Carbon Fluxes in Potato (Solanum tuberosum) Remain Stable in Cell Cultures Exposed to Nutritional Phosphate Deficiency
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Plant Material
2.2. Isotopic Labeling Experiments
2.3. Statistics
3. Results
3.1. Tracer Uptake by +Pi and −Pi Cells
3.2. Metabolization of [U-14C]Suc by +Pi and −Pi Cells
3.3. Labeling of +Pi and −Pi Cells with NaH14CO3
3.4. Analysis of the Interaction between Cell Culture Age and C Fluxes
4. Discussion
4.1. Sugar Uptake Is Maintained in Potato Cell Cultures Subjected to Pi Deficiency
4.2. Sugar Catabolic Fluxes Are Similar in +Pi and −Pi Cells
4.3. Metabolism of NaH14CO3 in +Pi and −Pi Cells Allows a Better Understanding of the Role of PEPC in the Stability of Anaplerotic C Flux under Pi Deficiency
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malhotra, H.; Vandana; Sharma, S.; Pandey, R. Phosphorus Nutrition: Plant Growth in Response to Deficiency and Excess. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 171–190. [Google Scholar]
- Bechtaoui, N.; Rabiu, M.K.; Raklami, A.; Oufdou, K.; Hafidi, M.; Jemo, M. Phosphate-Dependent Regulation of Growth and Stresses Management in Plants. Front. Plant Sci. 2021, 12, 679916. [Google Scholar] [CrossRef]
- Raghothama, K.G. Phosphate acquisition. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 665–693. [Google Scholar] [CrossRef]
- Nussaume, L.; Kanno, S.; Javot, H.; Marin, E.; Pochon, N.; Ayadi, A.; Nakanishi, T.M.; Thibaud, M.-C. Phosphate Import in Plants: Focus on the PHT1 Transporters. Front. Plant Sci. 2011, 2, 83. [Google Scholar] [CrossRef] [PubMed]
- López-Arredondo, D.L.; Leyva-González, M.A.; González-Morales, S.I.; López-Bucio, J.; Herrera-Estrella, L. Phosphate nutrition: Improving low phosphate tolerance in crops. Annu. Rev. Plant Biol. 2014, 65, 95–123. [Google Scholar] [CrossRef] [PubMed]
- Cordell, D.; Drangert, J.-O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. Hum. Policy Dimens. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Reijnders, L. Phosphorus resources, their depletion and conservation, a review. Resour. Conserv. Recycl. 2014, 93, 32–49. [Google Scholar] [CrossRef]
- Wu, P.; Ma, L.; Hou, X.; Wang, M.; Wu, Y.; Liu, F.; Deng, X.W. Phosphate starvation triggers distinct alterations of genome expression in Arabidopsis roots and leaves. Plant Physiol. 2003, 132, 1260–1271. [Google Scholar] [CrossRef]
- Chiou, T.J.; Aung, K.; Lin, S.I.; Wu, C.C.; Chiang, S.F.; Su, C.L. Regulation of phosphate homeostasis by microRNA in Arabidopsis. Plant Cell 2006, 18, 412–421. [Google Scholar] [CrossRef]
- Veljanovski, V.; Vanderbeld, B.; Knowles, V.L.; Snedden, W.A.; Plaxton, W.C. Biochemical and molecular characterization of AtPAP26, a vacuolar purple acid phosphatase up-regulated in phosphate-deprived arabidopsis suspension cells and seedlings. Plant Physiol. 2006, 142, 1282–1293. [Google Scholar] [CrossRef]
- Li, K.P.; Xu, C.Z.; Li, Z.X.; Zhang, K.W.; Yang, A.F.; Zhang, J.R. Comparative proteome analyses of phosphorus responses in maize (Zea mays L.) roots of wild-type and a low-P-tolerant mutant reveal root characteristics associated with phosphorus efficiency. Plant J. 2008, 55, 927–939. [Google Scholar] [CrossRef]
- Alexova, R.; Millar, A.H. Proteomics of phosphate use and deprivation in plants. Proteomics 2013, 13, 609–623. [Google Scholar] [CrossRef]
- Gregory, A.L.; Hurley, B.A.; Tran, H.T.; Valentine, A.J.; She, Y.-M.; Knowles, V.L.; Plaxton, W.C. In vivo regulatory phosphorylation of the phosphoenolpyruvate carboxylase AtPPC1 in phosphate-starved Arabidopsis thaliana. Biochem. J. 2009, 420, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Li, K.P.; Xu, C.Z.; Fan, W.M.; Zhang, H.L.; Hou, J.J.; Yang, A.F.; Zhang, K.W. Phosphoproteome and proteome analyses reveal low-phosphate mediated plasticity of root developmental and metabolic regulation in maize (Zea mays L.). Plant Physiol. Biochem. 2014, 83, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.T.; Hurley, B.A.; Plaxton, W.C. Feeding hungry plants: The role of purple acid phosphatases in phosphate nutrition. Plant Sci. 2010, 179, 14–27. [Google Scholar] [CrossRef]
- Abel, S. Phosphate scouting by root tips. Curr. Opin. Plant Biol. 2017, 39, 168–177. [Google Scholar] [CrossRef]
- del Pozo, J.C.; Allona, I.; Rubio, V.; Leyva, A.; de la Pena, A.; Aragoncillo, C.; Paz-Ares, J. A type 5 acid phosphatase gene from Arabidopsis thaliana is induced by phosphate starvation and by some other types of phosphate mobilising/oxidative stress conditions. Plant J. 1999, 19, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Bozzo, G.G.; Raghothama, K.G.; Plaxton, W.C. Purification and characterization of two secreted purple acid phosphatase isozymes from phosphate-starved tomato (Lycopersicon esculentum) cell cultures. Eur. J. Biochem. 2002, 269, 6278–6286. [Google Scholar] [CrossRef]
- Plaxton, W.C.; Tran, H.T. Metabolic adaptations of phosphate-starved plants. Plant Physiol. 2011, 156, 1006–1015. [Google Scholar] [CrossRef]
