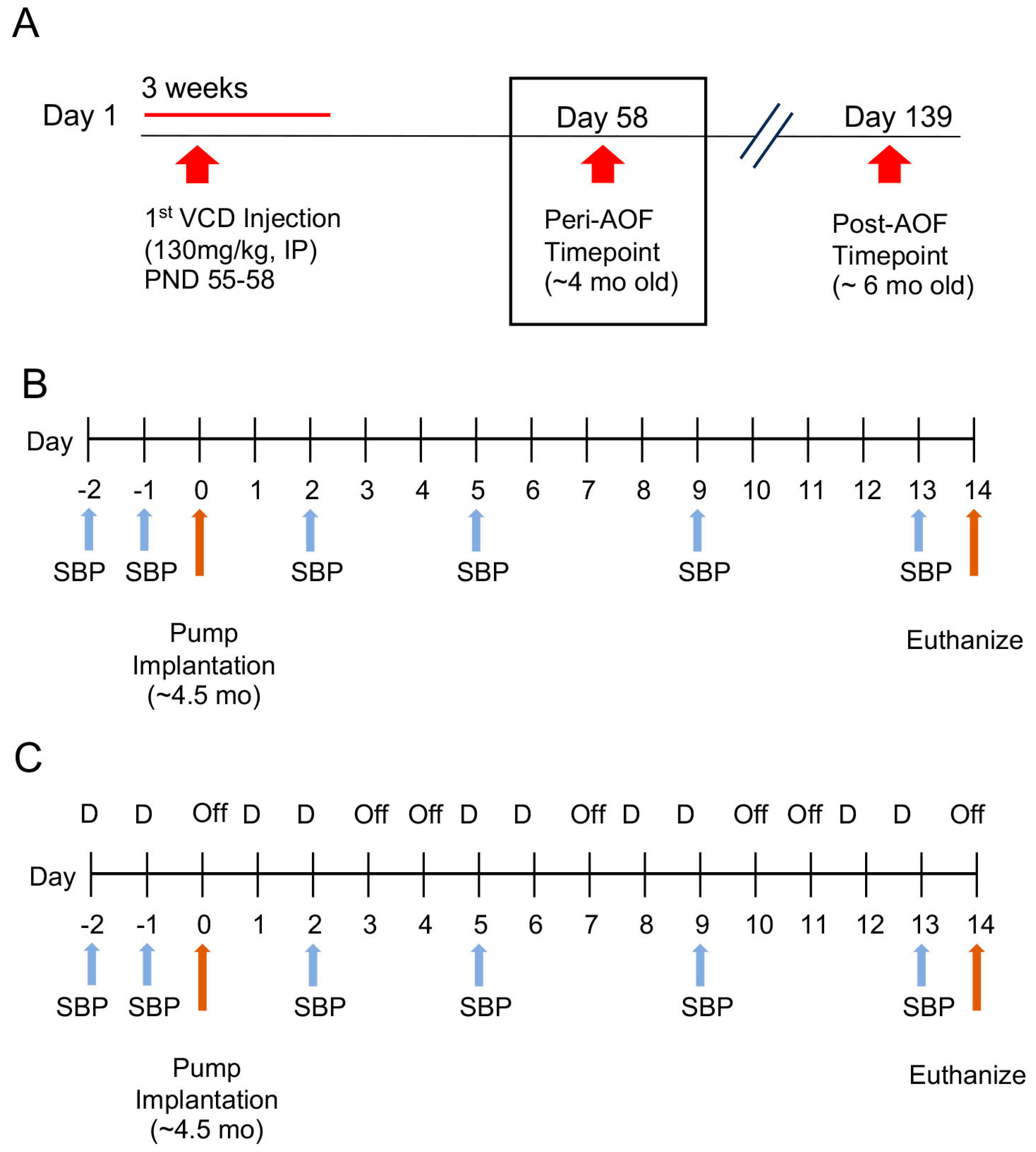
2. Materials and Methods
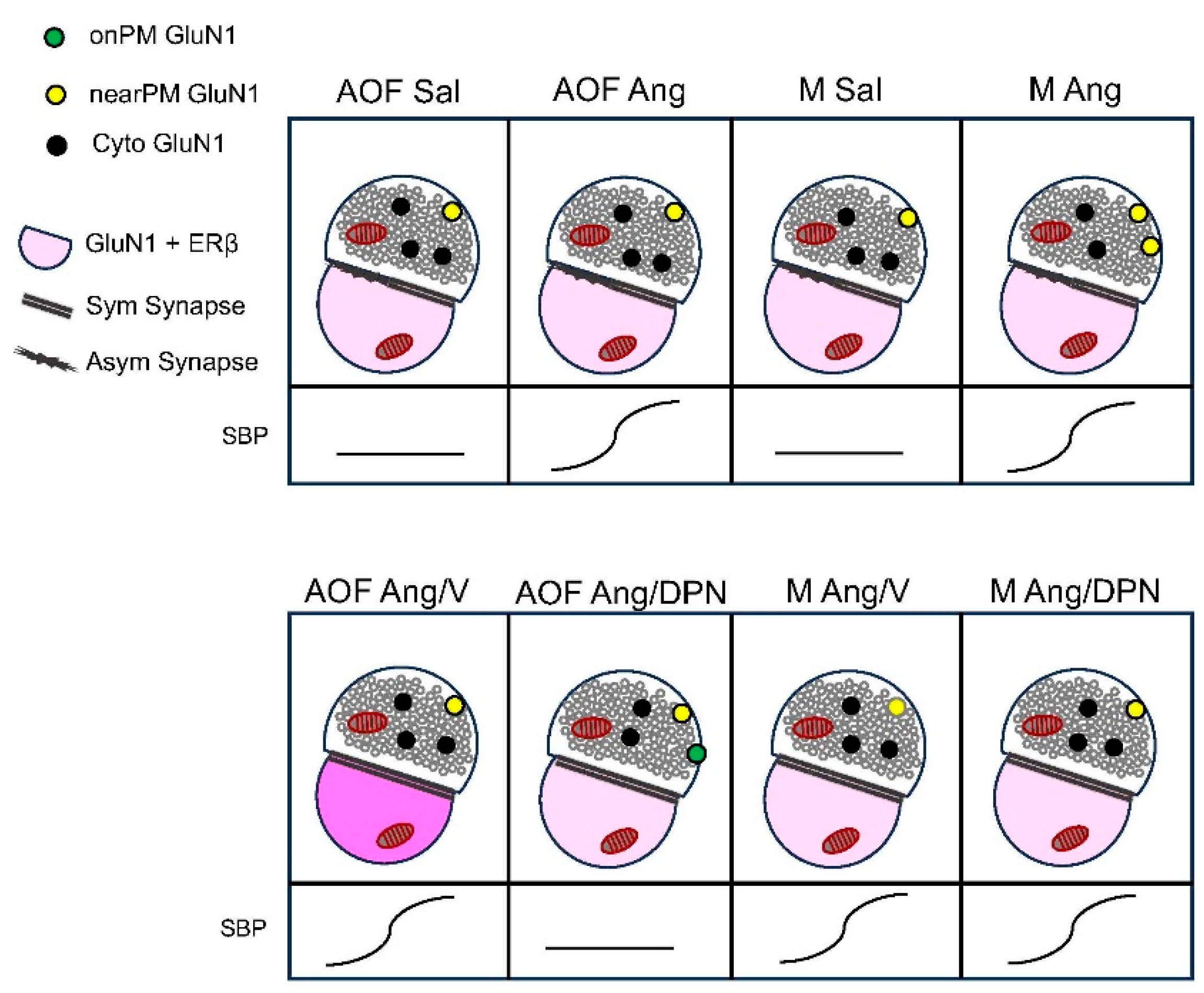
3. Results
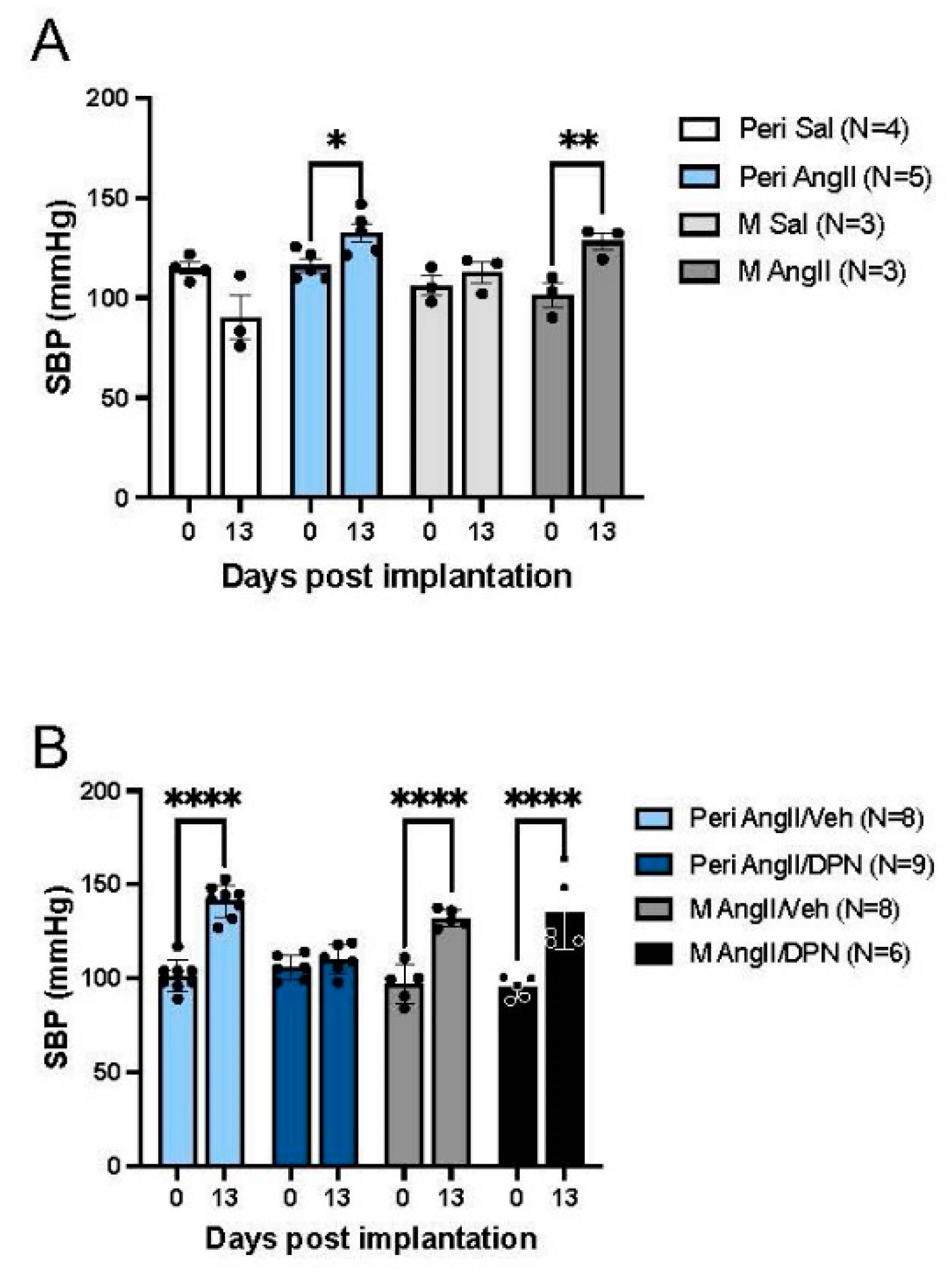
3.1. Blood Pressure in AngII-Infused Peri-AOF and Male Mice without or with Cyclic ERß Agonist Administration
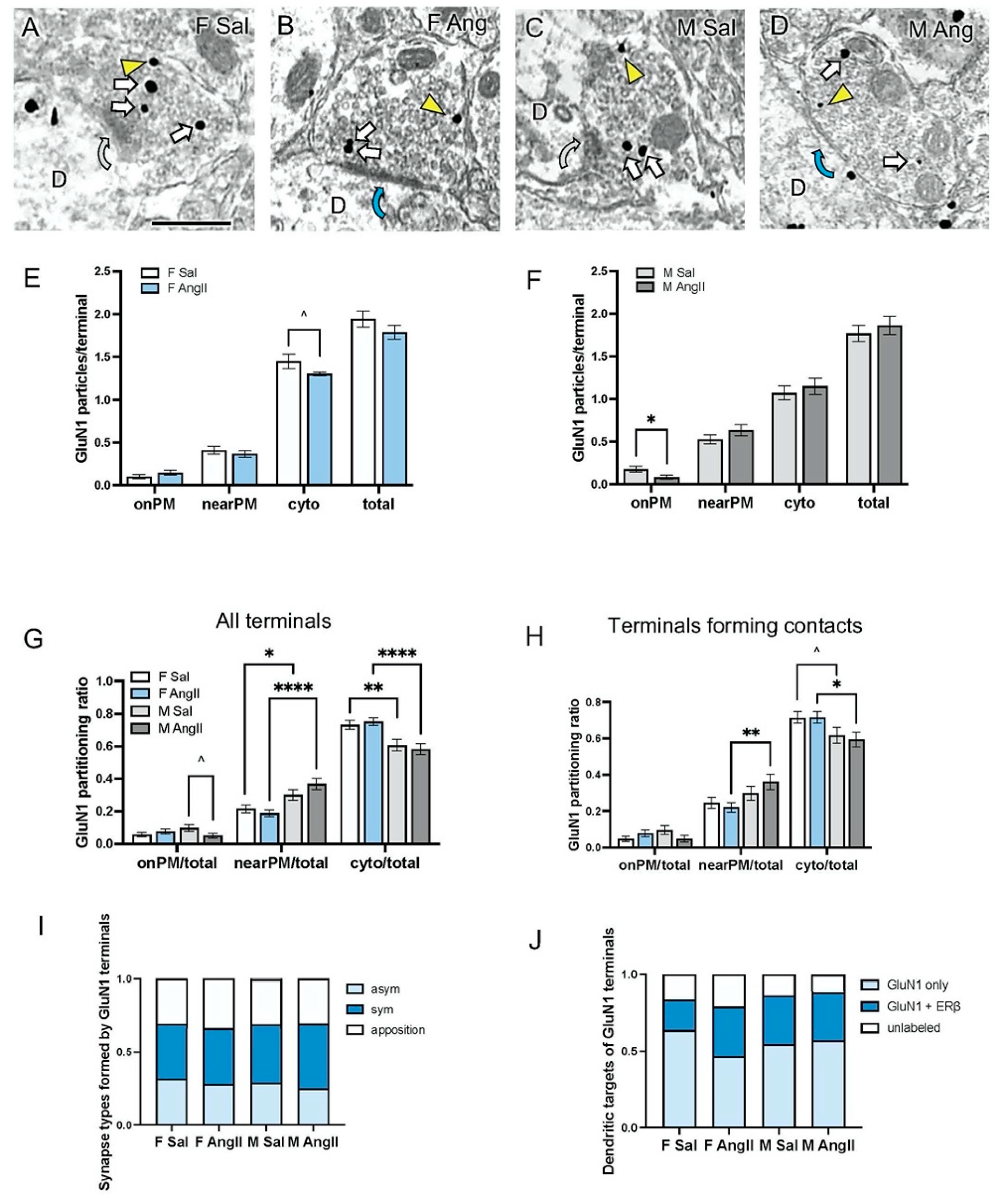
3.2. Presynaptic GluN1 in Mice Infused with Sal or Ang
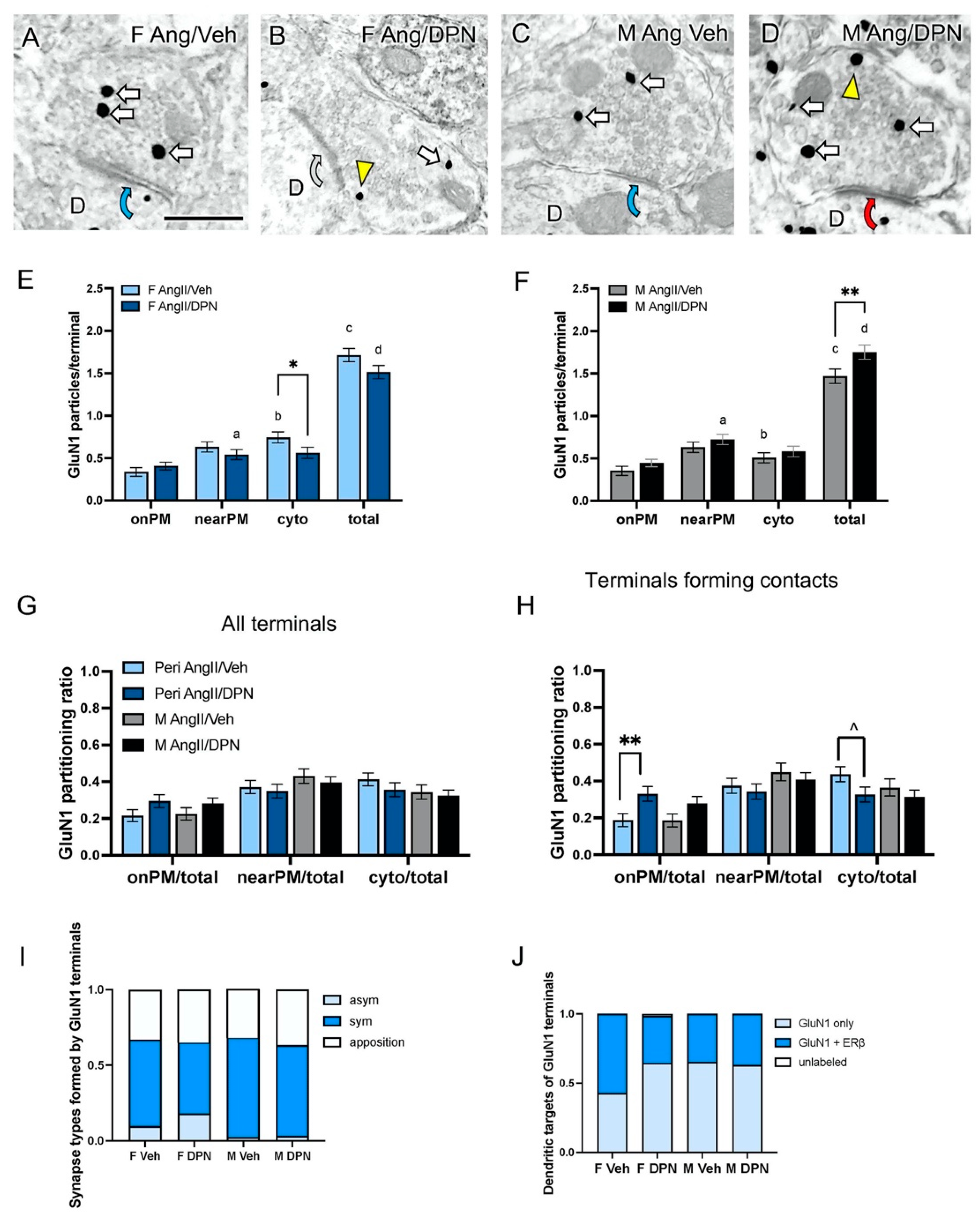
3.3. Presynaptic GluN1 in Ang-Infused Mice Co-Administered DPN
4. Discussion
5. Conclusions and Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gerdts, E.; Sudano, I.; Brouwers, S.; Borghi, C.; Bruno, R.M.; Ceconi, C.; Cornelissen, V.; Diévart, F.; Ferrini, M.; Kahan, T.; et al. Sex differences in arterial hypertension. Eur. Heart J. 2022, 43, 4777–4788. [Google Scholar] [CrossRef] [PubMed]
- D’Ignazio, T.; Grand, S.; Bérubé, L.; Forcillo, J.; Pacheco, C. Hypertension across a Woman’s lifespan. Maturitas 2023, 168, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Colafella, K.M.M.; Denton, K.M. Sex-specific differences in hypertension and associated cardiovascular disease. Nat. Rev. Nephrol. 2018, 14, 185–201. [Google Scholar] [CrossRef]
- Wenger, N.K.E.A. Hypertension Across a Woman’s Life Cycle. J. Am. Coll. Cardiol. 2018, 71, 1797–1813. [Google Scholar] [CrossRef]
- Van Kempen, T.; Dodos, M.; Woods, C.; Marques-Lopes, J.; Justice, N.; Iadecola, C.; Pickel, V.; Glass, M.; Milner, T. Sex differences in NMDA GluN1 plasticity in rostral ventrolateral medulla neurons containing corticotropin-releasing factor type 1 receptor following slow-pressor angiotensin II hypertension. Neuroscience 2015, 307, 83–97. [Google Scholar] [CrossRef]
- Wang, G.; Woods, C.; Johnson, M.A.; Milner, T.A.; Glass, M.J. Angiotensin II infusion results in both hypertension and increased AMPA GluA1 signaling in hypothalamic paraventricular nucleus of male but not female mice. Neuroscience 2022, 485, 129–144. [Google Scholar] [CrossRef]
- Xue, B.; Pamidimukkala, J.; Hay, M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am. J. Physiol. 2005, 288, H2177–H2184. [Google Scholar] [CrossRef]
- Marques-Lopes, J.; Van Kempen, T.; Waters, E.M.; Pickel, V.M.; Iadecola, C.; Milner, T.A. Slow-pressor angiotensin II hypertension and concomitant dendritic NMDA receptor trafficking in estrogen receptor beta-containing neurons of the mouse hypothalamic paraventricular nucleus are sex and age dependent. J. Comp. Neurol. 2014, 522, 3075–3090. [Google Scholar] [CrossRef] [PubMed]
