Evaluation of the Anti-Inflammatory/Immunomodulatory Effect of Teucrium montanum L. Extract in Collagen-Induced Arthritis in Rats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction
2.2. LC-MS Analysis
2.3. Experimental Animals
2.4. Induction and Clinical Assessment of CIA
2.5. Experimental Design
2.6. Serum Collection
2.7. Cell Preparation
2.8. Paw Culture and Secreted Cytokines Determination
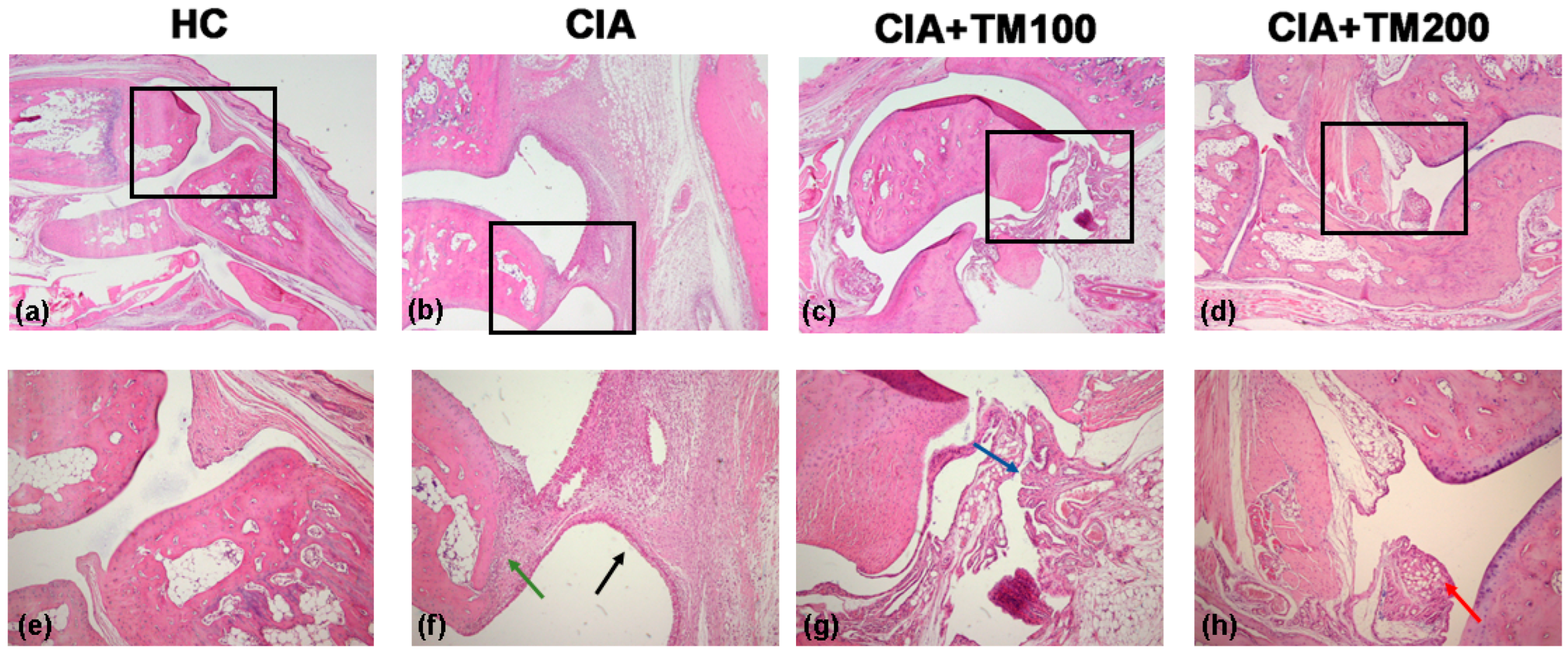
2.9. Histopathological Analysis
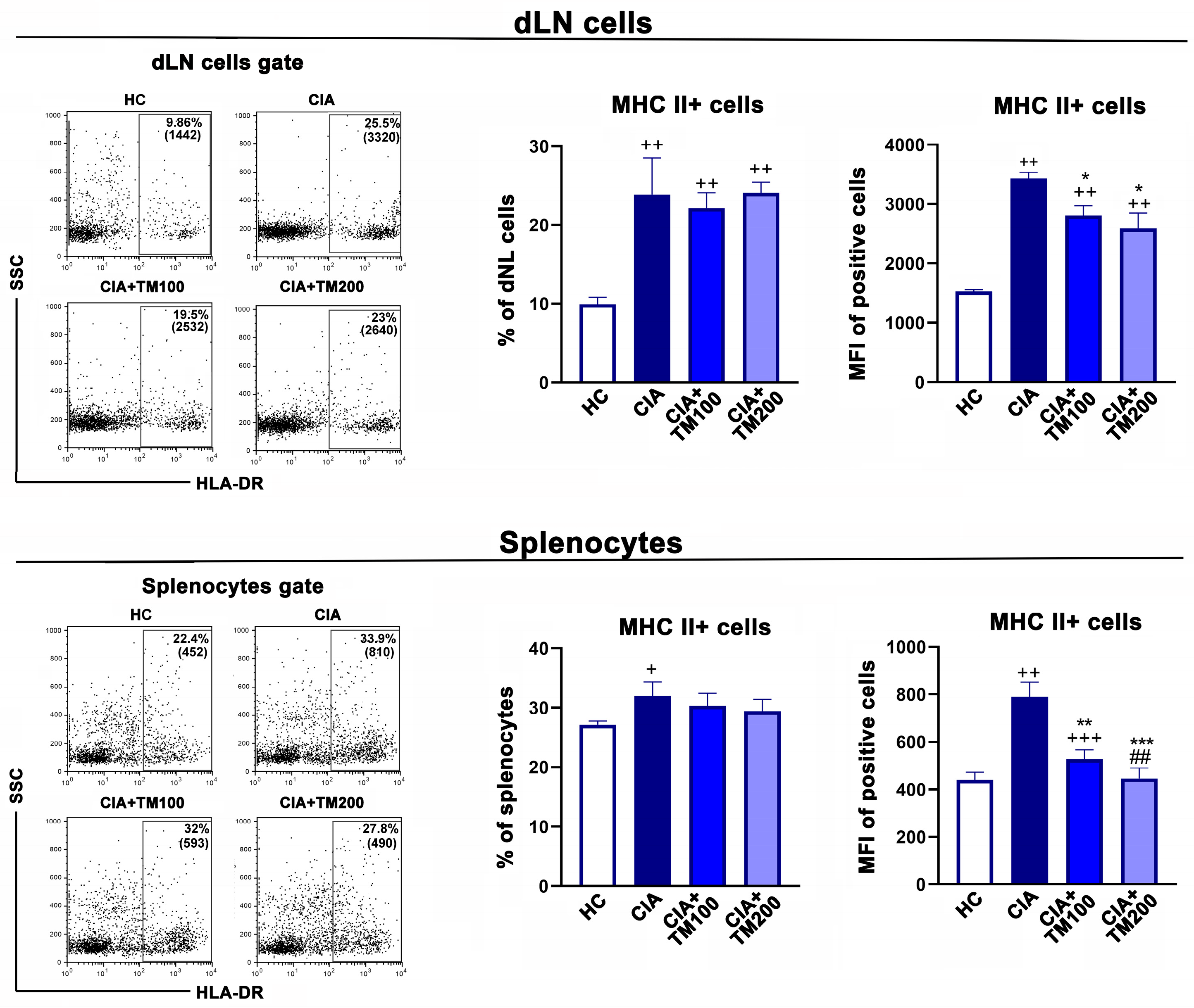
2.10. Cell Immunostaining and Flow Cytometry Analysis
2.11. Anti-CII Antibody ELISA
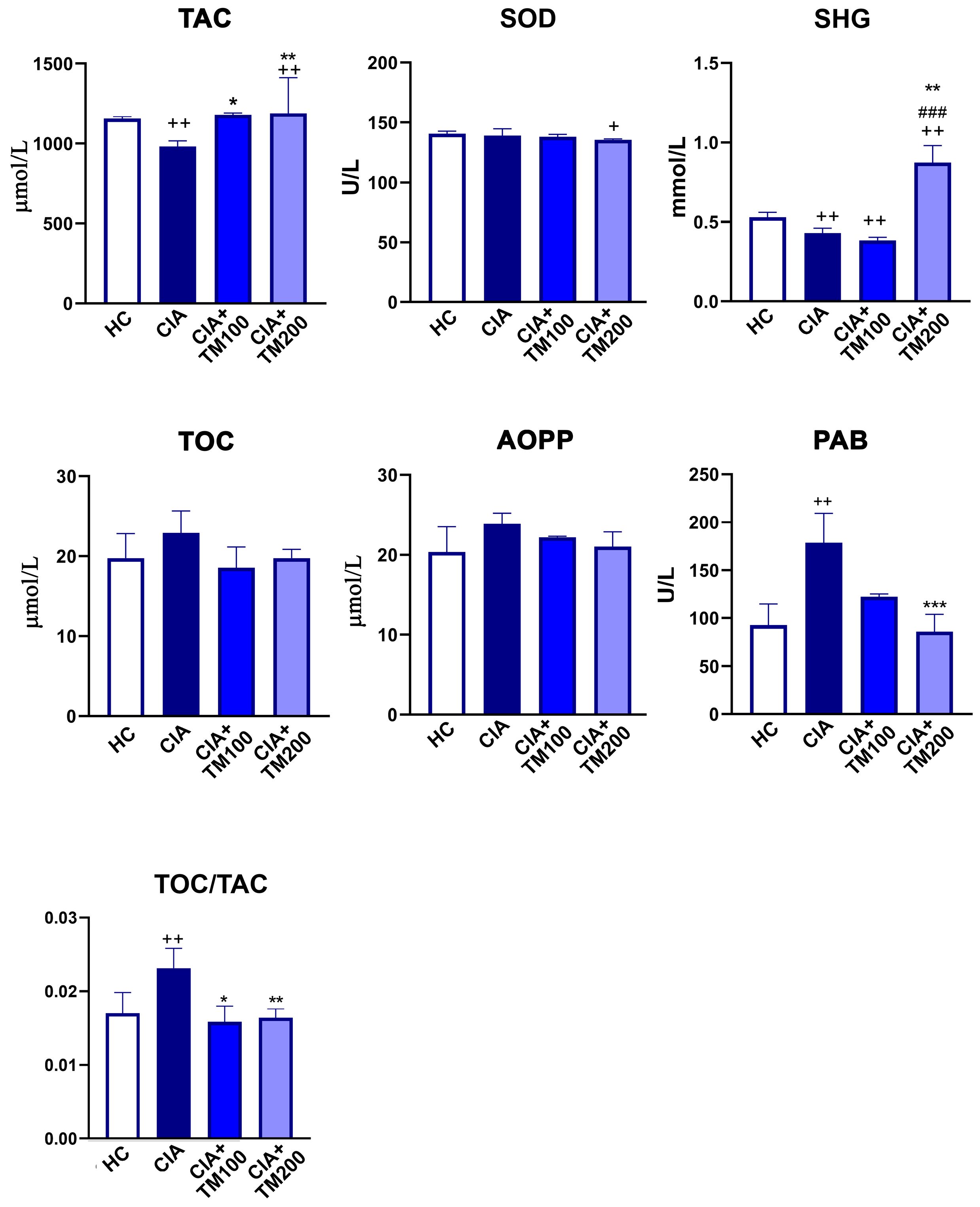
2.12. Oxidative Stress Analysis
2.13. Aspartate Aminotransferase and Alanine Aminotransferase Determination
2.14. Statistical Analysis
3. Results
3.1. Composition of T. montanum Extract
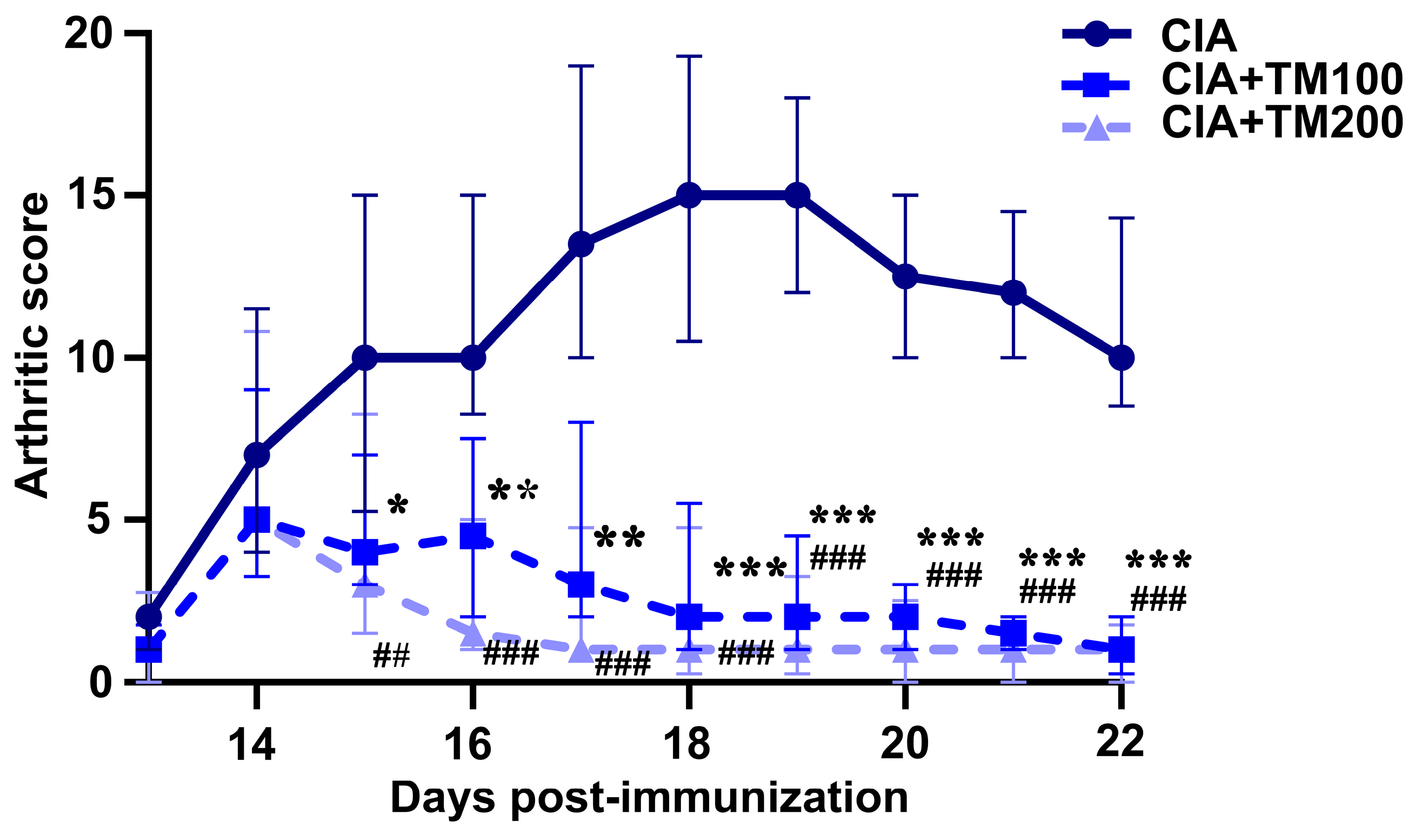
3.2. Extract T. montanum Improved Clinical and Histopathological Signs of Arthritis in CIA Rats
3.3. T. montanum Extract Promoted Anti-Oxidant Activity in CIA Rats
3.4. T. montanum Extract Reduced Production of Pro-Inflammatory Cytokines While Increased Production of Anti-Inflammatory Cytokines in Cia-Rats
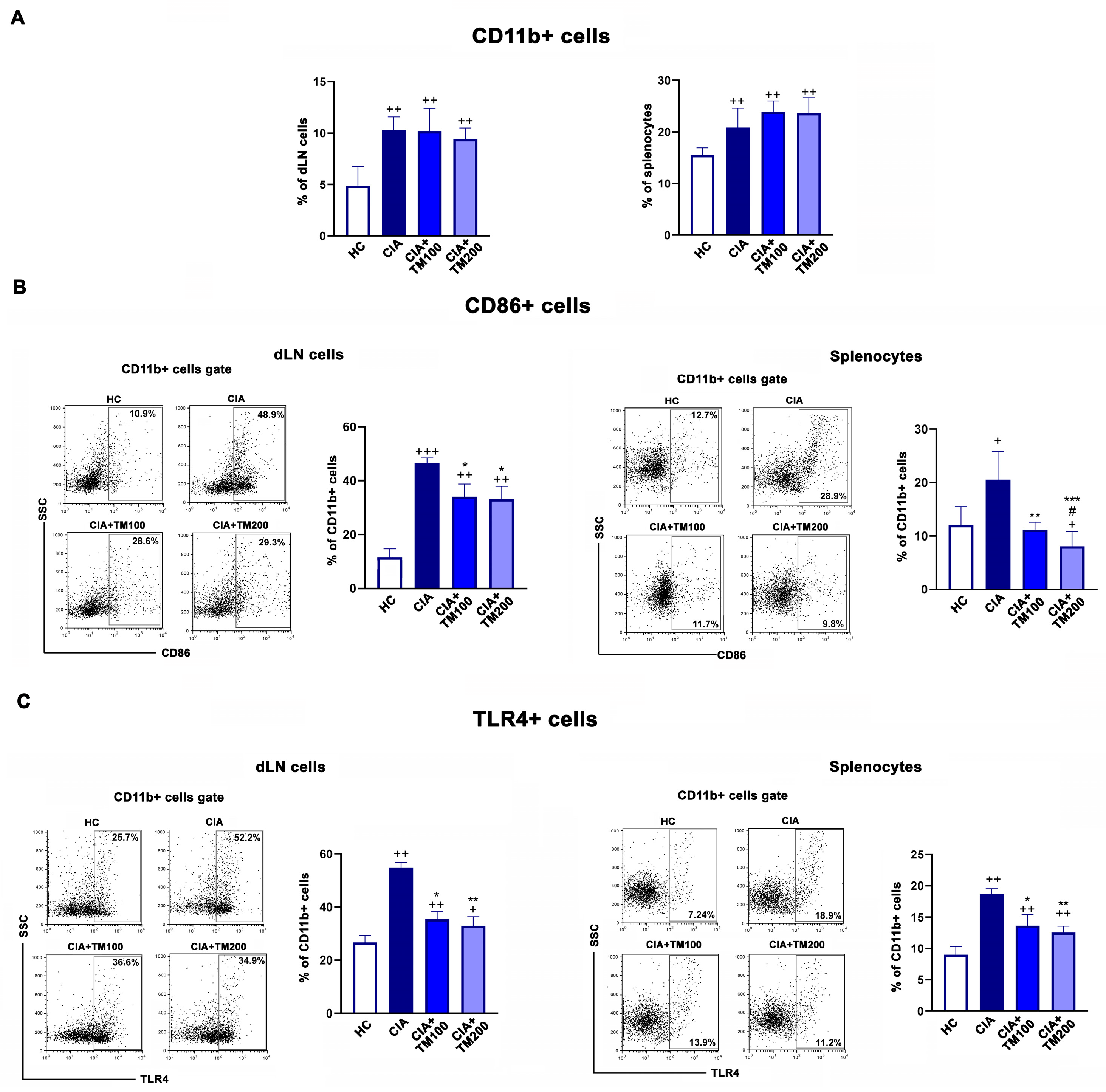
3.5. T. montanum Extract Affected Activation Status of Antigen-Presenting Cells in Secondary Lymphoid Organs
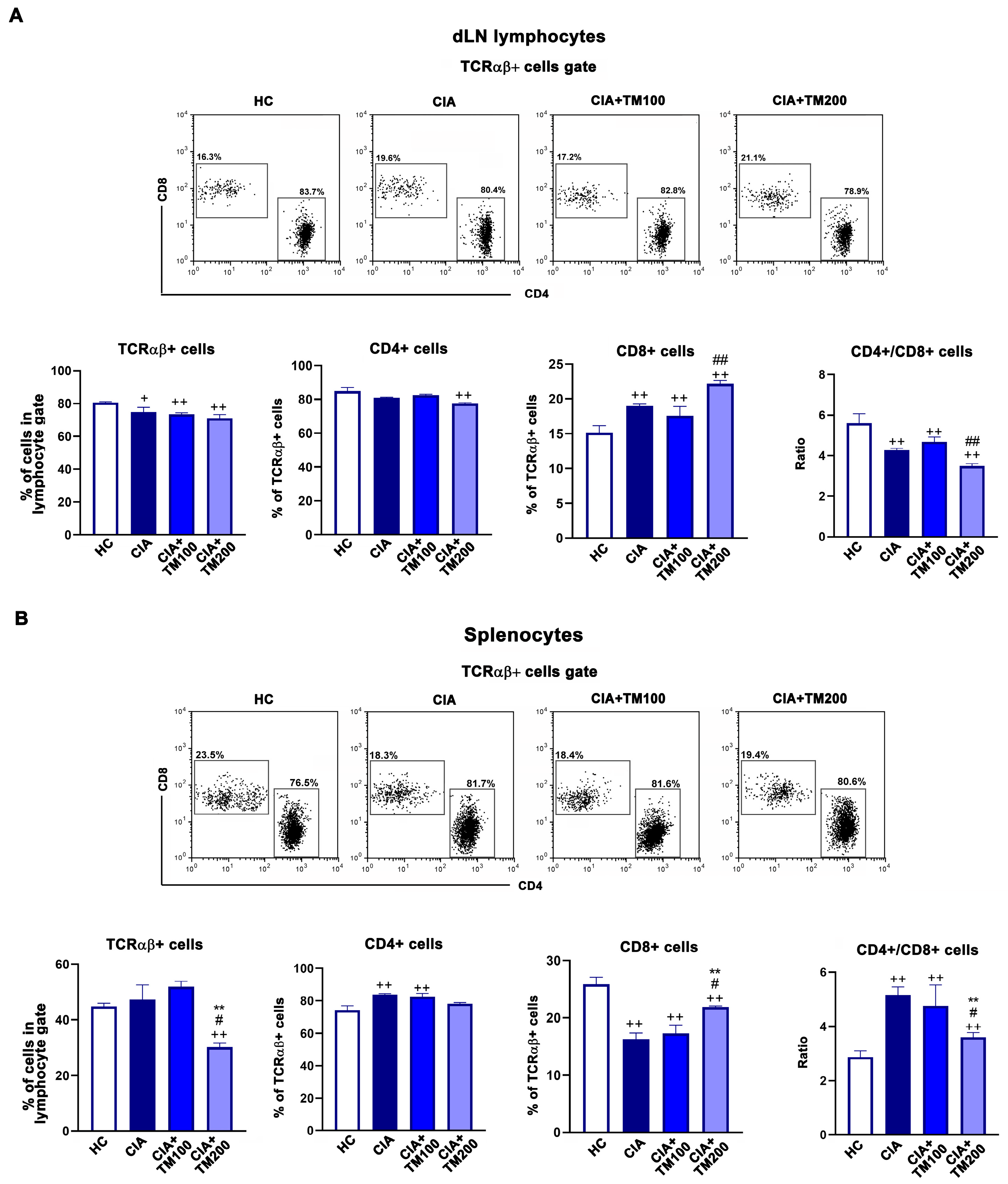
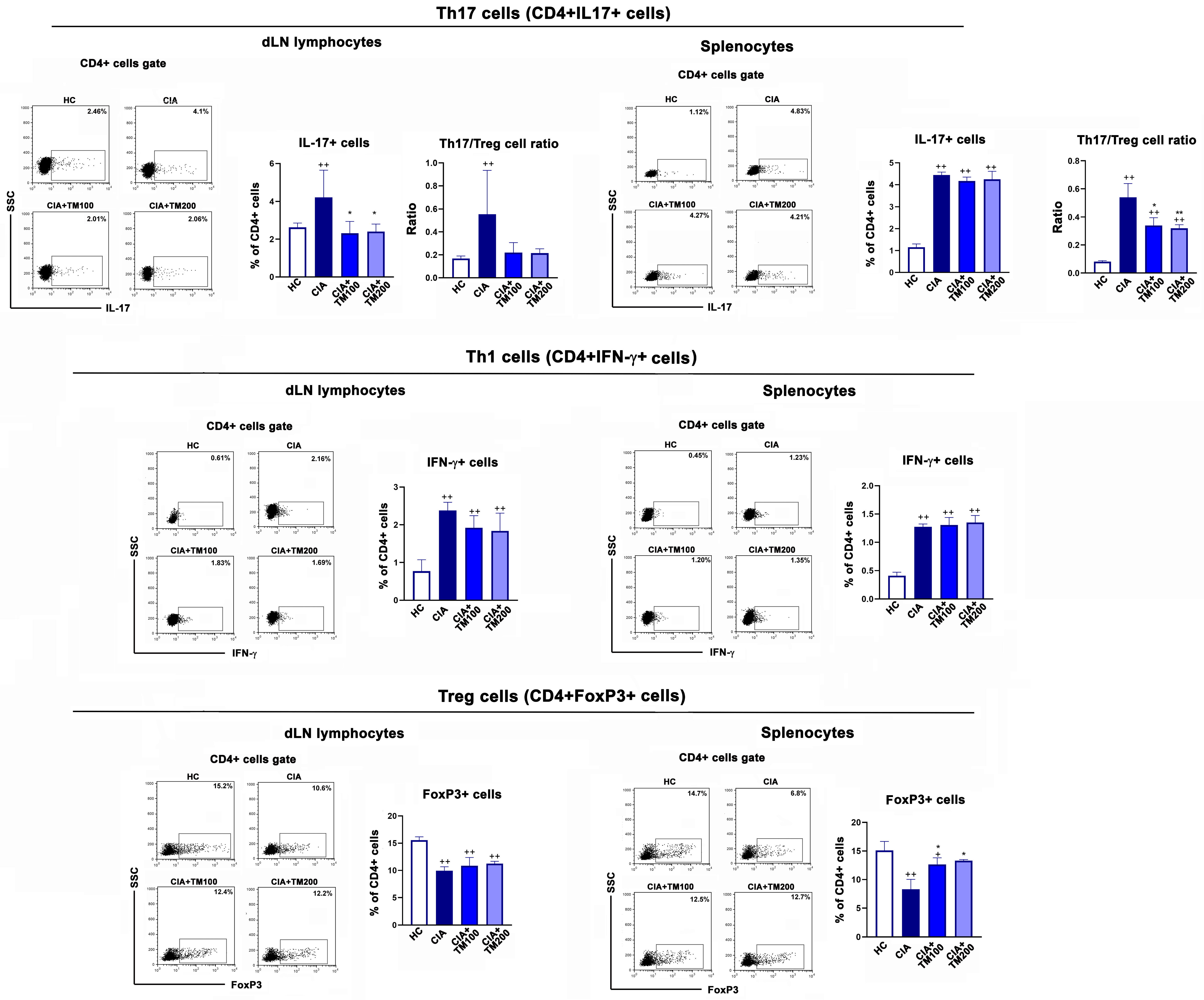
3.6. T. montanum Extract Affected T-Cell Mediated Response in CIA Rats
3.7. T. montanum Extract Reduced Circulating Level of Anti-CII Antibodies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Black, R.J.; Cross, M.; Haile, L.M.; Culbreth, G.T.; Steinmetz, J.D.; Hagins, H.; A Kopec, J.; Brooks, P.M.; Woolf, A.D.; Ong, K.L.; et al. Global, regional, and national burden of rheumatoid arthritis, 1990–2020, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e594–e610. [Google Scholar] [CrossRef] [PubMed]
- Alivernini, S.; Firestein, G.S.; McInnes, I.B. The pathogenesis of rheumatoid arthritis. Immunity 2022, 55, 2255–2270. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.-L.; Payandeh, Z.; Mohammadkhani, Z.; Mubarak, S.M.H.; Zakeri, A.; Alagheband Bahrami, A.; Brockmueller, A.; Shakibaei, M. Recent Advances in Understanding the Pathogenesis of Rheumatoid Arthritis: New Treatment Strategies. Cells 2021, 10, 3017. [Google Scholar] [CrossRef] [PubMed]
- Holers, V.M.; Demoruelle, M.K.; Kuhn, K.A.; Buckner, J.H.; Robinson, W.H.; Okamoto, Y.; Norris, J.M.; Deane, K.D. Rheumatoid arthritis and the mucosal origins hypothesis: Protection turns to destruction. Nat. Rev. Rheumatol. 2018, 14, 542–557. [Google Scholar] [CrossRef]
- Kollias, G.; Papadaki, P.; Apparailly, F.; Vervoordeldonk, M.J.; Holmdahl, R.; Baumans, V.; Desaintes, C.; Di Santo, J.; Distler, J.; Garside, P.; et al. Animal models for arthritis: Innovative tools for prevention and treatment. Ann. Rheumatol. Dis. 2011, 70, 1357–1362. [Google Scholar] [CrossRef]
- James, E.A.; Rieck, M.; Pieper, J.; Gebe, J.A.; Yue, B.B.; Tatum, M.; Peda, M.; Sandin, C.; Klareskog, L.; Malmström, V.; et al. Citrulline Specific Th1 Cells Are Increased in Rheumatoid Arthritis and Their Frequency Is Influenced by Disease Duration and Therapy. Arthritis Rheumatol. 2014, 66, 1712–1722. [Google Scholar] [CrossRef]
