The Altered Proteomic Landscape in Renal Tubular Epithelial Cells under High Oxalate Stimulation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Protein Extraction
2.3. Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS) Analysis in Data Independent Acquisition (DIA) Mode
2.4. Database Search and Quantitative Data Analysis
2.5. Functional Enrichment Analysis
2.6. Construction of Protein–Protein Interaction (PPI) Network
2.7. Real-Time Quantitative PCR (RT-qPCR)
2.8. Western Blot (WB)
2.9. Validation of DEPs in the Public Proteome Dataset
3. Results
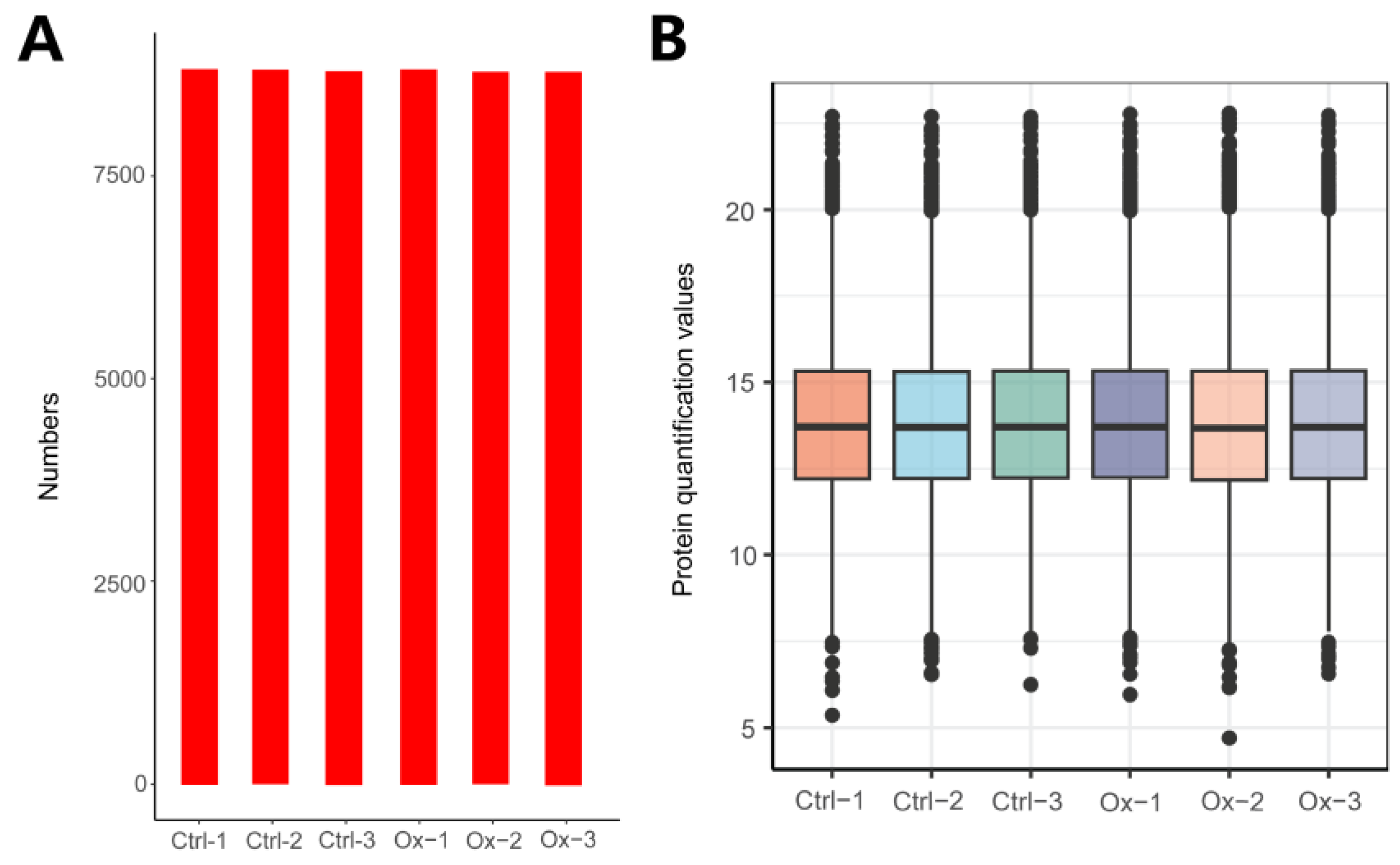
3.1. Overview of Protein Identification
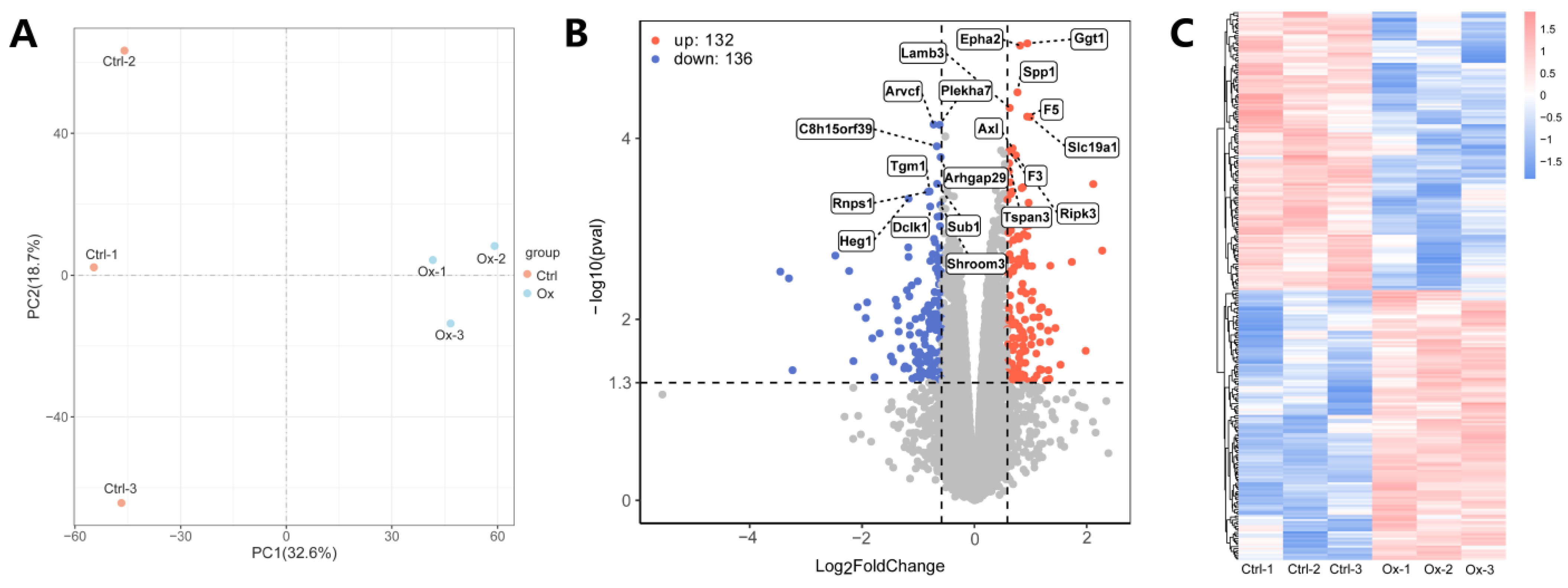
3.2. Identification of DEPs
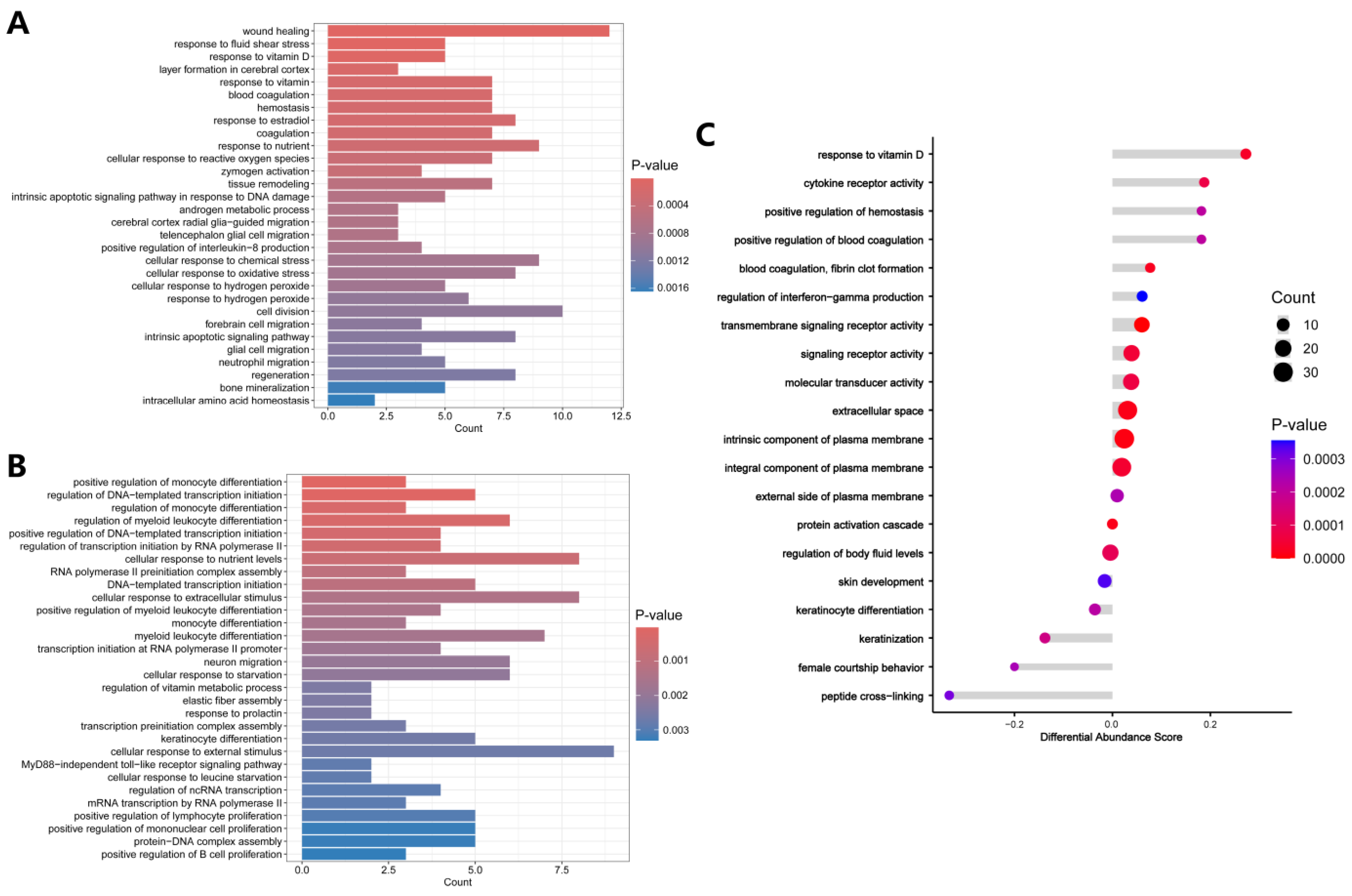
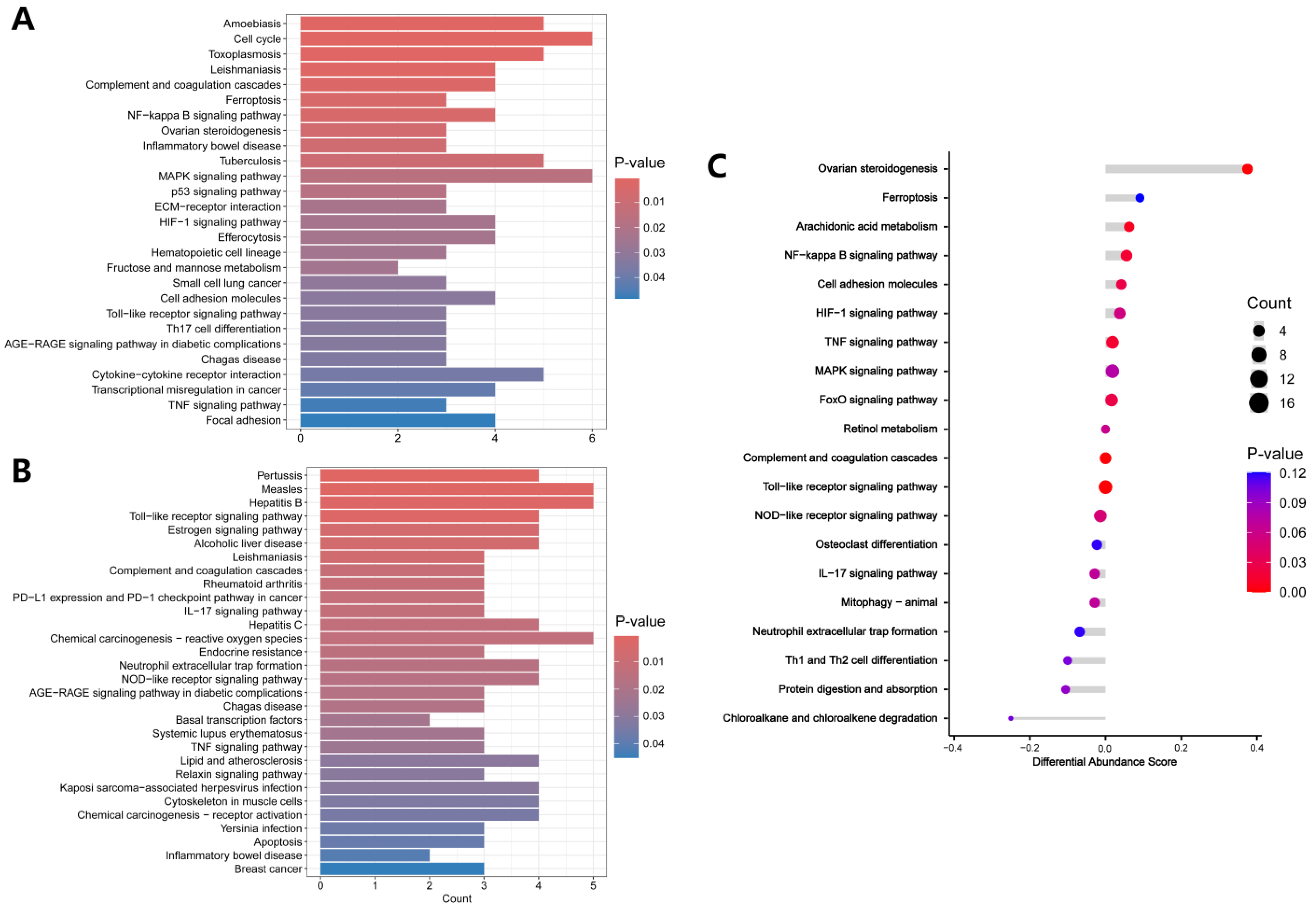
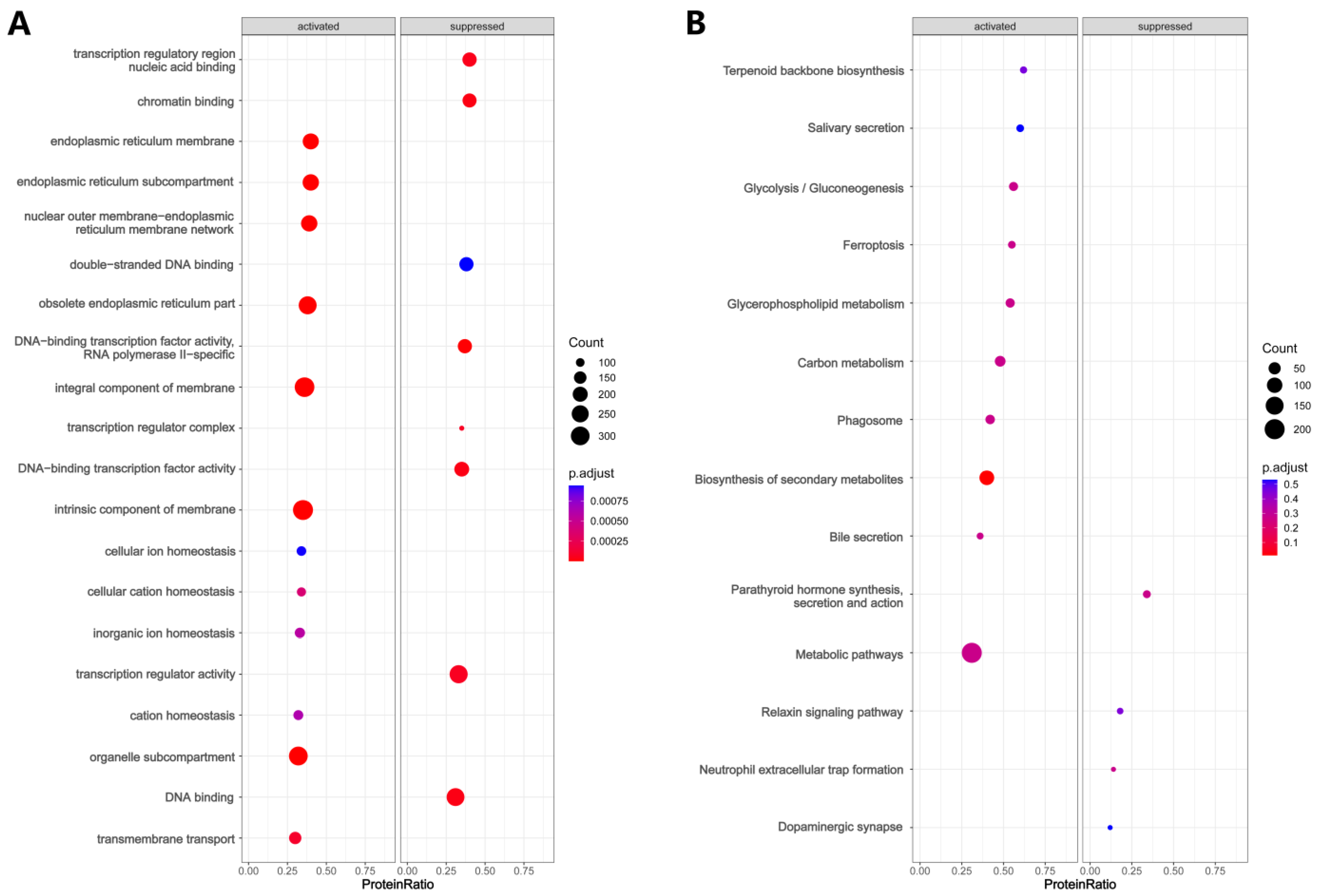
3.3. Functional Enrichment Analysis
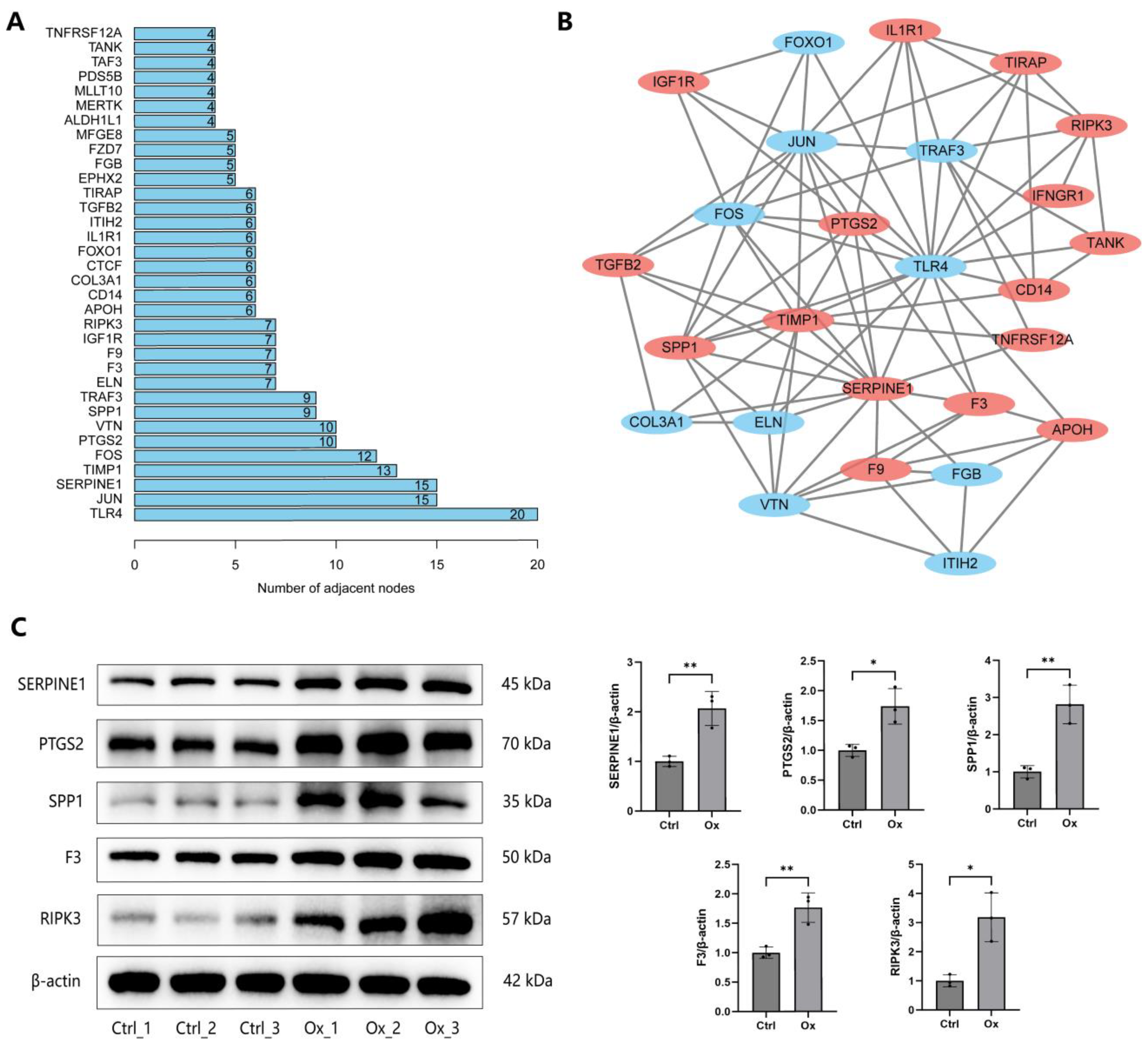
3.4. Construction of PPI Network
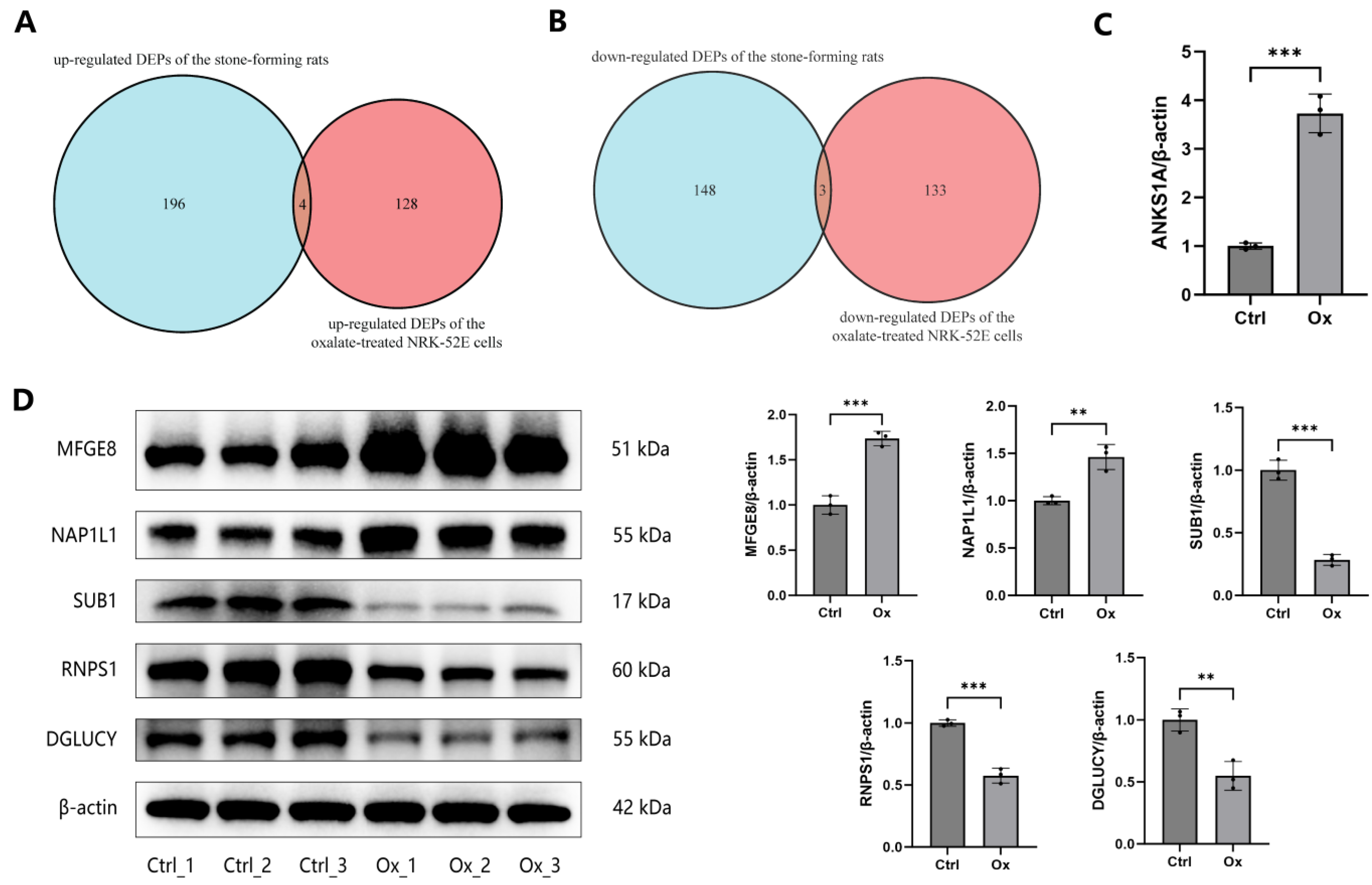
3.5. Validation of DEPs
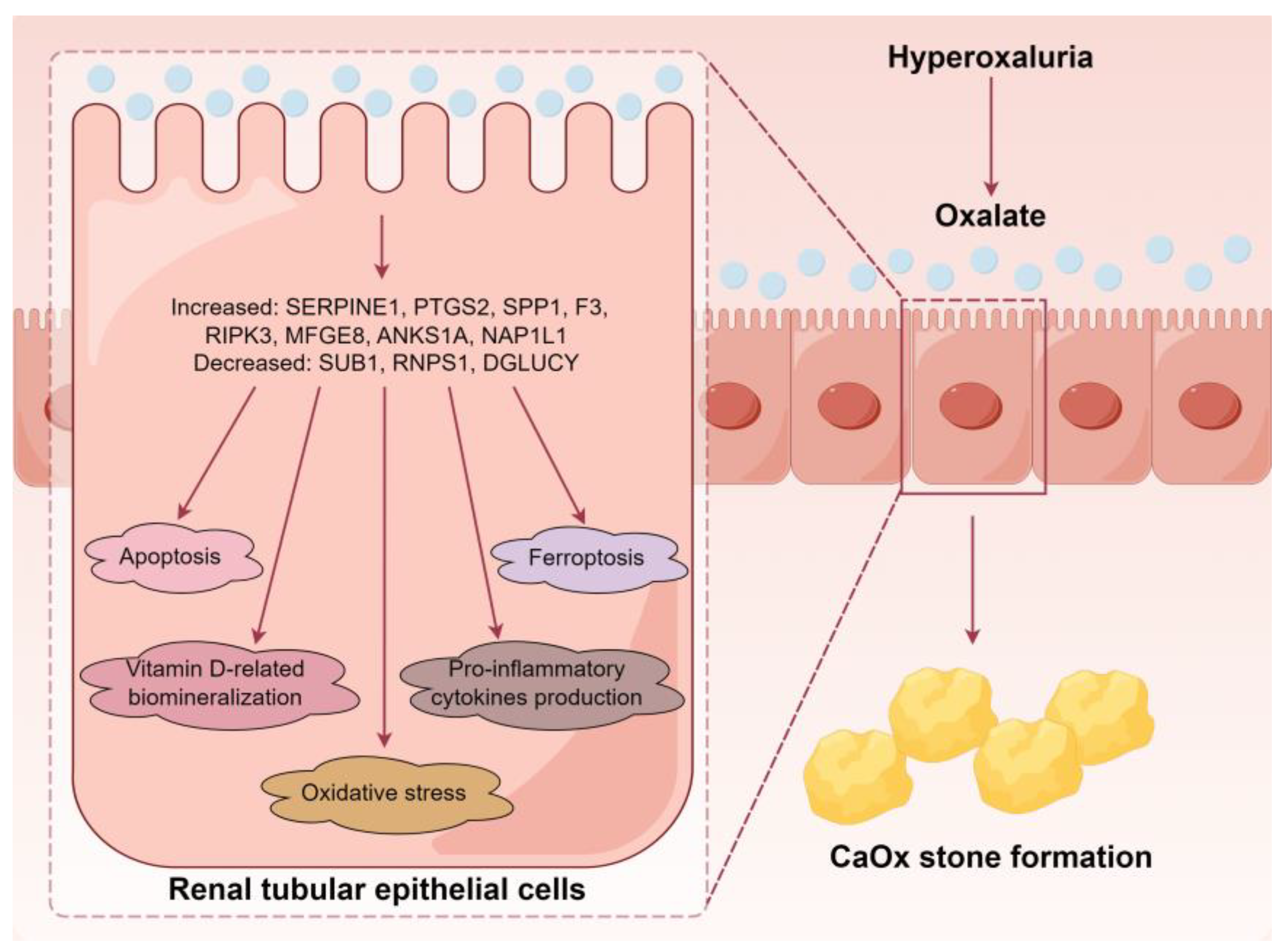
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, S.R.; Pearle, M.S.; Robertson, W.G.; Gambaro, G.; Canales, B.K.; Doizi, S.; Traxer, O.; Tiselius, H.G. Kidney stones. Nat. Rev. Dis. Primers 2016, 2, 16008. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, K.P.; Narula, S.; Kakkar, M.; Tandon, C. Nephrolithiasis: Molecular mechanism of renal stone formation and the critical role played by modulators. Biomed. Res. Int. 2013, 2013, 292953. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, B.; Ürekli, H.M.; Atta, M.G. Primary and secondary hyperoxaluria: Understanding the enigma. World J. Nephrol. 2015, 4, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Saylor, B.T.; Wang, W.; Peck, A.B.; Khan, S.R. Apocynin-treatment reverses hyperoxaluria induced changes in NADPH oxidase system expression in rat kidneys: A transcriptional study. PLoS ONE 2012, 7, e47738. