A549 as an In Vitro Model to Evaluate the Impact of Microplastics in the Air
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Micro- and Nanoplastics
1.2. A549
2. Methods
Search Strategy and Study Selection
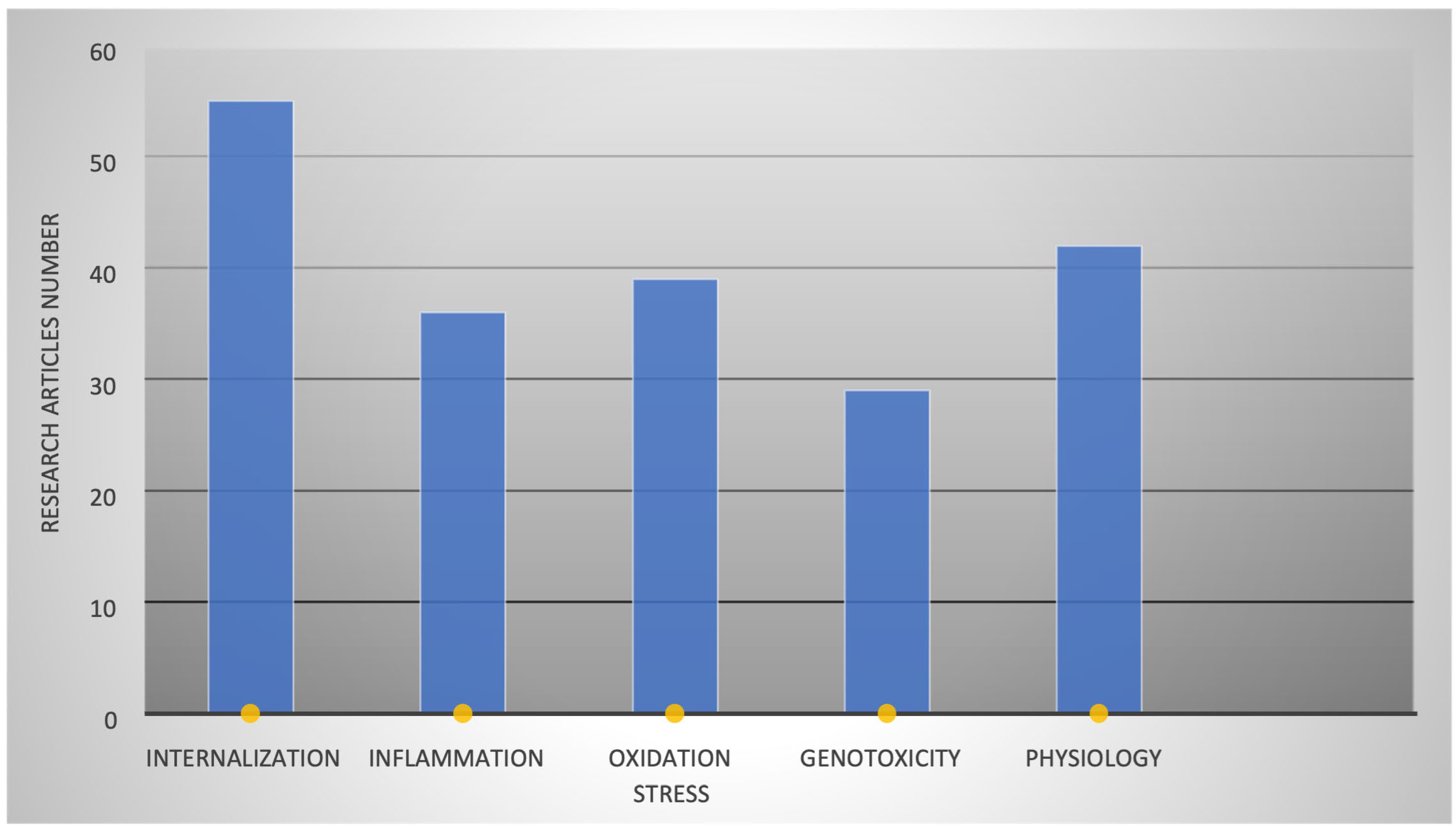
3. Results
3.1. PS-NP Types and Effects on Various Organs
3.2. Physiochemical Properties Effects on PS-NP Internalization
3.2.1. Size of PS Particles Affecting Toxicity
3.2.2. Surface Modifications Effects on Toxicity
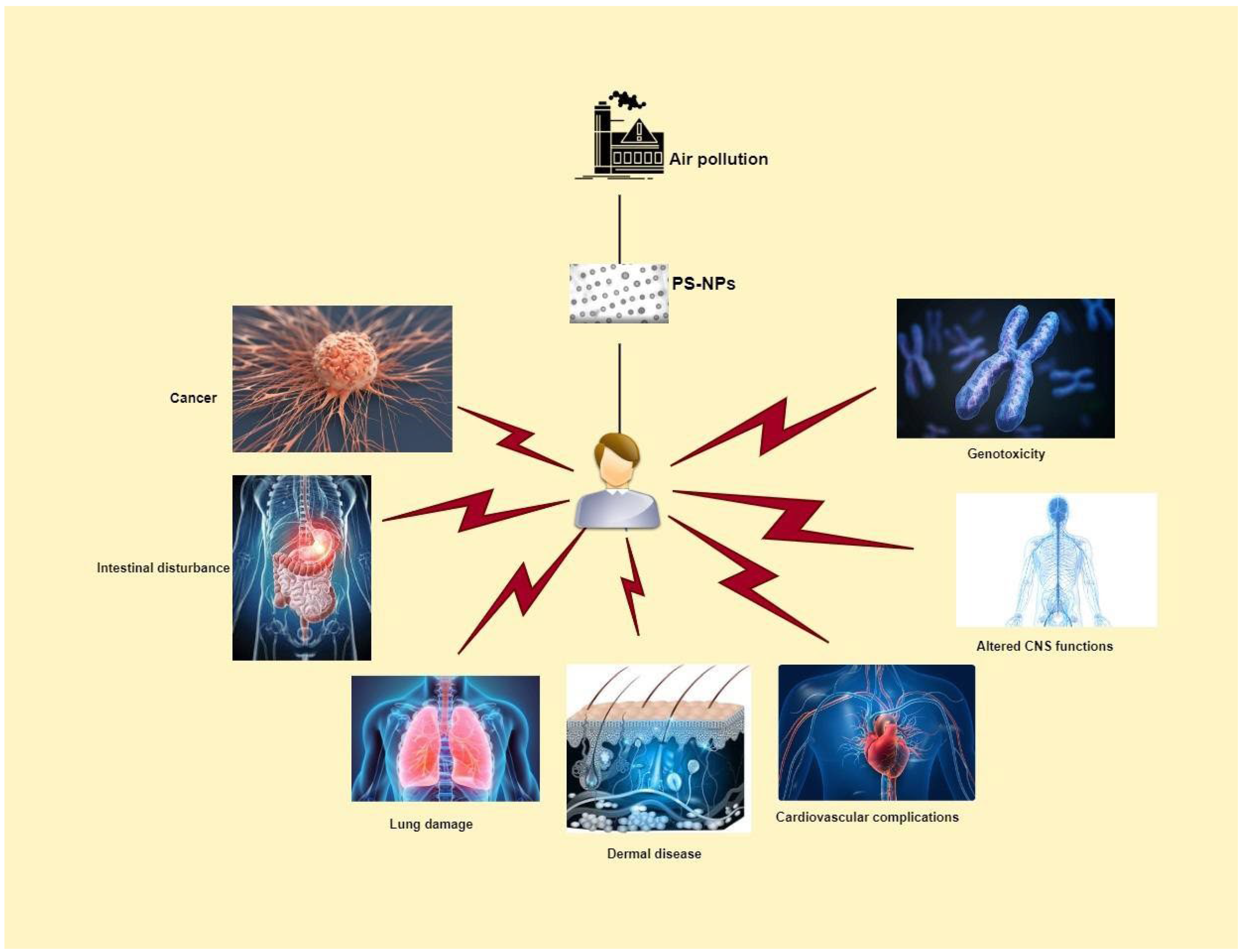
4. Effects of PS-NPs on Human Health
4.1. Genotoxicity
4.2. Endocytosis and Exocytosis of PS in A549 Cells
4.3. Oxidation Stress Caused by PS
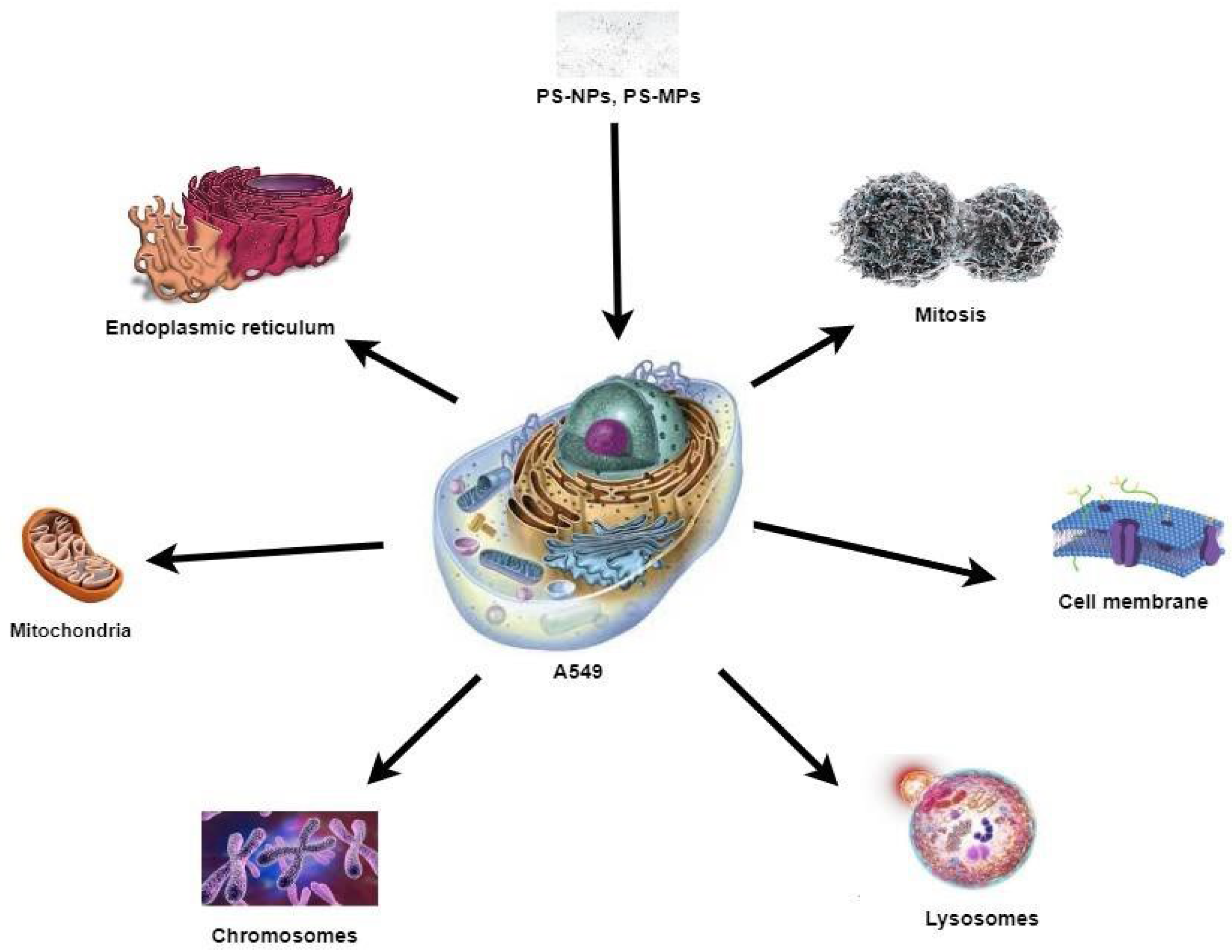
4.4. Morphological Changes in A549 Cells on PS-NPs Exposure
4.5. Inflammation Caused by PS
4.6. PS Effects on Epithelial–Mesenchymal Transition (EMT)
4.7. Accumulation of PS-NPs in the Brain
4.8. Cytotoxicity Studies of PS Particles
Is One Cell Culture Model Enough to Assess the Microplastic Toxicity?
5. Discussion
6. Conclusions
7. Suggestions for Future Research
Author Contributions
Funding
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- da Costa, J.P. Micro-and nanoplastics in the environment: Research and policymaking. Curr. Opin. Environ. Sci. Health 2018, 1, 12–16. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Jung, S.W.; Shim, W.J. Combined effects of UV exposure duration and mechanical abrasion on microplastic fragmentation by polymer type. Environ. Sci. Technol. 2017, 51, 4368–4376. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Fischer, E.K. Microplastic abundance in atmospheric deposition within the Metropolitan area of Hamburg, Germany. Sci. Total Environ. 2019, 685, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Ji, X.; Ma, Y.; Lv, B.; Huang, W.; Zhu, X.; Fang, M.; Wang, Q.; Wang, X.; Dahlgren, R. Airborne microplastics in indoor and outdoor environments of a coastal city in Eastern China. J. Hazard. Mater. 2021, 417, 126007. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shao, L.; Wang, W.; Zhang, M.; Feng, X.; Li, W.; Zhang, D. Airborne fiber particles: Types, size and concentration observed in Beijing. Sci. Total Environ. 2020, 705, 135967. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Brookson, C.; Bikker, J.; Djuric, N.; Earn, A.; Bucci, K.; Athey, S.; Huntington, A.; McIlwraith, H.; Munno, K. Rethinking microplastics as a diverse contaminant suite. Environ. Toxicol. Chem. 2019, 38, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Laskar, N.; Kumar, U. Plastics and microplastics: A threat to environment. Environ. Technol. Innov. 2019, 14, 100352. [Google Scholar] [CrossRef]