- Kotake, S.; Yago, T.; Kobashigawa, T.; Nanke, Y. The Plasticity of Th17 Cells in the Pathogenesis of Rheumatoid Arthritis. J. Clin. Med. 2017, 6, 67. [Google Scholar] [CrossRef]
- Luo, P.; Wang, P.; Xu, J.; Hou, W.; Xu, P.; Xu, K.; Liu, L. Immunomodulatory role of T helper cells in rheumatoid arthritis. Bone Jt. Res. 2022, 11, 426–438. [Google Scholar] [CrossRef]
- Noack, M.; Miossec, P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun. Rev. 2014, 3, 668–677. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Chen, Y.; Liu, H.; Zhang, S.; Yin, G.; Xie, Q. Augmenting regulatory T cells: New therapeutic strategy for rheumatoid arthritis. Front. Immunol. 2024, 15, 1312919. [Google Scholar] [CrossRef]
- Martinez-Gamboa, L.; Brezinschek, H.P.; Burmester, G.R.; Dorner, T. Immunopathologic role of B lymphocytes in rheumatoid arthritis: Rationale of B cell-directed therapy. Autoimmun. Rev. 2006, 5, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheumatol. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Fraenkel, L.; Bathon, J.M.; England, B.R.; St Clair, E.W.; Arayssi, T.; Carandang, K.; Deane, K.D.; Genovese, M.; Huston, K.K.; Kerr, G.; et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2021, 73, 924–939. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Anzaghe, M.; Schülke, S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells 2020, 9, 880. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Kim, Y.R.; Min, Y.; Zhao, Y.; Do, K.; Son, Y.O. Natural Plant Extracts and Compounds for Rheumatoid Arthritis Therapy. Medicina 2021, 57, 266. [Google Scholar] [CrossRef]
- Lindler, B.N.; Long, K.E.; Taylor, N.A.; Lei, W. Use of Herbal Medications for Treatment of Osteoarthritis and Rheumatoid Arthritis. Medicines 2020, 7, 67. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Du, K.; Liang, C.; Wang, S.; Owusu Boadi, E.; Li, J.; Pang, X.; He, J.; Chang, Y.X. Traditional herbal medicine: Therapeutic potential in rheumatoid arthritis. J. Ethnopharmacol. 2021, 279, 114368. [Google Scholar] [CrossRef]
- Yu, G.M.; Zhou, L.F.; Zeng, B.X.; Huang, J.J.; She, X.J. The antioxidant effect of triptolide contributes to the therapy in a collagen-induced arthritis rat model. Redox Rep. 2021, 26, 197–202. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Zhu, W.; Ma, C.; Ruan, J.; Long, H.; Wang, Y. Sinomenine inhibits the progression of rheumatoid arthritis by regulating the secretion of inflammatory cytokines and monocyte/macrophage subsets. Front. Immunol. 2018, 9, 2228. [Google Scholar] [CrossRef]
- Feng, Z.T.; Yang, T.; Hou, X.Q.; Wu, H.Y.; Feng, J.T.; Ou, B.J.; Cai, S.J.; Li, J.; Mei, Z.G. Sinomenine mitigates collagen-induced arthritis mice by inhibiting angiogenesis. Biomed. Pharmacother. 2019, 113, 108759. [Google Scholar] [CrossRef]
- Sung, S.; Kwon, D.; Um, E.; Kim, B. Could Polyphenols Help in the Control of Rheumatoid Arthritis? Molecules 2019, 24, 1589. [Google Scholar] [CrossRef] [PubMed]
- Nho, J.H.; Kim, A.H.; Jung, H.K.; Lee, M.J.; Jang, J.H.; Yang, B.D.; Lee, H.J.; Lee, K.H.; Woo, K.W.; Cho, H.W. Water Extract of Acori Graminei Rhizoma Attenuates Features of Rheumatoid Arthritis in DBA/1 Mice. Evid. Based Complement. Altern. Med. 2019, 2019, 3637453. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Li, Q.; Liu, J.; Wang, H.; Li, X.; Wüntrang, D.; Liu, C.; Zhao, Q.; Yao, R.; Meng, X.; et al. Ethyl acetate extract of Tibetan medicine Rhamnella gilgitica ameliorated type II collagen-induced arthritis in rats via regulating JAK-STAT signaling pathway. J. Ethnopharmacol. 2021, 267, 113514. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, N.; Lakusic, B.; Ristic, M. Composition of the Essential Oils of Seven Teucrium Species from Serbia and Montenegro. J. Essent. Oil Res. 2001, 13, 163–165. [Google Scholar] [CrossRef]