- Van Kempen, T.A.; Marques-Lopes, J.; Glass, M.J.; Milner, T.A. Sex differences in neural regulation of hypertension. In Arterial Hypertension and Brain as an End Organ; Girouard, H., Ed.; Research Signpost/Transworld Research Network: Scarborough, ON, Canada, 2016. [Google Scholar]
- Van Kempen, T.A.; Milner, T.A.; Waters, E.M. Accelerated ovarian failure: A novel, chemically induced animal model of menopause. Brain Res. 2011, 1379, 176–187. [Google Scholar] [CrossRef]
- Marques-Lopes, J.; Van Kempen, T.A.; Milner, T.A. Rodent Models of Ovarian Failure. In Conn’s Handbook of Models for Human Aging, 2nd ed.; Jeffery Ram, P.M.C., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Chapter 60; pp. 831–843. [Google Scholar]
- Van Kempen, T.A.; Gorecka, J.; Gonzalez, A.D.; Soeda, F.; Milner, T.A.; Waters, E.M. Characterization of neural estrogen signaling and neurotrophic changes in the accelerated ovarian failure mouse model of menopause. Endocrinology 2014, 155, 3610–3623. [Google Scholar] [CrossRef]
- Brooks, H.L.; Pollow, D.P.; Hoyer, P.B. The VCD Mouse Model of Menopause and Perimenopause for the Study of Sex Differences in Cardiovascular Disease and the Metabolic Syndrome. Physiology 2016, 31, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini, A.R.; Kararigas, G. Estrogen-related mechanisms in sex differences of hypertension and target organ damage. Biol. Sex Differ. 2020, 11, 31. [Google Scholar] [CrossRef]
- Lerman, L.O.; Kurtz, T.W.; Touyz, R.M.; Ellison, D.H.; Chade, A.R.; Crowley, S.D.; Mattson, D.L.; Mullins, J.J.; Osborn, J.; Eirin, A.; et al. Animal Models of Hypertension: A Scientific Statement From the American Heart Association. Hypertension 2019, 73, e87–e120. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Li, L.; Riazi, S.; Halagappa, V.K.; Ecelbarger, C.M. Sex and age result in differential regulation of the renal thiazide-sensitive NaCl cotransporter and the epithelial sodium channel in angiotensin II-infused mice. Am. J. Nephrol. 