- Myers, I.; Maynard, R. Polluted air—Outdoors and indoors. Occup. Med. 2005, 55, 432–438. [Google Scholar] [CrossRef]
- Gasperi, J.; Dris, R.; Mirande-Bret, C.; Mandin, C.; Langlois, V.; Tassin, B. First overview of microplastics in indoor and outdoor air. In Proceedings of the 15th EuCheMS International Conference on Chemistry and the Environment, Leipzig, Germany, 20–24 September 2015. [Google Scholar]
- Kooi, M.; Reisser, J.; Slat, B.; Ferrari, F.F.; Schmid, M.S.; Cunsolo, S.; Brambini, R.; Noble, K.; Sirks, L.-A.; Linders, T.E. The effect of particle properties on the depth profile of buoyant plastics in the ocean. Sci. Rep. 2016, 6, 33882. [Google Scholar] [CrossRef]
- Besseling, E.; Wegner, A.; Foekema, E.M.; Van Den Heuvel-Greve, M.J.; Koelmans, A.A. Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L.). Environ. Sci. Technol. 2013, 47, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Lehner, R.; Weder, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Emergence of nanoplastic in the environment and possible impact on human health. Environ. Sci. Technol. 2019, 53, 1748–1765. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.; Hollman, P.C.; Peters, R.J. Potential health impact of environmentally released micro-and nanoplastics in the human food production chain: Experiences from nanotoxicology. Environ. Sci. Technol. 2015, 49, 8932–8947. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Halimu, G.; Zhang, Q.; Song, Y.; Fu, X.; Li, Y.; Li, Y.; Zhang, H. Internalization and toxicity: A preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci. Total Environ. 2019, 694, 133794. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, K.; Zhang, B.; Ye, Y.; Zhang, Q.; Jiang, W. Cellular internalization and release of polystyrene microplastics and nanoplastics. Sci. Total Environ. 2021, 779, 146523. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, J.; Qiu, G.; Ying, J.; Du, Z.; Xiang, T.; Wong, K.Y.; Srivastava, G.; Zhu, X.-F.; Mok, T.S. Epigenomic characterization of a p53-regulated 3p22. 