- Duff, S.M.G.; Moorhead, G.B.; Lefebvre, D.D.; Plaxton, W.C. Phosphate starvation inducible ‘bypasses’ of adenylate and phosphate dependent glycolytic enzymes in Brassica nigra suspension cells. Plant Physiol. 1989, 90, 1275–1278. [Google Scholar] [CrossRef]
- Johnson, J.F.; Allan, D.L.; Vance, C.P. Phosphorus Stress-Induced Proteoid Roots Show Altered Metabolism in Lupinus albus. Plant Physiol. 1994, 104, 657–665. [Google Scholar] [CrossRef]
- Uhde-Stone, C.; Gilbert, G.; Johnson, J.M.F.; Litjens, R.; Zinn, K.E.; Temple, S.J.; Vance, C.P.; Allan, D.L. Acclimation of white lupin to phosphorus deficiency involves enhanced expression of genes related to organic acid metabolism. Plant Soil 2003, 248, 99–116. [Google Scholar] [CrossRef]
- Le Roux, M.R.; Ward, C.L.; Botha, F.C.; Valentine, A.J. Routes of pyruvate synthesis in phosphorus-deficient lupin roots and nodules. New Phytol. 2006, 169, 399–408. [Google Scholar] [CrossRef]
- He, J.Z.; Dorion, S.; Lacroix, M.; Rivoal, J. Sustained substrate cycles between hexose phosphates and free sugars in phosphate-deficient potato (Solanum tuberosum) cell cultures. Planta 2019, 249, 1319–1336. [Google Scholar] [CrossRef]
- Masakapalli, S.K.; Bryant, F.M.; Kruger, N.J.; Ratcliffe, R.G. The metabolic flux phenotype of heterotrophic Arabidopsis cells reveals a flexible balance between the cytosolic and plastidic contributions to carbohydrate oxidation in response to phosphate limitation. Plant J. 2014, 78, 964–977. [Google Scholar] [CrossRef]
- O’Leary, B.; Park, J.; Plaxton, W.C. The remarkable diversity of plant PEPC (phosphoenolpyruvate carboxylase): Recent insights into the physiological functions and post-translational controls of non-photosynthetic PEPCs. Biochem. J. 2011, 436, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Stevens, G.G.; Pérez-Fernández, M.A.; Morcillo, R.J.L.; Kleinert, A.; Hills, P.; Brand, D.J.; Steenkamp, E.T.; Valentine, A.J. Roots and Nodules Response Differently to P Starvation in the Mediterranean-Type Legume Virgilia divaricata. Front. Plant Sci. 2019, 10, 73. [Google Scholar] [CrossRef]
- Lan, P.; Li, W.; Schmidt, W. Complementary Proteome and Transcriptome Profiling in Phosphate-deficient Arabidopsis Roots Reveals Multiple Levels of Gene Regulation. Mol. Cell. Proteom. 2012, 11, 1156–1166. [Google Scholar] [CrossRef]
- Birch, P.R.J.; Bryan, G.; Fenton, B.; Gilroy, E.M.; Hein, I.; Jones, J.T.; Prashar, A.; Taylor, M.A.; Torrance, L.; Toth, I.K. Crops that feed the world 8: Potato: Are the trends of increased global production sustainable? Food Secur. 2012, 4, 477–508. [Google Scholar] [CrossRef]
- Koch, M.; Naumann, M.; Pawelzik, E.; Gransee, A.; Thiel, H. The importance of nutrient management for potato production Part I: Plant nutrition and yield. Potato Res. 2020, 63, 97–119. [Google Scholar] [CrossRef]
- Rosen, C.J.; Bierman, P.M. Potato yield and tuber set as affected by phosphorus fertilization. Am. J. Potato Res. 2008, 85, 110–120. [Google Scholar] [CrossRef]
- Dorion, S.; Rivoal, J. Quantification of uridine 5’-diphosphate (UDP)-glucose by high-performance liquid chromatography and its application to nonradioactive assay for nucleoside diphosphate kinase using UDP-glucose pyrophosphorylase as a coupling enzyme. Anal. Biochem. 2003, 323, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Dorion, S.; Clendenning, A.; Jeukens, J.; Salas, J.J.; Parveen, N.; Haner, A.A.; Law, R.D.; Martinez-Force, E.; Rivoal, J. A large decrease of cytosolic triosephosphate isomerase in transgenic potato roots affects the distribution of carbon in primary metabolism. Planta 2012, 236, 1177–1190. [Google Scholar] [CrossRef] [PubMed]
- Rivoal, J.; Hanson, A.D. Evidence for a large and sustained glycolytic flux to lactate in anoxic roots of some members of the halophytic genus Limonium. Plant Physiol. 1993, 101, 553–560. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dorion, S.; Clendenning, A.; Rivoal, J. Engineering the expression level of cytosolic nucleoside diphosphate kinase in transgenic Solanum tuberosum roots alters growth, respiration and carbon metabolism. Plant J. 2017, 89, 914–926. [Google Scholar] [CrossRef] [PubMed]
- Claeyssen, E.; Dorion, S.; Clendenning, A.; He, J.Z.; Wally, O.; Chen, J.; Auslender, E.L.; Moisan, M.-C.; Jolicoeur, M.; Rivoal, J. The futile cycling of hexose phosphates could account for the fact that hexokinase exerts a high control on glucose phosphorylation but not on glycolytic rate in transgenic potato (Solanum tuberosum) Roots. PLoS ONE 2013, 8, e53898. [Google Scholar] [CrossRef]
- Rolletschek, H.; Borisjuk, L.; Radchuk, R.; Miranda, M.; Heim, U.; Wobus, U.; Weber, H. Seed-specific expression of a bacterial phosphoenolpyruvate carboxylase in Vicia narbonensis increases protein content and improves carbon economy. Plant Biotechnol. J. 2004, 2, 211–219. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 17 July 2023).