2009, 30, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Marques-Lopes, J.; Tesfaye, E.; Israilov, S.; Van Kempen, T.A.; Wang, G.; Glass, M.J.; Pickel, V.M.; Iadecola, C.; Waters, E.M.; Milner, T.A. Redistribution of NMDA Receptors in Estrogen-Receptor-beta-Containing Paraventricular Hypothalamic Neurons following Slow-Pressor Angiotensin II Hypertension in Female Mice with Accelerated Ovarian Failure. Neuroendocrinology 2017, 104, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Milner, T.A.; Contoreggi, N.H.; Yu, F.; Johnson, M.A.; Wang, G.; Woods, C.; Mazid, S.; Van Kempen, T.A.; Waters, E.M.; McEwen, B.S.; et al. Estrogen receptor β contributes to both hypertension and hypothlamic plasticity in a mouse model of perimenopause. J. Neurosci. 2021, 41, 5190–5205. [Google Scholar] [CrossRef]
- Sellers, K.J.; Erli, F.; Raval, P.; Watson, I.A.; Chen, D.; Srivastava, D.P. Rapid modulation of synaptogenesis and spinogenesis by 17beta-estradiol in primary cortical neurons. Front. Cell. Neurosci. 2015, 9, 137. [Google Scholar] [CrossRef]
- Glass, M.J.; Wang, G.; Coleman, C.G.; Chan, J.; Ogorodnik, E.; Van Kempen, T.A.; Milner, T.A.; Butler, S.D.; Young, C.N.; Davisson, R.L.; et al. NMDA receptor plasticity in the hypothalamic paraventricular nucleus contributes to the elevated blood pressure produced by angiotensin II. J. Neurosci. 2015, 35, 9558–9567. [Google Scholar] [CrossRef]
- Kim, K.; Shin, W.; Kang, M.; Lee, S.; Kim, D.; Kang, R.; Jung, Y.; Cho, Y.; Yang, E.; Kim, H.; et al. Presynaptic PTPσ regulates postsynaptic NMDAreceptor function through direct adhesion-independent mechanisms. eLife 2020, 9, e54224. [Google Scholar] [CrossRef]
- Bertocchi, I.; Rocha-Almeida, F.; Romero-Barragán, M.T.; Cambiaghi, M.; Carretero-Guillén, A.; Botta, P.; Dogbevia, G.K.; Treviño, M.; Mele, P.; Oberto, A.; et al. Pre- and postsynaptic N-methyl-D-aspartatereceptors are required for sequential printing of fear memory engrams. iScience 2023, 26, 108050. [Google Scholar] [CrossRef]
- Abrahamsson, T.; Chou, C.Y.C.; Li, S.Y.; Mancino, A.; Costa, R.P.; Brock, J.A.; Nuro, E.; Buchanan, K.A.; Elgar, D.; Blackman, A.V.; et al. Differential Regulation of Evoked and Spontaneous Release by Presynaptic NMDA Receptors. Neuron 2017, 96, 839–855. [Google Scholar] [CrossRef]
- Enoki, R.; Hu, Y.L.; Hamilton, D.; Fine, A. Expression of long-term plasticity at individual synapses in hippocampus is graded, bidirectional, and mainly presynaptic: Optical quantal analysis. Neuron 2009, 62, 242–253. [Google Scholar] [CrossRef]
- Humeau, Y.; Shaban, H.; Bissière, S.; Lüthi, A. Presynaptic induction of heterosynaptic associative plasticity in the mammalian brain. Nature 2003, 426, 841–845. [Google Scholar] [CrossRef]