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Q.; Li, C.; Lu, Y.; Hu, H.; Qin, B.; Xun, Y.; Zhu, Y.; Wu, Y.; Zhang, J.; et al. Inhibiting inflammation and modulating oxidative stress in oxalate-induced nephrolithiasis with the Nrf2 activator dimethyl fumarate. Free Radic. Biol. Med. 2019, 134, 9–22. [Google Scholar] [CrossRef]
- Wu, Y.; Xun, Y.; Zhang, J.; Hu, H.; Qin, B.; Wang, T.; Wang, S.; Li, C.; Lu, Y. Resveratrol Attenuates Oxalate-Induced Renal Oxidative Injury and Calcium Oxalate Crystal Deposition by Regulating TFEB-Induced Autophagy Pathway. Front. Cell Dev. Biol. 2021, 9, 638759. [Google Scholar] [CrossRef]
- Phizicky, E.; Bastiaens, P.I.; Zhu, H.; Snyder, M.; Fields, S. Protein analysis on a proteomic scale. Nature 2003, 422, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, M.X.; Xu, C.Z.; Zhang, Y.; Deng, Q.; Sun, R.; Hu, Q.Y.; Zhang, S.P.; Zhang, J.W.; Liang, H. Comprehensive study of altered proteomic landscape in proximal renal tubular epithelial cells in response to calcium oxalate monohydrate crystals. BMC Urol. 2020, 20, 136. [Google Scholar] [CrossRef]
- Greenacre, M.; Groenen, P.J.F.; Hastie, T.; D’Enza, A.I.; Markos, A.; Tuzhilina, E. Principal component analysis. Nat. Rev. Methods Primers 2022, 2, 100. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.; Yan, L.; Kerr, P.G.; Zhang, S.; Wu, Y. Electron transport, light energy conversion and proteomic responses of periphyton in photosynthesis under exposure to AgNPs. J. Hazard. Mater. 2021, 401, 123809. [Google Scholar] [CrossRef]
- López-Grueso, M.J.; Lagal, D.J.; García-Jiménez, Á.F.; Tarradas, R.M.; Carmona-Hidalgo, B.; Peinado, J.; Requejo-Aguilar, R.; Bárcena, J.A.; Padilla, C.A. Knockout of PRDX6 induces mitochondrial dysfunction and cell cycle arrest at G2/M in HepG2 hepatocarcinoma cells. Redox Biol. 2020, 37, 101737. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Deng, Q.; Gu, Y.; Li, M.; Zhang, Y.; Hu, Q.; Zhang, S.; Wang, X.; Liang, H. Proteomics and transcriptomics profiling reveals distinct aspects of kidney stone related genes in calculi rats. BMC Genom. 2023, 24, 127. [Google Scholar]
- Demoulin, N.; Aydin, S.; Gillion, V.; Morelle, J.; Jadoul, M. Pathophysiology and Management of Hyperoxaluria and Oxalate Nephropathy: A Review. Am. J. Kidney Dis. 2022, 79, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Wu, W.; Hu, H.; Yu, X.; Wang, S.; Zhu, J.; Zhang, J. The role of reactive oxygen species derived from different NADPH oxidase isoforms and mitochondria in oxalate-induced oxidative stress and cell injury. Urolithiasis 2022, 50, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Q.; Xu, C.; Lu, Y.; Hu, H.; Qin, B.; Wang, Y.; He, D.; Li, C.; Yu, X.; et al. MitoTEMPO Prevents Oxalate Induced Injury in NRK-52E Cells via Inhibiting Mitochondrial Dysfunction and Modulating Oxidative Stress. Oxid. Med. Cell Longev. 2017, 2017, 7528090. [Google Scholar] [CrossRef]
- Thamilselvan, V.; Menon, M.; Thamilselvan, S. Oxalate-induced activation of PKC-alpha and -delta regulates NADPH oxidase-mediated oxidative injury in renal tubular epithelial cells. Am. J. Physiol. Renal Physiol. 2009, 297, F1399–F1410. [Google Scholar] [CrossRef]
- Abhishek, A.; Benita, S.; Kumari, M.; Ganesan, D.; Paul, E.; Sasikumar, P.; Mahesh, A.; Yuvaraj, S.; Ramprasath, T.; Selvam, G.S. Molecular analysis of oxalate-induced endoplasmic reticulum stress mediated apoptosis in the pathogenesis of kidney stone disease. J. Physiol. Biochem. 2017, 73, 561–573. [Google Scholar] [CrossRef]
- Ming, S.; Tian, J.; Ma, K.; Pei, C.; Li, L.; Wang, Z.; Fang, Z.; Liu, M.; Dong, H.; Li, W.; et al. Oxalate-induced apoptosis through ERS-ROS-NF-κB signalling pathway in renal tubular epithelial cell. Mol. Med. 2022, 28, 88. [Google Scholar] [CrossRef]