2 tumor suppressor that inhibits STAT3 phosphorylation via protein docking and is frequently methylated in esophageal and other carcinomas. Theranostics 2018, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Pilou, M.; Vaquero-Moralejo, C.; Jaén, M.; Lopez De Ipiña Peña, J.; Neofytou, P.; Housiadas, C. Modeling of occupational exposure to accidentally released manufactured nanomaterials in a production facility and calculation of internal doses by inhalation. Int. J. Occup. Environ. Health 2016, 22, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Liu, Y.; Zhang, T.; Zhang, F.; Ren, H.; Zhang, Y. Analysis of microplastics in human feces reveals a correlation between fecal microplastics and inflammatory bowel disease status. Environ. Sci. Technol. 2021, 56, 414–421. [Google Scholar] [CrossRef]
- Jung, B.-K.; Han, S.-W.; Park, S.-H.; Bae, J.-S.; Choi, J.; Ryu, K.-Y. Neurotoxic potential of polystyrene nanoplastics in primary cells originating from mouse brain. Neurotoxicology 2020, 81, 189–196. [Google Scholar] [CrossRef]
- Yin, K.; Lu, H.; Zhang, Y.; Hou, L.; Meng, X.; Li, J.; Zhao, H.; Xing, M. Secondary brain injury after polystyrene microplastic-induced intracerebral hemorrhage is associated with inflammation and pyroptosis. Chem.-Biol. Interact. 2022, 367, 110180. [Google Scholar] [CrossRef]
- Shan, S.; Zhang, Y.; Zhao, H.; Zeng, T.; Zhao, X. Polystyrene nanoplastics penetrate across the blood-brain barrier and induce activation of microglia in the brain of mice. Chemosphere 2022, 298, 134261. [Google Scholar] [CrossRef] [PubMed]
- Mazurais, D.; Ernande, B.; Quazuguel, P.; Severe, A.; Huelvan, C.; Madec, L.; Mouchel, O.; Soudant, P.; Robbens, J.; Huvet, A. Evaluation of the impact of polyethylene microbeads ingestion in European sea bass (Dicentrarchus labrax) larvae. Mar. Environ. Res. 2015, 112, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Stroh, A.; Anderka, O.; Pfeiffer, K.; Yagi, T.; Finel, M.; Ludwig, B.; Schägger, H. Assembly of respiratory complexes I, III, and IV into NADH oxidase supercomplex stabilizes complex I in Paracoccus denitrificans. J. Biol. Chem. 2004, 279, 5000–5007. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Durántez Jiménez, P.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar] [CrossRef]