- Grubešić, R.; Kremer, D.; Vladimir-Knežević, S.; Rodríguez, J. Analysis of polyphenols, phytosterols, and bitter principles in Teucrium L. species. Cent. Eur. J. Biol. 2012, 7, 542–550. [Google Scholar] [CrossRef]
- Sailović, P.; Odžaković, B.; Bodroža, D.; Vulić, J.; Čanadanović-Brunet, J.; Zvezdanović, J.; Danilović, B. Polyphenolic Composition and Antimicrobial, Antioxidant, Anti-Inflammatory, and Antihyperglycemic Activity of Different Extracts of Teucrium montanum from Ozren Mountain. Antibiotics 2024, 13, 358. [Google Scholar] [CrossRef]
- Jarić, S.; Mitrović, M.; Pavlović, P. Ethnobotanical Features of Teucrium Species. In Teucrium Species: Biology and Applications, 1st ed.; Stanković, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 111–142. [Google Scholar] [CrossRef]
- Li, J.J.; Ma, S.; Wang, Y.; Wang, M.; Li, M.; Gao, C.; Zhang, L.; Li, Y.; Liu, Y.; Dajić Stevanović, Z.; et al. Teucrium montanum extract drives effector and memory differentiation of CD8+ T cells. Biomed. Res. Ther. 2023, 10, 6023–6034. [Google Scholar] [CrossRef]
- Vukovic, N.; Milosevic, T.; Sukdolak, S.; Solujic, S. Antimicrobial Activities of Essential Oil and Methanol Extract of Teucrium montanum. Evid. Based Complement. Altern. Med. 2007, 4 (Suppl. S1), 17–20. [Google Scholar] [CrossRef] [PubMed]
- Dinić, J.; Novaković, M.; Pešić, M. Potential for cancer treatment: Natural products from the Balkans. In Biodiversity and Biomedicine: Our Future; Ozturk, M., Egamberdieva, D., Pešić, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 137–159. ISBN 9780128195413. [Google Scholar]
- Aćimović, M.; Stanković Jeremić, J.; Miljković, A.; Rat, M.; Lončar, B. Screening of Volatile Compounds, Traditional and Modern Phytotherapy Approaches of Selected Non-Aromatic Medicinal Plants (Lamiaceae, Lamioideae) from Rtanj Mountain, Eastern Serbia. Molecules 2023, 28, 4611. [Google Scholar] [CrossRef]
- Candela, R.G.; Rosselli, S.; Bruno, M.; Fontana, G. A Review of the Phytochemistry, Traditional Uses and Biological Activities of the Essential Oils of Genus Teucrium. Planta Medica 2021, 87, 432–479. [Google Scholar] [CrossRef]
- de Boer, Y.S.; Sherker, A.H. Herbal and Dietary Supplement-Induced Liver Injury. Clin. Liver Dis. 2017, 21, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Dag, M.S.; Aydinli, M.; Ozturk, Z.A.; Turkbeyler, I.H.; Koruk, I.; Savas, M.C.; Koruk, M.; Kadayifci, A. Drug- and herb-induced liver injury: A case series from a single center. Turk. J. Gastroenterol. 2014, 25, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Šteigerová, M.; Šíma, M.; Slanař, O. Pathogenesis of Collagen-Induced Arthritis: Role of Immune Cells with Associated Cytokines and Antibodies, Comparison with Rheumatoid Arthritis. Folia Biol. 2023, 69, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijević, M.; Arsenović-Ranin, N.; Bufan, B.; Nacka-Aleksić, M.; Lazarević Macanović, M.; Milovanović, P.; Đurić, M.; Sopta, J.; Leposavić, G. Collagen-induced arthritis in Dark Agouti rats as a model for study of immunological sexual dimorphisms in the human disease. Exp. Mol. Pathol. 2018, 105, 10–22. [Google Scholar] [CrossRef]