- Schmidt, C.C.; Tong, R.; Emptage, N.J. GluN2A- and GluN2B-containing pre-synaptic N-methyl-d-aspartate receptors differentially regulate action potential-evoked Ca2+ influx via modulation of SK channels. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2024, 379, 20230222. [Google Scholar] [CrossRef]
- Fink, K.; Bönisch, H.; Göthert, M. Presynaptic NMDA receptors stimulate noradrenaline release in the cerebral cortex. Eur. J. Pharmacol. 1990, 185, 115–117. [Google Scholar] [CrossRef]
- Farb, C.R.; Aoki, C.; Ledoux, J.E. Differential localization of NMDA and AMPA receptor subunits in the lateral and basal nuclei of the amygdala: A light and electron microscopic study. J. Comp. Neurol. 1995, 362, 86–108. [Google Scholar] [CrossRef]
- Gracy, K.N.; Pickel, V.M. Comparative ultrastructural localization of the NMDAR1 glutamate receptor in the rat basolateral amygdala and bed nucleus of the stria terminalis. J. Comp. Neurol. 1995, 362, 71–85. [Google Scholar] [CrossRef]
- Bidoret, C.; Ayon, A.; Barbour, B.; Casado, M. Presynaptic NR2A-containing NMDAreceptors implement a high-pass filter synaptic plasticity rule. Proc. Natl. Acad. Sci. USA 2009, 106, 14126–14131. [Google Scholar] [CrossRef]
- Schonewille, M.; Girasole, A.E.; Rostaing, P.; Mailhes-Hamon, C.; Ayon, A.; Nelson, A.B.; Triller, A.; Casado, M.; De Zeeuw, C.I.; Bouvier, G.; et al. NMDARs in granule cells contribute to parallel fiber-Purkinje cell synaptic plasticity and motor learning. Proc. Natl. Acad. Sci. USA 2021, 118, e2102635118. [Google Scholar] [CrossRef]
- Wang, J.K.; Andrews, H.; Thukral, V. Presynaptic glutamate receptors regulate noradrenaline release from isolated nerve terminals. J. Neurochem. 1992, 58, 204–211. [Google Scholar] [CrossRef]
- Milner, T.A.; Thompson, L.I.; Wang, G.; Kievits, J.A.; Martin, E.; Zhou, P.; McEwen, B.S.; Pfaff, D.W.; Waters, E.M. Distribution of estrogen receptor beta containing cells in the brains of bacterial artificial chromosome transgenic mice. Brain Res. 2010, 1351, 74–96. [Google Scholar] [CrossRef][Green Version]
- Gong, S.; Zheng, C.; Doughty, M.L.; Losos, K.; Didkovsky, N.; Schambra, U.B.; Nowak, N.J.; Joyner, A.; Leblanc, G.; Hatten, M.E.; et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 2003, 425, 917–925. [Google Scholar] [CrossRef]
- Harsh, V.; Schmidt, P.J.; Rubinow, D.R. The menopause transition: The next neuroendocrine frontier. Expert Rev. Neurother. 2007, 7 (Suppl. S1), S7–S10. [Google Scholar] [CrossRef]
