Simple Summary
Filter feeders can retain environmental DNA (eDNA) within their bodies, making them potential eDNA samplers. In this study, eDNA from the gut contents of Asian clams (Corbicula fluminea) was used to identify biodiversity in estuarine ecosystems. Various organisms, such as fish, copepods, and green algae, were detected, representing a wide range of habitats. Of the 20 families detected (except for Fungi and terrestrial taxa), 8 families were also documented in the conventional field survey, enabling the identification of an operational taxonomic unit (OTU) of migratory fish that are challenging to observe directly. These results support the potential application of C. fluminea as a supplementary tool for investigating the biodiversity of aquatic ecosystems.
Abstract
Environmental DNA (eDNA) extracted from the gut contents of filter feeders can be used to identify biodiversity in aquatic ecosystems. In this study, we used eDNA from the gut contents of the Asian clam Corbicula fluminea to examine biodiversity within estuarine ecosystem. Field sampling was conducted at three points in the Nakdong River Estuary, which is characterised by closed estuarine features resulting from the presence of an estuarine barrage. The collected C. fluminea samples were dissected to separate the gut contents, and the extracted eDNA was amplified using 18S V9 primer targeting all eukaryote-derived DNA. The amplified DNA was sequenced using a next-generation sequencing (NGS) technique, and a BLASTn search was performed based on the National Centre for Biotechnology Information (NCBI) database for taxa identification. We obtained 23 unique operational taxonomic units (OTUs), including fish (approximately 8.70%), copepods (approximately 17.39%), and green algae (approximately 21.74%), representing a wide range of habitats. Furthermore, 8 out of the 20 families were identified through comparisons with reference data from conventional field surveys, and the OTUs of elusive migratory fish were detected. The results support the application of C. fluminea as an eDNA sampler for supplementary biodiversity monitoring.
1. Introduction
Estuaries are highly complex and dynamic ecosystems that provide multifaceted habitats, such as tidal mudflats, sandbars, marshes, and transition zones, for numerous organisms [1,2,3]. However, estuaries are often subjected to serious threats from anthropogenic impacts, including overexploitation, reclamation, pollution, and barrage construction, leading to rapid declines in habitats and biodiversity [4,5]. Therefore, monitoring and detecting critical changes in estuarine ecosystems are important [6]. Conventional aquatic species monitoring methods that capture or rely on direct detection are time-consuming and labour-intensive [7,8]. Morphological identification using the naked eye or a microscope is skill-dependent and can lead to misidentification [9]. Furthermore, the dynamic environment and high biodiversity of estuarine ecosystems complicate monitoring [10].
Environmental DNA (eDNA) metabarcoding is a promising alternative to traditional monitoring methods [11]. This molecular technique enables the identification of an entire community from a single environmental sample (e.g., water, soil, air, faeces, or gut contents) without directly observing or capturing organisms. Through polymerase chain reaction (PCR) using universal or group specific primers and next-generation sequencing (NGS) techniques, researchers can identify the presence of various organisms, including rare, elusive, or endangered species [11,12]. This method provides a non-invasive and efficient way to assess biodiversity in different habitats [13]. Consequently, studies employing eDNA metabarcoding for biodiversity assessment have attracted significant attention in recent years and have explored various potential eDNA sources such as biofilms, faeces, or gut contents [14,15,16,17,18,19].
Filter feeders, which filter water and ingest organic particles, can accumulate eDNA within their bodies without an artificial filtering process and can be used to identify biodiversity in aquatic ecosystems [18,19,20]. Their unique and effective feeding habits make them potential eDNA samplers [18,21]. In particular, bivalves such as clams and mussels are widely distributed across aquatic ecosystems, including lakes, rivers, and estuaries, demonstrating broader applicability than other filter feeders [22]. However, there are few studies that have extracted eDNA from bivalves, and their potential has rarely been investigated, especially in specific ecosystems such as closed estuaries.
In this study, we conducted a first-of-its-kind investigation applying Corbicula fluminea to eDNA metabarcoding for biodiversity monitoring in a closed estuary, using the Nakdong River Estuary as a case study. This estuary exemplifies an artificially regulated ecosystem owing to the presence of an estuarine barrage, which was constructed in 1987 and reopened in 2020 to restore a brackish ecosystem [23]. The bivalve C. fluminea is widely distributed in the Nakdong River Estuary. We hypothesised that eDNA analysis of C. fluminea gut contents could reveal the biodiversity of the Nakdong River Estuary.
To test this hypothesis, we analysed eDNA from the gut contents of C. fluminea collected from the Nakdong River Estuary. DNA metabarcoding using 18S V9 primers was employed to explore comprehensive biodiversity of the ecosystem. We investigated the composition of detected taxa by analysing parameters such as the number of OTUs, frequency of occurrence (FOO), and relative read abundance (RRA). We subsequently reviewed the applicability of C. fluminea as an eDNA sampler through comparison with the actual biological monitoring report conducted at the Nakdong River Estuary.
2. Materials and Methods
2.1. Study Site
The Nakdong River is the second largest river system in South Korea and maintains a well-developed estuarine system (35°05′ N, 128°55′ E). It is also recognised as an important biodiversity conservation area, including winter bird habitats and stopover sites on the East Asia–Australasian Flyway [24]. Another important feature of the estuary is its flood control activities, primarily through an estuarine barrage built in 1987 which divides brackish areas into distinct freshwater and saline zones. This division is believed to influence biodiversity changes [23], necessitating thorough and efficient monitoring methods to address growing concerns regarding regional biodiversity protection. In the present study, we selected three points within the brackish area with different salinity levels (Figure 1). Points 1, 2, and 3 were located approximately 2.0 km, 2.7 km, and 3.9 km from the estuarine barrage, respectively. C. fluminea is widely distributed in this area, as they have been continuously released for fishery resources by the Busan Marine Fisheries Resources Research Institute [25].
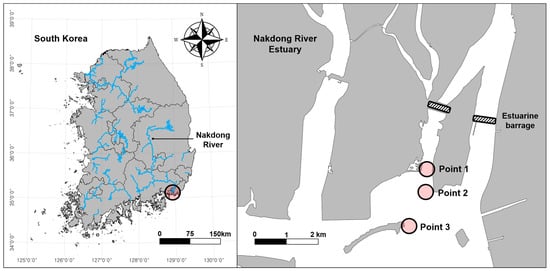
Figure 1.
Map of the sampling points in the Nakdong River Estuary.