- Dimitrijević, M.; Arsenović-Ranin, N.; Kosec, D.; Bufan, B.; Nacka-Aleksić, M.; Pilipović, I.; Leposavić, G. Sexual dimorphism in Th17/Treg axis in lymph nodes draining inflamed joints in rats with collagen-induced arthritis. Brain Behav. Immun. 2019, 76, 198–214. [Google Scholar] [CrossRef]
- Bevaart, L.; Vervoordeldonk, M.J.; Tak, P.P. Evaluation of therapeutic targets in animal models of arthritis: Howdoesit relate to rheumatoid arthritis. Arthritis Rheumatol. 2010, 62, 2192–2205. [Google Scholar] [CrossRef]
- Luross, J.A.; Williams, N.A. The genetic and immunopathological processes underlying collagen-induced arthritis. Immunology 2001, 103, 407–416. [Google Scholar] [CrossRef]
- Elferjane, M.R.; Milutinović, V.; Jovanović Krivokuća, M.; Taherzadeh, M.J.; Pietrzak, W.; Marinković, A.; Jovanović, A.A. Vaccinium myrtillus L. Leaf Waste as a Source of Biologically Potent Compounds: Optimization of Polyphenol Extractions, Chemical Profile, and Biological Properties of the Extracts. Pharmaceutics 2024, 16, 740. [Google Scholar] [CrossRef]
- International Conference on Harmonisation (ICH) 2005. ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures: Text and Methodology Q2 (R1); ICH: Geneva, Switzerland, 2005. [Google Scholar]
- Hou, W.; Meng, L.; Tian, L.; Zhu, W.; Jiang, C.; Lu, S. A systematic comparison between collagen-induced arthritis and pristane-induced arthritis in Dark Agouti rats. Clin. Exp. Rheumatol. 2010, 28, 532–538. [Google Scholar] [PubMed]
- Hawkins, P.; Armstrong, R.; Boden, T.; Garside, P.; Knight, K.; Lilley, E.; Seed, M.; Wilkinson, M.; Williams, R.O. Applying refinement to the use of mice and rats in rheumatoid arthritis research. Inflammopharmacology 2015, 23, 131–150. [Google Scholar] [CrossRef]
- Al-Naemi, H.A.; Alasmar, R.M.; Al-Ghanim, K. Alcoholic extracts of Teucrium polium exhibit remarkable anti-inflammatory activity: In vivo study. Biomol. Biomed. 2024, 24, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Alamdari, D.H.; Paletas, K.; Pegiou, T.; Sarigianni, M.; Befani, C.; Koliakos, G. A novel assay for the evaluation of the prooxidant-antioxidant balance, before and after antioxidant vitamin administration in type II diabetes patients. Clin. Biochem. 2007, 40, 248–254. [Google Scholar] [CrossRef]
- Kotur-Stevuljevic, J.; Bogavac-Stanojevic, N.; Jelic-Ivanovic, Z.; Stefanovic, A.; Gojkovic, T.; Joksic, J.; Sopic, M.; Gulan, B.; Janac, J.; Milosevic, S. Oxidative stress and paraoxonase 1 status in acute ischemic stroke patients. Atherosclerosis 2015, 241, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V.; Friedlander, M.; Capeillere-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Kaur, G.; Sharma, A.; Bhatnagar, A. Role of oxidative stress in pathophysiology of rheumatoid arthritis: Insights into NRF2-KEAP1 signalling. Autoimmunity 2021, 54, 385–397. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef]
- Jang, S.; Kwon, E.-J.; Jooha Lee, J. Rheumatoid Arthritis: Pathogenic Roles of Diverse Immune Cells. Int. J. Mol. Sci. 2022, 23, 905. [Google Scholar] [CrossRef]
