Association between Combination Antiretroviral Therapy and Telomere Length in People Living with Human Immunodeficiency Virus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/data/gho/data/themes/hiv-aids (accessed on 1 February 2023).
- May, M.T.; Gompels, M.; Delpech, V.; Porter, K.; Orkin, C.; Kegg, S.; Hay, P.; Johnson, M.; Palfreeman, A.; Gilson, R.; et al. Impact on life expectancy of HIV-1 positive individuals of CD+ cell count and viral load response to antiretroviral therapy. AIDS 2014, 28, 1193–1202. [Google Scholar] [CrossRef]
- Marcus, J.L.; Chao, C.R.; Leyden, W.A.; Xu, L.; Quesenberry, C.P., Jr.; Klein, D.B.; Towner, W.J.; Horberg, M.A.; Silverberg, M.J. Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. J. Acquir. Immune Defic. Syndr. 2016, 73, 39–46. [Google Scholar] [CrossRef]
- Deeks, S.G.; Lewin, S.R.; Havlir, D.V. The end of AIDS: HIV infection as a chronic disease. Lancet 2013, 382, 1525–1533. [Google Scholar] [CrossRef]
- Gueler, A.; Moser, A.; Calmy, A.; Günthard, H.F.; Bernasconi, E.; Furrer, H.; Fux, C.A.; Battegay, M.; Cavassini, M.; Vernazza, P.; et al. Life expectancy in HIV-positive persons in Switzerland: Matched comparison with general population. AIDS 2017, 31, 427–436. [Google Scholar] [CrossRef]
- Weller, I.V.; Williams, I.G. ABC of AIDS. Antiretroviral drugs. BMJ 2001, 322, 1410–1412. [Google Scholar] [CrossRef]
- European AIDS Clinical Society. Available online: https://www.eacsociety.org/media/guidelines-11.1_final_09-10.pdf (accessed on 12 February 2023).
- Lorenzo-Redondo, R.; Fryer, H.R.; Bedford, T.; Kim, E.Y.; Archer, J.; Pond, S.L.K.; Chung, Y.S.; Penugonda, S.; Chipman, J.; Fletcher, C.V.; et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 2016, 530, 51–56. [Google Scholar] [CrossRef]
- Deeks, S.G. HIV infection, inflammation, immunosenescence, and aging. Annu. Rev. Med. 2011, 62, 141–155. [Google Scholar] [CrossRef]
- De Francesco, D.; Wit, F.W.; Bürkle, A.; Oehlke, S.; Kootstra, N.A.; Winston, A.; Franceschi, C.; Garagnani, P.; Pirazzini, C.; Libert, C.; et al. Do people living with HIV experience greater age advancement than their HIV-negative counterparts? AIDS 2019, 33, 259–268. [Google Scholar] [CrossRef]
- Rickabaugh, T.M.; Baxter, R.M.; Sehl, M.; Sinsheimer, J.S.; Hultin, P.M.; Hultin, L.E.; Quach, A.; Martínez-Maza, O.; Horvath, S.; Vilain, E.; et al. Acceleration of age-associated methylation patterns in HIV-1-infected adults. PLoS ONE 2015, 10, e0119201. [Google Scholar] [CrossRef]
- Lagathu, C.; Cossarizza, A.; Béréziat, V.; Nasi, M.; Capeau, J.; Pinti, M. Basic science and pathogenesis of ageing with HIV. AIDS 2017, 31, 105–119. [Google Scholar] [CrossRef]
- Turner, K.J.; Vasu, V.; Griffin, D.K. Telomere Biology and Human Phenotype. Cells 2019, 8, 73. [Google Scholar] [CrossRef]
- Armstrong, C.A.; Tomita, K. Fundamental mechanisms of telomerase action in yeasts and mammals: Understanding telomeres and telomerase in cancer cells. Open Biol. 2017, 7, 160338. [Google Scholar] [CrossRef]
- Zanet, D.L.; Thorne, A.; Singer, J.; Maan, E.J.; Sattha, B.; Le Campion, A.; Soudeyns, H.; Pick, N.; Murray, M.; Money, D.M.; et al. Association between Short Leukocyte Telomere Length and HIV Infection in a Cohort Study: No Evidence of a Relationship with Antiretroviral Therapy. Clin. Infect. Dis. 2014, 58, 1322–1332. [Google Scholar] [CrossRef]
- Dragović, G.; Andjić, M.; Toljić, B.; Jevtović, D.; Lukić, R.; de Luka, S.; Trbovich, A.; Milašin, J. Correlation between metabolic syndrome and relative telomere length shortening in HIV/AIDS patients on combined antiretroviral therapy. Exp. Gerontol. 2021, 147, 111269. [Google Scholar] [CrossRef]
