Emergence and Progression of Behavioral Motor Deficits and Skeletal Muscle Atrophy across the Adult Lifespan of the Rat
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Behavioral Testing
2.2.1. Open Field Activity
2.2.2. Crossing a Wire Mesh Screen
2.2.3. Beam Balance
2.2.4. Walking Track Analysis
2.2.5. Gait Score
2.3. Experimental Endpoint
2.4. Histology
2.4.1. Counting of Myofibers and Myonuclei with a Central Position
2.4.2. Immunohistochemistry
2.5. Fiber Typing and Stereology
2.6. RNA Extraction and qPCR
2.7. Surgical Intervention
2.8. Statistical Analyses and Models
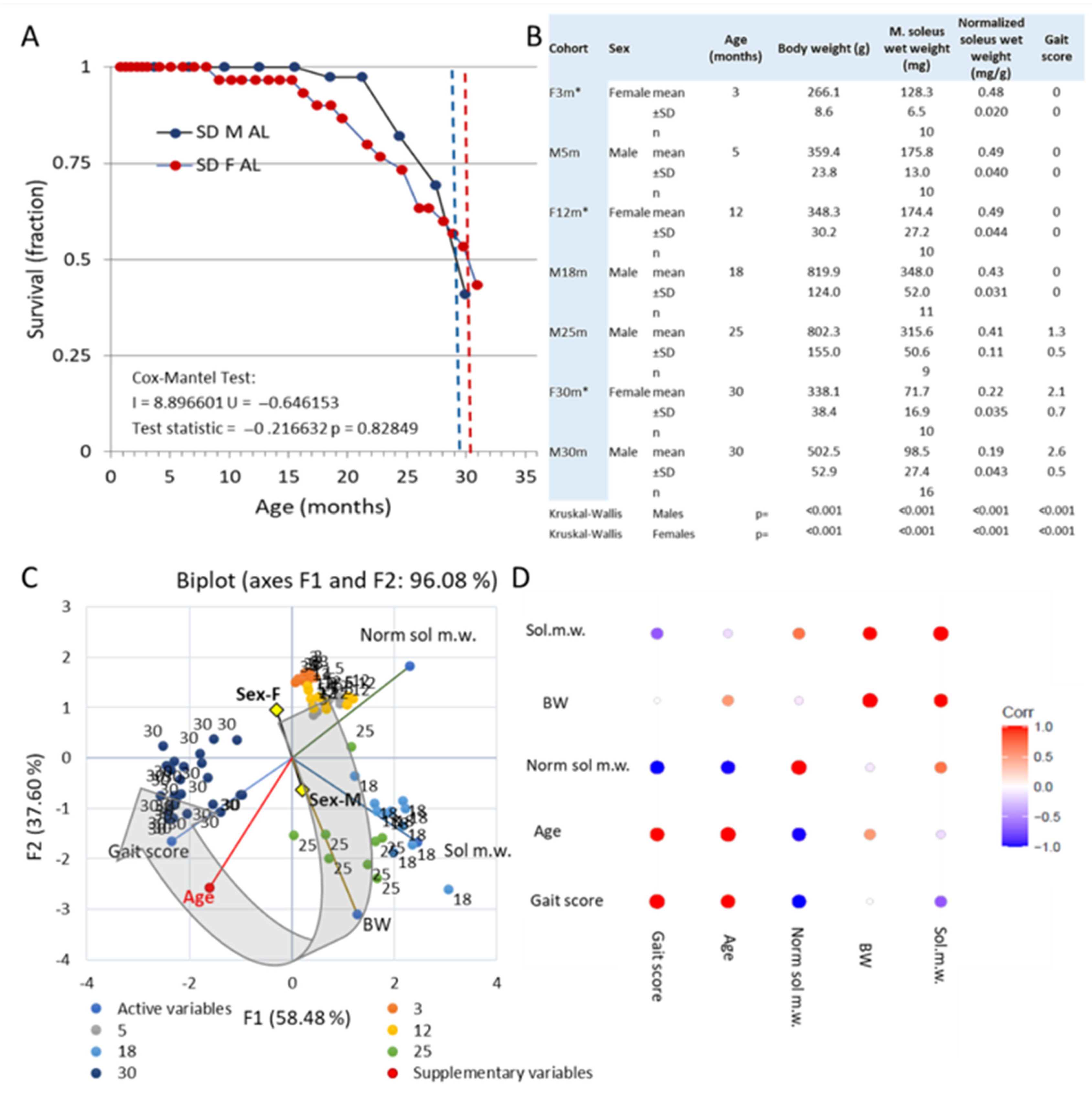
3. Results
3.1. Survival and Gross Observations
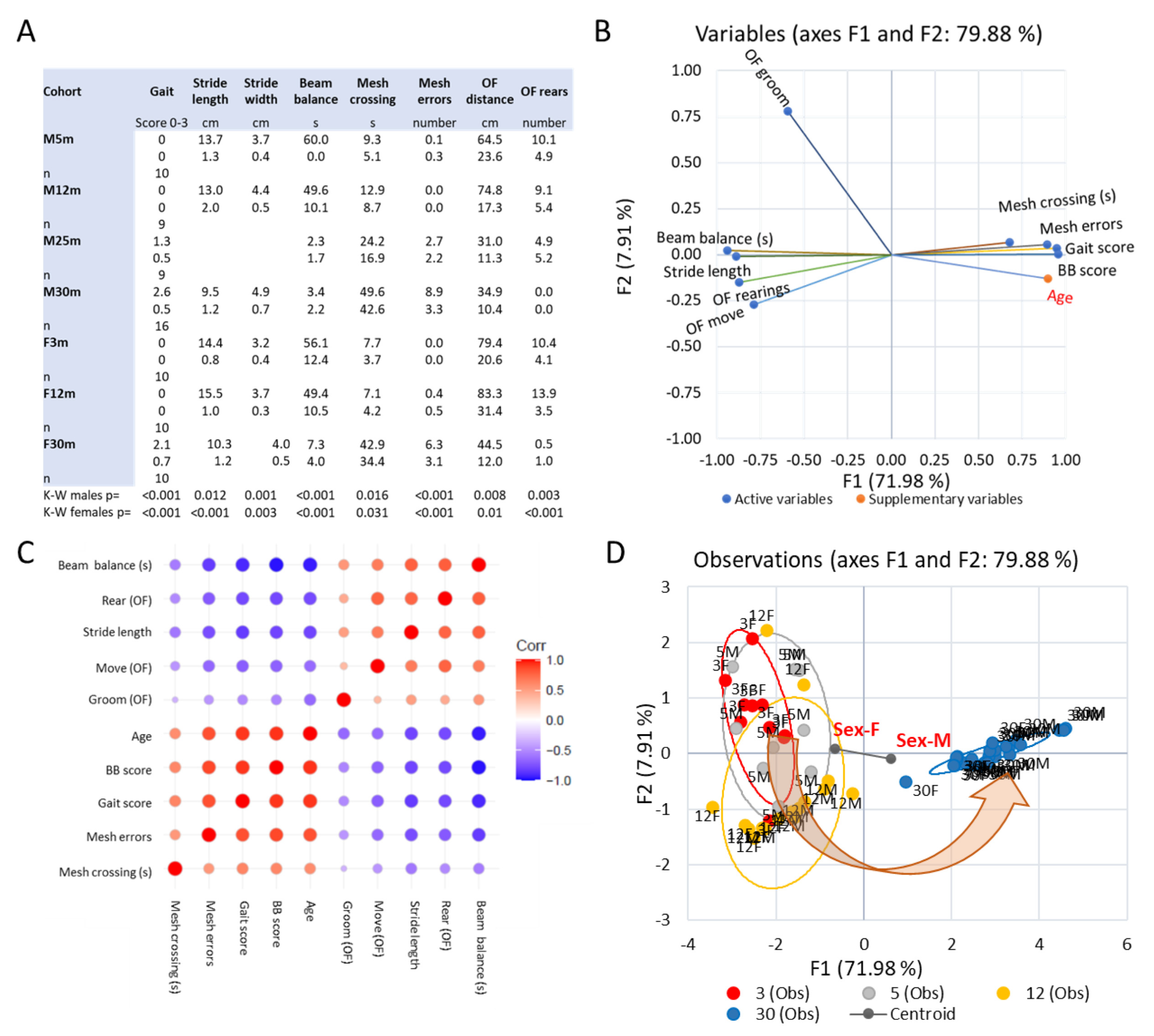
3.2. Emergence of Behavioral Motor Impairments of Aging Rats
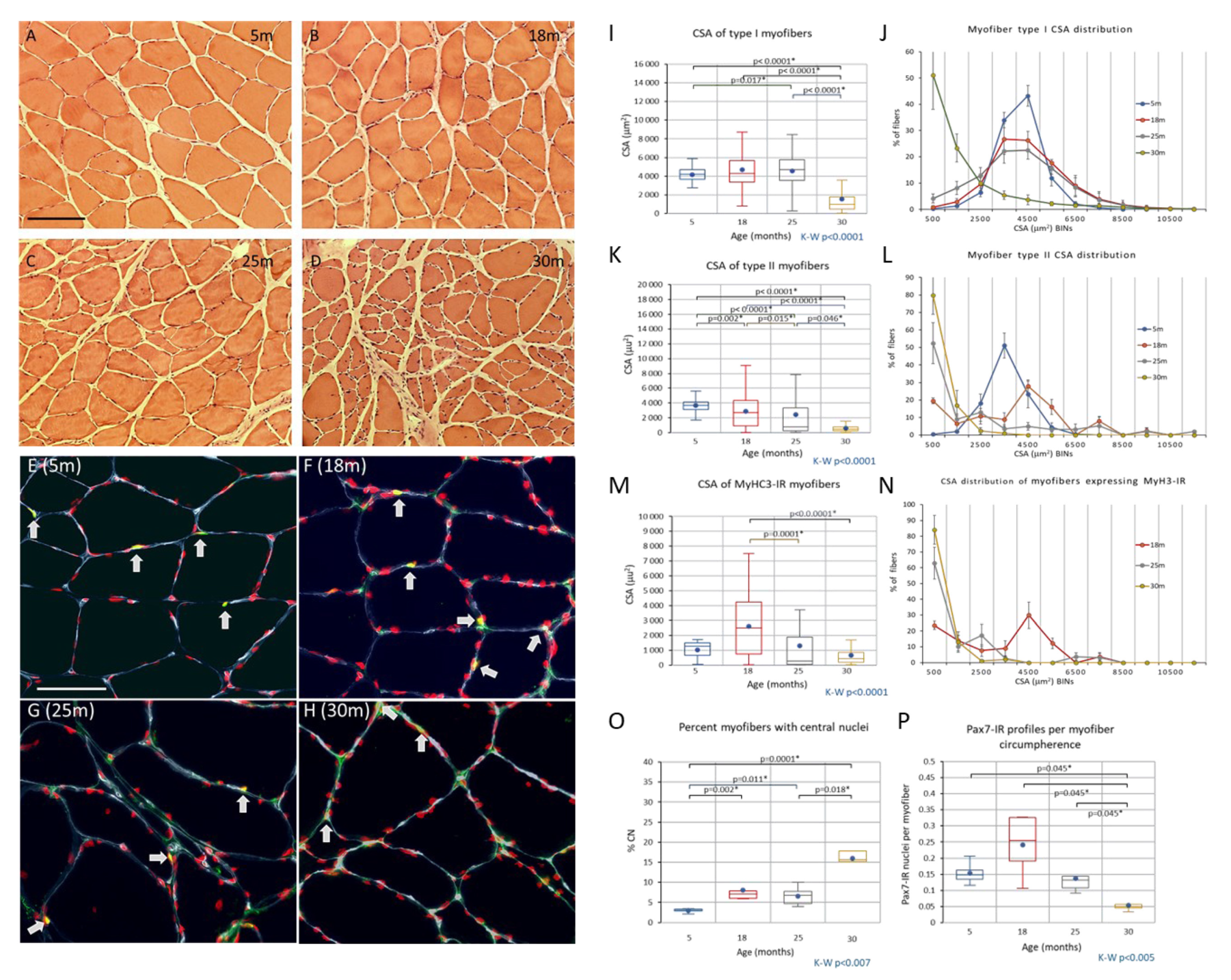