- Yang, J.; Wu, W.; Amier, Y.; Li, X.; Wan, W.; Xun, Y.; Yu, X. Ferroptosis and its emerging role in kidney stone formation. Mol. Biol. Rep. 2024, 51, 314. [Google Scholar] [CrossRef]
- He, Z.; Liao, W.; Song, Q.; Li, B.; Liu, J.; Xiong, Y.; Song, C.; Yang, S. Role of ferroptosis induced by a high concentration of calcium oxalate in the formation and development of urolithiasis. Int. J. Mol. Med. 2021, 47, 289–301. [Google Scholar] [CrossRef]
- Song, Q.; Liao, W.; Chen, X.; He, Z.; Li, D.; Li, B.; Liu, J.; Liu, L.; Xiong, Y.; Song, C.; et al. Oxalate Activates Autophagy to Induce Ferroptosis of Renal Tubular Epithelial Cells and Participates in the Formation of Kidney Stones. Oxid. Med. Cell Longev. 2021, 2021, 6630343. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Song, C.; He, Z.; Song, Q.; Song, T.; Liu, J.; Xiong, Y.; Su, X.; Zhou, J.; Yang, S.; et al. Protective efficacy of Schizandrin B on ameliorating nephrolithiasis via regulating GSK3β/Nrf2 signaling-mediated ferroptosis in vivo and in vitro. Int. Immunopharmacol. 2023, 117, 110042. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, J.; Zhu, P.; Hu, S.; Ren, J. Ripk3 regulates cardiac microvascular reperfusion injury: The role of IP3R-dependent calcium overload, XO-mediated oxidative stress and F-action/filopodia-based cellular migration. Cell Signal. 2018, 45, 12–22. [Google Scholar] [CrossRef]
- Liu, Z.M.; Chen, Q.X.; Chen, Z.B.; Tian, D.F.; Li, M.C.; Wang, J.M.; Wang, L.; Liu, B.H.; Zhang, S.Q.; Li, F.; et al. RIP3 deficiency protects against traumatic brain injury (TBI) through suppressing oxidative stress, inflammation and apoptosis: Dependent on AMPK pathway. Biochem. Biophys. Res. Commun. 2018, 499, 112–119. [Google Scholar] [CrossRef]
