A Novel Dual-Function Redox Modulator Relieves Oxidative Stress and Anti-Angiogenic Response in Placental Villus Explant Exposed to Hypoxia—Relevance for Preeclampsia Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Human Placental Villous Explant Cultures and Sample Collection
2.3. Antioxidant Assay
2.4. Mitochondrial ROS Measurements
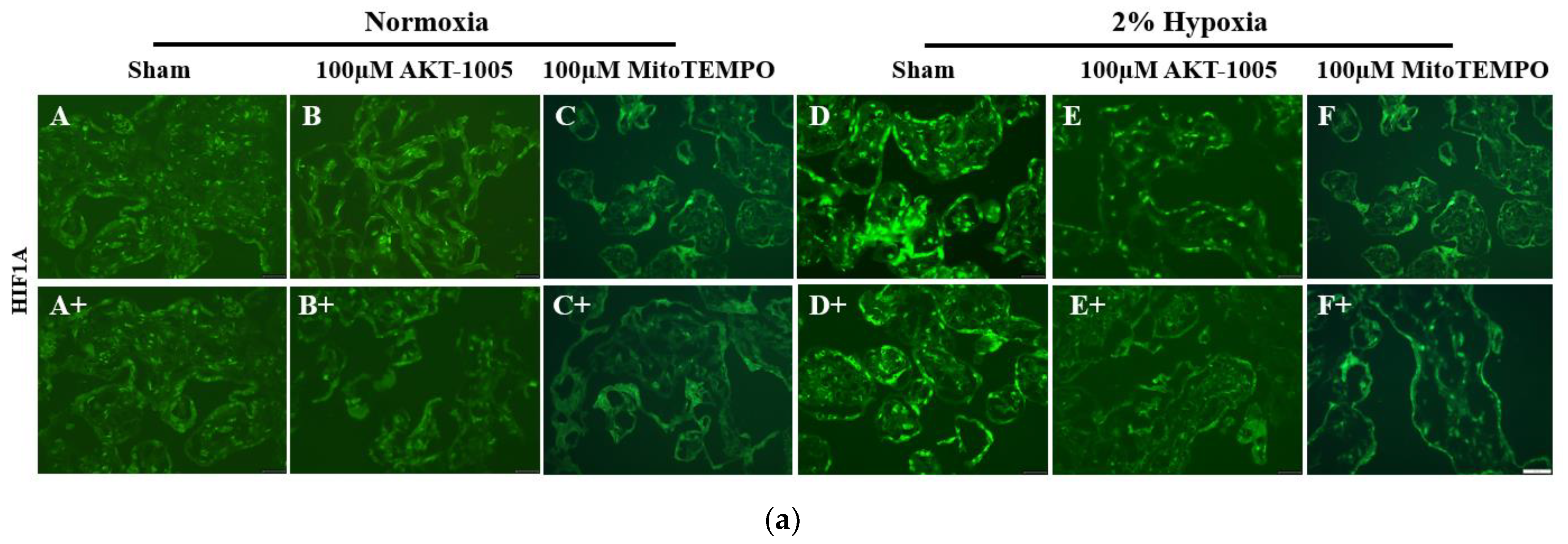
2.5. HIF1A and CK8 Immunofluorescence
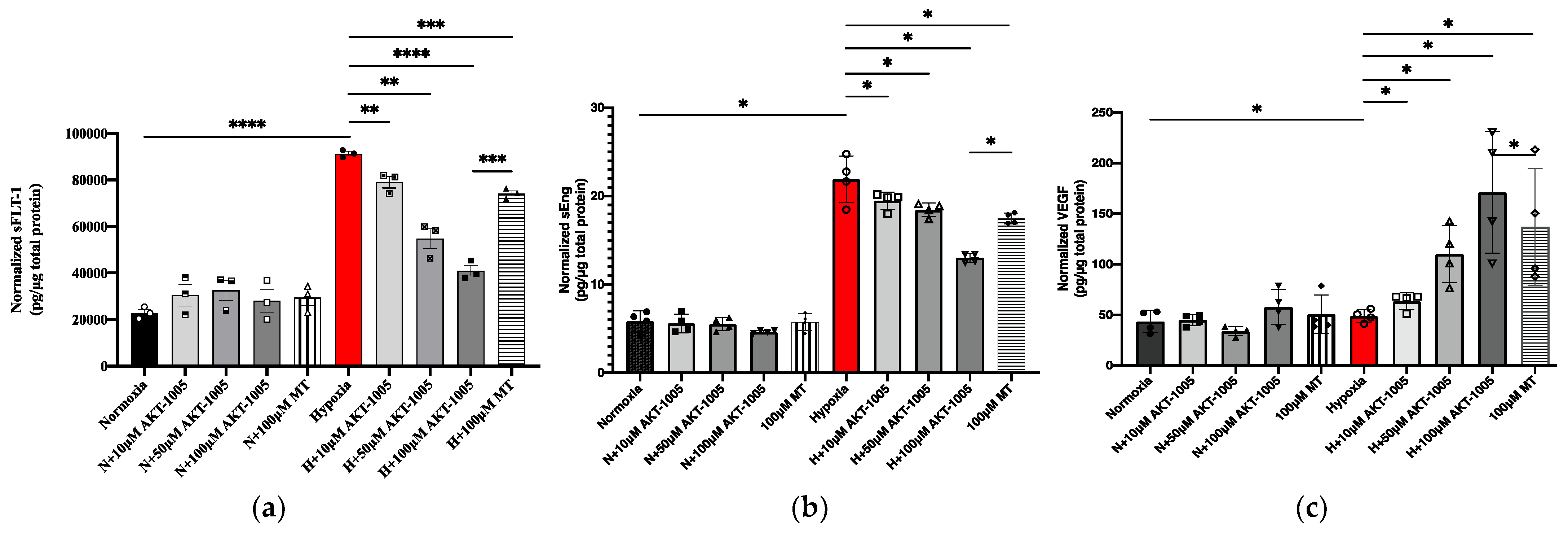
2.6. sFLT1, sEng, and VEGF Enzyme-Linked Immunosorbent Assay (ELISA)
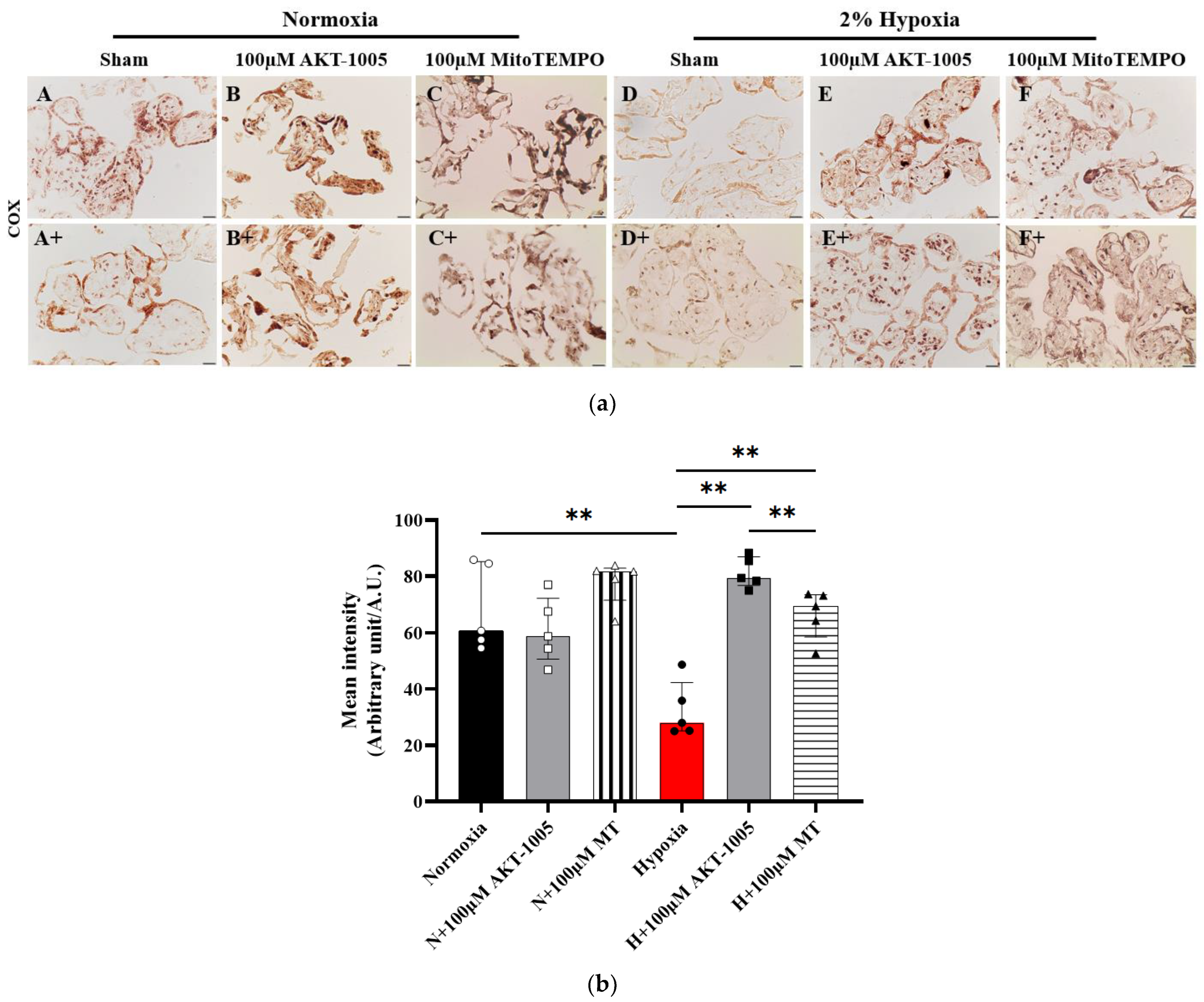
2.7. COX In Situ Enzyme Chemistry
2.8. Statistics
3. Results
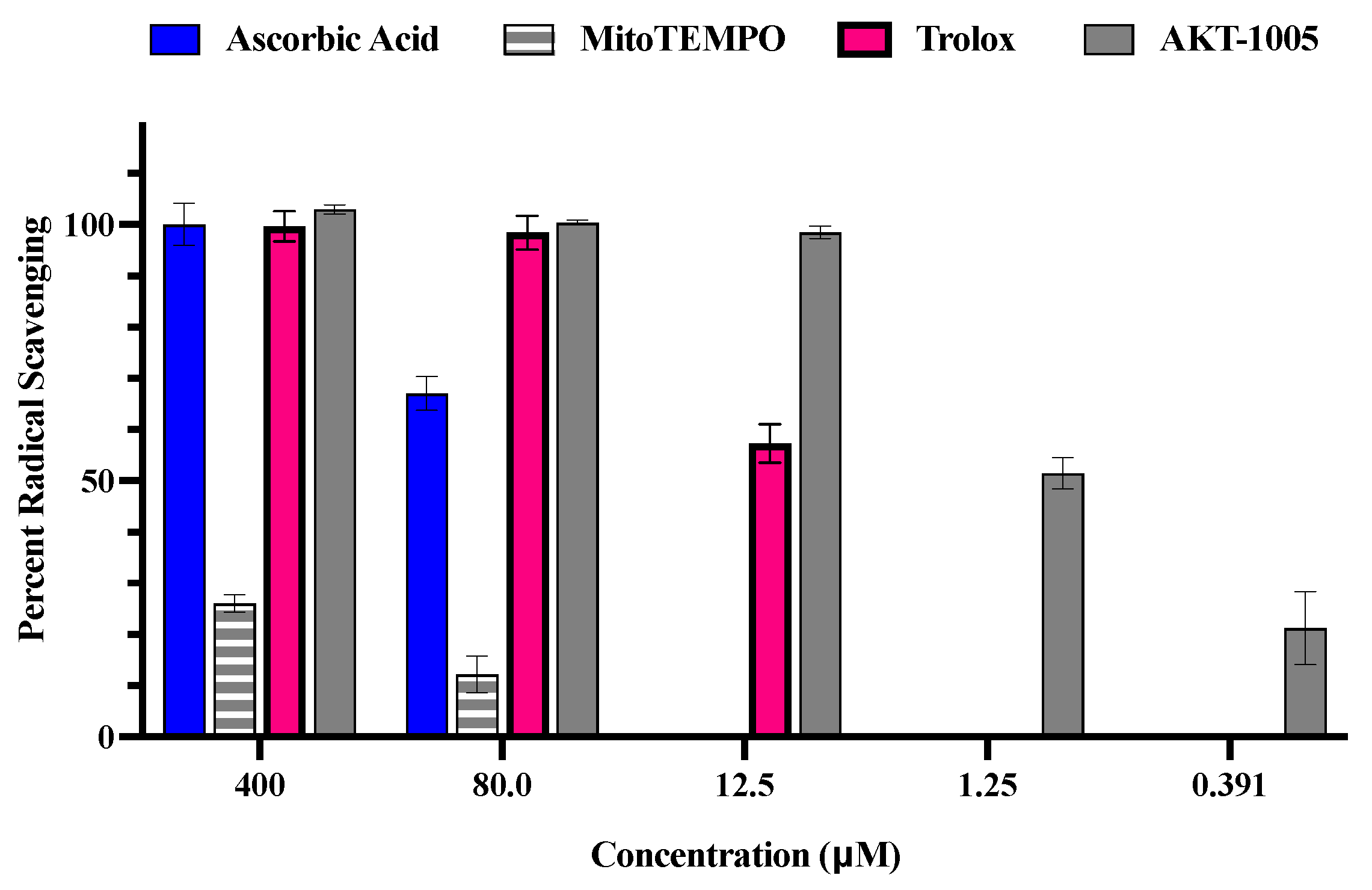
3.1. The Oxygen Radical Antioxidant Capacity (ORAC) Assay
3.2. The Human Placental Explant Is an Efficient Culture System, and It Responds to Hypoxic Stimuli, as Occurs in Human Preeclampsia Disease
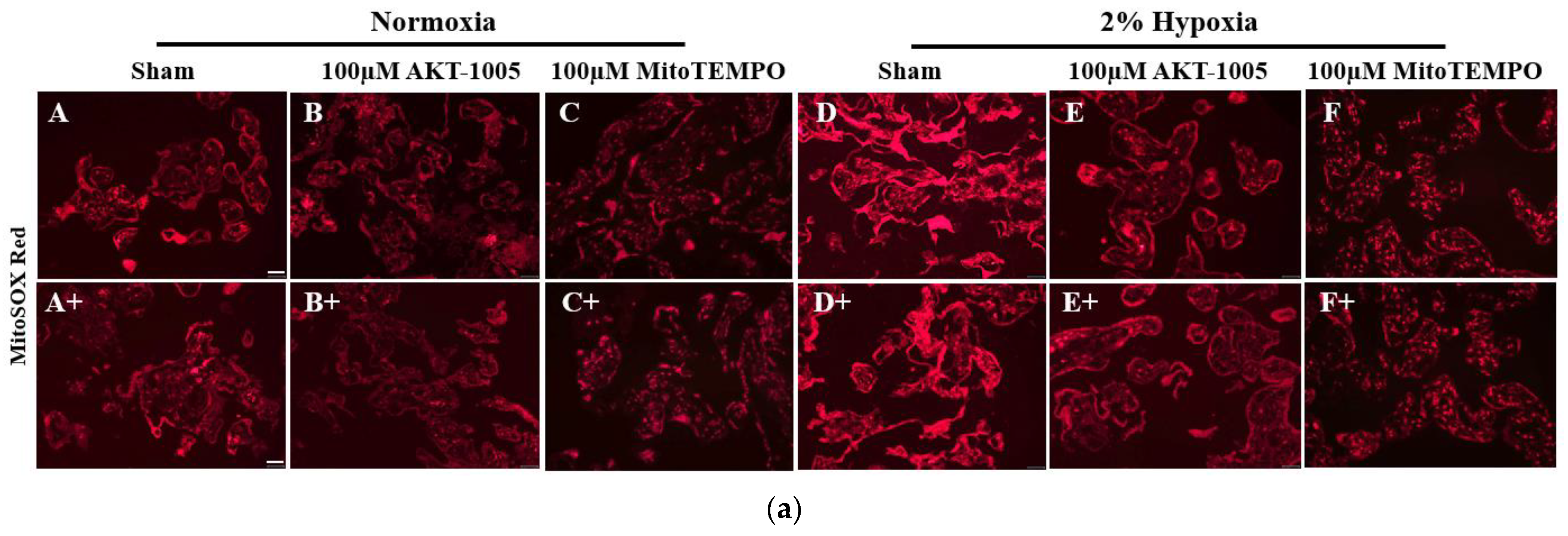
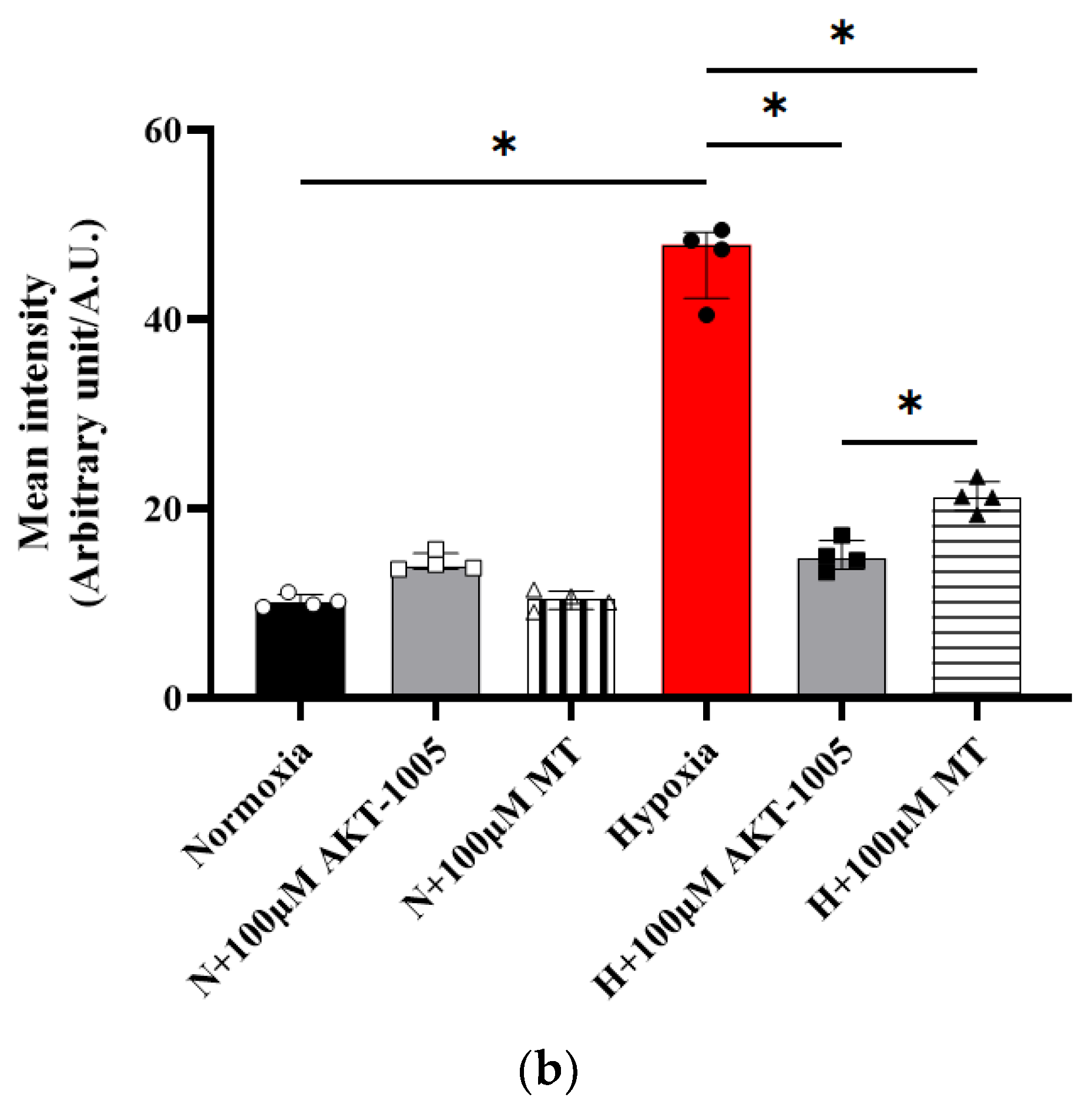
3.3. AKT-1005 Dose-Dependently Reduced Mitochondria-Derived ROS Production in Hypoxia-Exposed Human Villous Explant
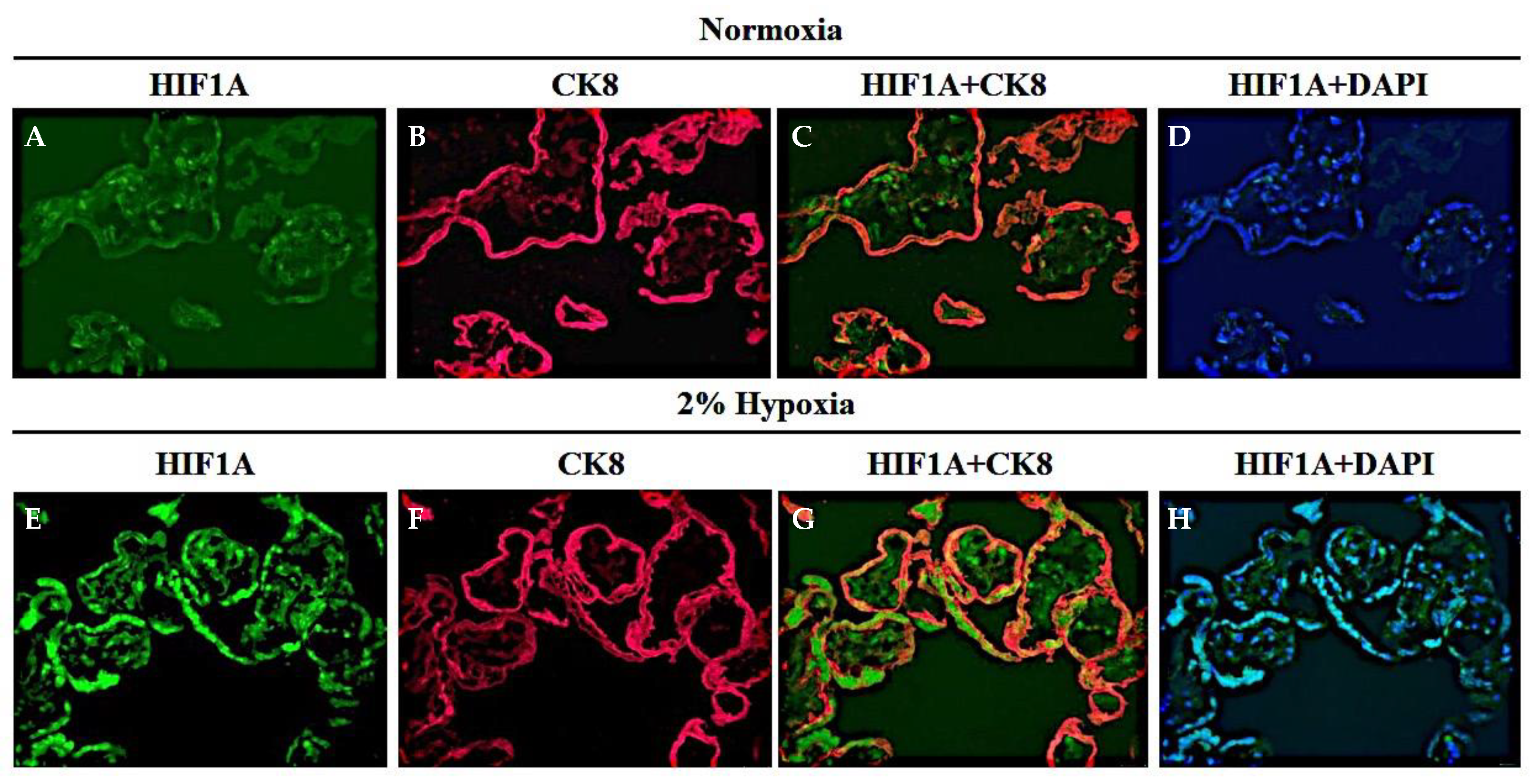
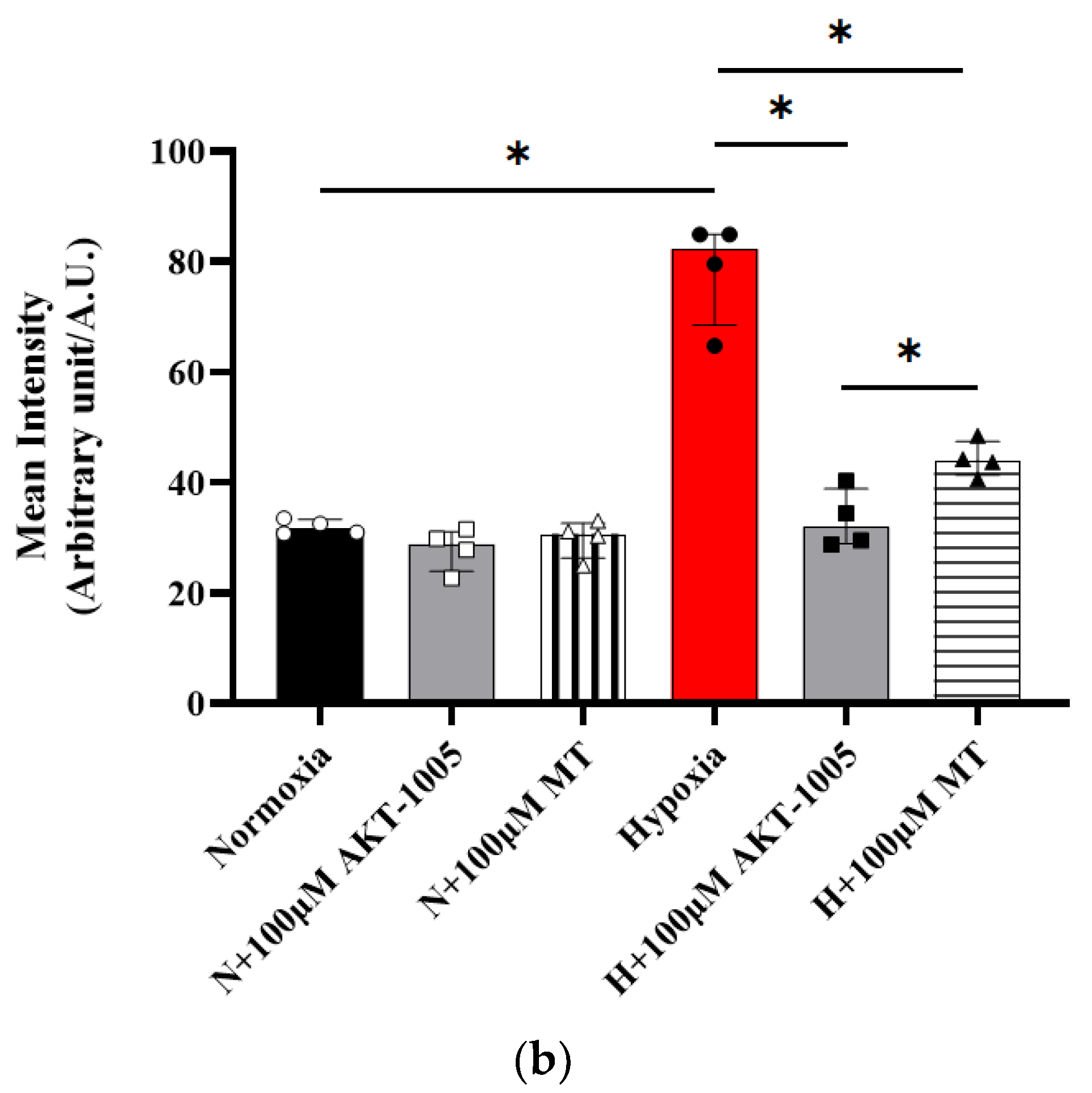
3.4. AKT-1005 Dose-Dependently Reduced HIF1A Transcription Factor Expression in Hypoxia-Exposed Villous Explant
3.5. Pretreatment with AKT-1005 Dose-Dependently Reduced Anti-Angiogenic Expression and Increased Pro-Angiogenic Response in Hypoxia-Exposed Villous Explant
3.6. Compound AKT-1005 Pre-Treatment Improved Mitochondrial COX Expression in 2% O2-Exposed Human Villous Explant
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ACOG. Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet. Gynecol. 2019, 133, e1–e25. [Google Scholar] [CrossRef]