- Jiménez, V.C.; Wit, F.W.; Joerink, M.; Maurer, I.; Harskamp, A.M.; Schouten, J.; Prins, M.; van Leeuwen, E.M.; Booiman, T.; Deeks, S.G.; et al. T-Cell Activation Independently Associates with Immune Senescence in HIV-Infected Recipients of Long-term Antiretroviral Treatment. J. Infect. Dis. 2016, 214, 216–225. [Google Scholar] [CrossRef]
- Blanco, J.R.; Jarrin, I.; Martinez, A.; Siles, E.; Larrayoz, I.M.; Cañuelo, A.; Gutierrez, F.; Gonzalez-Garcia, J.; Vidal, F.; Moreno, S. Shorter telomere length predicts poorer immunological recovery in virologically suppressed HIV-1-infected patients treated with combined antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2015, 68, 21–29. [Google Scholar] [CrossRef]
- O’Callaghan, N.J.; Bull, C.; Fenech, M. Elevated plasma magnesium and calcium may be associated with shorter telomeres in older South Australian women. J. Nutr. Health Aging 2014, 18, 131–136. [Google Scholar] [CrossRef]
- Brown, L.A.; Jin, J.; Ferrell, D.; Sadic, E.; Obregon, D.; Smith, A.J.; Tan, J.; Giunta, B. Efavirenz promotes β-secretase expression and increased Aβ1-40,42 via oxidative stress and reduced microglial phagocytosis: Implications for HIV associated neurocognitive disorders (HAND). PLoS ONE 2014, 9, e95500. [Google Scholar] [CrossRef]
- Surnar, B.; Shah, A.S.; Park, M.; Kalathil, A.A.; Kamran, M.Z.; Ramirez Jaime, R.; Toborek, M.; Nair, M.; Kolishetti, N.; Dhar, S. Brain-Accumulating Nanoparticles for Assisting Astrocytes to Reduce Human Immunodeficiency Virus and Drug Abuse-Induced Neuroinflammation and Oxidative Stress. ACS Nano 2021, 15, 15741–15753. [Google Scholar] [CrossRef]
- Dong, Q.; Oh, J.; Yi, J.K.; Kim, R.H.; Shin, K.; Mitsuyasu, R.; Park, N.H.; Kang, M.K. Efavirenz induces autophagy and aberrant differentiation in normal human keratinocytes. Int. J. Mol. Med. 2013, 31, 1305–1312. [Google Scholar] [CrossRef]
- Tutton, S.; Lieberman, P.M. A role for p53 in telomere protection. Mol. Cell Oncol. 2016, 4, e1143078. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hukezalie, K.R.; Thumati, N.R.; Côté, H.C.; Wong, J.M. In vitro and ex vivo inhibition of human telomerase by anti-HIV nucleoside reverse transcriptase inhibitors (NRTIs) but not by non-NRTIs. PLoS ONE 2012, 7, e47505. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.B.; Bedi, H.; Gomez, R.; Khan, A.; Meciszewski, T.; Aalinkeel, R.; Khoo, T.C.; Sharikova, A.V.; Khmaladze, A.; Mahajan, S.D. Telomere Length Shortening in Microglia: Implication for Accelerated Senescence and Neurocognitive Deficits in HIV. Vaccines 2021, 9, 721. [Google Scholar] [CrossRef] [PubMed]
- Stella-Ascariz, N.; Montejano, R.; Rodriguez-Centeno, J.; Alejos, B.; Schwimmer, C.; Bernardino, J.I.; Rodes, B.; Allavena, C.; Hoffmann, C.; Gisslén, M.; et al. Blood Telomere Length Changes After Ritonavir-Boosted Darunavir Combined with Raltegravir or Tenofovir-Emtricitabine in Antiretroviral-Naive Adults Infected with HIV-1. J. Infect. Dis. 2018, 218, 1523–1530. [Google Scholar] [CrossRef]
- Rodríguez-Centeno, J.; Esteban-Cantos, A.; Montejano, R.; Stella-Ascariz, N.; De Miguel, R.; Mena-Garay, B.; Saiz-Medrano, G.; Alejos, B.; Jiménez-González, M.; Bernardino, J.I.; et al. Effects of tenofovir on telomeres, telomerase and T cell maturational subset distribution in long-term aviraemic HIV-infected adults. J. Antimicrob. Chemother. 2022, 77, 1125–1132. [Google Scholar] [CrossRef]
- Alejos, B.; Stella-Ascariz, N.; Montejano, R.; Rodriguez-Centeno, J.; Schwimmer, C.; Bernardino, J.I.; Rodes, B.; Esser, S.; Goujard, C.; Sarmento-Castro, R.; et al. Determinants of blood telomere length in antiretroviral treatment-naïve HIV-positive participants enrolled in the NEAT 001/ANRS 143 clinical trial. HIV Med. 2019, 20, 691–698. [Google Scholar] [CrossRef]
- National Library of Medicine. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513289/ (accessed on 17 June 2023).
