The Origin Recognition Complex: From Origin Selection to Replication Licensing in Yeast and Humans
Abstract
:Simple Summary
Abstract
1. Introduction
2. Replication Licensing in Cell Cycle and Developmental Regulation
3. The Origin Recognition Complex Is Conserved in Structure but Diverged in DNA Binding Properties
4. The cryoEM Structure of Yeast ORC Bound to Origin DNA
5. Yeast Orc4 Insertion Helix Encodes Sequence-Specific Binding
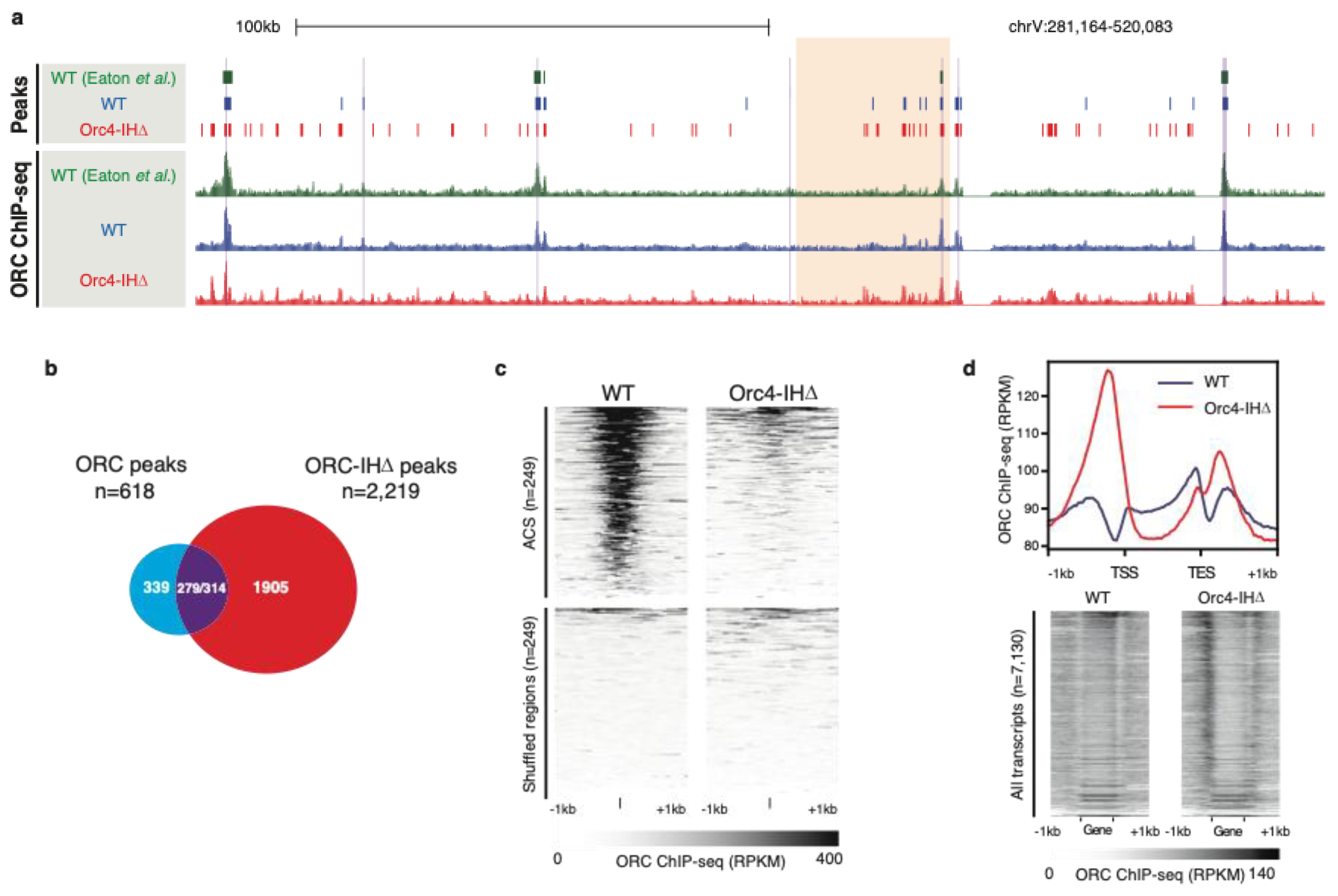
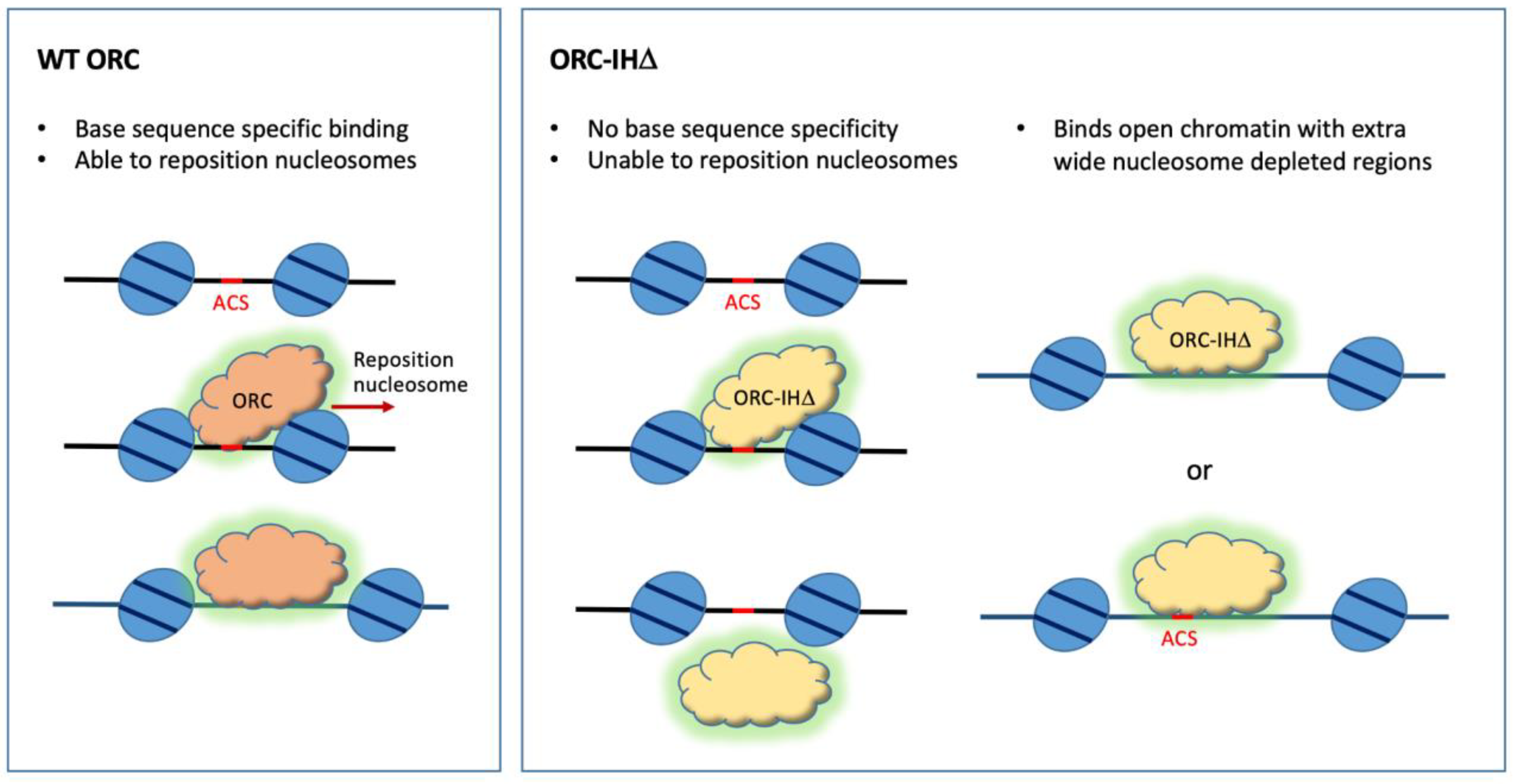
6. Humanizing the Yeast ORC by Removing the 19 Amino Acid Insertion Helix of Orc4
7. Interdependence of ORC and the Nucleosomal Context in Origin Site Selection
8. The Importance of Site-Specific Replication Initiation in Yeast and in Humans
9. Pre-RC Assembly in Yeast and in Humans
10. Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Li, N.; Zhai, Y.; Zhang, Y.; Li, W.; Yang, M.; Lei, J.; Tye, B.K.; Gao, N. Structure of the eukaryotic MCM complex at 3.8 A. Nature 2015, 524, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lam, W.H.; Zhai, Y.; Cheng, J.; Cheng, E.; Zhao, Y.; Gao, N.; Tye, B.K. Structure of the origin recognition complex bound to DNA replication origin. Nature 2018, 559, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.K.; Cheung, M.F.; Li, J.; Zhao, Y.; Lam, W.H.; Ho, V.; Rohs, R.; Zhai, Y.; Leung, D.; Tye, B.K. Humanizing the yeast origin recognition complex. Nat. Commun. 2021, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dong, J.; Wang, W.; Yu, D.; Fan, X.; Hui, Y.C.; Lee, C.S.K.; Lam, W.H.; Alary, N.; Yang, Y.; et al. The human pre-replication complex is an open complex. Cell 2023, 186, 98–111.e121. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.K.; Weibeta, M.; Hamperl, S. Where and when to start: Regulating DNA replication origin activity in eukaryotic genomes. Nucleus 2023, 14, 2229642. [Google Scholar] [CrossRef] [PubMed]
- Yeeles, J.T.; Deegan, T.D.; Janska, A.; Early, A.; Diffley, J.F. Regulated eukaryotic DNA replication origin firing with purified proteins. Nature 2015, 519, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Devbhandari, S.; Jiang, J.; Kumar, C.; Whitehouse, I.; Remus, D. Chromatin Constrains the Initiation and Elongation of DNA Replication. Mol. Cell 2017, 65, 131–141. [Google Scholar] [CrossRef]
- Heller, R.C.; Kang, S.; Lam, W.M.; Chen, S.; Chan, C.S.; Bell, S.P. Eukaryotic origin-dependent DNA replication in vitro reveals sequential action of DDK and S-CDK kinases. Cell 2011, 146, 80–91. [Google Scholar] [CrossRef]
- On, K.F.; Beuron, F.; Frith, D.; Snijders, A.P.; Morris, E.P.; Diffley, J.F. Prereplicative complexes assembled in vitro support origin-dependent and independent DNA replication. EMBO J. 2014, 33, 605–620. [Google Scholar] [CrossRef]
