Dynamics of Bacterial Diversity and Functions with Physicochemical Properties in Different Phases of Pig Manure Composting
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Composting and Sampling
2.2. Physicochemical Parameters Analysis
2.3. DNA Extraction and 16S rRNA Amplification, Sequencing
2.4. Statistical Data and Analysis
3. Results and Discussion
3.1. Changes in Physicochemical Properties in Composting
3.2. Changes in Bacterial Community Alpha Diversity in Composting
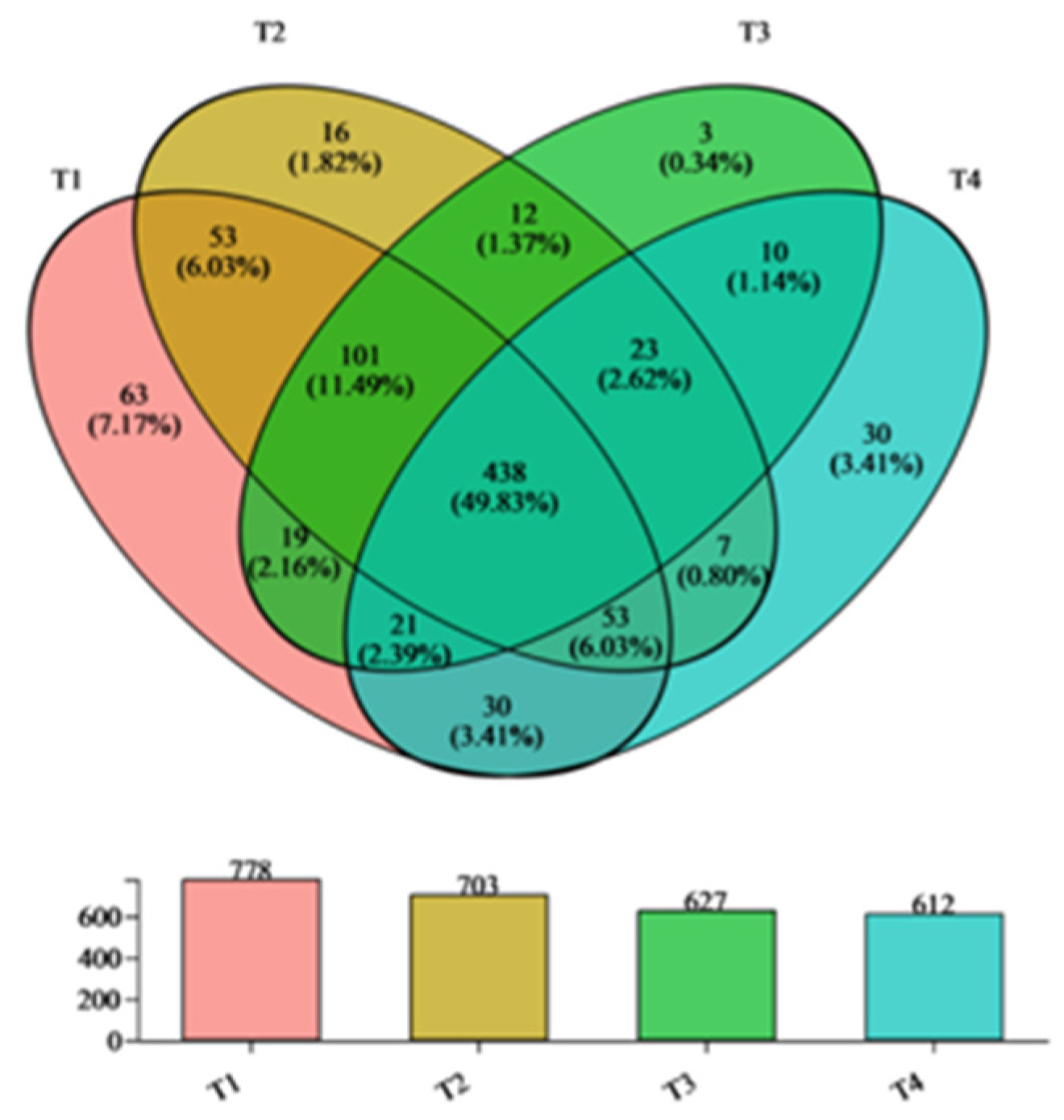
3.3. Beta Diversity Analysis of Bacterial Communities
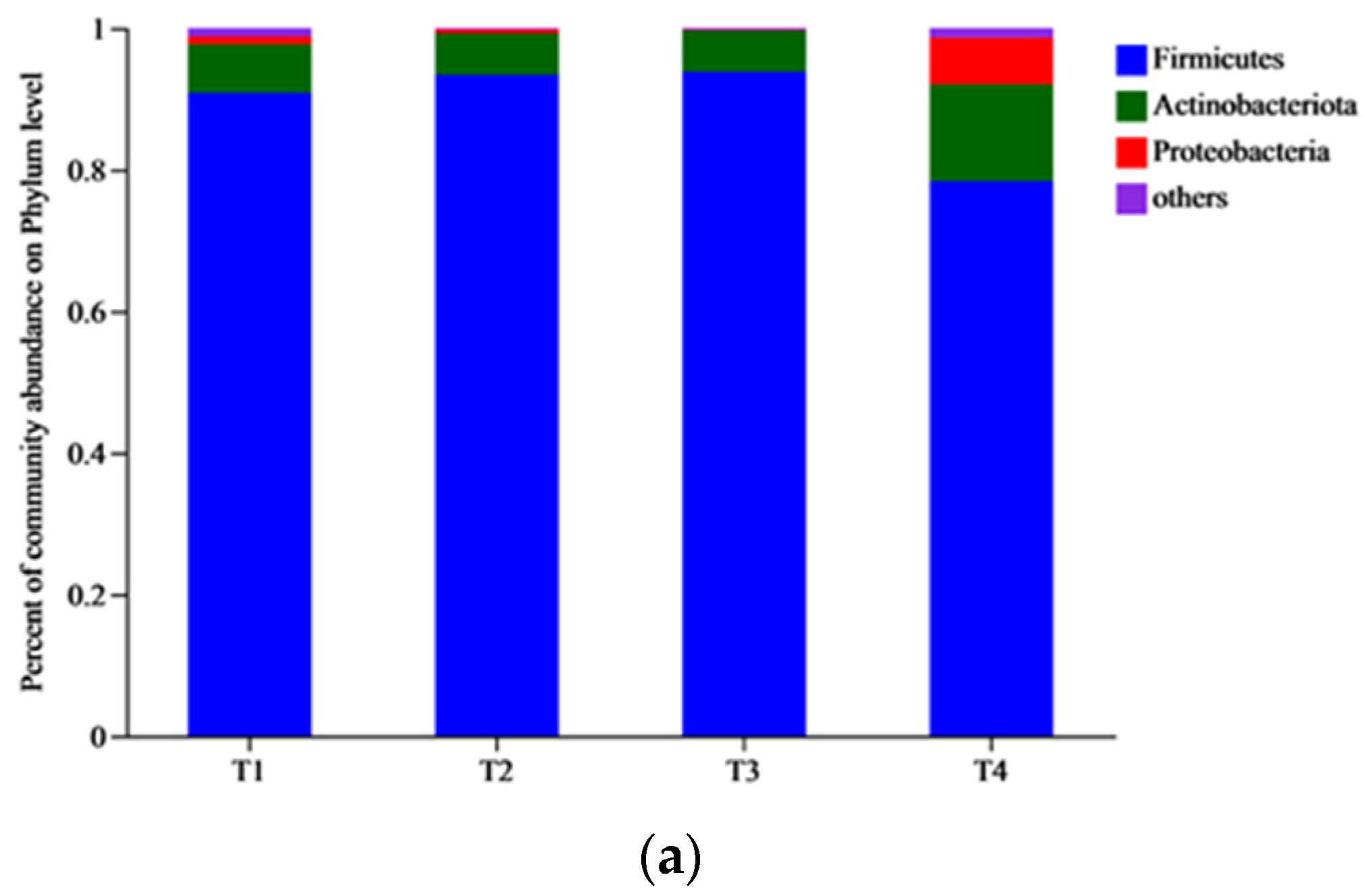
3.4. Changes in the Composition of Bacterial Communities
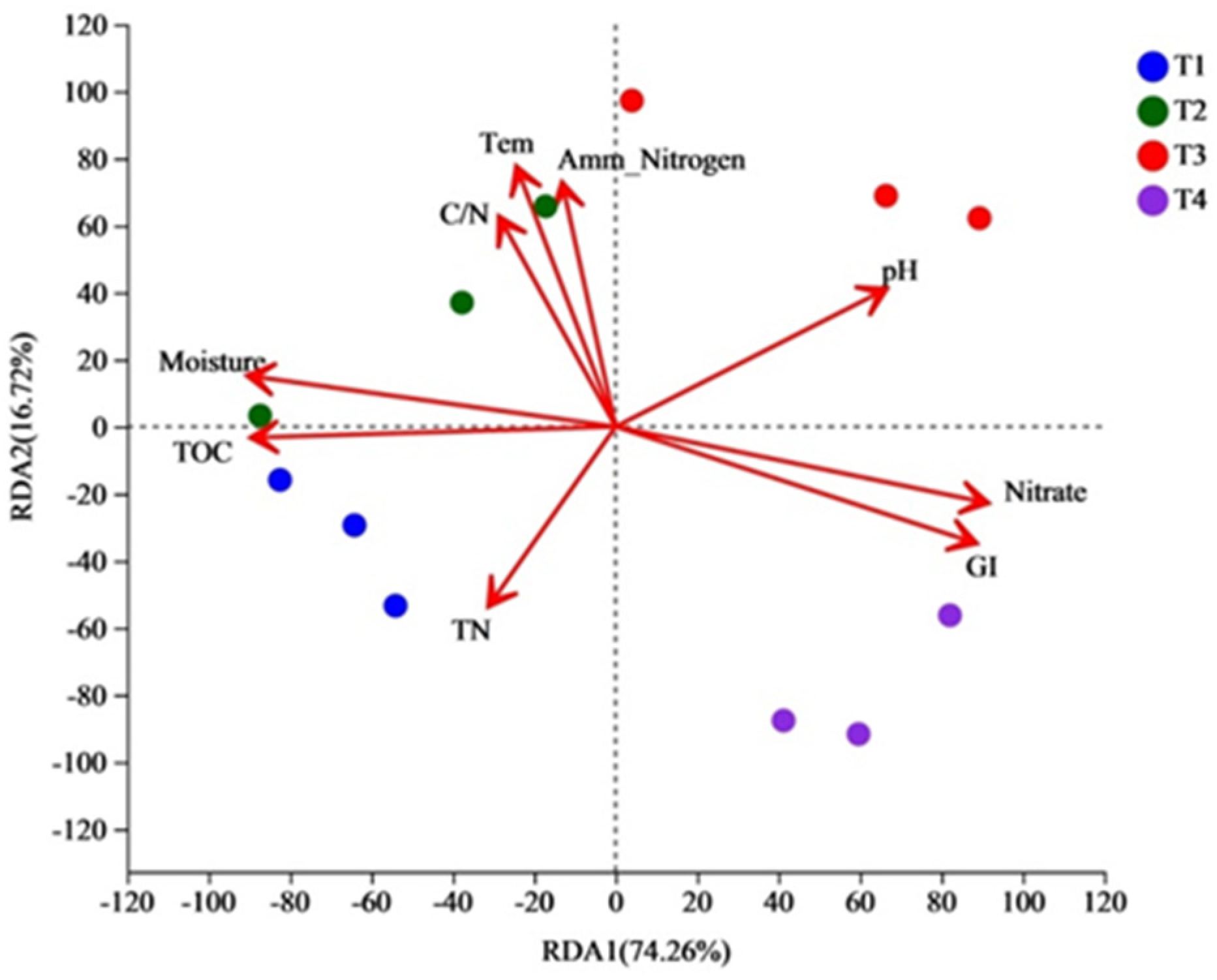
3.5. Correlation between Bacterial Community and Physicochemical Properties
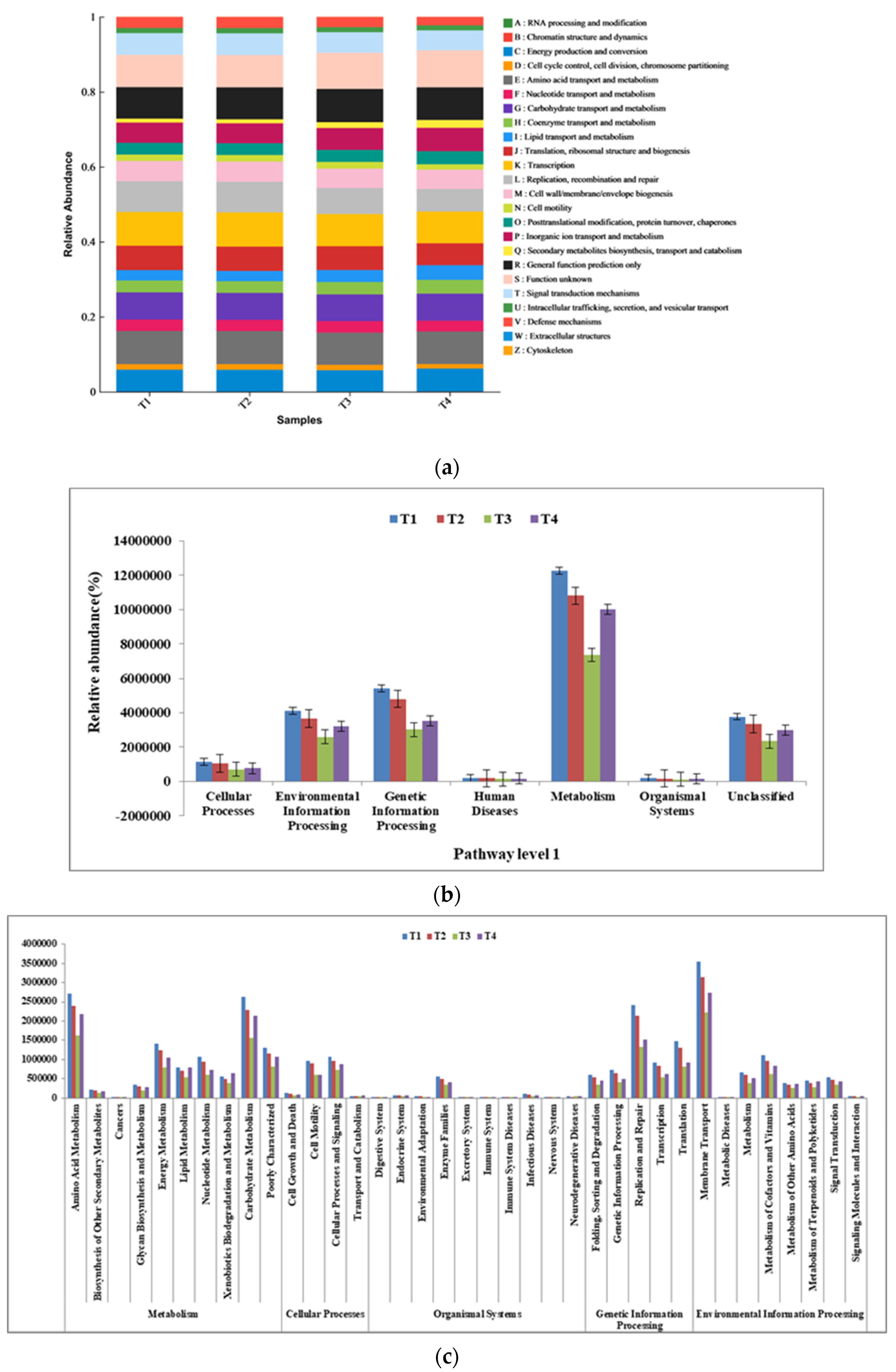
3.6. Bacteria’s Function in Composting
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wu, H.W.; Sun, X.Q.; Liang, B.W.; Chen, J.B.; Zhou, X.F. Analysis of livestock and poultry manure pollution in China and its treatment and resource utilization. J. Agro-Environ. Sci. 2020, 39, 1168–1176. [Google Scholar]
- Yan, Z.W.; Yang, F.Y.; Gao, X.Z.; Chen, J.; Li, S.H.; Li, G.X.; Luo, W.H. Effect of sulfur-containing additives on methane and odor emissions during pig manure composting. J. Agro-Environ. Sci. 2021, 40, 2448–2455. [Google Scholar]
- Fan, H.; Liao, J.; Abass, O.K.; Liu, L.; Huang, X.; Wei, L.; Li, J.; Xie, W.; Liu, C. Effects of compost characteristics on nutrient retention and simultaneous pollutant immobilization and degradation during co-composting process. Bioresour. Technol. 