- Prasad, K. C-reactive protein increases oxygen radical generation by neutrophils. J. Cardiovasc. Pharmacol. Ther. 2004, 9, 203–209. [Google Scholar] [CrossRef]
- Wong, J.Y.; De Vivo, I.; Lin, X.; Fang, S.C.; Christiani, D.C. The relationship between inflammatory biomarkers and telomere length in an occupational prospective cohort study. PLoS ONE 2014, 9, e87348. [Google Scholar] [CrossRef]
- Mazidi, M.; Shekoohi, N.; Katsiki, N.; Rakowski, M.; Mikhailidis, D.P.; Banach, M. Serum anti-inflammatory and inflammatory markers have no causal impact on telomere length: A Mendelian randomization study. Arch. Med. Sci. 2021, 17, 739–751. [Google Scholar] [CrossRef]
- Boccard, V.; Paolisso, G. The association between statins and telomere shortening. Clin. Lipidol. 2017, 9, 311–315. [Google Scholar] [CrossRef]
- Zeng, J.B.; Liu, H.B.; Ping, F.; Li, W.; Li, Y.X. Insulin treatment affects leukocyte telomere length in patients with type 2 diabetes: 6-year longitudinal study. J. Diabetes Complicat. 2019, 33, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Genis-Mendoza, A.D. Telomere Shortening in Three Diabetes Mellitus Types in a Mexican Sample. Biomedicines 2023, 11, 730. [Google Scholar] [CrossRef]
- Wang, J.; Dong, X.; Cao, L.; Sun, Y.; Qiu, Y.; Zhang, Y.; Cao, R.; Covasa, M.; Zhong, L. Association between telomere length and diabetes mellitus: A meta-analysis. J. Int. Med. Res. 2016, 44, 1156–1173. [Google Scholar] [CrossRef] [PubMed]
- Gielen, M.; Hageman, G.J.; Antoniou, E.E.; Nordfjall, K.; Mangino, M.; Balasubramanyam, M.; de Meyer, T.; Hendricks, A.E.; Giltay, E.J.; Hunt, S.C.; et al. Body mass index is negatively associated with telomere length: A collaborative cross-sectional meta-analysis of 87 observational studies. Am. J. Clin. Nutr. 2018, 108, 453–475. [Google Scholar] [CrossRef]
- Charm, S.E.; Landau, S.; Williams, B.; Horowitz, B.; Prince, A.M.; Pascual, D. High-temperature short-time heat inactivation of HIV and other viruses in human blood plasma. Vox Sang. 1992, 62, 12–20. [Google Scholar] [CrossRef] [PubMed]
- GE Healthcare Life Sciences. Available online: https://kersnikova.org/wp-content/uploads/2022/03/1.-Isolation-of-mononuclear-cells-_-basic-protocol_-GE-Healthcare.pdf (accessed on 6 May 2023).
- Miller, S.A.; Dykes, D.D.; Polesky, H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef]
- Lorenz, T.C. Polymerase chain reaction: Basic protocol plus troubleshooting and optimization strategies. J. Vis. Exp. 2012, 63, e3998. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef]
- Agencija za Akreditaciju Zdravstvenih Ustanova Republike Srbije. Available online: http://www.azus.gov.rs/wp-content/uploads/2011/04/Vodic-za-dijagnostikovanje-i-lecenje-lipidskih-poremecaja1.pdf (accessed on 17 June 2023).