2.2. Water Quality Survey
A water quality survey was conducted in September 2021 concurrently with the sampling of C. fluminea. Water samples were collected from the surface layer (at a depth of approximately 0.5 m) using a 10 L polypropylene bucket. Dissolved oxygen (DO, mg L−1, %) and water temperature (°C) were measured using a YSI 550A dissolved oxygen instrument (YSI, Yellow Springs, OH, USA). The pH levels were determined using a YSI Model 60 handheld pH–temperature system (YSI, Yellow Springs, OH, USA). Electrical conductivity (μS cm−1) and salinity (ppt) were assessed using a YSI Pro30 conductivity meter (YSI, Yellow Springs, OH, USA). Following the field survey, water samples were transported to the laboratory in refrigerated storage for turbidity and alkalinity analyses. Turbidity (NTU) was measured using an APERA TN500 portable white light turbidity meter (APERA, Columbus, OH, USA), and alkalinity (mg L−1) was determined using the neutralisation method in accordance with standard procedures [26].
2.3. C. fluminea Sampling and Pretreatment
Corbicula fluminea samples were collected using a fishing dredge 123 cm wide and 22 cm high (Figure A1). The dredge net was made of polyethylene and was 320 cm long with an 11 × 11 mm mesh size. The collected C. fluminea samples were placed in separate polyethylene bags at each point and transported to the laboratory for refrigerated storage. The samples were stored at −80 °C until further analysis.
From the collected C. fluminea samples, 10 individuals per point were randomly selected (totalling 30 mature individuals; shell length (cm), 2.0–3.0; shell height (cm), 1.9–2.4; shell width (cm), 1.1–1.5; and total weight (g), 2.990–6.051) [27]. The gut was eviscerated and dissected to obtain its contents. During dissection, scalpels, tweezers, and scissors were flame-sterilised between samples to minimise contamination. The extracted gut contents were placed in 1.5 mL microtubes separately (n = 30) and stored at −20 °C until further analysis.
2.4. DNA Extraction and Amplification
The gut contents from the C. fluminea samples were homogenised using sterilised homogeniser pestle, and genomic DNA was extracted using DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. The extracted DNA samples were stored at −20 °C.
Two consecutive PCR steps were performed for the next-generation sequencing (NGS) process. Throughout the entire PCR process, both negative and positive controls were employed. The first PCR was performed using primer sets that amplify the V9 regions of 18S rRNA (18S V9 primer) targeting universal eukaryotes. The forward primer sequence is 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCCTGCCHTTTGTACACAC-3′, and the reverse is 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGCCTTCYGCAGGTTCACCTAC-3′. We used AccuPower HotStart PCR PreMix (Bioneer, Deajeon, Republic of Korea), and the volume of the PCR reaction solution was 20 µL−1 (DNA template 1 µL, forward primer 1 µL, reverse primer 1 µL, and distilled water 17 µL). The PCR conditions consist of 1 cycle of initial denaturation (94 °C, 10 min) and 35 cycles of denaturation (94 °C, 1 min), annealing (50 °C, 1.5 min), extension (72 °C, 1 min) and 1 cycle of final extension (72 °C, 10 min). After first PCR, we confirmed the size of the products through 1.5% agarose gel electrophoresis and stored them at −20 °C.
The second PCR was performed using KAPA HiFi HotStart ReadyMix (KAPA Biosystems, Wilmington, MA, USA) and Nextera XT Index Kit v2 (Illumina, San Diego, CA, USA). The volume of the PCR reaction solution was 25 µL−1 (first PCR product 2.5 µL, Forward index 2.5 µL, Reverse index 2.5 µL, KAPA mix 12.5 µL, distilled water 5 µL). The PCR conditions consist of 1 cycle of initial denaturation (95 °C, 3 min) and 10 cycles of denaturation (95 °C, 30 s), annealing (55 °C, 30 s), extension (72 °C, 30 s), and 1 cycle of final extension (72 °C, 5 min). Subsequently, we confirmed the size of the products through 1.5% agarose gel electrophoresis and stored them at −20 °C.
The PCR products were purified by a beads clean-up process using AMPure XP Reagent (Beckman Coulter, Indianapolis, IN, USA), and then pooled in equal concentration (10 nM) using a DeNovix QFX Fluorometer and a DeNovix dsDNA Ultra High Sensitivity Assay (DeNovix, Wilmington, DE, USA) according to the manufacturer’s protocol. The generated library was stored at −20 °C until DNA sequencing.
2.5. DNA Sequence Analysis
We sequenced library samples using NGS and performed taxonomic identification. The library was sequenced on an Illumina iSeq platform (Illumina, San Diego, CA, USA), and data processing was performed using USEARCH (v11.0.667) [28]. Demultiplexed raw sequences (FASTQ files) were merged into one sequence, allowing a maximum of ten mismatches. Merged reads with expected errors > 1.0 were discarded after quality filtering. The remaining sequences were dereplicated and clustered into OTUs at a 97% OTU cut-off value, removing chimeric and singleton sequences.
The resulting OTU sequences were searched in the National Centre for Biotechnology Information (NCBI) database (Release 255.0; July 2023) using BLASTn [29]. A list of the 200 taxa for each OTU with the highest max score was obtained. The OTUs were provisionally identified based on identity percentages, with OTUs exhibiting an identity of 97% or higher assigned at the species level, whereas the remaining (90–97%) were assigned at the genus or family level. Subsequently, we referenced the biodiversity database of South Korea (National Institute of Biological Resources) [30] to ascertain the presence of the taxa within the nation’s territory. In cases wherein the taxa were not confirmed in the reference, we considered either the higher taxonomic ranks of the taxa or second-score taxa. The OTUs identified as C. fluminea were considered ‘self-DNA’ and excluded from the subsequent process. The obtained sequences were deposited in the NCBI repository under accession number SAMN35796656.
Next, we categorised the identified OTUs according to taxonomic classifications and examined habitat environments, based on the World Register of Marine Species (WoRMS) database (Table A1) [31]. For Hygrobates sp., as it was not found in the WoRMs database, we used the NCBI taxonomy browser [32] and additional references instead [33].
2.6. Statistical Analysis and Reference Data
To indicate the overall taxa composition detected in the gut contents of C. fluminea, the number of OTUs was presented based on the taxonomic categories, and two dietary metrics were used, FOO and RRA [34]. The FOO refers to the proportion of samples in which a particular taxon was detected. FOO of a taxon indicates how frequently it appears across all the samples in a study. A high FOO for a taxon suggests that it is widespread and common in the study area, whereas a low FOO indicates that it is less prevalent. The RRA represents the proportion of sequence reads that belonging to a specific taxon relative to the total number of reads obtained during the sequencing process. A high RRA for a taxon indicates that it is abundant and represented by a large number of reads, whereas a low RRA suggests that it is less abundant in the sample.