- Kurat, C.F.; Yeeles, J.T.P.; Patel, H.; Early, A.; Diffley, J.F.X. Chromatin Controls DNA Replication Origin Selection, Lagging-Strand Synthesis, and Replication Fork Rates. Mol. Cell 2017, 65, 117–130. [Google Scholar] [CrossRef]
- Yeeles, J.T.P.; Janska, A.; Early, A.; Diffley, J.F.X. How the Eukaryotic Replisome Achieves Rapid and Efficient DNA Replication. Mol. Cell 2017, 65, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Azmi, I.F.; Watanabe, S.; Maloney, M.F.; Kang, S.; Belsky, J.A.; MacAlpine, D.M.; Peterson, C.L.; Bell, S.P. Nucleosomes influence multiple steps during replication initiation. Elife 2017, 6, e22512. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Diffley, J.F.X. The Initiation of Eukaryotic DNA Replication. Annu. Rev. Biochem. 2022, 91, 107–131. [Google Scholar] [CrossRef] [PubMed]
- Stillman, B. The remarkable gymnastics of ORC. eLife 2022, 11, e76475. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.C.R.; Locke, J.; Greiwe, J.F.; Diffley, J.F.X.; Costa, A. Mechanism of head-to-head MCM double-hexamer formation revealed by cryo-EM. Nature 2019, 575, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Friedman, L.J.; Gelles, J.; Bell, S.P. A helicase-tethered ORC flip enables bidirectional helicase loading. eLife 2021, 10, e74282. [Google Scholar] [CrossRef] [PubMed]
- Sheu, Y.J.; Stillman, B. The Dbf4-Cdc7 kinase promotes S phase by alleviating an inhibitory activity in Mcm4. Nature 2010, 463, 113–117. [Google Scholar] [CrossRef]
- Cheng, J.; Li, N.; Huo, Y.; Dang, S.; Tye, B.K.; Gao, N.; Zhai, Y. Structural Insight into the MCM double hexamer activation by Dbf4-Cdc7 kinase. Nat. Commun. 2022, 13, 1396. [Google Scholar] [CrossRef]
- Saleh, A.; Noguchi, Y.; Aramayo, R.; Ivanova, M.E.; Stevens, K.M.; Montoya, A.; Sunidhi, S.; Carranza, N.L.; Skwark, M.J.; Speck, C. The structural basis of Cdc7-Dbf4 kinase dependent targeting and phosphorylation of the MCM2-7 double hexamer. Nat. Commun. 2022, 13, 2915. [Google Scholar] [CrossRef]
- Greiwe, J.F.; Miller, T.C.R.; Locke, J.; Martino, F.; Howell, S.; Schreiber, A.; Nans, A.; Diffley, J.F.X.; Costa, A. Structural mechanism for the selective phosphorylation of DNA-loaded MCM double hexamers by the Dbf4-dependent kinase. Nat. Struct. Mol. Biol. 2022, 29, 10–20. [Google Scholar] [CrossRef]
- Gillespie, P.J.; Blow, J.J. DDK: The Outsourced Kinase of Chromosome Maintenance. Biology 2022, 11, 877. [Google Scholar] [CrossRef] [PubMed]