2018, 275, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Fang, C.; Sun, X.; He, X.; Han, L.; Huang, G. Bacterial community succession during pig manure and wheat straw aerobic composting covered with a semi- permeable membrane under slight positive pressure. Bioresour. Technol. 2018, 259, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Li, C.N.; Su, M.; Yao, T.; Han, Q.Q.; Liang, J.J.; Ran, F.; Liu, Z.Y.; Liu, Y.Z.; Chai, S.J.; Gun, S.B. Effects of microbial inoculation on compost physical and chemical properties and dominant bacterial communities during composting of pig manure. J. Plant Nutr. Fertil. 2020, 26, 1600–1611. [Google Scholar]
- Ren, X.; Awasthi, M.K.; Wang, Q.; Zhao, J.; Li, R.; Tu, Z.; Chen, H.; Awasthi, S.K.; Zhang, Z. New insight of tertiary-amine modified bentonite amendment on the nitrogen transformation and volatile fatty acids during the chicken manure composting. Bioresour. Technol. 2018, 266, 524–531. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Changes in physical, chemical, and microbiological properties during the two–stage co–composting of green waste with spent mushroom compost and biochar. Bioresour. Technol. 2014, 171, 274–284. [Google Scholar] [CrossRef]
- Lu, Y.X.; Gao, P.; Cui, X.W.; Lu, H.L.; Chen, D.X.; Long, S.P.; Peng, F.Y. Study on nitrogen transformation and related microbial community changes during the composting process of Chinese medicinal herbal residues. Res. Agric. Mod. 2018, 39, 527–534. [Google Scholar]
- Zhang, H.B.; Men, H.B.; Shen, Y.J.; Zhao, L.X. Research progress on microbial aerobic composting. J. Agric. Sci. Technol. 2017, 19, 1–8. [Google Scholar]
- Zaffiri, L.; Gardner, J.; Toledo-Pereyra, L.H. History of Antibiotics. From Salvarsan to Cephalosporins. J. Investig. Surg. 2012, 25, 67–77. [Google Scholar] [CrossRef]