- Ministarstvo Zdravlja Republike Srbije. Available online: https://www.zdravlje.gov.rs/view_file.php?file_id=552&cache=sr (accessed on 17 June 2023).
- Ministarstvo Zdravlja Republike Srbije. Available online: https://www.zdravlje.gov.rs/view_file.php?file_id=667&cache=sr (accessed on 17 June 2023).
- Vens, C. Encyclopedia of Systems Biology; Springer: New York, NY, USA, 2013; ISBN 978-1-4419-9863-7. [Google Scholar]
- Kursa, M.B.; Rudnicki, W.R. Feature Selection with the Boruta Package. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef]
- FSA: Simple Fisheries Stock Assessment Methods. Available online: https://github.com/droglenc/FSA (accessed on 6 May 2023).
- The Comprehensive R Archive Network. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 6 May 2023).
- Auld, E.; Lin, J.; Chang, E.; Byanyima, P.; Ayakaka, I.; Musisi, E.; Worodria, W.; Davis, J.L.; Segal, M.; Blackburn, E.; et al. HIV Infection Is Associated with Shortened Telomere Length in Ugandans with Suspected Tuberculosis. PLoS ONE 2016, 11, e0163153. [Google Scholar] [CrossRef]
- Montejano, R.; Stella-Ascariz, N.; Monge, S.; Bernardino, J.I.; Pérez-Valero, I.; Montes, M.L.; Valencia, E.; Martín-Carbonero, L.; Moreno, V.; González-García, J.; et al. Impact of Antiretroviral Treatment Containing Tenofovir Difumarate on the Telomere Length of Aviremic HIV-Infected Patients. J. Acquir. Immune Defic. Syndr. 2017, 76, 102–109. [Google Scholar] [CrossRef]
- Chalouni, M.; Rodriguez-Centeno, J.; Samri, A.; Blanco, J.; Stella-Ascariz, N.; Wallet, C.; Knobel, H.; Zucman, D.; Alejos Ferreras, B.; Autran, B.; et al. Correlation between blood telomere length and CD4+ CD8+ T-cell subsets changes 96 weeks after initiation of antiretroviral therapy in HIV-1-positive individuals. PLoS ONE 2020, 15, e0230772. [Google Scholar] [CrossRef]
- Pathai, S.; Lawn, S.D.; Gilbert, C.E.; McGuinness, D.; McGlynn, L.; Weiss, H.A.; Port, J.; Christ, T.; Barclay, K.; Wood, R.; et al. Accelerated biological ageing in HIV-infected individuals in South Africa: A case-control study. AIDS 2013, 27, 2375–2384. [Google Scholar] [CrossRef]
- Leeansyah, E.; Cameron, P.U.; Solomon, A.; Tennakoon, S.; Velayudham, P.; Gouillou, M.; Spelman, T.; Hearps, A.; Fairley, C.; de Smit, V.; et al. Inhibition of telomerase activity by human immunodeficiency virus (HIV) nucleos(t)ide reverse transcriptase inhibitors: A potential factor contributing to HIV-associated accelerated aging. J. Infect. Dis. 2013, 207, 1157–1165. [Google Scholar] [CrossRef]
- Montejano, R.; Stella-Ascariz, N.; Monge, S.; Bernardino, J.I.; Pérez-Valero, I.; Montes, M.L.; Valencia, E.; Martín-Carbonero, L.; Moreno, V.; González-Garcia, J.; et al. Impact of Nucleos(t)ide Reverse Transcriptase Inhibitors on Blood Telomere Length Changes in a Prospective Cohort of Aviremic HIV-Infected Adults. J. Infect. Dis. 2018, 218, 1531–1540. [Google Scholar] [CrossRef]
- Schoepf, I.C.; Thorball, C.W.; Ledergerber, B.; Kootstra, N.A.; Reiss, P.; Raffenberg, M.; Engel, T.; Braun, D.L.; Hasse, B.; Thurnheer, C.; et al. Telomere Length Declines in Persons with Human Immunodeficiency Virus Before Antiretroviral Therapy Start but Not after Viral Suppression: A Longitudinal Study over >17 Years. J. Infect. Dis. 2022, 225, 1581–1591. [Google Scholar] [CrossRef]
- Raffenberg, M.; Engel, T.; Schoepf, I.C.; Kootstra, N.A.; Reiss, P.; Braun, D.L.; Thorball, C.W.; Fellay, J.; Kouyos, R.D.; Ledergerber, B.; et al. Impact of Delaying Antiretroviral Treatment During Primary Human Immunodeficiency Virus Infection on Telomere Length. J. Infect. Dis. 2021, 224, 1775–1784. [Google Scholar] [CrossRef]
- Clinton Health Access Initiative. Available online: https://chai19.wpenginepowered.com/wp-content/uploads/2022/12/2022-CHAI-HIV-Market-Report-12.8.22.pdf (accessed on 6 February 2023).