- Péret, B.; Clément, M.; Nussaume, L.; Desnos, T. Root developmental adaptation to phosphate starvation: Better safe than sorry. Trends Plant Sci. 2011, 16, 442–450. [Google Scholar] [CrossRef]
- Veneklaas, E.J.; Lambers, H.; Bragg, J.; Finnegan, P.M.; Lovelock, C.E.; Plaxton, W.C.; Price, C.A.; Scheible, W.R.; Shane, M.W.; White, P.J.; et al. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol. 2012, 195, 306–320. [Google Scholar] [CrossRef]
- O’Rourke, J.A.; Yang, S.S.; Miller, S.S.; Bucciarelli, B.; Liu, J.Q.; Rydeen, A.; Bozsoki, Z.; Uhde-Stone, C.; Tu, Z.J.; Allan, D.; et al. An RNA-Seq Transcriptome Analysis of Orthophosphate-Deficient White Lupin Reveals Novel Insights into Phosphorus Acclimation in Plants. Plant Physiol. 2013, 161, 705–724. [Google Scholar] [CrossRef]
- Baker, A.; Ceasar, S.A.; Palmer, A.J.; Paterson, J.B.; Qi, W.; Muench, S.P.; Baldwin, S.A. Replace, reuse, recycle: Improving the sustainable use of phosphorus by plants. J. Exp. Bot. 2015, 66, 3523–3540. [Google Scholar] [CrossRef]
- Paul, M.J.; Stitt, M. Effects of nitrogen and phosphorus deficiencies an levels of carbohydrates, respiratory enzymes and metabolites in seedlings of tobacco and their response to exogenous sucrose. Plant Cell Environ. 1993, 16, 1047–1057. [Google Scholar] [CrossRef]
- Morcuende, R.; Bari, R.; Gibon, Y.; Zheng, W.; Pant, B.D.; Bläsing, O.; Usadel, B.; Czechowski, T.; Udvardi, M.K.; Stitt, M.; et al. Genome-wide reprogramming of metabolism and regulatory networks of Arabidopsis in response to phosphorus. Plant Cell Environ. 2007, 30, 85–112. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Roessner, U.; Eickmeier, I.; Genc, Y.; Callahan, D.L.; Shirley, N.; Langridge, P.; Bacic, A. Metabolite profiling reveals distinct changes in carbon and nitrogen metabolism in phosphate-deficient barley plants (Hordeum vulgare L.). Plant Cell Physiol. 2008, 49, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Hoffland, E.; Vandenboogaard, R.; Nelemans, J.; Findenegg, G. Biosynthesis and root exudation of citric and malic-acids in phosphate-starved rape plants. New Phytol. 1992, 122, 675–680. [Google Scholar] [CrossRef]