- Tuomela, M.; Vikman, M.; Hatakka, A.; Itävaara, M. Biodegradation of lignin in compost environment a review. Bioresour. Technol. 2000, 72, 169–183. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Chen, H.Y.; Wang, Q.; Liu, T.; Duan, Y.; Awasthi, S.K.; Ren, X.; Tu, Z.; Li, J.; Zhao, J.; et al. Succession of bacteria diversity in the poultry manure composted mixed with clay: Studies upon its dynamics and associations with physicochemical and gaseous parameters. Bioresour. Technol. 2018, 267, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Awasthi, S.K.; Liu, T.; Verma, S.; Wang, Q.; Chen, H.; Ren, X.; Zhang, Z.; Awasthi, M.K. Positive impact of biochar alone and combined with bacterial consortium amendment on improvement of bacterial community during cow manure composting. Bioresour. Technol. 2019, 280, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Piceno, Y.M.; Pecora-Black, G.; Kramer, S.; Roy, M.; Reid, F.C.; Dubinsky, E.A.; Andersen, G.L. Bacterial community structure transformed after thermophilically composting human waste in Haiti. PLoS ONE 2017, 12, e0177626. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Mao, H.; Wang, Z.; Tian, Y. Succession of organics metabolic function of bacterial community in swine manure composting. J. Hazard. Mater. 2018, 360, 471–480. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Li, J.; Kumar, S.; Awasthi, S.K.; Wang, Q.; Chen, H.Y.; Wang, M.J.; Ren, X.N.; Zhang, Z.Q. Effects of biochar amendment on bacterial and fungal diversity for co-composting of gelatin industry sludge mixed with organic fraction of municipal solid waste. Bioresour. Technol. 2017, 246, 214–223. [Google Scholar] [CrossRef]
- Wang, K.; Yin, X.B.; Mao, H.L.; Chu, C.; Tian, Y. Changes in structure and function of fungal community in cow manure composting. Bioresour. Technol. 2018, 255, 123–130. [Google Scholar] [CrossRef]