- Steegers, E.A.; von Dadelszen, P.; Duvekot, J.J.; Pijnenborg, R. Pre-eclampsia. Lancet 2010, 376, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.W. Maternal-placental interactions of oxidative stress and antioxidants in preeclampsia. Semin. Reprod. Endocrinol. 1998, 16, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Myatt, L.; Cui, X. Oxidative stress in the placenta. Histochem. Cell Biol. 2004, 122, 369–382. [Google Scholar] [CrossRef]
- Myatt, L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta 2010, 31, S66–S69. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. Placental oxidative stress: From miscarriage to preeclampsia. J. Soc. Gynecol. Investig. 2004, 11, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Hubel, C.A. Oxidative stress in the pathogenesis of preeclampsia. Proc. Soc. Exp. Biol. Med. 1999, 222, 222–235. [Google Scholar] [CrossRef]
- Covarrubias, A.E.; Lecarpentier, E.; Lo, A.; Salahuddin, S.; Gray, K.J.; Karumanchi, S.A.; Zsengellér, Z.K. AP39, a Modulator of Mitochondrial Bioenergetics, Reduces Antiangiogenic Response and Oxidative Stress in Hypoxia-Exposed Trophoblasts: Relevance for Preeclampsia Pathogenesis. Am. J. Pathol. 2019, 189, 104–114. [Google Scholar] [CrossRef]
- Zsengellér, Z.K.; Rajakumar, A.; Hunter, J.T.; Salahuddin, S.; Rana, S.; Stillman, I.E.; Ananth Karumanchi, S. Trophoblast mitochondrial function is impaired in preeclampsia and correlates negatively with the expression of soluble fms-like tyrosine kinase 1. Pregnancy Hypertens. 2016, 6, 313–319. [Google Scholar] [CrossRef]