- Neumann, G.; Massonneau, A.; Martinoia, E.; Römheld, V. Physiological adaptations to phosphorus deficiency during proteoid root development in white lupin. Planta 1999, 208, 373–382. [Google Scholar] [CrossRef]
- Neumann, G.; Römheld, V. Root excretion of carboxylic acids and protons in phosphorus-deficient plants. Plant Soil 1999, 211, 121–130. [Google Scholar] [CrossRef]
- Ryan, P.R.; Delhaize, E.; Jones, D.L. Function and mechanism of organic anion exudation from plant roots. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 527–560. [Google Scholar] [CrossRef]
- Johnson, J.F.; Allan, D.L.; Vance, C.P.; Weiblen, G. Root Carbon Dioxide Fixation by Phosphorus-Deficient Lupinus albus (Contribution to Organic Acid Exudation by Proteoid Roots). Plant Physiol. 1996, 112, 19–30. [Google Scholar] [CrossRef]
- Le Roux, M.; Phiri, E.; Khan, W.; Şakiroğlu, M.; Valentine, A.; Khan, S. Expression of novel cytosolic malate dehydrogenases (cMDH) in Lupinus angustifolius nodules during phosphorus starvation. J. Plant Physiol. 2014, 171, 1609–1618. [Google Scholar] [CrossRef]
- Liang, C.; Wang, J.; Zhao, J.; Tian, J.; Liao, H. Control of phosphate homeostasis through gene regulation in crops. Curr. Opin. Plant Biol. 2014, 21, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Plaxton, W.C.; Podestà, F.E. The functional organization and control of plant respiration. Crit. Rev. Plant Sci. 2006, 25, 159–198. [Google Scholar] [CrossRef]
- Ding, Y.; Tang, Y.; Kwok, C.K.; Zhang, Y.; Bevilacqua, P.C.; Assmann, S.M. In vivo genome-wide profiling of RNA secondary structure reveals novel regulatory features. Nature 2013, 505, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Rivoal, J.; Hanson, A.D. Metabolic control of anaerobic glycolysis—Overexpression of lactate dehydrogenase in transgenic tomato roots supports the Davies-Roberts hypothesis and points to a critical role for lactate secretion. Plant Physiol. 