- Zhang, S.; Li, R.; Dong, W.; Yang, H.; Zhang, L.; Chen, Y.; Wang, W.; Li, C.; Wu, Y.; Ye, Z.; et al. RIPK3 mediates renal tubular epithelial cell apoptosis in endotoxin-induced acute kidney injury. Mol. Med. Rep. 2019, 20, 1613–1620. [Google Scholar] [CrossRef]
- Lai, K.; Wang, J.; Lin, S.; Chen, Z.; Lin, G.; Ye, K.; Yuan, Y.; Lin, Y.; Zhong, C.Q.; Wu, J.; et al. Sensing of mitochondrial DNA by ZBP1 promotes RIPK3-mediated necroptosis and ferroptosis in response to diquat poisoning. Cell Death Differ. 2024, 31, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Martin-Sanchez, D.; Ruiz-Andres, O.; Poveda, J.; Carrasco, S.; Cannata-Ortiz, P.; Sanchez-Niño, M.D.; Ruiz Ortega, M.; Egido, J.; Linkermann, A.; Ortiz, A.; et al. Ferroptosis, but Not Necroptosis, Is Important in Nephrotoxic Folic Acid-Induced AKI. J. Am. Soc. Nephrol. 2017, 28, 218–229. [Google Scholar] [CrossRef]
- Yuan, K.; Hu, D.; Mo, X.; Zeng, R.; Wu, B.; Zhang, Z.; Hu, R.; Wang, C. Novel diagnostic biomarkers of oxidative stress, immune- infiltration characteristics and experimental validation of SERPINE1 in colon cancer. Discov. Oncol. 2023, 14, 206. [Google Scholar] [CrossRef]
- Wang, T.; Lu, H.; Li, D.; Huang, W. TGF-β1-Mediated Activation of SERPINE1 is Involved in Hemin-Induced Apoptotic and Inflammatory Injury in HT22 Cells. Neuropsychiatr. Dis. Treat. 2021, 17, 423–433. [Google Scholar] [CrossRef]
- Hagan, S.; Khurana, N.; Chandra, S.; Abdel-Mageed, A.B.; Mondal, D.; Hellstrom, W.J.; Sikka, S.C. Differential expression of novel biomarkers (TLR-2, TLR-4, COX-2, and Nrf-2) of inflammation and oxidative stress in semen of leukocytospermia patients. Andrology 2015, 3, 848–855. [Google Scholar] [CrossRef]