- Duan, Y.; Awasthi, S.K.; Liu, T.; Chen, H.; Zhang, Z.; Wang, Q.; Ren, X.; Tu, Z.; Awasthi, M.K.; Taherzadeh, M.J. Dynamics of fungal diversity and interactions with environmental elements in response to wheat straw biochar amended poultry manure composting. Bioresour. Technol. 2019, 274, 410–417. [Google Scholar] [CrossRef]
- Galitskaya, P.; Biktasheva, L.; Saveliev, A.; Tatiana, G.; Eugenia, B.; Svetlana, S. Fungal and bacterial successions in the process of co-composting of organic wastes as revealed by 454 pyrosequencing. PLoS ONE 2017, 12, e0186051. [Google Scholar] [CrossRef]
- Mao, H.; Lv, Z.; Sun, H.; Li, R.H.; Zhai, B.N.; Wang, Z.H.; Awasthi, M.K.; Wang, Q.; Zhou, L. Improvement of biochar and bacterial powder addition on gaseous emission and bacterial community in pig manure compost. Bioresour. Technol. 2018, 258, 195–202. [Google Scholar] [CrossRef]
- Neher, D.A.; Weicht, T.R.; Bates, S.T.; Leff, J.W.; Fierer, N. Changes in bacterial and fungal communities across compost recipes, preparation methods, and composting times. PLoS ONE 2013, 8, e79512. [Google Scholar] [CrossRef]
- Duan, H.; Ji, M.; Chen, A.; Zhang, B.G.; Shi, J.P.; Liu, L.; Li, X.; Sun, J.S. Evaluating the impact of rice husk on successions of bacterial and fungal communities during cow manure composting. Environ. Technol. Innov. 2021, 24, 102084. [Google Scholar] [CrossRef]
- Sun, Z.Y.; Zhang, J.; Zhong, X.Z.; Li, T.; Tang, Y.Q.; Kida, K.J. Production of nitrate-rich compost from the solid fraction of dairy manure by a lab-scale composting system. Waste Manag. 2016, 51, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gao, Z.; Shi, W.; Li, L.; Tian, R.; Huang, J.; Lin, R.; Wang, B.; Zhou, B. Material conversion, microbial community composition and metabolic functional succession during green soybean hull composting. Bioresour. Technol. 2020, 316, 123823. [Google Scholar] [CrossRef]