1994, 106, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Geigenberger, P.; Stitt, M. Flux an important, but neglected, component of functional genomics. Curr. Opin. Plant Biol. 2005, 8, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Gaudinier, A.; Tang, M.; Kliebenstein, D.J. Transcriptional networks governing plant metabolism. Curr. Plant Biol. 2015, 3–4, 56–64. [Google Scholar] [CrossRef]
- Cheung, C.Y.M.; Williams, T.C.R.; Poolman, M.G.; Fell, D.A.; Ratcliffe, R.G.; Sweetlove, L.J. A method for accounting for maintenance costs in flux balance analysis improves the prediction of plant cell metabolic phenotypes under stress conditions. Plant J. 2013, 75, 1050–1061. [Google Scholar] [CrossRef]
- Dong, N.Q.; Sun, Y.W.; Guo, T.; Shi, C.L.; Zhang, Y.M.; Kan, Y.; Xiang, Y.H.; Zhang, H.; Yang, Y.B.; Li, Y.C.; et al. UDP-glucosyltransferase regulates grain size and abiotic stress tolerance associated with metabolic flux redirection in rice. Nat. Commun. 2020, 11, 2629. [Google Scholar] [CrossRef]
- Qian, X.; Zhang, Y.; Lun, D.S.; Dismukes, G.C. Rerouting of Metabolism into Desired Cellular Products by Nutrient Stress: Fluxes Reveal the Selected Pathways in Cyanobacterial Photosynthesis. ACS Synth. Biol. 2018, 7, 1465–1476. [Google Scholar] [CrossRef]
- Heydarizadeh, P.; Veldl, B.; Huang, B.; Lukomska, E.; Wielgosz-Collin, G.; Couzinet-Mossion, A.; Bougaran, G.; Marchand, J.; Schoefs, B. Carbon Orientation in the Diatom Phaeodactylum tricornutum: The Effects of Carbon Limitation and Photon Flux Density. Front. Plant Sci. 2019, 10, 471. [Google Scholar] [CrossRef]
- Wang, Q.D.; Hu, J.K.; Hu, H.F.; Li, Y.; Xiang, M.L.; Wang, D.Z. Integrated eco-physiological, biochemical, and molecular biological analyses of selenium fortification mechanism in alfalfa. Planta 2022, 256, 114. [Google Scholar] [CrossRef] [PubMed]
- Sulieman, S.; Abdelrahman, M.; Tran, L.S.P. Carbon metabolic adjustment in soybean nodules in response to phosphate limitation: A metabolite perspective. Environ. Exp. Bot. 2022, 196, 104810. [Google Scholar] [CrossRef]