- Song, Q.; Feng, Y.B.; Wang, L.; Shen, J.; Li, Y.; Fan, C.; Wang, P.; Yu, S.Y. COX-2 inhibition rescues depression-like behaviors via suppressing glial activation, oxidative stress and neuronal apoptosis in rats. Neuropharmacology 2019, 160, 107779. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, S.Y.; Song, Y.; Wang, Y.; Wan, Z.W.; Sun, P.; Yu, X.M.; Deng, B.; Zeng, K.H. Resveratrol Protects Müller Cells Against Ferroptosis in the Early Stage of Diabetic Retinopathy by Regulating the Nrf2/GPx4/PTGS2 Pathway. Mol. Neurobiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Suen, J.L.; Liu, C.C.; Lin, Y.S.; Tsai, Y.F.; Juo, S.H.; Chou, Y.H. Urinary chemokines/cytokines are elevated in patients with urolithiasis. Urol. Res. 2010, 38, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Pan, Y.; Li, Q.; Zhang, L.; Duan, L.; Xu, Y.; Cao, J.; Cui, X.; Huang, Y. Landscape of peripheral immunity in patients with upper urinary tract urolithiasis and the underlying correlations with renal function. BMC Urol. 2024, 24, 169. [Google Scholar] [CrossRef]
- Singhto, N.; Thongboonkerd, V. Exosomes derived from calcium oxalate-exposed macrophages enhance IL-8 production from renal cells, neutrophil migration and crystal invasion through extracellular matrix. J. Proteom. 2018, 185, 64–76. [Google Scholar] [CrossRef]
- Marshall, L.J.; Ramdin, L.S.; Brooks, T.; PC, D.P.; Shute, J.K. Plasminogen activator inhibitor-1 supports IL-8-mediated neutrophil transendothelial migration by inhibition of the constitutive shedding of endothelial IL-8/heparan sulfate/syndecan-1 complexes. J. Immunol. 2003, 171, 2057–2065. [Google Scholar] [CrossRef]
- Hjortoe, G.M.; Petersen, L.C.; Albrektsen, T.; Sorensen, B.B.; Norby, P.L.; Mandal, S.K.; Pendurthi, U.R.; Rao, L.V. Tissue factor-factor VIIa-specific up-regulation of IL-8 expression in MDA-MB-231 cells is mediated by PAR-2 and results in increased cell migration. Blood 2004, 103, 3029–3037. [Google Scholar] [CrossRef]
- Taguchi, K.; Okada, A.; Unno, R.; Hamamoto, S.; Yasui, T. Macrophage Function in Calcium Oxalate Kidney Stone Formation: A Systematic Review of Literature. Front. Immunol. 2021, 12, 673690. [Google Scholar] [CrossRef]