- Kong, W.; Sun, B.; Zhang, J.; Zhang, Y.T.; Gu, L.K.; Bao, L.J.; Liu, S.X. Metagenomic analysis revealed the succession of microbiota and metabolic function in corncob composting for preparation of cultivation medium for Pleurotus ostreatus. Bioresour. Technol. 2020, 306, 123156. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Z.; Zhong, X.; Huang, Y.; Tan, L.; Tang, Y. Maturity and microbial community structure dynamics during simulated windrow composting of solid fraction of dairy manure. Chin. J. Appl. Environ. Biol. 2016, 22, 6. [Google Scholar]
- Guo, H.; Zhu, C.; Geng, B.; Liu, X.; Ye, J.; Tian, Y.L.; Peng, X.W. Improved fermentation performance in an expanded ectopic fermentation system inoculated with thermophilic bacteria. Bioresour. Technol. 2015, 198, 867–875. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, K.; Wang, J.; Wang, Z.; Pan, R.; Wang, Q.; Li, S.; Kumar, S.; Zhang, Z.; Li, R. Potential of biochar integrated manganese sulfate for promoting pig manure compost humification and its biological mechanism. Bioresour. Technol. 2022, 357, 127350. [Google Scholar] [CrossRef]
- Zhao, X.L.; Luo, Y.L.; Yu, X.; Yu, X.; Jia, H.T.; Li, J.J.; Liu, H.P. Impact of temperature and straw adding ratio on contents of nutrients in cow dung aerobic compost. Chin. J. Environ. Eng. 2014, 8, 7. [Google Scholar]
- Xie, G.; Kong, X.; Kang, J.; Su, N.; Fei, J.C.; Luo, G.W. Fungal community succession contributes to product maturity during the co-composting of chicken manure and crop residues. Bioresour. Technol. 2021, 328, 124845. [Google Scholar] [CrossRef]