- Ruan, Y.-L. Sucrose Metabolism: Gateway to Diverse Carbon Use and Sugar Signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef] [PubMed]
- Komor, E.; Thom, M.; Maretzki, A. The mechanism of sugar uptake by sugarcane suspension cells. Planta 1981, 153, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Botha, F.C.; O’Kennedy, M.M. Carbohydrate utilisation by cell suspension cultures of Phaseolus vulgaris. Physiol. Plant. 1998, 102, 429–436. [Google Scholar] [CrossRef]
- Krook, J.; Vreugdenhil, D.; van der Plas, L.H.W. Uptake and phosphorylation of glucose and fructose in Daucus carota cell suspensions are differently regulated. Plant Physiol. Biochem. 2000, 38, 603–612. [Google Scholar] [CrossRef]
- Conde, C.; Agasse, A.; Glissant, D.; Tavares, R.; Gerós, H.; Delrot, S. Pathways of Glucose Regulation of Monosaccharide Transport in Grape Cells. Plant Physiol. 2006, 141, 1563–1577. [Google Scholar] [CrossRef]
- Felker, F.C.; Goodwin, J.C. Sugar uptake by maize endosperm suspension cultures. Plant Physiol. 1988, 88, 1235–1239. [Google Scholar] [CrossRef]
- Etxeberria, E.; Baroja-Fernandez, E.; Munoz, F.J.; Pozueta-Romero, J. Sucrose-inducible endocytosis as a mechanism for nutrient uptake in heterotrophic plant cells. Plant Cell Physiol. 2005, 46, 474–481. [Google Scholar] [CrossRef]
- Menges, M.; Hennig, L.; Gruissem, W.; Murray, J.A.H. Genome-wide gene expression in an Arabidopsis cell suspension. Plant Mol. Biol. 2003, 53, 423–442. [Google Scholar] [CrossRef]
- Ganie, A.H.; Ahmad, A.; Pandey, R.; Aref, I.M.; Yousuf, P.Y.; Ahmad, S.; Iqbal, M. Metabolite profiling of low-P tolerant and low-P sensitive maize genotypes under phosphorus starvation and restoration conditions. PLoS ONE 2015, 10, e0129520. [Google Scholar] [CrossRef]