- Wang, Y.; Walsh, S.W. Placental mitochondria as a source of oxidative stress in pre-eclampsia. Placenta 1998, 19, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Vaka, V.R.; McMaster, K.M.; Cunningham, M.W.; Ibrahim, T.; Hazlewood, R.; Usry, N.; Cornelius, D.C.; Amaral, L.M.; LaMarca, B. Role of Mitochondrial Dysfunction and Reactive Oxygen Species in Mediating Hypertension in the Reduced Uterine Perfusion Pressure Rat Model of Preeclampsia. Hypertension 2018, 72, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.R.; Tong, S.; Kaitu’u-Lino, T.J. Placental-specific sFLT-1: Role in pre-eclamptic pathophysiology and its translational possibilities for clinical prediction and diagnosis. Mol. Hum. Reprod. 2017, 23, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.D.; Zsengeller, Z.K.; Khankin, E.V.; Lo, A.S.; Rajakumar, A.; DuPont, J.J.; McCurley, A.; Moss, M.E.; Zhang, D.; Clark, C.D.; et al. Soluble fms-like tyrosine kinase 1 promotes angiotensin II sensitivity in preeclampsia. J. Clin. Investig. 2016, 126, 2561–2574. [Google Scholar] [CrossRef] [PubMed]
- Johal, T.; Lees, C.C.; Everett, T.R.; Wilkinson, I.B. The nitric oxide pathway and possible therapeutic options in pre-eclampsia. Br. J. Clin. Pharmacol. 2014, 78, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Meher, S.; Duley, L. Nitric oxide for preventing pre-eclampsia and its complications. Cochrane Database Syst. Rev. 2007, 2007, CD006490. [Google Scholar] [CrossRef]
- Neri, I.; Monari, F.; Sgarbi, L.; Berardi, A.; Masellis, G.; Facchinetti, F. L-arginine supplementation in women with chronic hypertension: Impact on blood pressure and maternal and neonatal complications. J. Matern. Fetal Neonatal Med. 2010, 23, 1456–1460. [Google Scholar] [CrossRef] [PubMed]
- Dikalova, A.E.; Kirilyuk, I.A.; Dikalov, S.I. Antihypertensive effect of mitochondria-targeted proxyl nitroxides. Redox Biol. 2015, 4, 355–362. [Google Scholar] [CrossRef]