- Scaglia, B.; Pognani, M.; Adani, F. Evaluation of hormone-like activity of the dissolved organic matter fraction (DOM) of compost and digestate. Sci. Total Environ. 2015, 514, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.N.; Wang, X.H.; Cao, Q.; Fu, X.C.; Fu, W.Y.; Ma, J.; Zhang, M. Analysis of Microbial Community Changes in Pig Excrement duringCompost Process Based on High-Throughput Sequencing Technology. J. Microbiol. 2018, 38, 21–26. [Google Scholar]
- Zhong, X.Z.; Li, X.X.; Zeng, Y.; Wang, S.P.; Sun, Z.Y.; Tang, Y.Q. Dynamic change of bacterial community during dairy manure composting process revealed by high-throughput sequencing and advanced bioinformatics tools. Bioresour. Technol. 2020, 306, 123091. [Google Scholar] [CrossRef]
- Wang, J.C.; Zhu, R.S.; Liu, X.H.; Sun, S.L.; Wang, H.Z.; Tang, Q.; Qi, B.; Huang, B.H. Effects of microbial agents on bacterial community composition during swine manure composting. Chin. J. Appl. Ecol. 2020, 31, 2449–2456. [Google Scholar]
- Wang, R.; Zhang, J.Y.; Sui, Q.W.; Wan, H.F.; Tong, J.; Chen, M.X.; Wei, Y.S.; Wei, D.B. Effect of red mud addition on tetracycline and copper resistance genes and microbial community during the full scale swine manure composting. Bioresour. Technol. 2016, 216, 1049–1057. [Google Scholar] [CrossRef]
- Bao, Y.; Feng, Y.; Qiu, C.; Zhang, J.W.; Wang, Y.M.; Lin, X.G. Organic matter- and temperature-driven deterministic assembly processes govern bacterial community composition and functionality during manure composting. Waste Manag. 2021, 131, 31–40. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.; Wen, Q. Effects of chlortetracycline on the fate of multi-antibiotic resistance genes and the microbial community during swine manure composting. Environ. Pollut. 2018, 237, 977–987. [Google Scholar] [CrossRef]