- Stone, V.; Johnston, H.; Clift, M.J. Air pollution, ultrafine and nanoparticle toxicology: Cellular and molecular interactions. IEEE Trans. Nanobiosci. 2007, 6, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Forte, M.; Iachetta, G.; Tussellino, M.; Carotenuto, R.; Prisco, M.; De Falco, M.; Laforgia, V.; Valiante, S. Polystyrene nanoparticles internalization in human gastric adenocarcinoma cells. Toxicol. Vitr. 2016, 31, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef]
- Salvi, S. Health effects of ambient air pollution in children. Paediatr. Respir. Rev. 2007, 8, 275–280. [Google Scholar] [CrossRef]
- Turner, M.C.; Krewski, D.; Diver, W.R.; Pope, C.A., III; Burnett, R.T.; Jerrett, M.; Marshall, J.D.; Gapstur, S.M. Ambient air pollution and cancer mortality in the cancer prevention study II. Environ. Health Perspect. 2017, 125, 087013. [Google Scholar] [CrossRef]
- Giard, D.J.; Aaronson, S.A.; Todaro, G.J.; Arnstein, P.; Kersey, J.H.; Dosik, H.; Parks, W.P. In vitro cultivation of human tumors: Establishment of cell lines derived from a series of solid tumors. J. Natl. Cancer Inst. 1973, 51, 1417–1423. [Google Scholar] [CrossRef]
- Arif, T.; Amsalem, Z.; Shoshan-Barmatz, V. Metabolic reprograming via silencing of mitochondrial VDAC1 expression encourages differentiation of cancer cells. Mol. Ther.-Nucleic Acids 2019, 17, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wang, Q.; Liao, X.; Ge, H.; Huo, G.; Zhang, L.; Chen, N.; Zhai, X.; Hong, Y.; Wang, L. Discovery of 6-(2-(dimethylamino)ethyl)-N-(5-fluoro-4-(4-fluoro-1-isopropyl-2-methyl-1H-benzo[d]imidazole-6-yl)pyrimidin-2-yl)-5,6,7,8-tetrahydro-1, 6-naphthyridin-2-amine as a highly potent cyclin-dependent kinase 4/6 inhibitor for treatment of cancer. Eur. J. Med. Chem. 2019, 178, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef] [PubMed]
- Kik, K.; Bukowska, B.; Sicińska, P. Polystyrene nanoparticles: Sources, occurrence in the environment, distribution in tissues, accumulation and toxicity to various organisms. Environ. Pollut. 2020, 262, 114297. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Manna, C.; Padha, S.; Verma, A.; Sharma, P.; Dhar, A.; Ghosh, A.; Bhattacharya, P. Micro (nano) plastics pollution and human health: How plastics can induce carcinogenesis to humans? Chemosphere 2022, 298, 134267. [Google Scholar] [CrossRef] [PubMed]