- Lohff, J.C.; Christian, P.J.; Marion, S.L.; Arrandale, A.; Hoyer, P.B. Characterization of cyclicity and hormonal profile with impending ovarian failure in a novel chemical-induced mouse model of perimenopause. Comp. Med. 2005, 55, 523–527. [Google Scholar]
- Mayer, L.P.; Devine, P.J.; Dyer, C.A.; Hoyer, P.B. The follicle-deplete mouse ovary produces androgen. Biol. Reprod. 2004, 71, 130–138. [Google Scholar] [CrossRef]
- Turner, C.D.; Bagnara, J.T. General Endocrinology; W.B. Saunders: Philadelphia, PA, USA, 1971. [Google Scholar]
- Morrison, J.H.; Brinton, R.D.; Schmidt, P.J.; Gore, A.C. Estrogen, menopause, and the aging brain: How basic neuroscience can inform hormone therapy in women. J. Neurosci. 2006, 26, 10332–10348. [Google Scholar] [CrossRef]
- Encinas, J.M.; Vaahtokari, A.; Enikolopov, G. Fluoxetine targets early progenitor cells in the adult brain. Proc. Natl. Acad. Sci. USA 2006, 103, 8233–8238. [Google Scholar] [CrossRef]
- Volkmann, K.; Chen, Y.Y.; Harris, M.P.; Wullimann, M.F.; Koster, R.W. The zebrafish cerebellar upper rhombic lip generates tegmental hindbrain nuclei by long-distance migration in an evolutionary conserved manner. J. Comp. Neurol. 2010, 518, 2794–2817. [Google Scholar] [CrossRef]
- Milner, T.A.; Waters, E.M.; Robinson, D.C.; Pierce, J.P. Degenerating processes identified by electron microscopic immunocytochemical methods. Methods Mol. Biol. 2011, 793, 23–59. [Google Scholar] [CrossRef]
- Oyola, M.G.; Thompson, M.K.; Handa, A.Z.; Handa, R.J. Distribution and chemical composition of estrogen receptor beta neurons in the paraventricular nucleus of the female and male mouse hypothalamus. J. Comp. Neurol. 2017, 525, 3666–3682. [Google Scholar] [CrossRef]
- Brose, N.; Huntley, G.W.; Stern-Bach, Y.; Sharma, G.; Morrison, J.H.; Heinemann, S.F. Differential assembly of coexpressed glutamate receptor subunits in neurons of rat cerebral cortex. J. Biolog. Chem. 1994, 269, 16780–16784. [Google Scholar] [CrossRef]
- Siegel, S.J.; Brose, N.; Janssen, W.G.; Gasic, G.P.; Jahn, R.; Heinemann, S.F.; Morrison, J.H. Regional, cellular, and ultrastructural distribution of N-methyl-D-aspartate receptor subunit 1 in monkey hippocampus. Proc. Natl. Acad. Sci. USA 1994, 91, 564–568. [Google Scholar] [CrossRef]