We examined the differences in the detected taxa composition between three sampling points using a Permutational Multivariate Analysis of Variance (PERMANOVA) performed with 999 random permutations. The analysis was conducted in PRIMER v7 (PRIMER-e, Auckland, New Zealand) on the basis of a Bray–Curtis dissimilarity matrix and using log(x + 1) transformed RRA data.
To assess the correspondence between taxa identified from the gut contents of C. fluminea and the actual biodiversity of the Nakdong River Estuary, we referred to a monitoring report published by the Korea Water Resources Corporation (K-water) [35]. The conventional field survey was conducted throughout 2021 (April, June, July, August, October, and November) in both the upstream and downstream areas of the estuarine barrage, encompassing a broader spatiotemporal range, including the timing and locations of our C. fluminea sampling (September 2021, downstream of the barrage). Reference data included the survey results for phytoplankton, zooplankton, benthic invertebrate, shellfish, fish, and vegetation. In the present study, we considered the data of phytoplankton, zooplankton and fish, aiming to ascertain whether the taxa detected in present study were also documented in the conventional field survey. Additionally, we referred only to the results of formal surveys conducted on a regular basis, and other additional data were subsidiarily considered.
3. Results
3.1. Water Parameters
The water parameters did not differ considerably among the sampling points, except for salinity and electrical conductivity (Table 1). Salinity values ranged from 10.0 to 14.2 ppt, indicating a brackish area. They increased in the following order: Points 1, 3, and 2, regardless of the distance from the estuarine barrage. Electrical conductivity exhibited a trend similar to salinity, as it was also affected by dissolved salts.

Table 1.
Table of water parameters for each sampling point in the Nakdong River Estuary.
3.2. eDNA from the Gut Contents of C. fluminea
The DNA metabarcoding analysis generated 17,272 paired-end reads from 30 samples. After quality filtering, 16,980 (98.3%) sequences were obtained, comprising 53 unique OTUs. Through a series of identification processes, 23 eukaryotic taxa were identified (belonged to 22 genera, 22 families, 17 orders, 15 classes, 12 phyla, and 5 kingdoms; Table 2) in 28 samples. We assigned 3 OTUs at the species level, 18 OTUs at the genus level, and 2 OTUs at the family level.

Table 2.
List of identified taxa in the gut contents of C. fluminea (n = 30) and BLASTn results of each taxon. The OTUs were taxonomically classified according to the WoRMS database.
The OTUs identified in the C. fluminea gut contents represented taxonomically diverse organisms (Figure 2A). Animalia accounted for the highest percentage (nine OTUs), followed by Chromista (six OTUs), Plantae (five OTUs), Fungi (two OTUs), and Protozoa (one OTU). The OTUs in the kingdom Animalia included three phyla, with copepods (Arthropoda) and fish (Chordata) accounting for 17.39% (4 OTUs) and 8.70% (2 OTUs) of the total 23 OTUs, respectively. The OTUs in the kingdom Chromista covered five phyla, with diatom (Bacillariophyta) representing the highest proportion (8.70%, 2 OTUs). All Plantae OTUs belonged to the phylum Chlorophyta (green algae).
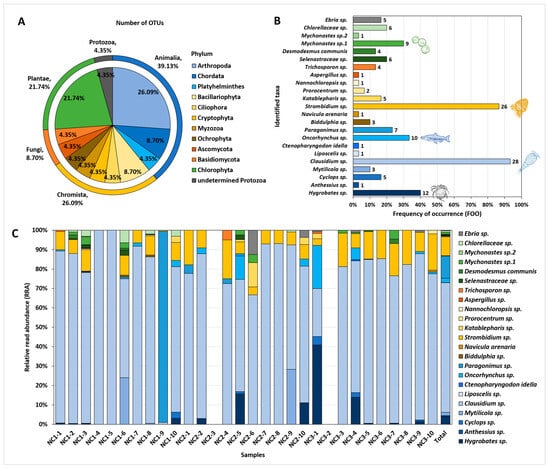
Figure 2.
Overall taxa composition detected from the gut contents of C. fluminea (n = 30). Each colour sector represents a different kingdom group (blue: Animalia, yellow: Chromista, orange: Fungi, green: Plantae, grey: Protozoa). (A) The number of OTUs represented as a proportion based on each phylum and kingdom group. (B) Frequency of occurrence (FOO) for each OTUs. The number at the edge of the bars refers to the number of C. fluminea samples in which the taxon detected. (C) Relative read abundance (RRA) for each OTU. The sample name represents each of the 10 samples collected from three different sampling points.
FOO and RRA demonstrated the detection frequency and relative abundance of each taxon recovered from C. flumina gut contents (Figure 2B,C). Clausidium sp. (copepods) exhibited the highest proportion in both FOO and RRA, being present in the greatest number of samples (93.33%) and displaying the most abundant DNA reads (66.78%). Subsequently, Strombidium sp. (ciliates) appeared in 86.67% of the samples, accounting for 9.67% of the DNA reads, whereas Hygrobates sp. (water mites) appeared in 40.00% of the samples, accounting 4.05% of the DNA reads. Additionally, Oncorhynchus sp. (salmon and trout; 33.33% FOO, 2.23% RRA), Mychonastes sp.1 (green algae; 30.00% FOO, 1.06% RRA), Paragonimus sp. (lung fluke; 23.33% FOO, 11.47% RRA), and Mytilicola sp. (parasitic copepods; 10.00% FOO, 1.63% RRA) displayed RRA values >1%.
Furthermore, the identified OTUs covered various habitat environments, including marine, brackish, freshwater, and terrestrial environments (Table A1). They comprised freshwater green algae (Desmodesmus communis), marine copepods (Clausidium sp., Anthessius sp.), and even terrestrial insects (Liposcelis sp.).
3.3. Comparison between Sampling Points
In the comparison of number of OTUs, RRA, and FOO, no marked difference was observed between the three sampling points (10 samples per sampling point). The Points 1, 2 and 3 included 19 OTUs (11 phyla, 4 kingdoms), 13 OTUs (8 phyla, 5 kingdoms), 16 OTUs (10 phyla, 5 kingdoms) of the total 23 OTUs, respectively (Figure 3A). At all sampling points, the kingdom Animaila accounted for ≥90% FOO and ≥85% RRA, whereas Chromista accounted for ≥80% FOO and ≥7% RRA (Figure 3B,C). Based on PERMANOVA, the three sampling points did not exhibit significant differences in their RRA data of total 23 taxa (Table A2; p > 0.05), suggesting that the composition of taxa detected from the C. fluminea gut contents was not different across the sampling points.