- Halimu, G.; Zhang, Q.; Liu, L.; Zhang, Z.; Wang, X.; Gu, W.; Zhang, B.; Dai, Y.; Zhang, H.; Zhang, C. Toxic effects of nanoplastics with different sizes and surface charges on epithelial-to-mesenchymal transition in A549 cells and the potential toxicological mechanism. J. Hazard. Mater. 2022, 430, 128485. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, K.; Bukowska, B.; Piwoński, I.; Foksiński, M.; Kisielewska, A.; Zarakowska, E.; Gackowski, D.; Sicińska, P. Polystyrene nanoparticles: The mechanism of their genotoxicity in human peripheral blood mononuclear cells. Nanotoxicology 2022, 16, 791–811. [Google Scholar] [CrossRef] [PubMed]
- Debbage, P.; Jaschke, W. Molecular imaging with nanoparticles: Giant roles for dwarf actors. Histochem. Cell Biol. 2008, 130, 845–875. [Google Scholar] [CrossRef]
- Iversen, T.-G.; Skotland, T.; Sandvig, K. Endocytosis and intracellular transport of nanoparticles: Present knowledge and need for future studies. Nano Today 2011, 6, 176–185. [Google Scholar] [CrossRef]
- Kihara, S.; Ashenden, A.; Kaur, M.; Glasson, J.; Ghosh, S.; van der Heijden, N.; Brooks, A.E.; Mata, J.P.; Holt, S.; Domigan, L.J. Cellular interactions with polystyrene nanoplastics—The role of particle size and protein corona. Biointerphases 2021, 16, 041001. [Google Scholar] [CrossRef]
- Shi, X.; Wang, X.; Huang, R.; Tang, C.; Hu, C.; Ning, P.; Wang, F. Cytotoxicity and genotoxicity of polystyrene micro-and nanoplastics with different size and surface modification in A549 cells. Int. J. Nanomed. 2022, 17, 4509–4523. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, J.; Chen, J.; Miao, X.; Li, G.; He, Q.; Xu, H.; Li, H.; Wei, Y. Cytotoxic effects of polystyrene nanoplastics with different surface functionalization on human HepG2 cells. Sci. Total Environ. 2020, 723, 138180. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, Y.; Jiao, Y.; Chen, Q.; Wu, D.; Yu, P.; Li, Y.; Cai, M.; Zhao, Y. Polystyrene nanoplastic induces ROS production and affects the MAPK-HIF-1/NFkB-mediated antioxidant system in Daphnia pulex. Aquat. Toxicol. 2020, 220, 105420. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Marion, M.; Jeong, B.-H.; Hoek, E.M. Crossflow membrane filtration of interacting nanoparticle suspensions. J. Membr. Sci. 2006, 284, 361–372. [Google Scholar] [CrossRef]
- Shi, X.; Huang, R.; Wang, X.; Tang, C.; Hu, C.; Wang, F. Cytotoxicity and Genotoxicity of Polystyrene Microplastics with Different Size and Surface Modification in A549 Human Lung Cells. 2021. Available online: https://ssrn.com/abstract=3935593 (accessed on 10 May 2023).