- Dimitrijević, M.; Arsenović-Ranin, N.; Bufan, B.; Nacka-Aleksić, M.; Kosec, D.; Pilipović, I.; Kotur-Stevuljević, J.; Simić Lj Sopta, J.; Leposavić, G. Sex-Based Differences in Monocytic Lineage Cells Contribute to More Severe Collagen-Induced Arthritis in Female Rats Compared with Male Rats. Inflammation 2020, 43, 2312–2331. [Google Scholar] [CrossRef]
- Odobasic, D.; Leech, M.T.; Xue, J.R.; Holdsworth, S.R. Distinct in vivo roles of CD80 and CD86 in the effector T-cell responses inducing antigen-induced arthritis. Immunology 2008, 124, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Edilova, M.I.; Akram, A.; Abdul-Sater, A.A. Innate immunity drives pathogenesis of rheumatoid arthritis. Biomed. J. 2021, 44, 172–182. [Google Scholar] [CrossRef]
- Goh, F.G.; Midwood, K.S. Intrinsic danger: Activation of Toll-like receptors in rheumatoid arthritis. Rheumatology 2012, 51, 7–23. [Google Scholar] [CrossRef]
- Ospelt, C.; Brentano, F.; Rengel, Y.; Stanczyk, J.; Kolling, C.; Tak, P.P.; Gay, R.E.; Gay, S.; Kyburz, D. Overexpression of toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis: Toll-like receptor expression in early and longstanding arthritis. Arthritis Rheumatol. 2008, 58, 3684–3692. [Google Scholar] [CrossRef]
- Tada, T.; Koarada, S.; Morito, F.; Mitamura, M.; Inoue, H.; Suematsu, R.; Ohta, A.; Miyake, K.; Nagasawa, K. Toll-like receptor homolog RP105 modulates the antigen-presenting cell function and regulates the development of collagen-induced arthritis. Arthritis Res. Ther. 2008, 10, R121. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H. Adaptive immunity in the joint of rheumatoid arthritis. Immunol. Med. 2022, 45, 1–11. [Google Scholar] [CrossRef]
- Hussein, M.R.; Fathi, N.A.; El-Din, A.M.E.; Hassan, H.I.; Abdullah, F.A.; Al-Hakeem, E.; Backer, E.A. Alterations of the CD4+, CD8+ T cell subsets, interleukins-1beta, IL-10, IL-17, tumor necrosis factor-alpha and soluble intercellular adhesion molecule-1 in rheumatoid arthritis and osteoarthritis: Preliminary observations. Pathol. Oncol. Res. 2008, 14, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Wang, Q.T.; Song, S.S.; Wu, Y.J.; Ma, Y.K.; Zhang, L.L.; Chen, J.Y.; Wu, H.X.; Jiang, L.; Wei, W. Combined use of etanercept and MTX restores CD4⁺/CD8⁺ ratio and Tregs in spleen and thymus in collagen-induced arthritis. Inflamm. Res. 2012, 61, 1229–1239. [Google Scholar] [CrossRef]
- Perng, O.A.; Aitken, M.; Rankin, A.L.; Garcia, V.; Kropf, E.; Erikson, J.; Garlick, D.S.; Caton, A.J. The degree of CD4+ T cell autoreactivity determines cellular pathways underlying inflammatory arthritis. J. Immunol. 2014, 192, 3043–3056. [Google Scholar] [CrossRef]
- Chemin, K.; Gerstner, C.; Malmström, V. Effector Functions of CD4+ T Cells at the Site of Local Autoimmune Inflammation—Lessons From Rheumatoid Arthritis. Front. Immunol. 2019, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yang, G.; Liu, Q.; Wang, S.; Cui, D. Function and Role of Regulatory T Cells in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 626193. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, M.S.; Curcic, M.G.; Zizic, J.B.; Topuzovic, M.D.; Solujic, S.R.; Markovic, S.D. Teucrium plant species as natural sources of novel anticancer compounds: Antiproliferative, proapoptotic and antioxidant properties. Int. J. Mol. Sci. 2011, 12, 4190–4205. [Google Scholar] [CrossRef] [PubMed]