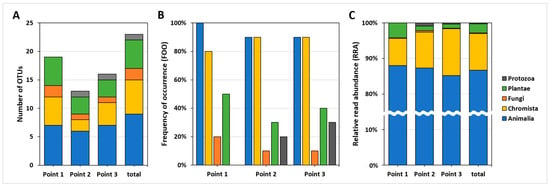
Figure 3.
Overall taxa composition (kingdom level) detected from the C. fluminea gut contents at each sampling point. (A) The number of OTUs at each sampling point based on kingdom group. (B) Frequency of occurrence (FOO) at each sampling point based on kingdom group. (C) Relative read abundance (RRA) at each sampling point based on kingdom group.
Additionally, we cross-checked the appearance of OTUs among the sampling points (Figure 4, the red circles). Of the total 23 detected taxa, 10 OTUs (10 families) appeared at all three sampling points, 5 OTUs (5 families) appeared at two points, and 8 OTUs (7 families) appeared at only one point. There were no remarkable taxonomical trends based on the commonness among the sampling points.
3.4. Comparison with Conventional Field Study
Upon comparing the taxa detected in C. fluminea gut contents (20 families, excluding fungi and terrestrial taxa) with the field survey results (123 families) at the family level, 7 families (3 at the genus level and 1 at the species level) were also identified in the field survey (Figure 4). These seven families were Cyclopidae (Cyclops sp.), Cyprinidae, Naviculaceae (Navicula arenaria), Prorocentraceae (Prorocentrum sp.), Selenastraceae, Scenedesmaceae, and Chlorellaceae. Conversely, taxa such as Clausidiidae (Clausidium sp.), Paragonimidae (Paragonimus sp.), and Strombidiidae (Strombidium sp.), which exhibited high FOO and RRA values, were not corroborated by the field survey. In addition, we noted Oncorhynchus sp., which was not found in general field surveys. According to the reference report, Oncorhynchus keta (Chum Salmon), which was not confirmed in regular surveys, was found during additional monitoring targeting only this species.
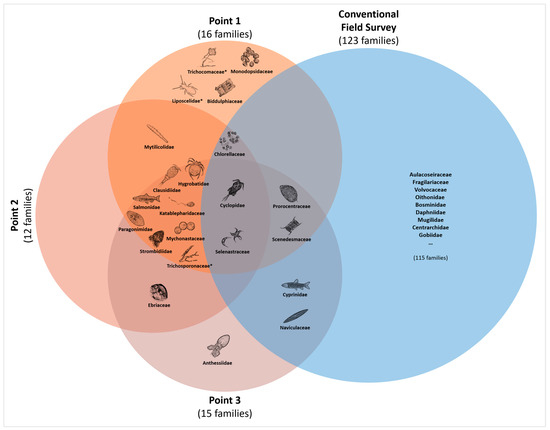

Figure 4.
Venn diagram illustrating the taxa detected at each sampling point and a conventional field survey based on family level. Fungi and terrestrial insect marked with * were not considered in the comparison with the field survey. Salmonidae was placed outside the blue circle (the field survey), as confirmed through a targeted monitoring focused on Chum Salmon (Oncorhynchus keta), rather than in a regular field survey.
4. Discussion
In the present study, we examined the potential use of C. fluminea as an eDNA sampler to assess the biodiversity of the Nakdong River Estuary. We extracted eDNA from the gut contents of C. fluminea and used the 18S V9 primer to investigate overall biodiversity at the study sites. The metabarcoding results recovered 23 taxa belonging to different classifications, including fish, copepods, diatoms, green algae, which represented a wide range of habitat environments. We also ascertained that out of total 20 families, 7 (35%) were also documented in the conventional field survey and detected elusive migratory fish and planktonic taxa that might be overlooked in the field survey.
4.1. Potential of C. fluminea as an eDNA Sampler for Supplementary Biodiversity Monitoring
An ideal eDNA sample for biodiversity monitoring should be capable of detecting a taxonomically wide range of organisms, efficiently providing an accurate reflection of habitat biodiversity [18]. In light of this perspective, our results support the potential of C. fluminea to supplement other monitoring methodologies, rather than completely replace them, by providing a perfect illustration of habitat biodiversity.
First, similar to general eDNA samples such as water and soil, eDNA from C. fluminea can recover a broad spectrum of taxa, including the species that are challenging to identify through morphological identification alone. These clams have an efficient filtering mechanism [36], indicating their potential as a biological indicator [37,38], and do not represent strong selectivity for food [39], enabling the detection of diverse species. In this study, we successfully identified a wide range of taxa from C. fluminea eDNA (Table 2). Notably, although Clausidium sp. and Strombidium sp. displayed high FOO and RRA values in our results, they were not observed in the field survey. Furthermore, ‘unidentified copepodites and nauplii’ consistently appeared as dominant or sub-dominant species throughout the entire field survey, highlighting difficulties in identifying these species. This represented that eDNA from C. fluminea is not limited to specific biological groups, suggesting the presence of unrecognized species, and overcomes and complements the identification limit of the field surveys.
Second, C. fluminea eDNA presents an accumulated record within their body over a relatively long period, and present the possibility of detecting rare species that can be overlooked in other monitoring methods. Conventional methods typically yield a ‘snapshot’ of the species present during the survey period, potentially missing species with limited populations (i.e., endangered species) or unique ecological traits (i.e., migratory fish) [7,8]. Water samples can encounter the same issue as the conventional surveys if the volume or duration of filtration is insufficient [40,41]. However, C. fluminea rarely leaves its habitat, and provides eDNA accumulated in the body for a longer time. This indicates the possibility of better detection of the missing species despite eDNA degradation within the digestive tract. In this study, we identified elusive migratory fish, Oncorhynchus sp., which were not found in conventional field survey. This result suggested that C. fluminea eDNA can serve as a puzzle to fill the missing parts of habitat biodiversity.