- Mercy, D.J.; Girigoswami, A.; Girigoswami, K. Relationship between urinary tract infections and serum vitamin D level in adults and children—A literature review. Mol. Biol. Rep. 2024, 51, 955. [Google Scholar] [CrossRef]
- Letavernier, E.; Daudon, M. Vitamin D, Hypercalciuria and Kidney Stones. Nutrients 2018, 10, 366. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, J.; Lu, Y.; Zhang, Z.; Qin, B.; Gao, H.; Wang, Y.; Zhu, J.; Wang, Q.; Zhu, Y.; et al. Association between Circulating Vitamin D Level and Urolithiasis: A Systematic Review and Meta-Analysis. Nutrients. 2017, 9, 301. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Wang, S.; He, D.; Cui, L.; Lu, Y.; Hu, H.; Qin, B.; Zhao, Z. Role of calcium in the regulation of bone morphogenetic protein 2, runt-related transcription factor 2 and Osterix in primary renal tubular epithelial cells by the vitamin D receptor. Mol. Med. Rep. 2015, 12, 2082–2088. [Google Scholar] [CrossRef]
- Daudon, M.; Bazin, D.; Letavernier, E. Randall’s plaque as the origin of calcium oxalate kidney stones. Urolithiasis 2015, 43 (Suppl. 1), 5–11. [Google Scholar] [CrossRef]
- Khan, S.R.; Rodriguez, D.E.; Gower, L.B.; Monga, M. Association of Randall plaque with collagen fibers and membrane vesicles. J. Urol. 2012, 187, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L.; Christensen, B.; Taleb, H.; Sørensen, E.S. Post-translational modification of osteopontin: Effects on in vitro hydroxyapatite formation and growth. Biochem. Biophys. Res. Commun. 2012, 419, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Chaikiawkeaw, D.; Khorattanakulchai, N.; Nammultriputtar, K.; Rattanapisit, K.; Everts, V.; Kubera, A.; Phoolcharoen, W.; Pavasant, P. Osteopontin induces osteogenic differentiation by human periodontal ligament cells via calcium binding domain-ALK-1 interaction. J. Periodontol. 2022, 93, e13–e23. [Google Scholar] [CrossRef]