- Douglas, M.E.; Ali, F.A.; Costa, A.; Diffley, J.F.X. The mechanism of eukaryotic CMG helicase activation. Nature 2018, 555, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Amasino, A.L.; Gupta, S.; Friedman, L.J.; Gelles, J.; Bell, S.P. Regulation of replication origin licensing by ORC phosphorylation reveals a two-step mechanism for Mcm2-7 ring closing. Proc. Natl. Acad. Sci. USA 2023, 120, e2221484120. [Google Scholar] [CrossRef] [PubMed]
- Liku, M.E.; Nguyen, V.Q.; Rosales, A.W.; Irie, K.; Li, J.J. CDK phosphorylation of a novel NLS-NES module distributed between two subunits of the Mcm2-7 complex prevents chromosomal rereplication. Mol. Biol. Cell 2005, 16, 5026–5039. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Makino, N.; Nagai, M.; Araki, H.; Ushimaru, T. CDK phosphorylation regulates Mcm3 degradation in budding yeast. Biochem. Biophys. Res. Commun. 2018, 506, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Baris, Y.; Taylor, M.R.G.; Aria, V.; Yeeles, J.T.P. Fast and efficient DNA replication with purified human proteins. Nature 2022, 606, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, A.B.; Kriegstein, H.J.; Hogness, D.S. The units of DNA replication in Drosophila melanogaster chromosomes. Cold Spring Harb. Symp. Quant Biol. 1974, 38, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, D.M. Making sense of eukaryotic DNA replication origins. Science 2001, 294, 96–100. [Google Scholar] [CrossRef]
- Farrell, J.A.; O’Farrell, P.H. From egg to gastrula: How the cell cycle is remodeled during the Drosophila mid-blastula transition. Annu. Rev. Genet. 2014, 48, 269–294. [Google Scholar] [CrossRef]
- Bleichert, F.; Balasov, M.; Chesnokov, I.; Nogales, E.; Botchan, M.R.; Berger, J.M. A Meier-Gorlin syndrome mutation in a conserved C-terminal helix of Orc6 impedes origin recognition complex formation. eLife 2013, 2, e00882. [Google Scholar] [CrossRef]
- Kuo, A.J.; Song, J.; Cheung, P.; Ishibe-Murakami, S.; Yamazoe, S.; Chen, J.K.; Patel, D.J.; Gozani, O. The BAH domain of ORC1 links H4K20me2 to DNA replication licensing and Meier-Gorlin syndrome. Nature 2012, 484, 115–119. [Google Scholar] [CrossRef] [PubMed]
- de Munnik, S.A.; Hoefsloot, E.H.; Roukema, J.; Schoots, J.; Knoers, N.V.; Brunner, H.G.; Jackson, A.P.; Bongers, E.M. Meier-Gorlin syndrome. Orphanet J. Rare Dis. 2015, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.P.; Stillman, B. ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex. Nature 1992, 357, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Struhl, K.; Stinchcomb, D.T.; Scherer, S.; Davis, R.W. High-frequency transformation of yeast: Autonomous replication of hybrid DNA molecules. Proc. Natl. Acad. Sci. USA 1979, 76, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.S.; Tye, B.K. Autonomously replicating sequences in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1980, 77, 6329–6333. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Theis, J.F.; Miller, J.; Nieduszynski, C.A.; Newlon, C.S.; Weinreich, M. Analysis of chromosome III replicators reveals an unusual structure for the ARS318 silencer origin and a conserved WTW sequence within the origin recognition complex binding site. Mol. Cell. Biol. 2008, 28, 5071–5081. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Stillman, B. Origins of DNA replication in eukaryotes. Mol. Cell 2023, 83, 352–372. [Google Scholar] [CrossRef]
- Dijkwel, P.A.; Hamlin, J.L. The Chinese hamster dihydrofolate reductase origin consists of multiple potential nascent-strand start sites. Mol. Cell. Biol. 1995, 15, 3023–3031. [Google Scholar] [CrossRef]
- Miotto, B.; Ji, Z.; Struhl, K. Selectivity of ORC binding sites and the relation to replication timing, fragile sites, and deletions in cancers. Proc. Natl. Acad. Sci. USA 2016, 113, E4810–E4819. [Google Scholar] [CrossRef]
- Rivera-Mulia, J.C.; Gilbert, D.M. Replication timing and transcriptional control: Beyond cause and effect-part III. Curr. Opin. Cell Biol. 2016, 40, 168–178. [Google Scholar] [CrossRef]
- Vouzas, A.E.; Gilbert, D.M. Replication timing and transcriptional control: Beyond cause and effect-part IV. Curr. Opin. Genet. Dev. 2023, 79, 102031. [Google Scholar] [CrossRef] [PubMed]
- Broach, J.R.; Li, Y.Y.; Feldman, J.; Jayaram, M.; Abraham, J.; Nasmyth, K.A.; Hicks, J.B. Localization and sequence analysis of yeast origins of DNA replication. Cold Spring Harb. Symp. Quant Biol. 1983, 47 Pt 2, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Lipford, J.R.; Bell, S.P. Nucleosomes positioned by ORC facilitate the initiation of DNA replication. Mol. Cell 2001, 7, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Eaton, M.L.; Galani, K.; Kang, S.; Bell, S.P.; MacAlpine, D.M. Conserved nucleosome positioning defines replication origins. Genes Dev. 2010, 24, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Riera, A.; Bai, L.; Sun, J.; Nandi, S.; Spanos, C.; Chen, Z.A.; Barbon, M.; Rappsilber, J.; Stillman, B.; et al. Structural basis of Mcm2-7 replicative helicase loading by ORC-Cdc6 and Cdt1. Nat. Struct. Mol. Biol. 2017, 24, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Bleichert, F.; Leitner, A.; Aebersold, R.; Botchan, M.R.; Berger, J.M. Conformational control and DNA-binding mechanism of the metazoan origin recognition complex. Proc. Natl. Acad. Sci. USA 2018, 115, E5906–E5915. [Google Scholar] [CrossRef] [PubMed]
- Chuang, R.Y.; Kelly, T.J. The fission yeast homologue of Orc4p binds to replication origin DNA via multiple AT-hooks. Proc. Natl. Acad. Sci. USA 1999, 96, 2656–2661. [Google Scholar] [CrossRef]