- Liang, H.L.; Sedlic, F.; Bosnjak, Z.; Nilakantan, V. SOD1 and MitoTEMPO partially prevent mitochondrial permeability transition pore opening, necrosis, and mitochondrial apoptosis after ATP depletion recovery. Free Radic. Biol. Med. 2010, 49, 1550–1560. [Google Scholar] [CrossRef]
- von Versen-Höynck, F.; Rajakumar, A.; Bainbridge, S.A.; Gallaher, M.J.; Roberts, J.M.; Powers, R.W. Human placental adenosine receptor expression is elevated in preeclampsia and hypoxia increases expression of the A2A receptor. Placenta 2009, 30, 434–442. [Google Scholar] [CrossRef]
- Brownfoot, F.C.; Hastie, R.; Hannan, N.J.; Cannon, P.; Tuohey, L.; Parry, L.J.; Senadheera, S.; Illanes, S.E.; Kaitu’u-Lino, T.J.; Tong, S. Metformin as a prevention and treatment for preeclampsia: Effects on soluble fms-like tyrosine kinase 1 and soluble endoglin secretion and endothelial dysfunction. Am. J. Obstet. Gynecol. 2016, 214, 356.e1–356.e15. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, A.; Cerdeira, A.S.; Rana, S.; Zsengeller, Z.; Edmunds, L.; Jeyabalan, A.; Hubel, C.A.; Stillman, I.E.; Parikh, S.M.; Karumanchi, S.A. Transcriptionally active syncytial aggregates in the maternal circulation may contribute to circulating soluble fms-like tyrosine kinase 1 in preeclampsia. Hypertension 2012, 59, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Pintye, D.; Sziva, R.E.; Mastyugin, M.; Török, M.; Jacas, S.; Lo, A.; Salahuddin, S.; Zsengellér, Z.K. Nitroxide—HMP—Protects Human Trophoblast HTR-8/SVneo Cells from H2O2-Induced Oxidative Stress by Reducing the HIF1A Signaling Pathway. Antioxidants 2023, 12, 1578. [Google Scholar] [CrossRef] [PubMed]