Third, this approach can be applied in various aquatic ecosystems, easily integrated with other biological monitoring methods. The bivalves are commonly distributed worldwide and exist in various habitats [42,43], so they offer extensive applicability and can be readily collected during field surveys, especially when they are included in the subject of comprehensive monitoring, or when the water is shallow. In other words, additional information can be obtained from the clams “simply picked up during the field sampling”. In particular, when investigating extensive ecosystems with multiple habitat characteristics (e.g., important wetlands or nature reserves), using such a readily accessible natural eDNA sampler may be a way to identify hidden biodiversity and enhance the efficiency of species discovery.
Fourth, eDNA retained within the C. fluminea may be less affected by external environmental factors. In this study, we measured and compared water quality parameters at sampling points and confirmed the difference in salinity. Salinity is one of the factors impacting the preservation of eDNA, with higher salinity levels correlating with elevated eDNA concentrations in water [44]. However, statistical analysis of species composition in each point (RRA data) showed no significant difference between the three sampling points (Table A2). Interestingly, even the sampling point with the highest salinity (point 2) showed the least number of OTUs. These results suggest the possibility that eDNA within C. fluminea may be less affected by several environmental factors, resulting in better preservation than other environmental samples.
4.2. Challenges of C. fluminea as an eDNA Sampler and Future Research
Our sample species, C. fluminea, is a typical benthic filter feeder [45], and the possibility of dominant detection of benthic organisms cannot be ruled out. Additionally, in the estuary where freshwater and seawater are mixed, seawater with high density often flows under the freshwater, forming a vertical salinity gradient in the brackish zone [46,47]. Salinity is an important factor that determines the distribution of aquatic organisms [48,49], suggesting that the unique characteristics of this habitat raise concerns about dominant detection of marine species which prefer saltwater. Therefore, when employing bivalves like C. fluminea as eDNA samplers, it is imperative to consider the traits of the selected species and the environmental conditions of the research site. Although our study did not demonstrate a pronounced bias toward benthic or marine organisms (Table 2 and Table A1 ), this issue may be a critical consideration when trying to study biodiversity through eDNA of bivalves.
There are few cases of eDNA extraction from bivalves for biodiversity monitoring. This has led to an absence of established protocols for initial sample processing methods, and several studies have shown distinct results depending on their sample treatment method. According to Heo et al. [50], the gut content of Asian clams (C. fluminea) recovered relatively fewer OTUs than pseudo-faeces. Jeunen et al. [20] reported that eDNA could not be obtained from the gills of blue mussels (Mytilus galloprovincialis). These results suggest that the sample processing method should be carefully selected, and that various methods need to be tried in future studies.
In present study, we assessed the potential of bivalves as an eDNA sampler. However, to fully understand their availability, comparison with water samples will be essential in further studies. Filtered water samples satisfy the conditions of the most ideal eDNA sample [11,18,51]. Therefore, future studies should investigate whether it is worth using C. fluminea eDNA compared to water samples under various experimental conditions and environments. In particular, it may be beneficial to explore scenarios wherein the analysis of water samples is considered challenging. For example, research in (1) sites where water flow is irregular, such as closed estuaries, affecting the dispersion and detection efficiency of eDNA [41,52]; and (2) sites where many particles and high turbidity cause filter clogging during water filtration [41,53,54] will provide a new perspective on the value of bivalves as an eDNA sampler.
4.3. Limitations in Molecular Analysis
DNA metabarcoding using faeces or gut contents usually poses the risk of excessive detection of self-DNA (i.e., DNA of the sample species itself) or the DNA of the species interacting with the sample species (for example, parasites and symbionts) [55,56,57]. It may be excessively amplified during DNA processing and detected in much larger amounts than prey DNA, leading to non-informative results [56,58]. Our study also identified ten OTUs of C. fluminea and one OTU of Mytilicola sp., which are known to be intestinal parasites of bivalves. Therefore, considering alternatives, such as blocking oligonucleotides that prevent self-DNA amplification, is necessary to avoid these biases in future studies.
The 18S rRNA V9 barcode is suitable for DNA metabarcoding for dietary analysis because of its ability to amplify degraded DNA and detect a relatively broad range of eukaryotic organisms [59,60]. However, because the targeting region is relatively short, the 18S rRNA region is considered limited for distinguishing taxonomically close species [61,62]. To compensate for this, we cross-referenced the database and confirmed the indigenous presence of each taxon. Subsequently, species that had never been documented in South Korea were excluded. This process minimised the risk of false-positives caused by short amplicons compared to a simple program that selects taxa with the most similar sequences from the BLASTn results. Nevertheless, in pursuit of finer-grained results at the species level and detection of newly introduced species, future studies must consider a variety of primer combinations, to ideally analyse the decomposed DNA from the sample species.
5. Conclusions
In this study, we explored the potential use of C. fluminea as an eDNA sampler to supplement the biodiversity monitoring of Nakdong River Estuary. Our analysis of the gut contents of C. fluminea has revealed a broad taxonomic spectrum of organisms and provided a chance to uncover hidden biodiversity that may be overlooked in conventional field surveys due to rarity or identification challenges. Additionally, we suggest that the ecological properties of bivalves including C. fluminea strongly support their suitability as eDNA samplers for supplementary biodiversity monitoring.
Author Contributions
Conceptualization, H.J.; methodology, H.J. and K.K.; investigation, K.K., J.-S.G., Y.L., D.H. and H.J; writing—original draft preparation, K.K., K.-S.J. and H.J.; writing—review and editing, G.-J.J.; visualization, K.K.; supervision, H.J.; project administration, H.J.; funding acquisition, H.J. All authors have read and agreed to the published version of the manuscript.
Funding
The results of this research were produced with the support of the Ministry of Education of the Republic of Korea and the National Research Foundation, through the Leaders in INdustry-university Cooperation 3.0 program (Project No. L23-0001).
Institutional Review Board Statement
Ethical review and approval were waived due to this study dealing with lower invertebrates (Asian clams (Corbicula fluminea)), meaning this study is not classified as involving humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the NCBI repository under accession number SAMN35796656.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
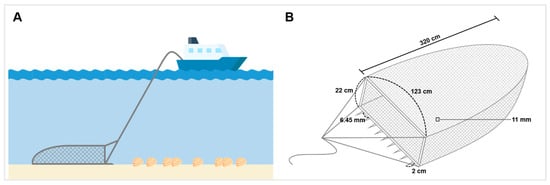
Figure A1.
Illustration of the C. flumina sampling method: (A) Schematic diagram of C. fluminea sampling using fishing dredge; (B) Schematic diagram of fishing dredge used for C. fluminea sampling.

Table A1.
Table of references for habitat environmental investigations.