- Beckerman, M.A.; Glass, M.J. The NMDA-NR1 receptor subunit and the mu-opioid receptor are expressed in somatodendritic compartments of central nucleus of the amygdala neurons projecting to the bed nucleus of the stria terminalis. Exp. Neurol. 2012, 234, 112–126. [Google Scholar] [CrossRef][Green Version]
- Hof, P.; Young, W.; Bloom, F.; Belinchenko, P.; Ceilo, M. Comparative Cytoarchitectonic Atlas of the C57BL/6 and 129/SV Mouse Brains, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Peters, A.; Palay, S.; Webster, H. The Fine Structure of the Nervous System; Oxford UP: New York, NY, USA, 1991. [Google Scholar]
- Boudin, H.; Pelaprat, D.; Rostene, W.; Pickel, V.M.; Beaudet, A. Correlative ultrastructural distribution of neurotensin receptor proteins and binding sites in the rat substantia nigra. J. Neurosci. 1998, 18, 8473–8484. [Google Scholar] [CrossRef]
- Haberstock-Debic, H.; Wein, M.; Barrot, M.; Colago, E.E.O.; Rahman, Z.; Neve, R.L.; Pickel, V.M.; Nestler, E.J.; von Zastrow, M.; Svingos, A.L. Morphine acutely regulates opioid receptor trafficking selectively in dendrites of nucleus accumbens neurons. J. Neurosci. 2003, 23, 4324–4332. [Google Scholar] [CrossRef][Green Version]
- Fernandez-Monreal, M.; Brown, T.C.; Royo, M.; Esteban, J.A. The balance between receptor recycling and trafficking toward lysosomes determines synaptic strength during long-term depression. J. Neurosci. 2012, 32, 13200–13205. [Google Scholar] [CrossRef]
- Pierce, J.P.; van Leyen, K.; McCarthy, J.B. Translocation machinery for synthesis of integral membrane and secretory proteins in dendritic spines. Nat. Neurosci. 2000, 3, 311–313. [Google Scholar] [CrossRef]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef]
- Dore, K.; Stein, I.S.; Brock, J.A.; Castillo, P.E.; Zito, K.; Sjöström, P.J. Unconventional NMDA Receptor Signaling. J. Neurosci. 2017, 37, 10800–10807. [Google Scholar] [CrossRef]
- Yang, Y.; Calakos, N. Presynaptic long-term plasticity. Front. Synaptic Neurosci. 2013, 5, 8. [Google Scholar] [CrossRef]
- Bouvier, G.; Bidoret, C.; Casado, M.; Paoletti, P. Presynaptic NMDA receptors: Roles and rules. Neuroscience 2015, 311, 322–340. [Google Scholar] [CrossRef]
- Paquet, M.; Smith, Y. Presynaptic NMDA receptor subunit immunoreactivity in GABAergic terminals in rat brain. J. Comp. Neurol. 2000, 423, 330–347. [Google Scholar] [CrossRef]