- Zsengeller, Z.K.; Ellezian, L.; Brown, D.; Horvath, B.; Mukhopadhyay, P.; Kalyanaraman, B.; Parikh, S.M.; Karumanchi, S.A.; Stillman, I.E.; Pacher, P. Cisplatin nephrotoxicity involves mitochondrial injury with impaired tubular mitochondrial enzyme activity. J. Histochem. Cytochem. 2012, 60, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Zsengellér, Z.K.; Rosen, S. The Use of Cytochrome C Oxidase Enzyme Activity and Immunohistochemistry in Defining Mitochondrial Injury in Kidney Disease. J. Histochem. Cytochem. 2016, 64, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Baier, A.; Kokel, A.; Horton, W.; Gizińska, E.; Pandey, G.; Szyszka, R.; Török, B.; Török, M. Organofluorine Hydrazone Derivatives as Multifunctional Anti-Alzheimer’s Agents with CK2 Inhibitory and Antioxidant Features. ChemMedChem 2021, 16, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Charlton, N.C.; Mastyugin, M.; Török, B.; Török, M. Structural Features of Small Molecule Antioxidants and Strategic Modifications to Improve Potential Bioactivity. Molecules 2023, 28, 1057. [Google Scholar] [CrossRef]
- Horton, W.; Peerannawar, S.; Török, B.; Török, M. Theoretical and experimental analysis of the antioxidant features of substituted phenol and aniline model compounds. Struct. Chem. 2019, 30, 23–35. [Google Scholar] [CrossRef]
- Sebaugh, J.L. Guidelines for accurate EC50/IC50 estimation. Pharm. Stat. 2011, 10, 128–134. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Nazarewicz, R.R.; Bikineyeva, A.; Hilenski, L.; Lassègue, B.; Griendling, K.K.; Harrison, D.G.; Dikalova, A.E. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid. Redox Signal 2014, 20, 281–294. [Google Scholar] [CrossRef]