- Mitreski, I.; Stanoeva, J.P.; Stefova, M.; Stefkov, G.; Kulevanova, S. Polyphenols in representative Teucrium species in the Flora of R. Macedonia: LC/DAD/ESI-MSn profile and content. Nat. Prod. Commun. 2014, 9, 1934578X1400900211. [Google Scholar] [CrossRef]
- Mandura Jarić, A.; Čikoš, A.; Pocrnić, M.; Aladić, K.; Jokić, S.; Šeremet, D.; Vojvodić Cebin, A.; Komes, D. Teucrium montanum L.—Unrecognized source of phenylethanoid glycosides: Green extraction approach and elucidation of Phenolic compounds via NMR and UHPLC-HR MS/MS. Antioxidants 2023, 12, 1903. [Google Scholar] [CrossRef]
- Galli, A.; Marciani, P.; Marku, A.; Ghislanzoni, S.; Bertuzzi, F.; Rossi, R.; Di Giancamillo, A.; Castagna, M.; Perego, C. Verbascoside Protects Pancreatic β-Cells against ER-Stress. Biomedicines 2020, 8, 582. [Google Scholar] [CrossRef]
- Lee, J.Y.; Woo Eun-Rhan Kang, K.W. Inhibition of lipopolysaccharide-inducible nitric oxide synthase expression by acteoside through blocking of AP-1 activation. J. Ethnopharmacol. 2005, 97, 561–566. [Google Scholar] [CrossRef]
- Alipieva, K.; Korkina, L.; Erdogan Orhan, I.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef]
- Seo, E.S.; Oh, B.K.; Pak, J.H.; Yim, S.-H.; Gurunathan, S.; Kim, Y.-P.; Lee, K.J. Acteoside improves survival in cecal ligation and puncture-induced septic mice via blocking of high mobility group box 1 release. Mol. Cells 2013, 35, 348–354. [Google Scholar] [CrossRef]
- Pastore, S.; Lulli, D.; Fidanza, P.; Potapovich, A.I.; Kostyuk, V.A.; De Luca, C.; Mikhal’chik, E.; Korkina, L.G. Plant polyphenols regulate chemokine expression and tissue repair in human keratinocytes through interaction with cytoplasmic and nuclear components of epidermal growth factor receptor system. Antioxid. Redox Signal 2012, 16, 314–328. [Google Scholar] [CrossRef]
- Speranza, L.; Franceschelli, S.; Pesce, M.; Reale, M.; Menghini, L.; Vinciguerra, I.; De Lutiis, M.A.; Felaco, M.; Grilli, A. Antiinflammatory effects in THP-1 cells treated with verbascoside. Phytother. Res. 2010, 24, 1398–1404. [Google Scholar] [CrossRef]
- Pastore, S.; Potapovich, A.; Kostyuk, V.; Mariani, V.; Lulli, D.; De Luca, C.; Korkina, L. Plant polyphenols effectively protect HaCaT cells from ultraviolet C-triggered necrosis and suppress inflammatory chemokine expression. Ann. N. Y. Acad. Sci. 2009, 1171, 305–311. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, A.; Caporali, S.; Di Daniele, N.; Rovella, V.; Cardillo, C.; Schinzari, F.; Minieri, M.; Pieri, M.; Candi, E.; Bernardini, S.; et al. Anti-Inflammatory and Proliferative Properties of Luteolin-7-O-Glucoside. Int. J. Mol. Sci. 2021, 22, 1321. [Google Scholar] [CrossRef]
- Cho, Y.-C.; Park, J.; Cho, S. Anti-Inflammatory and Anti-Oxidative Effects of luteolin-7-O-glucuronide in LPS-Stimulated Murine Macrophages through TAK1 Inhibition and Nrf2 Activation. Int. J. Mol. Sci. 2020, 21, 2007. [Google Scholar] [CrossRef]
- Da Fonseca, L.J.S.; Nunes-Souza, V.; Goulart, M.O.F.; Rabelo, L.A. Oxidative Stress in Rheumatoid Arthritis: What the Future Might Hold regarding Novel Biomarkers and Add-On Therapies. Oxidative Med. Cell Longev. 2019, 2019, 7536805. [Google Scholar] [CrossRef] [PubMed]
- Quiñonez-Flores, C.M.; González-Chávez, S.A.; Del Río Nájera, D.; Pacheco-Tena, C. Oxidative Stress Relevance in the Pathogenesis of the Rheumatoid Arthritis: A Systematic Review. Biomed. Res. Int. 2016, 2016, 6097417. [Google Scholar] [CrossRef] [PubMed]