- Contoreggi, N.H.; Mazid, S.; Goldstein, L.B.; Park, J.; Ovalles, A.C.; Waters, E.M.; Glass, M.J.; Milner, T.A. Sex and age influence gonadal steroid hormone receptor distributions relative to estrogen receptor β-containing neurons in the mouse hypothalamic paraventricular nucleus. J. Comp. Neurol. 2021, 529, 2283–2310. [Google Scholar] [CrossRef]
- Woods, C.; Contoreggi, N.H.; Johnson, M.A.; Milner, T.A.; Wang, G.; Glass, M.J. Estrogen receptor beta activity contributes to both tumor necrosis factor alpha expression in the hypothalamic paraventricular nucleus and the resistance to hypertension following angiotensin II in female mice. Neurochem. Int. 2022, 161, 105420. [Google Scholar] [CrossRef]
- Neubauer, F.B.; Min, R.; Nevian, T. Presynaptic NMDA receptors influence Ca(2+) dynamics by interacting with voltage-dependent calcium channels during the induction of long-term depression. Neural Plast. 2022, 2022, 2900875. [Google Scholar] [CrossRef]
- Lituma, P.J.; Kwon, H.B.; Alviña, K.; Luján, R.; Castillo, P.E. Presynaptic NMDA receptors facilitate short-term plasticity and BDNF release at hippocampal mossy fiber synapses. eLife 2021, 10, e66612. [Google Scholar] [CrossRef]
- Pittaluga, A. Presynaptic release-regulating NMDA receptors in isolated nerve terminals: A narrative review. Br. J. Pharmacol. 2021, 178, 1001–1017. [Google Scholar] [CrossRef]
- Li, Y.F.; Cornish, K.G.; Patel, K.P. Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ. Res. 2003, 93, 990–997. [Google Scholar] [CrossRef]
- Ma, H.; Chen, S.-R.; Chen, H.; Li, L.; Li, D.-P.; Zhou, J.-J.; Pan, H.-L. α2δ-1 Is Essential for Sympathetic Output and NMDA Receptor Activity Potentiated by Angiotensin II in the Hypothalamus. J. Neurosci. 2018, 38, 6388–6398. [Google Scholar] [CrossRef]
- Zhang, K.; Patel, K.P. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: Role of GABA. Am. J. Physiol. 1998, 275, R728–R734. [Google Scholar] [CrossRef]
- Allen, A.M. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension 2002, 39, 275–280. [Google Scholar] [CrossRef]
- Cruz, J.C.; Machado, B.H. GABA and nitric oxide in the PVN are involved in arterial pressure control but not in the chemoreflex responses in rats. Auton. Neurosci. 2009, 146, 47–55. [Google Scholar] [CrossRef]
- Grzęda, E.; Schlicker, E.; Toczek, M.; Zalewska, I.; Baranowska-Kuczko, M.; Malinowska, B. CB(1) receptor activation in the rat paraventricular nucleus induces bi-directional cardiovascular effects via modification of glutamatergic and GABAergic neurotransmission. Naunyn. Schmiedebergs. Arch. Pharmacol. 2017, 390, 25–35. [Google Scholar] [CrossRef]