- Jeon, S.; Clavadetscher, J.; Lee, D.-K.; Chankeshwara, S.V.; Bradley, M.; Cho, W.-S. Surface charge-dependent cellular uptake of polystyrene nanoparticles. Nanomaterials 2018, 8, 1028. [Google Scholar] [CrossRef] [PubMed]
- Deville, S.; Penjweini, R.; Smisdom, N.; Notelaers, K.; Nelissen, I.; Hooyberghs, J.; Ameloot, M. Intracellular dynamics and fate of polystyrene nanoparticles in A549 Lung epithelial cells monitored by image (cross-) correlation spectroscopy and single particle tracking. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2015, 1853, 2411–2419. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Cheng, Y.; Chen, Z.; Liu, T.; Yin, L.; Pu, Y.; Liang, G. In vitro evaluation of nanoplastics using human lung epithelial cells, microarray analysis and co-culture model. Ecotoxicol. Environ. Saf. 2021, 226, 112837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, S.; Ge, Y.; Wan, X.; Zhu, Y.; Li, J.; Yin, L.; Pu, Y.; Liang, G. Polystyrene nanoplastics induce lung injury via activating oxidative stress: Molecular insights from bioinformatics analysis. Nanomaterials 2022, 12, 3507. [Google Scholar] [CrossRef]
- Martin, D.; Brun, C.; Remy, E.; Mouren, P.; Thieffry, D.; Jacq, B. GOToolBox: Functional analysis of gene datasets based on Gene Ontology. Genome Biol. 2004, 5, R101. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Furumichi, M.; Tanabe, M.; Hirakawa, M. KEGG for representation and analysis of molecular networks involving diseases and drugs. Nucleic Acids Res. 2010, 38, D355–D360. [Google Scholar] [CrossRef]
- Madrigal-Matute, J.; Fernandez-Laso, V.; Sastre, C.; Llamas-Granda, P.; Egido, J.; Martin-Ventura, J.L.; Zalba, G.; Blanco-Colio, L.M. TWEAK/Fn14 interaction promotes oxidative stress through NADPH oxidase activation in macrophages. Cardiovasc. Res. 2015, 108, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xie, Z.; Xu, J.; Feng, Z. TWEAK/Fn14 axis in respiratory diseases. Clin. Chim. Acta 2020, 509, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Liu, J.; Wu, H.; Zhang, Q.; Tang, X.-R.; Li, D.; Li, C.-S.; Liu, Y.; Cao, A.; Wang, H. Endocytosis, Distribution, and Exocytosis of Polystyrene Nanoparticles in Human Lung Cells. Nanomaterials 2022, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Battaglia, G.; Golestanian, R. The effect of interactions on the cellular uptake of nanoparticles. Phys. Biol. 2011, 8, 046002. [Google Scholar] [CrossRef] [PubMed]
- Varela, J.A.; Bexiga, M.G.; Åberg, C.; Simpson, J.C.; Dawson, K.A. Quantifying size-dependent interactions between fluorescently labeled polystyrene nanoparticles and mammalian cells. J. Nanobiotechnol. 2012, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, T.; Varela, J.; Lynch, I.; Salvati, A.; Dawson, K.A. Effects of transport inhibitors on the cellular uptake of carboxylated polystyrene nanoparticles in different cell lines. PLoS ONE 2011, 6, e24438. [Google Scholar] [CrossRef] [PubMed]
- Sipos, A.; Kim, K.-J.; Sioutas, C.; Crandall, E.D. Evidence for nanoparticle-induced lysosomal dysfunction in lung adenocarcinoma (A549) cells. Int. J. Mol. Sci. 2019, 20, 5253. [Google Scholar] [CrossRef]
- Goodman, K.E.; Hare, J.T.; Khamis, Z.I.; Hua, T.; Sang, Q.-X.A. Exposure of human lung cells to polystyrene microplastics significantly retards cell proliferation and triggers morphological changes. Chem. Res. Toxicol. 2021, 34, 1069–1081. [Google Scholar] [CrossRef]
- Hou, B.; Wang, F.; Liu, T.; Wang, Z. Reproductive toxicity of polystyrene microplastics: In vivo experimental study on testicular toxicity in mice. J. Hazard. Mater. 2021, 405, 124028. [Google Scholar] [CrossRef]