- Jia, Q.; Huang, Z.; Wang, G.; Sun, X.; Wu, Y.; Yang, B.; Yang, T.; Liu, J.; Li, P.; Li, J. Osteopontin: An important protein in the formation of kidney stones. Front. Pharmacol. 2022, 13, 1036423. [Google Scholar] [CrossRef]
- Chiang, H.Y.; Chu, P.H.; Chen, S.C.; Lee, T.H. MFG-E8 promotes osteogenic transdifferentiation of smooth muscle cells and vascular calcification by regulating TGF-β1 signaling. Commun. Biol. 2022, 5, 364. [Google Scholar] [CrossRef]
- Zhao, T.; He, X.; Liang, X.; Kellum, A.H., Jr.; Tang, F.; Yin, J.; Guo, S.; Wang, Y.; Gao, Z.; Wang, Y. HMGB3 and SUB1 Bind to and Facilitate the Repair of N(2)-Alkylguanine Lesions in DNA. J. Am. Chem. Soc. 2024, 146, 22553–22562. [Google Scholar] [CrossRef]
- Viegas, M.H.; Gehring, N.H.; Breit, S.; Hentze, M.W.; Kulozik, A.E. The abundance of RNPS1, a protein component of the exon junction complex, can determine the variability in efficiency of the Nonsense Mediated Decay pathway. Nucleic Acids Res. 2007, 35, 4542–4551. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.-Y.; Qin, B.-L. The Altered Proteomic Landscape in Renal Tubular Epithelial Cells under High Oxalate Stimulation. Biology 2024, 13, 814. https://doi.org/10.3390/biology13100814
Hong S-Y, Qin B-L. The Altered Proteomic Landscape in Renal Tubular Epithelial Cells under High Oxalate Stimulation. Biology. 2024; 13(10):814. https://doi.org/10.3390/biology13100814
Chicago/Turabian StyleHong, Sen-Yuan, and Bao-Long Qin. 2024. "The Altered Proteomic Landscape in Renal Tubular Epithelial Cells under High Oxalate Stimulation" Biology 13, no. 10: 814. https://doi.org/10.3390/biology13100814
APA StyleHong, S.-Y., & Qin, B.-L. (2024). The Altered Proteomic Landscape in Renal Tubular Epithelial Cells under High Oxalate Stimulation. Biology, 13(10), 814. https://doi.org/10.3390/biology13100814