- Wang, G.; Coleman, C.G.; Chan, J.; Faraco, G.; Marques-Lopes, J.; Milner, T.A.; Guruju, M.R.; Anrather, J.; Davisson, R.L.; Iadecola, C.; et al. Angiotensin II slow-pressor hypertension enhances NMDA currents and NOX2-dependent superoxide production in hypothalamic paraventricular neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R1096-106. [Google Scholar] [CrossRef]
- Xue, B.; Singh, M.; Guo, F.; Hay, M.; Johnson, A.K. Protective actions of estrogen on angiotensin II-induced hypertension: Role of central nitric oxide. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1638–H1646. [Google Scholar] [CrossRef]
- Gingerich, S.; Krukoff, T.L. Estrogen in the paraventricular nucleus attenuates L-glutamate-induced increases in mean arterial pressure through estrogen receptor beta and NO. Hypertension 2006, 48, 1130–1136. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommer, G.; Rodríguez López, C.; Hirschkorn, A.; Calimano, G.; Marques-Lopes, J.; Milner, T.A.; Glass, M.J. Estrogen Receptor Beta Agonist Influences Presynaptic NMDA Receptor Distribution in the Paraventricular Hypothalamic Nucleus Following Hypertension in a Mouse Model of Perimenopause. Biology 2024, 13, 819. https://doi.org/10.3390/biology13100819
Sommer G, Rodríguez López C, Hirschkorn A, Calimano G, Marques-Lopes J, Milner TA, Glass MJ. Estrogen Receptor Beta Agonist Influences Presynaptic NMDA Receptor Distribution in the Paraventricular Hypothalamic Nucleus Following Hypertension in a Mouse Model of Perimenopause. Biology. 2024; 13(10):819. https://doi.org/10.3390/biology13100819
Chicago/Turabian StyleSommer, Garrett, Claudia Rodríguez López, Adi Hirschkorn, Gianna Calimano, Jose Marques-Lopes, Teresa A. Milner, and Michael J. Glass. 2024. "Estrogen Receptor Beta Agonist Influences Presynaptic NMDA Receptor Distribution in the Paraventricular Hypothalamic Nucleus Following Hypertension in a Mouse Model of Perimenopause" Biology 13, no. 10: 819. https://doi.org/10.3390/biology13100819
APA StyleSommer, G., Rodríguez López, C., Hirschkorn, A., Calimano, G., Marques-Lopes, J., Milner, T. A., & Glass, M. J. (2024). Estrogen Receptor Beta Agonist Influences Presynaptic NMDA Receptor Distribution in the Paraventricular Hypothalamic Nucleus Following Hypertension in a Mouse Model of Perimenopause. Biology, 13(10), 819. https://doi.org/10.3390/biology13100819