Table A1.
Table of references for habitat environmental investigations.
| Phylum | Genus + Species | Habitat Environments |
|---|---|---|
| Arthropoda | Hygrobates sp. | marine, fresh 1 [33] |
| Anthessius sp. | marine | |
| Cyclops sp. | fresh | |
| Mytilicola sp. | marine | |
| Clausidium sp. | marine | |
| Liposcelis sp. | terrestrial | |
| Chordata | Ctenopharyngodon idella | brackish, fresh |
| Oncorhynchus sp. | marine, brackish, fresh | |
| Platyhelminthes | Paragonimus sp. | fresh, terrestrial |
| Bacillariophyta | Biddulphia sp. | marine |
| Navicula arenaria | marine, brackish | |
| Ciliophora | Strombidium sp. | marine, brackish, fresh |
| Cryptophyta | Katablepharis sp. | marine, fresh, terrestrial |
| Myzozoa | Prorocentrum sp. | marine, brackish, fresh |
| Ochrophyta | Nannochloropsis sp. | marine, brackish, fresh |
| Ascomycota | Aspergillus sp. | marine, brackish, fresh, terrestrial |
| Basidiomycota | Trichosporon sp. | marine, terrestrial |
| Chlorophyta | Selenastraceae sp. | fresh, terrestrial |
| Desmodesmus communis | fresh | |
| Mychonastes sp.1 | marine, fresh, terrestrial | |
| Mychonastes sp.2 | marine, fresh, terrestrial | |
| Chlorellaceae sp. | marine, brackish, fresh, terrestrial | |
| undetermined | Ebria sp. | marine, brackish |
1 Hygrobates sp. was not found in the WoRMs database, we used additional references instead.

Table A2.
Permutational Multivariate Analysis of Variance (PERMANOVA) results of three sampling points based on Bray–Curtis dissimilarities of RRA data with log(x + 1) transformation.
Table A2.
Permutational Multivariate Analysis of Variance (PERMANOVA) results of three sampling points based on Bray–Curtis dissimilarities of RRA data with log(x + 1) transformation.
| Source | df | SS | MS | Pseudo-F | P (Perm) | Unique Perms |
|---|---|---|---|---|---|---|
| Sampling sites | 2 | 2140.2 | 1070.1 | 0.74744 | 0.822 | 997 |
| Residuals | 27 | 38,655 | 1431.7 | |||
| Total | 29 | 40,795 |
df = degree of freedom; SS = sum of square; MS = mean sum of square; perm = permutation.
References
- Levin, L.A.; Boesch, D.F.; Covich, A.; Dahm, C.; Erséus, C.; Ewel, K.C.; Kneib, R.T.; Moldenke, A.; Palmer, M.A.; Snelgrove, P.; et al. The Function of Marine Critical Transition Zones and the Importance of Sediment Biodiversity. Ecosystems 2001, 4, 430–451. [Google Scholar] [CrossRef]
- John, W.; Day, J.; Crump, B.C.; Kemp, W.M.; Yáñez-Arancibia, A. Estuarine Ecology, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- McLusky, D.S.; Elliott, M. The Estuarine Ecosystem: Ecology, Threats and Management; OUP Oxford: Oxford, UK, 2004. [Google Scholar]
- Lotze, H.K.; Lenihan, H.S.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.G.; Kay, M.C.; Kidwell, S.M.; Kirby, M.X.; Peterson, C.H.; Jackson, J.B.C. Depletion, Degradation, and Recovery Potential of Estuaries and Coastal Seas. Science 2006, 312, 1806–1809. [Google Scholar] [CrossRef]
- Kennish, M.J. Environmental threats and environmental future of estuaries. Environ. Conserv. 2002, 29, 78–107. [Google Scholar] [CrossRef]
- Cloern, J.E.; Jassby, A.D. Drivers of change in estuarine-coastal ecosystems: Discoveries from four decades of study in San Francisco Bay. Rev. Geophys. 2012, 50, RG4001. [Google Scholar] [CrossRef]
- Hobbs, J.; Bright, D. Environmental DNA: Implementation for Resource Development Projects in BC and Beyond; University of British Columbia Library: Vancouver, BC Canada, 2016. [Google Scholar]
- Zimmermann, J.; Glöckner, G.; Jahn, R.; Enke, N.; Gemeinholzer, B. Metabarcoding vs. morphological identification to assess diatom diversity in environmental studies. Mol. Ecol. Resour. 2015, 15, 526–542. [Google Scholar] [CrossRef]
- Ko, H.-L.; Wang, Y.-T.; Chiu, T.-S.; Lee, M.-A.; Leu, M.-Y.; Chang, K.-Z.; Chen, W.-Y.; Shao, K.-T. Evaluating the Accuracy of Morphological Identification of Larval Fishes by Applying DNA Barcoding. PLoS ONE 2013, 8, e53451. [Google Scholar] [CrossRef] [PubMed]
- Polanco, F.A.; Mutis Martinezguerra, M.; Marques, V.; Villa-Navarro, F.; Borrero Pérez, G.H.; Cheutin, M.-C.; Dejean, T.; Hocdé, R.; Juhel, J.-B.; Maire, E.; et al. Detecting aquatic and terrestrial biodiversity in a tropical estuary using environmental DNA. Biotropica 2021, 53, 1606–1619. [Google Scholar] [CrossRef]
- Ruppert, K.M.; Kline, R.J.; Rahman, M.S. Past, present, and future perspectives of environmental DNA (eDNA) metabarcoding: A systematic review in methods, monitoring, and applications of global eDNA. Glob. Ecol. Conserv. 2019, 17, e00547. [Google Scholar] [CrossRef]