- Zhou, Y.W.; Zhang, Y.H.; Shen, Y.J.; Ding, J.T.; Fan, S.Y.; Jia, Y.M.; Wang, H.H.; Cheng, Q.Y.; Li, D.Y.; Zhang, A.Q. Effect of the initial moisture content on the maturity extent and the microbial community structure of the aerobic compost with human excrement. Chin. J. Environ. Eng. 2022, 16, 4108–4120. [Google Scholar]
- Yao, S.T.; Lu, G.; Deng, Y.; Fan, Y.J.; Zhou, H.K.; Dang, N.; Wang, Y.; Zhang, H.; Yan, H. Effects of simulated warming on the composition and diversity of soil prokaryotic communities in Alpine grasslands. Acta Agrestia Sin. 2021, 29, 27–34. [Google Scholar]
- Hernández-Lara, A.; Ros, M.; Cuartero, J.; Bustamante, M.Á.; Moral, R.; Andreu-Rodríguez, F.J.; Fernández, J.A.; Egea-Gilabert, C.; Pascual, J.A. Bacterial and fungal community dynamics during different stages of agro-industrial waste composting and its relationship with compost suppressiveness. Sci. Total Environ. 2022, 805, 150330. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Penton, C.R.; Liu, C.; Tao, C.Y.; Deng, X.H.; Ou, Y.N.; Liu, H.J.; Li, R. Patterns of fungal community succession triggered by C/N ratios during composting. J. Hazard. Mater. 2021, 401, 123344. [Google Scholar] [CrossRef] [PubMed]



| Samples | Temperature (°C) | Moisture (%) | TOC (% TS) | TN (% TS) | NO3−-N (mg ·kg−1 TS) | NH4+-N (mg ·kg−1 TS) | C_N | pH | GI (%) |
|---|---|---|---|---|---|---|---|---|---|
| T1 | 39.43 ± 1.12 c | 57.65 ± 1.83 a | 49.34 ± 0.26 a | 3.2 ± 0.103 a | 0.058 ± 0.002 d | 0.37 ± 0.082 d | 15.34 ± 0.71 b | 8.41 ± 0.04 d | 16.63 ± 1.52 d |
| T2 | 59.67 ± 1.47 a | 50.62 ± 1.76 b | 47.44 ± 0.19 b | 2.75 ± 0.78 d | 0.398 ± 0.011 c | 3.71 ± 0.146 a | 17.26 ± 0.84 a | 8.85 ± 0.07 b | 25.87 ± 2.35 c |
| T3 | 59.63 ± 1.52 ab | 43.19 ± 0.92 c | 42.82 ± 0.36 c | 2.84 ± 0.058 c | 0.989 ± 0.026 b | 2.19 ± 0.086 b | 16.12 ± 0.47 c | 8.93 ± 0.03 a | 43.24 ± 2.88 b |