- Brown, D.M.; Wilson, M.R.; MacNee, W.; Stone, V.; Donaldson, K. Size-dependent proinflammatory effects of ultrafine polystyrene particles: A role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol. Appl. Pharmacol. 2001, 175, 191–199. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Módis, K.; Gerő, D.; Erdélyi, K.; Szoleczky, P.; DeWitt, D.; Szabo, C. Cellular bioenergetics is regulated by PARP1 under resting conditions and during oxidative stress. Biochem. Pharmacol. 2012, 83, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Senft, D.; Ze’ev, A.R. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem. Sci. 2015, 40, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.; Baek, J.Y.; Koo, J.; Park, S.; Ryu, Y.-K.; Kim, K.-S.; Zhang, S.; Chung, C.; Dogan, R.; Choi, H.-S. Maternal exposure to polystyrene nanoplastics causes brain abnormalities in progeny. J. Hazard. Mater. 2022, 426, 127815. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhu, J.; Zhou, X.; Pan, D.; Nan, S.; Yin, R.; Lei, Q.; Ma, N.; Zhu, H.; Chen, J. Polystyrene micro-and nano-particle coexposure injures fetal thalamus by inducing ROS-mediated cell apoptosis. Environ. Int. 2022, 166, 107362. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.; Kim, D.; Kim, H.-Y.; Jeong, S.W.; Lee, S.-G.; Kim, H.-C.; Lee, Y.-J.; Kwon, M.K.; Hwang, J.-S.; Han, J.E. Microglial phagocytosis of polystyrene microplastics results in immune alteration and apoptosis in vitro and in vivo. Sci. Total Environ. 2022, 807, 150817. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yi, X.; Zhou, H.; Chi, T.; Li, W.; Yang, K. Combined effect of polystyrene microplastics and dibutyl phthalate on the microalgae Chlorella pyrenoidosa. Environ. Pollut. 2020, 257, 113604. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Tang, J.; Liu, R.; Wang, L. Toxicity in vitro reveals potential impacts of microplastics and nanoplastics on human health: A review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 3863–3895. [Google Scholar] [CrossRef]
- Lodovici, M.; Bigagli, E. Oxidative stress and air pollution exposure. J. Toxicol. 2011, 2011, 487074. [Google Scholar] [CrossRef]
- MacNee, W. Oxidative stress and lung inflammation in airways disease. Eur. J. Pharmacol. 2001, 429, 195–207. [Google Scholar] [CrossRef]
- Hwang, S.-H.; Thielbeer, F.; Jeong, J.; Han, Y.; Chankeshwara, S.V.; Bradley, M.; Cho, W.-S. Dual contribution of surface charge and protein-binding affinity to the cytotoxicity of polystyrene nanoparticles in nonphagocytic A549 cells and phagocytic THP-1 cells. J. Toxicol. Environ. Health Part A 2016, 79, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Stock, V.; Böhmert, L.; Dönmez, M.H.; Lampen, A.; Sieg, H. An inverse cell culture model for floating plastic particles. Anal. Biochem. 2020, 591, 113545. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Vallabani, S.; Bordes, R.; Bhattacharya, K.; Fadeel, B. Development of microfluidic, serum-free bronchial epithelial cells-on-a-chip to facilitate a more realistic in vitro testing of nanoplastics. Front. Toxicol. 2021, 3, 735331. [Google Scholar] [CrossRef] [PubMed]
- Lehner, R.; Wohlleben, W.; Septiadi, D.; Landsiedel, R.; Petri-Fink, A.; Rothen-Rutishauser, B. A novel 3D intestine barrier model to study the immune response upon exposure to microplastics. Arch. Toxicol. 2020, 94, 2463–2479. [Google Scholar] [CrossRef] [PubMed]
- Meindl, C.; Öhlinger, K.; Zrim, V.; Steinkogler, T.; Fröhlich, E. Screening for effects of inhaled nanoparticles in cell culture models for prolonged exposure. Nanomaterials 2021, 11, 606. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Palmberg, L. Air-liquid interface: Relevant in vitro models for investigating air pollutant-induced pulmonary toxicity. Toxicol. Sci. 2018, 164, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, K.; Ekvall, M.T.; Hansson, L.-A.; Linse, S.; Malmendal, A.; Cedervall, T. Altered behavior, physiology, and metabolism in fish exposed to polystyrene nanoparticles. Environ. Sci. Technol. 2015, 49, 553–561. [Google Scholar] [CrossRef]