- Trnka, J.; Blaikie, F.H.; Logan, A.; Smith, R.A.; Murphy, M.P. Antioxidant properties of MitoTEMPOL and its hydroxylamine. Free Radic. Res. 2009, 43, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Stratta, P.; Canavese, C.; Porcu, M.; Dogliani, M.; Todros, T.; Garbo, E.; Belliardo, F.; Maina, A.; Marozio, L.; Zonca, M. Vitamin E supplementation in preeclampsia. Gynecol. Obstet. Investig. 1994, 37, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Myatt, L.; Spong, C.Y.; Thom, E.A.; Hauth, J.C.; Leveno, K.J.; Pearson, G.D.; Wapner, R.J.; Varner, M.W.; Thorp, J.M.; et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N. Engl. J. Med. 2010, 362, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Lorzadeh, N.; Kazemirad, Y.; Kazemirad, N. Investigating the preventive effect of vitamins C and E on preeclampsia in nulliparous pregnant women. J. Perinat. Med. 2020, 48, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, A.; Brandon, H.M.; Daftary, A.; Ness, R.; Conrad, K.P. Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta 2004, 25, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Caniggia, I.; Winter, J.L. Adriana and Luisa Castellucci Award lecture 2001. Hypoxia inducible factor-1: Oxygen regulation of trophoblast differentiation in normal and pre-eclamptic pregnancies—A review. Placenta 2002, 23, S47–S57. [Google Scholar] [CrossRef] [PubMed]
- Baltajian, K.; Hecht, J.L.; Wenger, J.B.; Salahuddin, S.; Verlohren, S.; Perschel, F.H.; Zsengeller, Z.K.; Thadhani, R.; Karumanchi, S.A.; Rana, S. Placental lesions of vascular insufficiency are associated with anti-angiogenic state in women with preeclampsia. Hypertens. Pregnancy 2014, 33, 427–439. [Google Scholar] [CrossRef]
- Maynard, S.E.; Min, J.Y.; Merchan, J.; Lim, K.H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef]