- Maeda, T.; Oyama, J.; Higuchi, Y.; Koyanagi, M.; Sasaki, M.; Arima, T.; Mimori, K.; Makino, N. The correlation between clinical laboratory data and telomeric status of male patients with metabolic disorders and no clinical history of vascular events. Aging Male 2011, 14, 21–26. [Google Scholar] [CrossRef]
- Babu, H.; Ambikan, A.T.; Gabriel, E.E.; Svensson Akusjärvi, S.; Palaniappan, A.N.; Sundaraj, V.; Mupanni, N.R.; Sperk, M.; Cheedarla, N.; Sridhar, R.; et al. Systemic Inflammation and the Increased Risk of Inflamm-Aging and Age-Associated Diseases in People Living with HIV on Long Term Suppressive Antiretroviral Therapy. Front. Immunol. 2019, 10, 1965. [Google Scholar] [CrossRef]
- Alomar, F.A.; Tian, C.; Bidasee, S.R.; Venn, Z.L.; Schroder, E.; Palermo, N.Y.; AlShabeeb, M.; Edagwa, B.J.; Payne, J.J.; Bidasee, K.R. HIV-Tat Exacerbates the Actions of Atazanavir, Efavirenz, and Ritonavir on Cardiac Ryanodine Receptor (RyR2). Int. J. Mol. Sci. 2022, 24, 274. [Google Scholar] [CrossRef]
- Loke, J.; MacLennan, D.H. Malignant hyperthermia and central core disease: Disorders of Ca2+ release channels. Am. J. Med. 1998, 104, 470–486. [Google Scholar] [CrossRef] [PubMed]
- Giannini, G.; Conti, A.; Mammarella, S.; Scrobogna, M.; Sorrentino, V. The ryanodine receptor/calcium channel genes are widely and differentially expressed in murine brain and peripheral tissues. J. Cell Biol. 1995, 128, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Alomar, F.A.; Tian, C.; Dash, P.K.; McMillan, J.M.; Gendelman, H.E.; Gorantla, S.; Bidasee, K.R. Efavirenz, atazanavir, and ritonavir disrupt sarcoplasmic reticulum Ca2+ homeostasis in skeletal muscles. Antivir. Res. 2021, 187, 104975. [Google Scholar] [CrossRef] [PubMed]