- Kong, D.; DePamphilis, M.L. Site-specific DNA binding of the Schizosaccharomyces pombe origin recognition complex is determined by the Orc4 subunit. Mol. Cell. Biol. 2001, 21, 8095–8103. [Google Scholar] [CrossRef]
- Kielkopf, C.L.; White, S.; Szewczyk, J.W.; Turner, J.M.; Baird, E.E.; Dervan, P.B.; Rees, D.C. A structural basis for recognition of A.T and T.A base pairs in the minor groove of B-DNA. Science 1998, 282, 111–115. [Google Scholar] [CrossRef]
- White, S.; Szewczyk, J.W.; Turner, J.M.; Baird, E.E.; Dervan, P.B. Recognition of the four Watson-Crick base pairs in the DNA minor groove by synthetic ligands. Nature 1998, 391, 468–471. [Google Scholar] [CrossRef]
- Dellino, G.I.; Cittaro, D.; Piccioni, R.; Luzi, L.; Banfi, S.; Segalla, S.; Cesaroni, M.; Mendoza-Maldonado, R.; Giacca, M.; Pelicci, P.G. Genome-wide mapping of human DNA-replication origins: Levels of transcription at ORC1 sites regulate origin selection and replication timing. Genome Res. 2013, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Petryk, N.; Kahli, M.; d’Aubenton-Carafa, Y.; Jaszczyszyn, Y.; Shen, Y.; Silvain, M.; Thermes, C.; Chen, C.L.; Hyrien, O. Replication landscape of the human genome. Nat. Commun. 2016, 7, 10208. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.; Park, S.; Shor, E.; Huebert, D.J.; Warren, C.L.; Ansari, A.Z.; Weinreich, M.; Eaton, M.L.; MacAlpine, D.M.; Fox, C.A. The conserved bromo-adjacent homology domain of yeast Orc1 functions in the selection of DNA replication origins within chromatin. Genes Dev. 2010, 24, 1418–1433. [Google Scholar] [CrossRef] [PubMed]
- Mas, A.M.; Goni, E.; Ruiz de Los Mozos, I.; Arcas, A.; Statello, L.; Gonzalez, J.; Blazquez, L.; Lee, W.T.C.; Gupta, D.; Sejas, A.; et al. ORC1 binds to cis-transcribed RNAs for efficient activation of replication origins. Nat. Commun. 2023, 14, 4447. [Google Scholar] [CrossRef] [PubMed]
- Chacin, E.; Reusswig, K.U.; Furtmeier, J.; Bansal, P.; Karl, L.A.; Pfander, B.; Straub, T.; Korber, P.; Kurat, C.F. Establishment and function of chromatin organization at replication origins. Nature 2023, 616, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Zhang, L.; Lv, M.; Wen, Z.; Zhang, W.; Chen, X.; Zhang, P.; Li, T.; Chang, L.; Jin, C.; et al. H2A.Z facilitates licensing and activation of early replication origins. Nature 2020, 577, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Kitsberg, D.; Selig, S.; Keshet, I.; Cedar, H. Replication structure of the human beta-globin gene domain. Nature 1993, 366, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Coster, G.; Diffley, J.F.X. Bidirectional eukaryotic DNA replication is established by quasi-symmetrical helicase loading. Science 2017, 357, 314–318. [Google Scholar] [CrossRef]