- Rajakumar, A.; Conrad, K.P. Expression, ontogeny, and regulation of hypoxia-inducible transcription factors in the human placenta. Biol. Reprod. 2000, 63, 559–569. [Google Scholar] [CrossRef]
- Nevo, O.; Soleymanlou, N.; Wu, Y.; Xu, J.; Kingdom, J.; Many, A.; Zamudio, S.; Caniggia, I. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1085–R1093. [Google Scholar] [CrossRef]
- Tal, R.; Shaish, A.; Barshack, I.; Polak-Charcon, S.; Afek, A.; Volkov, A.; Feldman, B.; Avivi, C.; Harats, D. Effects of hypoxia-inducible factor-1alpha overexpression in pregnant mice: Possible implications for preeclampsia and intrauterine growth restriction. Am. J. Pathol. 2010, 177, 2950–2962. [Google Scholar] [CrossRef] [PubMed]
- Haram, K.; Mortensen, J.H.; Myking, O.; Magann, E.F.; Morrison, J.C. The role of oxidative stress, adhesion molecules and antioxidants in preeclampsia. Curr. Hypertens. Rev. 2019, 15, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Maloyan, A.; Mele, J.; Muralimanohara, B.; Myatt, L. Measurement of mitochondrial respiration in trophoblast culture. Placenta 2012, 33, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; West, N.E.; Pillai, R.; Taggart, D.P.; Channon, K.M. Nitric oxide modulates superoxide release and peroxynitrite formation in human blood vessels. Hypertension 2002, 39, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ahmed, A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ. Res. 2004, 95, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.J.; Lam, C.; Qian, C.; Yu, K.F.; Maynard, S.E.; Sachs, B.P.; Sibai, B.M.; Epstein, F.H.; Romero, R.; Thadhani, R.; et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N. Engl. J. Med. 2006, 355, 992–1005. [Google Scholar] [CrossRef]