- Veselinovic, M.; Barudzic, N.; Vuletic, M.; Zivkovic, V.; Tomic-Lucic, A.; Djuric, D.; Jakovljevic, V. Oxidative stress in rheumatoid arthritis patients: Relationship to diseases activity. Mol. Cell Biochem. 2014, 391, 225–232. [Google Scholar] [CrossRef]
- Smallwood, M.J.; Nissim, A.; Knight, A.R.; Whiteman, M.; Haigh, R.; Winyard, P. Oxidative stress in autoimmune rheumatic diseases. Free Radic. Biol. Med. 2018, 125, 3–14. [Google Scholar] [CrossRef]
- Mititelu, R.R.; Pădureanu, R.; Docea, A.O.; Calina, D.; Băcănoiu, M.; Pădureanu, V.; Barbulescu, A.L.; Buga, A.M. Inflammatory and Oxidative Stress Markers—Mirror Tools in Rheumatoid Arthritis. Biomedicines 2020, 8, 125. [Google Scholar] [CrossRef]
- Ghosh, N.; Pathak, S.; Malsawmdawngkimi Kumar, G.; Gull, A. Natural Products and Traditional Herbal Medicines as Managerial Therapies to Combat Rheumatoid Arthritis. Clin. Transl. Metab. 2024, 22, 2. [Google Scholar] [CrossRef]
- Alam, J.; Jantan, I.; Abbas Bukhari, S.N. Rheumatoid arthritis: Recent advances on its etiology, role of cytokines and pharmacotherapy. Biomed. Pharmacother. 2017, 92, 615–633. [Google Scholar] [CrossRef] [PubMed]
- Madel, M.B.; Ibanez, L.; Wakkach, A.; de Vries, T.J.; Teti, A.; Apparailly, F.; Blin-Wakkach, C. Immune function and diversity of osteoclasts in normal and pathological conditions. Front. Immunol. 2019, 10, 1408. [Google Scholar] [CrossRef]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef]
- Abdolghaffari, A.H.; Baghaei, A.; Moayer, F.; Esmaily, H.; Baeeri, M.; Monsef-Esfahani, H.R.; Hajiaghaee, R.; Abdollahiet, M. On the benefit of Teucrium in murine colitis through improvement of toxic inflammatory mediators. Hum. Exp. Toxicol. 2010, 29, 287–295. [Google Scholar] [CrossRef]
- Bandola-Simon, J.; Roche, P.A. Regulation of MHC class II and CD86 expression by March-I in immunity and disease. Curr. Opin. Immunol. 2023, 82, 102325. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.M.; Majidi, J.; Baradaran, B.; Yousefi, M. Toll-Like Receptors in the Pathogenesis of Autoimmune Diseases. Adv. Pharm. Bull. 2015, 5, 605–614. [Google Scholar] [CrossRef]
- Huang, Q.-Q.; Pope, R.M. Role of Toll like receptors in rheumatoid arthritis. Curr. Rheumatol. Rep. 2009, 11, 357–364. [Google Scholar] [CrossRef]
- Abdollahi-Roodsaz, S.; Joosten, L.A.B.; Roelofs, M.F.; Radstake, T.R.D.J.; Matera, G.; Popa, C.; van der Meer, J.W.M.; Netea, M.G.; van den Berg, W.B. Inhibition of Toll-like Receptor 4 Breaks the Inflammatory Loop in Autoimmune Destructive Arthritis. Arthritis Rheumatol. 2007, 56, 2957–2967. [Google Scholar] [CrossRef]
- Gill, R.; Tsung, A.; Billiar, T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic. Biol. Med. 2010, 48, 1121–1132. [Google Scholar] [CrossRef]
- Chahal, D.S.; Sivamani, R.K.; Isseroff, R.R.; Dasu, M.R. Plant-Based Modulation of Toll-like Receptors: An Emerging Therapeutic Model. Phytother. Res. 2013, 27, 1423–1438. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, K.M.; Matsuno, H.; Tsuji, H.; Tunru, I. CD4+ T cells from collagen-induced arthritic mice are essential to transfer arthritis into severe combined immunodeficient mice. Clin. Exp. Immunol. 1994, 97, 212. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Chen, J. Changes in peripheral blood T lymphocyte subsets predict disease progression in patients with rheumatoid arthritis. Am. J. Transl. Res. 2022, 14, 1068–1075. [Google Scholar] [PubMed]