- Ganier, O.; Prorok, P.; Akerman, I.; Mechali, M. Metazoan DNA replication origins. Curr. Opin. Cell Biol. 2019, 58, 134–141. [Google Scholar] [CrossRef]
- Tian, M.; Wang, Z.; Su, Z.; Shibata, E.; Shibata, Y.; Dutta, A.; Zang, C. Integrative analysis of DNA replication origins and ORC/MCM binding sites in human cells reveals a lack of overlap. bioRxiv 2023. [Google Scholar] [CrossRef]
- Sanchez, H.; McCluskey, K.; van Laar, T.; van Veen, E.; Asscher, F.M.; Solano, B.; Diffley, J.F.X.; Dekker, N.H. DNA replication origins retain mobile licensing proteins. Nat. Commun. 2021, 12, 1908. [Google Scholar] [CrossRef] [PubMed]
- Abid Ali, F.; Renault, L.; Gannon, J.; Gahlon, H.L.; Kotecha, A.; Zhou, J.C.; Rueda, D.; Costa, A. Cryo-EM structures of the eukaryotic replicative helicase bound to a translocation substrate. Nat. Commun. 2016, 7, 10708. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, Y.; Yuan, Z.; Bai, L.; Schneider, S.; Zhao, G.; Stillman, B.; Speck, C.; Li, H. Cryo-EM structure of Mcm2-7 double hexamer on DNA suggests a lagging-strand DNA extrusion model. Proc. Natl. Acad. Sci. USA 2017, 114, E9529–E9538. [Google Scholar] [CrossRef] [PubMed]
- Remus, D.; Beuron, F.; Tolun, G.; Griffith, J.D.; Morris, E.P.; Diffley, J.F. Concerted loading of Mcm2-7 double hexamers around DNA during DNA replication origin licensing. Cell 2009, 139, 719–730. [Google Scholar] [CrossRef]
- Gros, J.; Kumar, C.; Lynch, G.; Yadav, T.; Whitehouse, I.; Remus, D. Post-licensing Specification of Eukaryotic Replication Origins by Facilitated Mcm2-7 Sliding along DNA. Mol. Cell 2015, 60, 797–807. [Google Scholar] [CrossRef]
- Das, S.P.; Rhind, N. How and why multiple MCMs are loaded at origins of DNA replication. Bioessays 2016, 38, 613–617. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tye, B.-K.; Zhai, Y. The Origin Recognition Complex: From Origin Selection to Replication Licensing in Yeast and Humans. Biology 2024, 13, 13. https://doi.org/10.3390/biology13010013
Tye B-K, Zhai Y. The Origin Recognition Complex: From Origin Selection to Replication Licensing in Yeast and Humans. Biology. 2024; 13(1):13. https://doi.org/10.3390/biology13010013
Chicago/Turabian StyleTye, Bik-Kwoon, and Yuanliang Zhai. 2024. "The Origin Recognition Complex: From Origin Selection to Replication Licensing in Yeast and Humans" Biology 13, no. 1: 13. https://doi.org/10.3390/biology13010013
APA StyleTye, B.-K., & Zhai, Y. (2024). The Origin Recognition Complex: From Origin Selection to Replication Licensing in Yeast and Humans. Biology, 13(1), 13. https://doi.org/10.3390/biology13010013