- Powe, C.E.; Levine, R.J.; Karumanchi, S.A. Preeclampsia, a disease of the maternal endothelium: The role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 2011, 123, 2856–2869. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pintye, D.; Sziva, R.E.; Mastyugin, M.; Young, B.C.; Jacas, S.; Török, M.; Salahuddin, S.; Jagtap, P.; Southan, G.J.; Zsengellér, Z.K. A Novel Dual-Function Redox Modulator Relieves Oxidative Stress and Anti-Angiogenic Response in Placental Villus Explant Exposed to Hypoxia—Relevance for Preeclampsia Therapy. Biology 2023, 12, 1229. https://doi.org/10.3390/biology12091229
Pintye D, Sziva RE, Mastyugin M, Young BC, Jacas S, Török M, Salahuddin S, Jagtap P, Southan GJ, Zsengellér ZK. A Novel Dual-Function Redox Modulator Relieves Oxidative Stress and Anti-Angiogenic Response in Placental Villus Explant Exposed to Hypoxia—Relevance for Preeclampsia Therapy. Biology. 2023; 12(9):1229. https://doi.org/10.3390/biology12091229
Chicago/Turabian StylePintye, Diana, Réka E. Sziva, Maxim Mastyugin, Brett C. Young, Sonako Jacas, Marianna Török, Saira Salahuddin, Prakash Jagtap, Garry J. Southan, and Zsuzsanna K. Zsengellér. 2023. "A Novel Dual-Function Redox Modulator Relieves Oxidative Stress and Anti-Angiogenic Response in Placental Villus Explant Exposed to Hypoxia—Relevance for Preeclampsia Therapy" Biology 12, no. 9: 1229. https://doi.org/10.3390/biology12091229
APA StylePintye, D., Sziva, R. E., Mastyugin, M., Young, B. C., Jacas, S., Török, M., Salahuddin, S., Jagtap, P., Southan, G. J., & Zsengellér, Z. K. (2023). A Novel Dual-Function Redox Modulator Relieves Oxidative Stress and Anti-Angiogenic Response in Placental Villus Explant Exposed to Hypoxia—Relevance for Preeclampsia Therapy. Biology, 12(9), 1229. https://doi.org/10.3390/biology12091229