| T4 | 38.53 ± 1.18 d | 37.11 ± 2.02 d | 39.24 ± 0.43 cd | 2.94 ± 0.063 b | 1.503 ± 0.089 a | 0.71 ± 0.033 c | 15.39 ± 0.32 cd | 8.84 ± 0.02 bc | 64.48 ± 2.76 a |
| Samples | Coverage/% | Simpson | Shannon | ACE | Chao1 |
|---|---|---|---|---|---|
| T1 | 99.776 ± 0.023 ab | 0.118 ± 0.017 a | 3.361 ± 0.145 b | 699.583 ± 53.312 a | 717.061 ± 39.267 a |
| T2 | 99.755 ± 0.035 cd | 0.108 ± 0.031 ab | 3.687 ± 0.212 a | 697.129 ± 36.721 ab | 650.47 ± 42.476 b |
| T3 | 99.758 ± 0.041 bc | 0.090 ± 0.01 c | 3.207 ± 0.201 c | 639.142 ± 42.335 c | 579.62 ± 25.261 c |
| T4 | 99.79 ± 0.016 a | 0.065 ± 0.022 d | 3.137 ± 0.163 cd | 617.102 ± 78.635 cd | 575.809 ± 56.012 cd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Li, J.; Yuan, H.; Che, Z.; Xue, L. Dynamics of Bacterial Diversity and Functions with Physicochemical Properties in Different Phases of Pig Manure Composting. Biology 2023, 12, 1197. https://doi.org/10.3390/biology12091197
Zhao X, Li J, Yuan H, Che Z, Xue L. Dynamics of Bacterial Diversity and Functions with Physicochemical Properties in Different Phases of Pig Manure Composting. Biology. 2023; 12(9):1197. https://doi.org/10.3390/biology12091197
Chicago/Turabian StyleZhao, Xu, Juan Li, Hongxia Yuan, Zongxian Che, and Lingui Xue. 2023. "Dynamics of Bacterial Diversity and Functions with Physicochemical Properties in Different Phases of Pig Manure Composting" Biology 12, no. 9: 1197. https://doi.org/10.3390/biology12091197
APA StyleZhao, X., Li, J., Yuan, H., Che, Z., & Xue, L. (2023). Dynamics of Bacterial Diversity and Functions with Physicochemical Properties in Different Phases of Pig Manure Composting. Biology, 12(9), 1197. https://doi.org/10.3390/biology12091197