- Benedicto, A.M.; Fuster-Martínez, I.; Tosca, J.; Esplugues, J.V.; Blas-García, A.; Apostolova, N. NNRTI and Liver Damage: Evidence of Their Association and the Mechanisms Involved. Cells 2021, 10, 1687. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.L.; Beland, F.A. Differential responses of human hepatocytes to the non-nucleoside HIV-1 reverse transcriptase inhibitor nevirapine. J. Toxicol. Sci. 2013, 38, 741–752. [Google Scholar] [CrossRef][Green Version]
- Shang, H.; Zhao, J.; Yao, J.; Wang, H.; Dong, J.; Liao, L. Nevirapine Increases Sodium/Iodide Symporter-Mediated Radioiodide Uptake by Activation of TSHR/cAMP/CREB/PAX8 Signaling Pathway in Dedifferentiated Thyroid Cancer. Front. Oncol. 2020, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Campbell, H.G.; Wiles, A.K.; Eccles, M.R.; Reddel, R.R.; Braithwaite, A.W.; Royds, J.A. PAX8 regulates telomerase reverse transcriptase and telomerase RNA component in glioma. Cancer Res. 2008, 68, 5724–5732. [Google Scholar] [CrossRef]
- Andreu-Sánchez, S.; Aubert, G.; Ripoll-Cladellas, A.; Henkelman, S.; Zhernakova, D.V.; Sinha, T.; Kurilshikov, A.; Cenit, M.C.; Bonder, M.J.; Franke, L.; et al. Genetic, parental and lifestyle factors influence telomere length. Commun. Biol. 2022, 5, 565. [Google Scholar] [CrossRef]
- Weng, N.P. Telomere and adaptive immunity. Mech. Ageing Dev. 2008, 129, 60–66. [Google Scholar] [CrossRef]
- Liew, C.C.; Ma, J.; Tang, H.C.; Zheng, R.; Dempsey, A.A. The peripheral blood transcriptome dynamically reflects system wide biology: A potential diagnostic tool. J. Lab. Clin. Med. 2006, 147, 126–132. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.; Bann, D.; Wiley, L.; Cooper, R.; Hardy, R.; Nitsch, D.; Martin-Ruiz, C.; Shiels, P.; Sayer, A.A.; Barbieri, M.; et al. Gender and telomere length: Systematic review and meta-analysis. Exp. Gerontol. 2014, 15, 15–27. [Google Scholar] [CrossRef] [PubMed]
| Reagent | DNA | Forward primer (telomeres) | Reverse primer (telomeres) | Forward primer (HBG) | Reverse primer (HBG) | 2 × Master Mix (Green/ ROX qPCR) | Water |
| Quantity | 10 ng | 1 μL | 1 μL | 1 μL | 1 μL | 12.5 μL | To a final volume of 25 μL |
| Primer | Sequence | Final Concentration | Annealing Temperature |
|---|---|---|---|
| TEL 1 forward | 5′ CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT 3′ | 600 nM | 56 °C |
| TEL 2 reverse | 5′ GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT 3′ | 600 nM | |
| HBG 1 forward | 5′ TCTGACACAACTGTGTTCACTAGC 3′ | 300 nM | |
| HBG 2 reverse | 5′ TCTGACACAACTGTGTTCACTAGC 3′ | 700 nM |
| Variable | Mean Value ± SD | p Values of Kendall’s Correlation Test with RTL |
|---|---|---|
| Age (years) | 42.70 ± 13.18 | p = 0.904 |
| BMI (kg/m2) | 24.51 ± 3.49 | p = 0.551 |
| CD4+ T-cells count (cells/μL) | 554 ± 259.68 | p = 0.536 |
| CRP (mg/L) | 5.57 ± 5.43 | p = 0.264 |
| HIV infection duration (months) | 92.42 ± 72.22 | p = 0.220 |
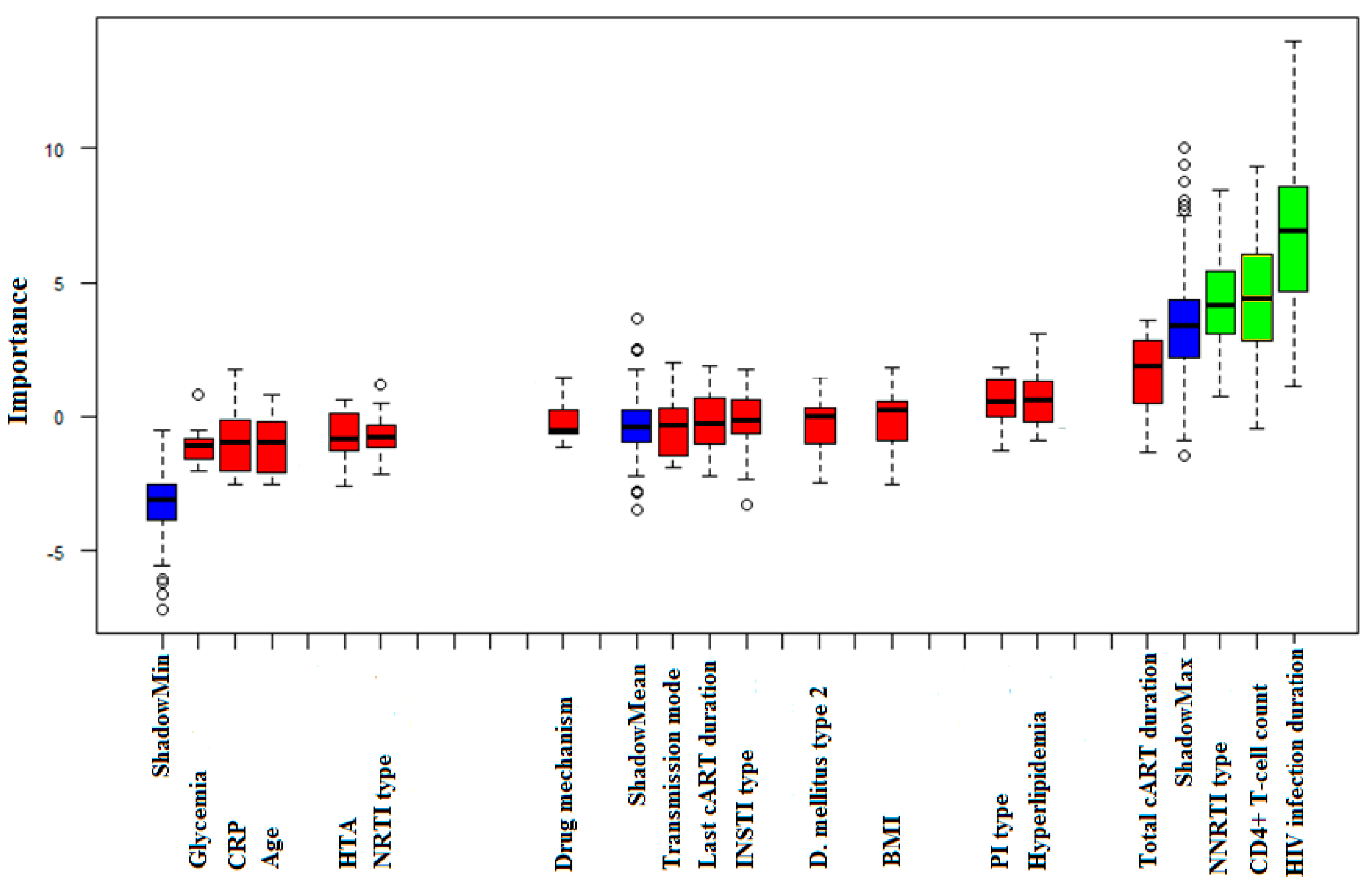
| Total cART duration (months) | 82.02 ± 64.81 | p = 0.095 |
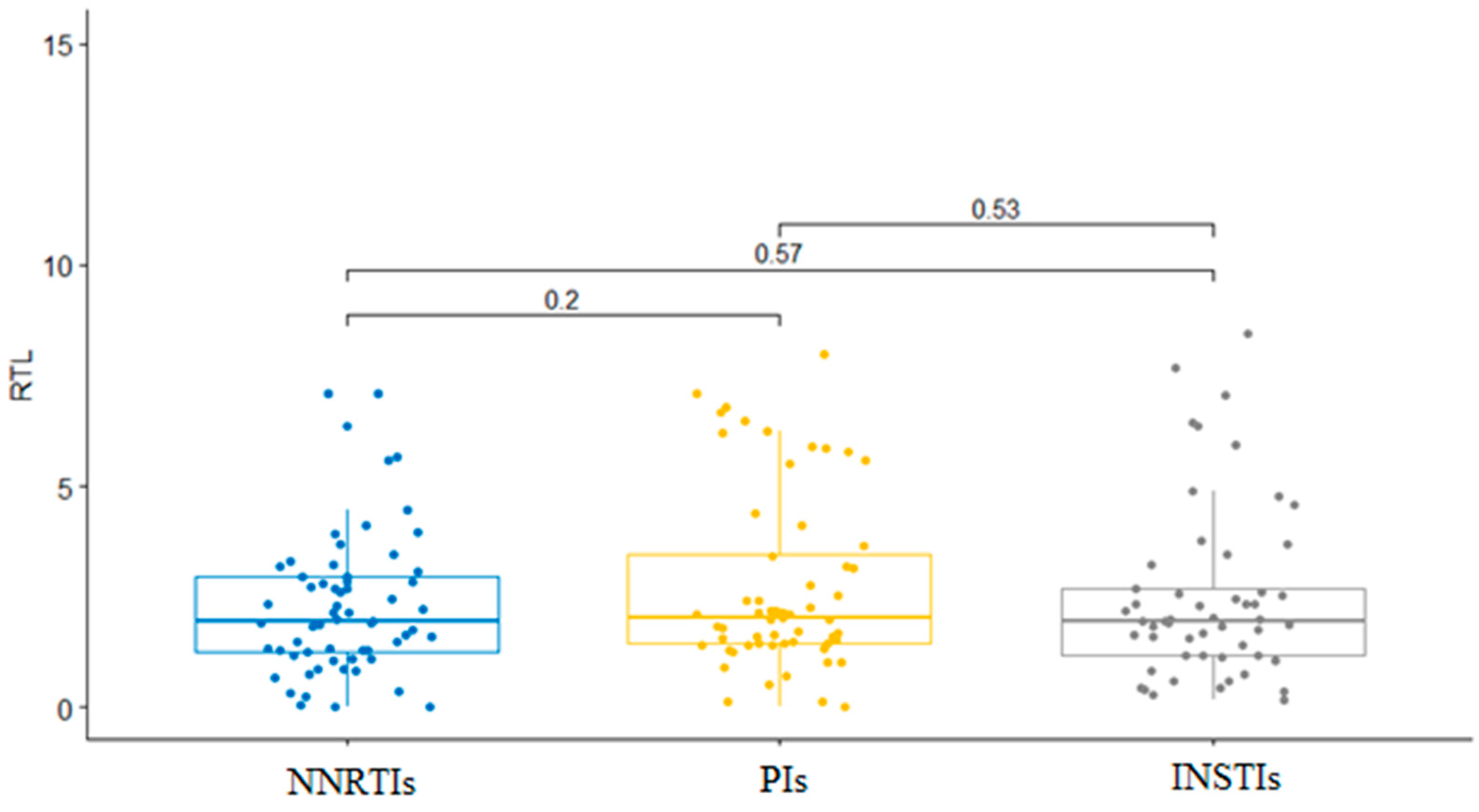
| cART Group | Regimen within the cART Group | Patients Receiving Regimen (%) | RTL (Mean Value ± SD) | Regimen’s Association with RTL |
|---|---|---|---|---|
| INSTIs | Dolutegravir | 40 (75.5%) | 2.69 ± 2.11 | p = 0.187 |
| Raltegravir | 13 (24.5%) | 1.87 ± 1.37 | ||