- Valentinuzzi, F.; Pii, Y.; Vigani, G.; Lehmann, M.; Cesco, S.; Mimmo, T. Phosphorus and iron deficiencies induce a metabolic reprogramming and affect the exudation traits of the woody plant Fragaria × ananassa. J. Exp. Bot. 2015, 66, 6483–6495. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.S.; Israel, D.W. Diurnal starch accumulation and utilization in phosphorus-deficient soybean plants. Plant Physiol. 1992, 98, 316–323. [Google Scholar] [CrossRef]
- Qiu, J.; Israel, D.W. Carbohydrate accumulation and utilization in soybean plants in response to altered phosphorus-nutrition. Physiol. Plant. 1994, 90, 722–728. [Google Scholar] [CrossRef]
- Ciereszko, I.; Barbachowska, A. Sucrose metabolism in leaves and roots of bean (Phaseolus vulgaris L.) during phosphate deficiency. J. Plant Physiol. 2000, 156, 640–644. [Google Scholar] [CrossRef]
- Rontein, D.; Dieuaide-Noubhani, M.; Dufourc, E.J.; Raymond, P.; Rolin, D. The metabolic architecture of plant cells. Stability of central metabolism and flexibility of anabolic pathways during the growth cycle of tomato cells. J. Biol. Chem. 2002, 277, 43948–43960. [Google Scholar] [CrossRef] [PubMed]
- Mori, S. A High Performance Liquid Chromatography-Radio-Organic Acid Analyzer for determining the specific activities of radioactive organic acids. Plant Cell Physiol. 1982, 23, 703–708. [Google Scholar] [CrossRef]
- Cramer, M.D.; Lewis, O.A.M.; Lips, S.H. Inorganic carbon fixation and metabolism in maize roots as affected by nitrate and ammonium nutrition. Physiol. Plant. 1993, 89, 632–639. [Google Scholar] [CrossRef]
- Wegner, A.; Meiser, J.; Weindl, D.; Hiller, K. How metabolites modulate metabolic flux. Curr. Opin. Biotechnol. 2015, 34, 16–22. [Google Scholar] [CrossRef]
- Clark, T.J.; Guo, L.; Morgan, J.; Schwender, J. Modeling plant metabolism: From network reconstruction to mechanistic models. Annu. Rev. Plant Biol. 2020, 71, 303–326. [Google Scholar] [CrossRef]
- De Nisi, P.; Zocchi, G. Phosphoenolpyruvate carboxylase in cucumber (Cucumis sativus L.) roots under iron deficiency: Activity and kinetic characterization. J. Exp. Bot. 2000, 51, 1903–1909. [Google Scholar] [CrossRef] [PubMed]