- Cai, L.; Wang, J.; Peng, J.; Tan, Z.; Zhan, Z.; Tan, X.; Chen, Q. Characteristic of microplastics in the atmospheric fallout from Dongguan city, China: Preliminary research and first evidence. Environ. Sci. Pollut. Res. 2017, 24, 24928–24935. [Google Scholar] [CrossRef]
- Shi, Q.; Tang, J.; Wang, L.; Liu, R.; Giesy, J.P. Combined cytotoxicity of polystyrene nanoplastics and phthalate esters on human lung epithelial A549 cells and its mechanism. Ecotoxicol. Environ. Saf. 2021, 213, 112041. [Google Scholar] [CrossRef]


| Polymer Type | PS Size | Biological Model | Results | References |
|---|---|---|---|---|
| PS-NPs | 25 nm, 70 nm | A549 cells | Upregulated inflammatory gene expression, lost cell membrane integrity, necrosis | [15] |
| PS-NPs | 20 nm | A549 cells | Membrane disruption | [41] |
| PS-NPs | 40 nm | BEAS-2B and HPAEpiC | Altered genetic expressions, redox imbalance-mediated inflammation leading to apoptosis | [49] |
| PS-NPs | 50 nm, 100 nm | A549 cells | Size- and charge-dependent cellular internalization of PS-NPs, uptake is energy-dependent which decreases at low temperature | [55] |
| PS-NPs | A549 cells | Oxidation stress | [59] | |
| PS-NPs | 1 µm and 10 µm | A549 | Cells lose close contact with neighboring cells and grow apart. Generation of Cytoskeletal features and ability to move at distant places, inhibition in proliferation | [60] |
| PS-NPs | 64 nm | A549 | Oxidative stress leading to IL-8 expression, stimulating neutrophil recruitment and inflammation. | [62] |
| PS-NPs | 20 nm, 50 nm | A549 | Endoplasmic reticulum stress, mitochondrial dysfunction, oxidative stress. | [37] |
| PS-NPs, PS-NH2, PS- COOH | 80 nm, 2 μm | A549 | Size-dependent internalization of PS particles, more internalization of surface-functionalized PS particles. | [42] |
| PS-NPs | 116 nm, 152 nm | A549 | Transport of PR-NPs in cell is assisted by microtubules and transported by lysosomes | [48] |
| PS-NPs | 200 nm | A549 | Zetapotential of PS shows negative correlation with toxicity. | [73] |
| PS-NPs, Carboxylated PS-NPs | 20 nm, 40 nm, 100 nm | A549 | Cellular uptake is size-dependent but 40 nm internalized more than 20 nm | [57] |
| fluorophore-conjugated polystyrene nanoparticles (F-PLNPs) | 82 nm | A549 | Cellular internalization shows positive correlation with zeta potential, surface charge of PS impacts greatly on cellular uptake | [47] |
| PS-MPs | 10 μm, 1 μm | A549 | Decreased in metabolic activity and proliferation rate of cells, changed cell morphology | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahzadi, C.; Di Serafino, A.; Aruffo, E.; Mascitelli, A.; Di Carlo, P. A549 as an In Vitro Model to Evaluate the Impact of Microplastics in the Air. Biology 2023, 12, 1243. https://doi.org/10.3390/biology12091243
Shahzadi C, Di Serafino A, Aruffo E, Mascitelli A, Di Carlo P. A549 as an In Vitro Model to Evaluate the Impact of Microplastics in the Air. Biology. 2023; 12(9):1243. https://doi.org/10.3390/biology12091243
Chicago/Turabian StyleShahzadi, Chman, Alessandra Di Serafino, Eleonora Aruffo, Alessandra Mascitelli, and Piero Di Carlo. 2023. "A549 as an In Vitro Model to Evaluate the Impact of Microplastics in the Air" Biology 12, no. 9: 1243. https://doi.org/10.3390/biology12091243
APA StyleShahzadi, C., Di Serafino, A., Aruffo, E., Mascitelli, A., & Di Carlo, P. (2023). A549 as an In Vitro Model to Evaluate the Impact of Microplastics in the Air. Biology, 12(9), 1243. https://doi.org/10.3390/biology12091243