| PIs | Darunavir + Ritonavir | 48 (80%) | 2.68 ± 1.99 | p = 0.790 |
| Fosamprenavir + Ritonavir | 9 (15%) | 2.82 ± 2.34 | ||
| Lopinavir + Ritonavir | 3 (5%) | 3.23 ± 2.33 | ||
| NNRTIs | Efavirenz | 53 (84.1%) | 2.10 ± 1.55 | p = 0.018 |
| Nevirapine | 10 (15.9%) | 3.33 ± 1.63 | ||
| NRTIs | Lamivudine + Abacavir | 111 (63.07%) | 2.44 ± 1.84 | p = 0.277 |
| Tenofovir + Emtricitabine | 60 (34.09%) | 2.00 ± 1.94 | ||
| Lamivudine + Zidovudine | 5 (2.84%) | 3.59 ± 1.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukic, E.; Milasin, J.; Toljic, B.; Jadzic, J.; Jevtovic, D.; Obradovic, B.; Dragovic, G. Association between Combination Antiretroviral Therapy and Telomere Length in People Living with Human Immunodeficiency Virus. Biology 2023, 12, 1210. https://doi.org/10.3390/biology12091210
Bukic E, Milasin J, Toljic B, Jadzic J, Jevtovic D, Obradovic B, Dragovic G. Association between Combination Antiretroviral Therapy and Telomere Length in People Living with Human Immunodeficiency Virus. Biology. 2023; 12(9):1210. https://doi.org/10.3390/biology12091210
Chicago/Turabian StyleBukic, Ena, Jelena Milasin, Bosko Toljic, Jelena Jadzic, Djordje Jevtovic, Bozana Obradovic, and Gordana Dragovic. 2023. "Association between Combination Antiretroviral Therapy and Telomere Length in People Living with Human Immunodeficiency Virus" Biology 12, no. 9: 1210. https://doi.org/10.3390/biology12091210
APA StyleBukic, E., Milasin, J., Toljic, B., Jadzic, J., Jevtovic, D., Obradovic, B., & Dragovic, G. (2023). Association between Combination Antiretroviral Therapy and Telomere Length in People Living with Human Immunodeficiency Virus. Biology, 12(9), 1210. https://doi.org/10.3390/biology12091210