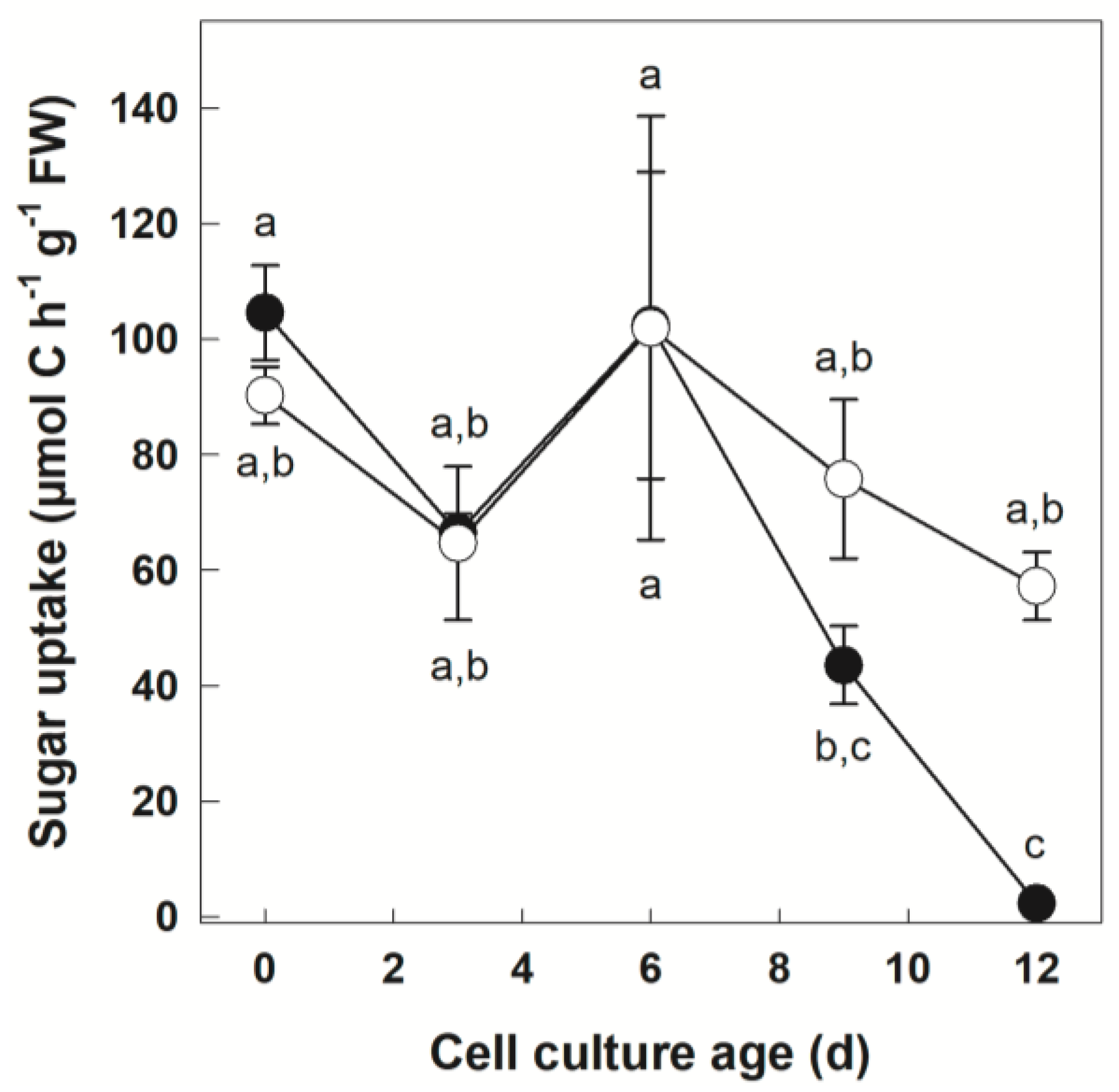
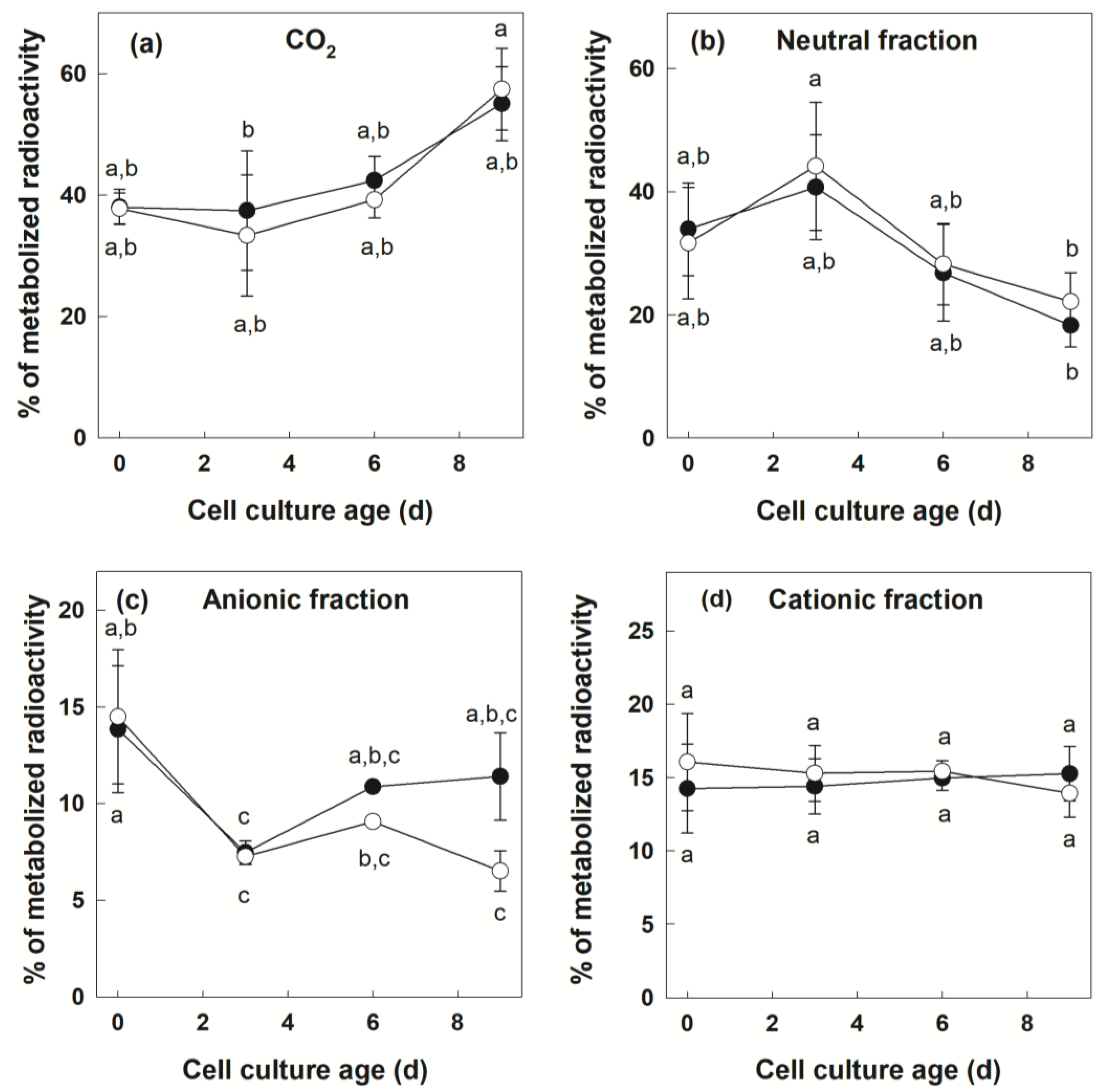
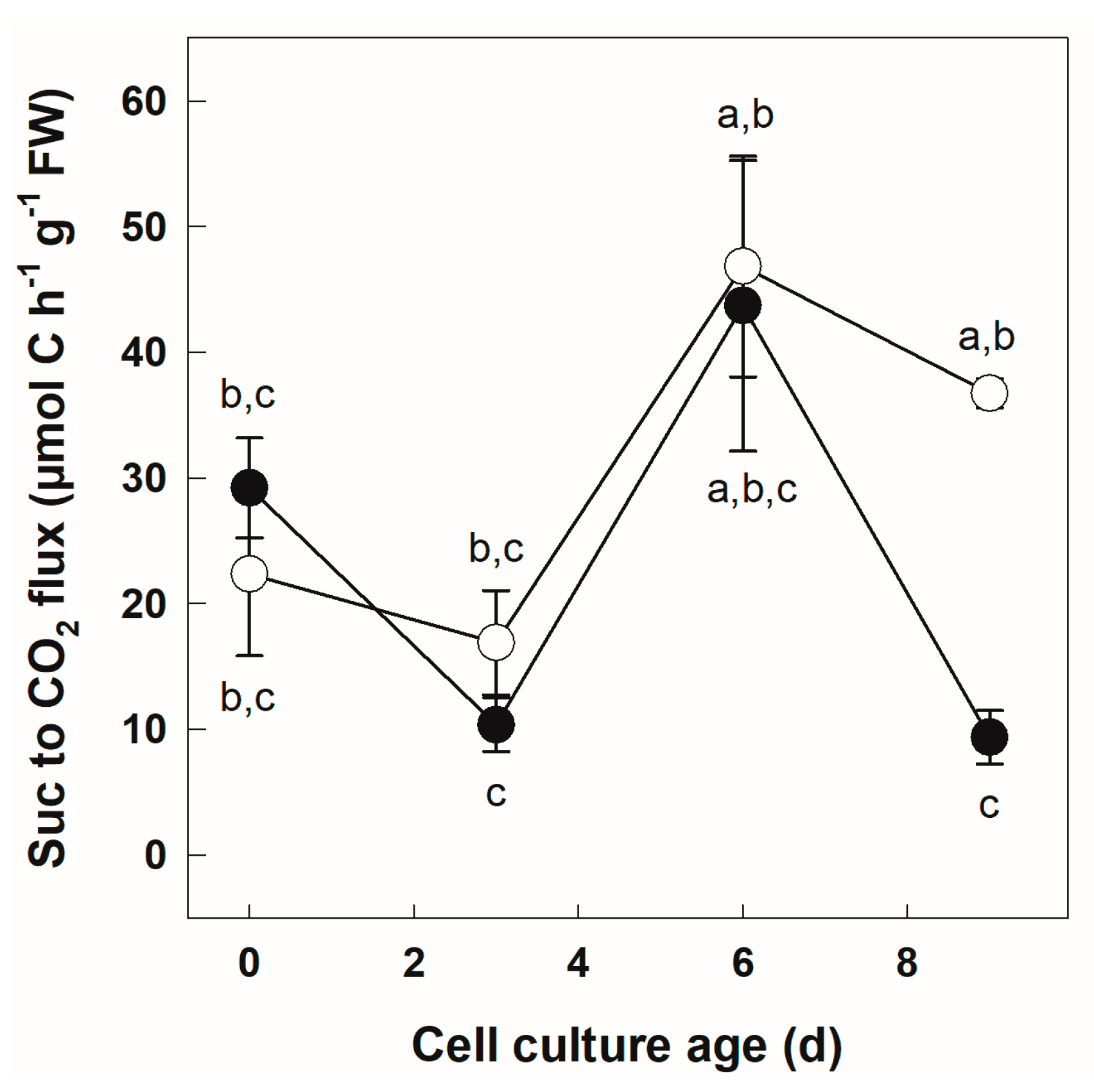
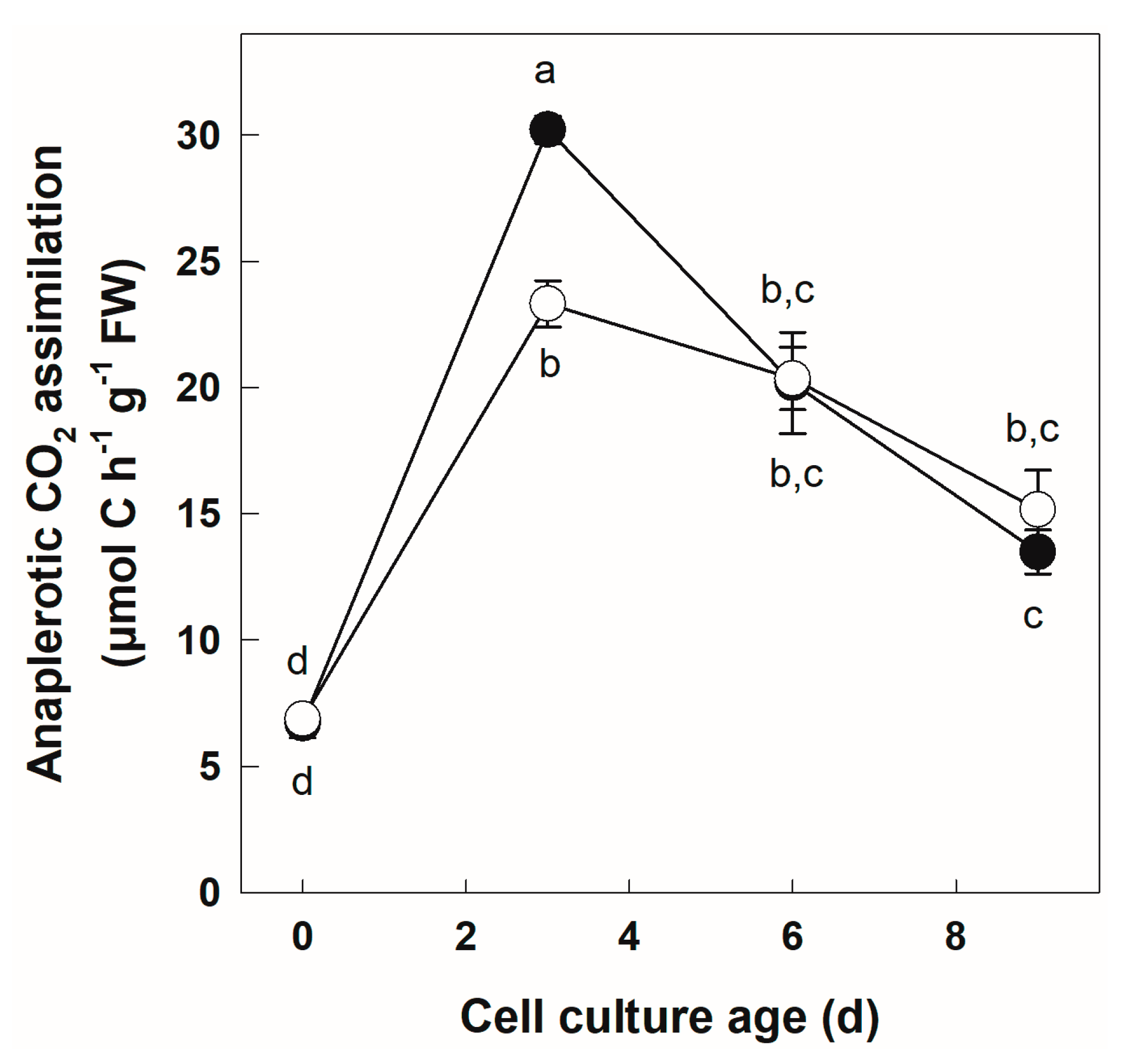
| Parameter | Cell Culture Age | Phosphate Regime | Phosphate Regime x Cell Culture Age | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| C uptake (μmol C h−1·g−1 FW) | 6.22 | 0.002 ** | 2.15 | 0.157 | 1.43 | 0.260 |
| % CO2 in metabolized [U-14C]Suc | 4.92 | 0.013 * | 1.33 | 0.2667 | 0.849 | 0.4874 |
| % Neutral fraction in metabolized [U-14C]Suc | 4.14 | 0.023 * | 0.74 | 0.401 | 0.214 | 0.8850 |
| % Anionic fraction in metabolized [U-14C]Suc | 5.46 | 0.008 ** | 2.60 | 0.126 | 0.41 | 0.743 |
| % Cationic fraction in metabolized [U-14C]Suc | 0.14 | 0.932 | 0.030 | 0.865 | 0.188 | 0.903 |
| Suc to CO2 flux (μmol C h−1 g−1 FW) | 11.16 | 3.37 × 10−4 *** | 3.22 | 0.0916 | 2.94 | 0.064 |
| Anaplerotic CO2 assimilation (μmol C h−1 g−1 FW) | 83.9 | 5.25 × 10−10 *** | 3.03 | 0.100 | 5.25 | 0.010 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.Z.; Dorion, S.; Carmona-Rojas, L.M.; Rivoal, J. Carbon Fluxes in Potato (Solanum tuberosum) Remain Stable in Cell Cultures Exposed to Nutritional Phosphate Deficiency. Biology 2023, 12, 1190. https://doi.org/10.3390/biology12091190
He JZ, Dorion S, Carmona-Rojas LM, Rivoal J. Carbon Fluxes in Potato (Solanum tuberosum) Remain Stable in Cell Cultures Exposed to Nutritional Phosphate Deficiency. Biology. 2023; 12(9):1190. https://doi.org/10.3390/biology12091190
Chicago/Turabian StyleHe, Jiang Zhou, Sonia Dorion, Laura Michell Carmona-Rojas, and Jean Rivoal. 2023. "Carbon Fluxes in Potato (Solanum tuberosum) Remain Stable in Cell Cultures Exposed to Nutritional Phosphate Deficiency" Biology 12, no. 9: 1190. https://doi.org/10.3390/biology12091190
APA StyleHe, J. Z., Dorion, S., Carmona-Rojas, L. M., & Rivoal, J. (2023). Carbon Fluxes in Potato (Solanum tuberosum) Remain Stable in Cell Cultures Exposed to Nutritional Phosphate Deficiency. Biology, 12(9), 1190. https://doi.org/10.3390/biology12091190