- Taberlet, P.; Bonin, A.; Zinger, L.; Coissac, E. Environmental DNA: For Biodiversity Research and Monitoring; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Beng, K.C.; Corlett, R.T. Applications of environmental DNA (eDNA) in ecology and conservation: Opportunities, challenges and prospects. Biodivers. Conserv. 2020, 29, 2089–2121. [Google Scholar] [CrossRef]
- Rivera, S.F.; Rimet, F.; Vasselon, V.; Vautier, M.; Domaizon, I.; Bouchez, A. Fish eDNA metabarcoding from aquatic biofilm samples: Methodological aspects. Mol. Ecol. Resour. 2022, 22, 1440–1453. [Google Scholar] [CrossRef]
- Tran, B.T.; Kim, K.-Y.; Heo, J.S.; Kim, K.-S.; Lee, H.J.; Park, T.G. Dietary analysis of three important mariculture species in South Korea using DNA metabarcoding in fecal samples. Aquac. Rep. 2023, 30, 101606. [Google Scholar] [CrossRef]
- Curran, T.G.; Browett, S.S.; O’Neill, D.; O’Hanlon, A.; O’Reilly, C.; Harrington, A.P.; McDevitt, A.D.; O’Meara, D.B. One bat’s waste is another man’s treasure: A DNA metabarcoding approach for the assessment of biodiversity and ecosystem services in Ireland using bat faeces. Biodivers. Conserv. 2022, 31, 2699–2722. [Google Scholar] [CrossRef]
- Nørgaard, L.; Olesen, C.R.; Trøjelsgaard, K.; Pertoldi, C.; Nielsen, J.L.; Taberlet, P.; Ruiz-González, A.; De Barba, M.; Iacolina, L. eDNA metabarcoding for biodiversity assessment, generalist predators as sampling assistants. Sci. Rep. 2021, 11, 6820. [Google Scholar] [CrossRef]
- Weber, S.; Junk, I.; Brink, L.; Wörner, M.; Künzel, S.; Veith, M.; Teubner, D.; Klein, R.; Paulus, M.; Krehenwinkel, H. Molecular diet analysis in mussels and other metazoan filter feeders and an assessment of their utility as natural eDNA samplers. Mol. Ecol. Resour. 2023, 23, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Siegenthaler, A.; Wangensteen, O.S.; Soto, A.Z.; Benvenuto, C.; Corrigan, L.; Mariani, S. Metabarcoding of shrimp stomach content: Harnessing a natural sampler for fish biodiversity monitoring. Mol. Ecol. Resour. 2019, 19, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Jeunen, G.-J.; Cane, J.S.; Ferreira, S.; Strano, F.; von Ammon, U.; Cross, H.; Day, R.; Hesseltine, S.; Ellis, K.; Urban, L.; et al. Assessing the utility of marine filter feeders for environmental DNA (eDNA) biodiversity monitoring. Mol. Ecol. Resour. 2023, 23, 771–786. [Google Scholar] [CrossRef]
- Mariani, S.; Baillie, C.; Colosimo, G.; Riesgo, A. Sponges as natural environmental DNA samplers. Curr. Biol. 2019, 29, R401–R402. [Google Scholar] [CrossRef]
- Gutiérrez, J.L.; Jones, C.G.; Strayer, D.L.; Iribarne, O.O. Mollusks as ecosystem engineers: The role of shell production in aquatic habitats. Oikos 2003, 101, 79–90. [Google Scholar] [CrossRef]
- Yoon, J.-D.; Jang, M.-H.; Jo, H.-B.; Jeong, K.-S.; Kim, G.-Y.; Joo, G.-J. Changes of fish assemblages after construction of an estuary barrage in the lower Nakdong River, South Korea. Limnology 2016, 17, 183–197. [Google Scholar] [CrossRef]
- Lee, C.-W.; Jang, J.-D.; Jeong, K.-S.; Kim, D.-K.; Joo, G.-J. Patterning habitat preference of avifaunal assemblage on the Nakdong River estuary (South Korea) using self-organizing map. Ecol. Inform. 2010, 5, 89–96. [Google Scholar] [CrossRef]
- Busan Marine Fisheries Resources Research Institute. Available online: https://www.busan.go.kr/fisheries/index (accessed on 16 August 2023).
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; Volume 10. [Google Scholar]
- Rak, A.; Khalid, N.; Syed Omar, S. The distribution and length size of Corbicula fluminea (ETAK) in Sungai Pergau at Gunung Reng. Int. J. Eng. Technol. 2018, 7, 279–281. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- National Institute of Biological Resources. Available online: https://species.nibr.go.kr/index.do (accessed on 21 July 2023).
- World Register of Marine Species (WoRMS). Available online: https://www.marinespecies.org/ (accessed on 21 July 2023).
- National Center for Biotechnology Information (NCBI). Taxonomy Browser. Available online: https://www.ncbi.nlm.nih.gov/datasets/taxonomy/tree/ (accessed on 21 July 2023).
- Pešić, V.; Jovanović, M.; Manović, A.; Zawal, A.; Bańkowska, A.; Broda, Ł.; Martin, P.; Dabert, M. Two new species from the Hygrobates nigromaculatus-complex (Acariformes, Hydrachnidia, Hygrobatidae), based on morphological and molecular evidence. Acarologia 2020, 60, 753–768. [Google Scholar] [CrossRef]
- Deagle, B.E.; Thomas, A.C.; McInnes, J.C.; Clarke, L.J.; Vesterinen, E.J.; Clare, E.L.; Kartzinel, T.R.; Eveson, J.P. Counting with DNA in metabarcoding studies: How should we convert sequence reads to dietary data? Mol. Ecol. 2019, 28, 391–406. [Google Scholar] [CrossRef] [PubMed]
- K-Water, Report of Water Quality and Aquatic Ecosystem Monitoring at Nakdong River Estuary; Korea Water Resources Corporation: Busan, Republic of Korea, 2022.