- Takayanagi, H. Interaction between the immune system and bone metabolism: An emerging field of osteoimmunology. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2007, 83, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Cai, B.; Huang, Z.-C.; Shi, Y.-Y.; Wang, L.-L. Disturbed Th17/Treg balance in patients with rheumatoid arthritis. Rheumatol. Int. 2012, 32, 2731–2736. [Google Scholar] [CrossRef]
- Wang, W.; Su, T.; Sun, H.; Cheng, H.; Jiang, C.; Guo, P.; Zhu, Z.; Fang, R.; He, F.; Ge, M.; et al. Regulating Th17/Treg Balance Contributes to the Therapeutic Effect of Ziyuglycoside I on Collagen-Induced Arthritis. Int. J. Mol. Sci. 2022, 23, 16105. [Google Scholar] [CrossRef]
- Fan, M.; Li, Y.; Yao, C.; Liu, X.; Liu, X.; Liu, J. Dihydroartemisinin derivative DC32 attenuates collagen-induced arthritis in mice by restoring the Treg/Th17 balance and inhibiting synovitis through down-regulation of IL-6. Int. Immunopharmacol. 2018, 65, 233–243. [Google Scholar] [CrossRef]


| No | Rt (min) | m/z (250 V) | λmax, nm | Compound | Content (%, g(100 g)−1 dw) * |
|---|---|---|---|---|---|
| 1 | 10.43 | 353 [M − H]−, 191, 179, 173 | 296, 328 | Chlorogenic acid | 0.317 ± 0.027 |
| 2 | 21.35 | 623 [M − H]−, 461, 315, 161 | 292, 334 | Verbascoside | 6.099 ± 0.349 |
| 3 | 21.35 | 593 [M − H]−, 285 | 254, 268, 350 | Luteolin 7-O-rutinoside | 0.915 ± 0.043 |
| 4 | 22.51 | 447 [M − H]−, 285 | 254, 268, 350 | Luteolin 7-O-glucoside | 0.344 ± 0.002 |
| 5 | 23.35 | 577 [M − H]−, 269 | 266, 338 | Apigenin 7-O-rutinoside | t.r. |
| 6 | 24.96 | 431 [M − H]−, 269 | 266, 336 | Apigenin 7-O-glucoside | 0.045 ± 0.003 |
| 7 | 29.49 | 285 [M − H]−, 256, 241, 151, 133 | 254, 268, 350 | Luteolin | 0.223 ± 0.032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bufan, B.; Marčetić, M.; Djuretić, J.; Ćuruvija, I.; Blagojević, V.; Božić, D.D.; Milutinović, V.; Janković, R.; Sopta, J.; Kotur-Stevuljević, J.; et al. Evaluation of the Anti-Inflammatory/Immunomodulatory Effect of Teucrium montanum L. Extract in Collagen-Induced Arthritis in Rats. Biology 2024, 13, 818. https://doi.org/10.3390/biology13100818
Bufan B, Marčetić M, Djuretić J, Ćuruvija I, Blagojević V, Božić DD, Milutinović V, Janković R, Sopta J, Kotur-Stevuljević J, et al. Evaluation of the Anti-Inflammatory/Immunomodulatory Effect of Teucrium montanum L. Extract in Collagen-Induced Arthritis in Rats. Biology. 2024; 13(10):818. https://doi.org/10.3390/biology13100818
Chicago/Turabian StyleBufan, Biljana, Mirjana Marčetić, Jasmina Djuretić, Ivana Ćuruvija, Veljko Blagojević, Dragana D. Božić, Violeta Milutinović, Radmila Janković, Jelena Sopta, Jelena Kotur-Stevuljević, and et al. 2024. "Evaluation of the Anti-Inflammatory/Immunomodulatory Effect of Teucrium montanum L. Extract in Collagen-Induced Arthritis in Rats" Biology 13, no. 10: 818. https://doi.org/10.3390/biology13100818
APA StyleBufan, B., Marčetić, M., Djuretić, J., Ćuruvija, I., Blagojević, V., Božić, D. D., Milutinović, V., Janković, R., Sopta, J., Kotur-Stevuljević, J., & Arsenović-Ranin, N. (2024). Evaluation of the Anti-Inflammatory/Immunomodulatory Effect of Teucrium montanum L. Extract in Collagen-Induced Arthritis in Rats. Biology, 13(10), 818. https://doi.org/10.3390/biology13100818