- Sousa, R.; Antunes, C.; Guilhermino, L. Ecology of the invasive Asian clam Corbicula fluminea (Müller, 1774) in aquatic ecosystems: An overview. Ann. Limnol.-Int. J. Limnol. 2008, 44, 85–94. [Google Scholar] [CrossRef]
- Su, L.; Cai, H.; Kolandhasamy, P.; Wu, C.; Rochman, C.M.; Shi, H. Using the Asian clam as an indicator of microplastic pollution in freshwater ecosystems. Environ. Pollut. 2018, 234, 347–355. [Google Scholar] [CrossRef]
- Li, Z.; He, X.; Feng, C. A review of freshwater benthic clams (Corbicula fluminea): Accumulation capacity, underlying physiological mechanisms and environmental applications. Sci. Total Environ. 2023, 857, 159431. [Google Scholar] [CrossRef] [PubMed]
- Way, C.M.; Hornbach, D.J.; Miller-Way, C.A.; Payne, B.S.; Miller, A.C. Dynamics of filter feeding in Corbicula fluminea (Bivalvia: Corbiculidae). Can. J. Zool. 1990, 68, 115–120. [Google Scholar] [CrossRef]
- Schabacker, J.C.; Amish, S.J.; Ellis, B.K.; Gardner, B.; Miller, D.L.; Rutledge, E.A.; Sepulveda, A.J.; Luikart, G. Increased eDNA detection sensitivity using a novel high-volume water sampling method. Environ. DNA 2020, 2, 244–251. [Google Scholar] [CrossRef]
- Sepulveda, A.J.; Schabacker, J.; Smith, S.; Al-Chokhachy, R.; Luikart, G.; Amish, S.J. Improved detection of rare, endangered and invasive trout in using a new large-volume sampling method for eDNA capture. Environ. DNA 2019, 1, 227–237. [Google Scholar] [CrossRef]
- Crespo, D.; Dolbeth, M.; Leston, S.; Sousa, R.; Pardal, M.Â. Distribution of Corbicula fluminea (Müller, 1774) in the invaded range: A geographic approach with notes on species traits variability. Biol. Invasions 2015, 17, 2087–2101. [Google Scholar] [CrossRef]
- Gosling, E. Bivalve Molluscs: Biology, Ecology and Culture; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Xin, Y.; Guo, Y.; Sun, M.; Yu, G.; Ma, Z.; Pei, K.; Qin, C. Optimal Conditions to Quantify the Relationship between eDNA Concentration and Biomass in Acanthopagrus latus. Water 2022, 14, 3521. [Google Scholar] [CrossRef]
- Lauritsen, D.D. Filter-Feeding in Corbicula fluminea and Its Effect on Seston Removal. J. N. Am. Benthol. Soc. 1986, 5, 165–172. [Google Scholar] [CrossRef]
- Telesh, I.V.; Khlebovich, V.V. Principal processes within the estuarine salinity gradient: A review. Mar. Pollut. Bull. 2010, 61, 149–155. [Google Scholar] [CrossRef]
- Cloern, J.E.; Jassby, A.D.; Schraga, T.S.; Nejad, E.; Martin, C. Ecosystem variability along the estuarine salinity gradient: Examples from long-term study of San Francisco Bay. Limnol. Oceanogr. 2017, 62, S272–S291. [Google Scholar] [CrossRef]
- Franco, T.P.; Neves, L.M.; Araújo, F.G. Better with more or less salt? The association of fish assemblages in coastal lagoons with different salinity ranges. Hydrobiologia 2019, 828, 83–100. [Google Scholar] [CrossRef]
- Matela, M.; Obolewski, K. Structural diagnosis of benthic invertebrate communities in relation to salinity gradient in Baltic coastal lake ecosystems using biological trait analysis. Sci. Rep. 2022, 12, 12750. [Google Scholar] [CrossRef]
- Heo, Y.-J.; Jo, H.; Kim, J.Y.; Kim, G.-Y.; Joo, G.-J.; Kim, H.-W. Application of DNA Metabarcoding for Identifying the Diet of Asian Clam (Corbicula fluminea, Müller, 1774). Sustainability 2023, 15, 441. [Google Scholar] [CrossRef]
- Valentini, A.; Taberlet, P.; Miaud, C.; Civade, R.; Herder, J.; Thomsen, P.F.; Bellemain, E.; Besnard, A.; Coissac, E.; Boyer, F.; et al. Next-generation monitoring of aquatic biodiversity using environmental DNA metabarcoding. Mol. Ecol. 2016, 25, 929–942. [Google Scholar] [CrossRef]
- Nagarajan, R.P.; Bedwell, M.; Holmes, A.E.; Sanches, T.; Acuña, S.; Baerwald, M.; Barnes, M.A.; Blankenship, S.; Connon, R.E.; Deiner, K.; et al. Environmental DNA Methods for Ecological Monitoring and Biodiversity Assessment in Estuaries. Estuaries Coasts 2022, 45, 2254–2273. [Google Scholar] [CrossRef]
- Hunter, M.E.; Ferrante, J.A.; Meigs-Friend, G.; Ulmer, A. Improving eDNA yield and inhibitor reduction through increased water volumes and multi-filter isolation techniques. Sci. Rep. 2019, 9, 5259. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lawson Handley, L.-J.; Read, D.S.; Hänfling, B. The effect of filtration method on the efficiency of environmental DNA capture and quantification via metabarcoding. Mol. Ecol. Resour. 2018, 18, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Vestheim, H.; Jarman, S.N. Blocking primers to enhance PCR amplification of rare sequences in mixed samples—A case study on prey DNA in Antarctic krill stomachs. Front. Zool. 2008, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Leray, M.; Agudelo, N.; Mills, S.C.; Meyer, C.P. Effectiveness of Annealing Blocking Primers versus Restriction Enzymes for Characterization of Generalist Diets: Unexpected Prey Revealed in the Gut Contents of Two Coral Reef Fish Species. PLoS ONE 2013, 8, e58076. [Google Scholar] [CrossRef]
- Blankenship, L.E.; Yayanos, A.A. Universal primers and PCR of gut contents to study marine invertebrate diets. Mol. Ecol. 2005, 14, 891–899. [Google Scholar] [CrossRef]
- Piñol, J.; Mir, G.; Gomez-Polo, P.; Agustí, N. Universal and blocking primer mismatches limit the use of high-throughput DNA sequencing for the quantitative metabarcoding of arthropods. Mol. Ecol. Resour. 2015, 15, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Amaral-Zettler, L.A.; McCliment, E.A.; Ducklow, H.W.; Huse, S.M. A Method for Studying Protistan Diversity Using Massively Parallel Sequencing of V9 Hypervariable Regions of Small-Subunit Ribosomal RNA Genes. PLoS ONE 2009, 4, e6372. [Google Scholar] [CrossRef]
- Albaina, A.; Aguirre, M.; Abad, D.; Santos, M.; Estonba, A. 18S rRNA V9 metabarcoding for diet characterization: A critical evaluation with two sympatric zooplanktivorous fish species. Ecol. Evol. 2016, 6, 1809–1824. [Google Scholar] [CrossRef]
- Hugerth, L.W.; Muller, E.E.L.; Hu, Y.O.O.; Lebrun, L.A.M.; Roume, H.; Lundin, D.; Wilmes, P.; Andersson, A.F. Systematic Design of 18S rRNA Gene Primers for Determining Eukaryotic Diversity in Microbial Consortia. PLoS ONE 2014, 9, e95567. [Google Scholar] [CrossRef]
- Questel, J.M.; Hopcroft, R.R.; DeHart, H.M.; Smoot, C.A.; Kosobokova, K.N.; Bucklin, A. Metabarcoding of zooplankton diversity within the Chukchi Borderland, Arctic Ocean: Improved resolution from multi-gene markers and region-specific DNA databases. Mar. Biodivers. 2021, 51, 4. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).