Changes to the Soleus Muscle: The Preclinical Phase
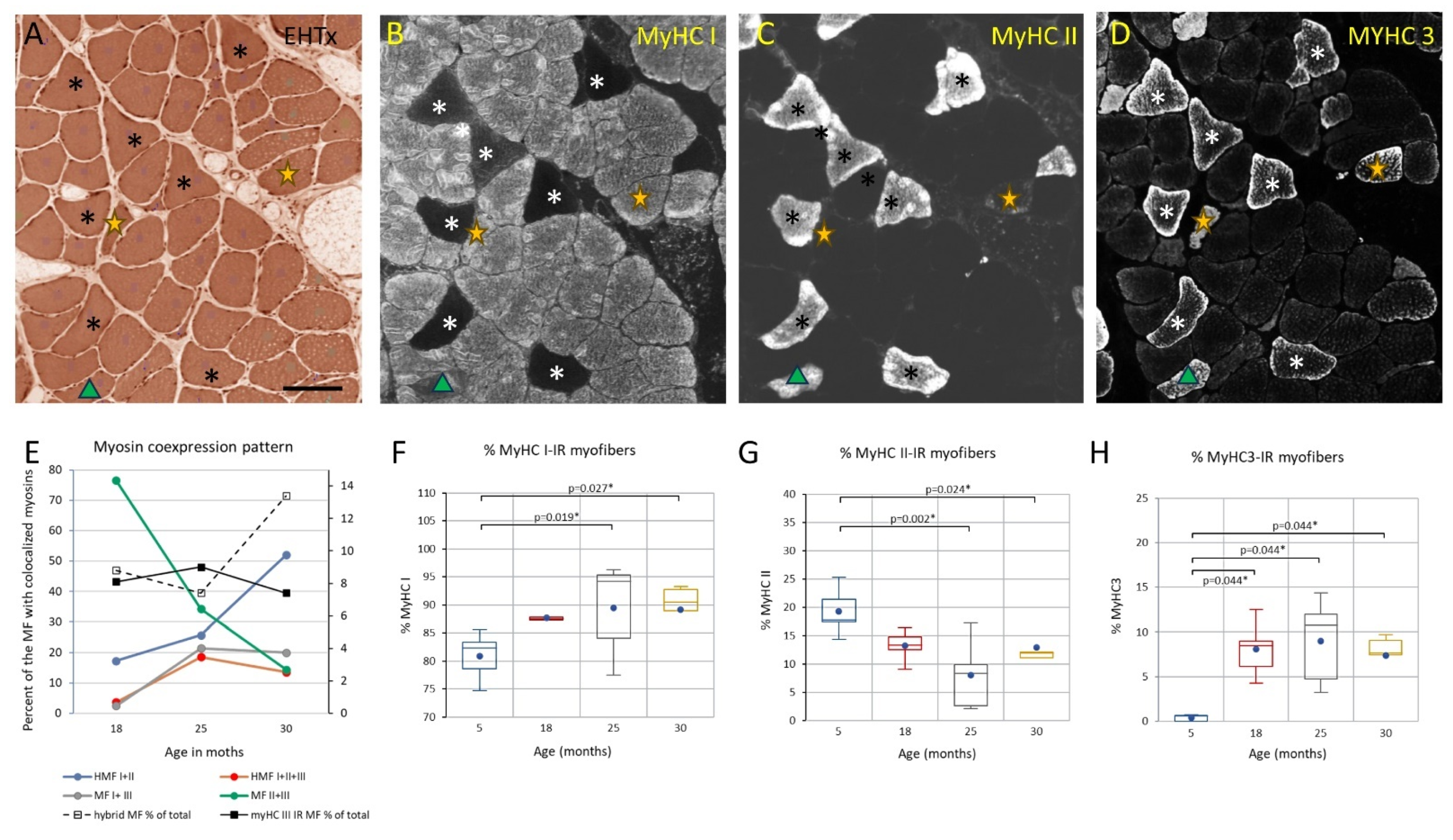
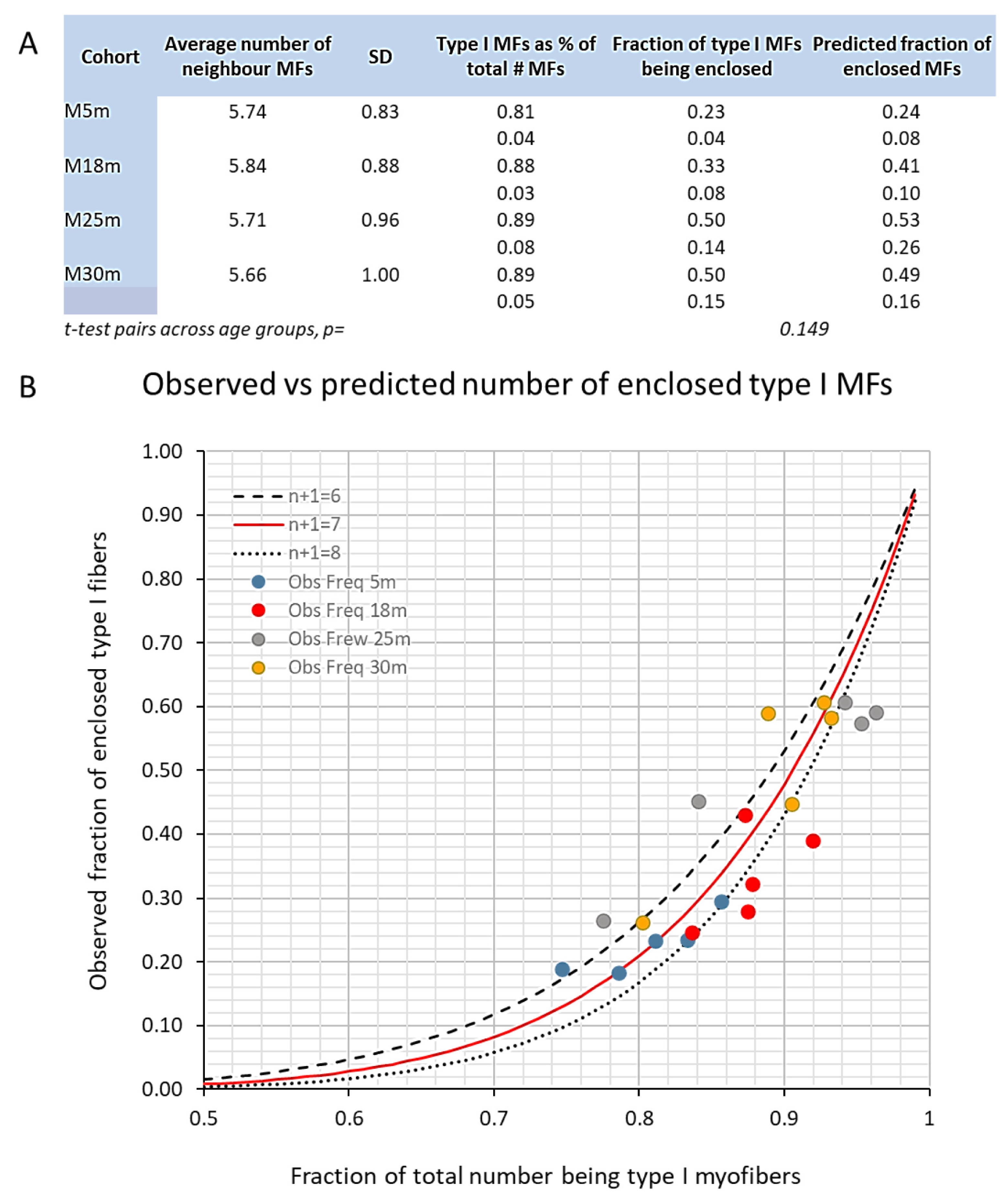
3.3. Changes to the Soleus Muscle: The Clinical Phase
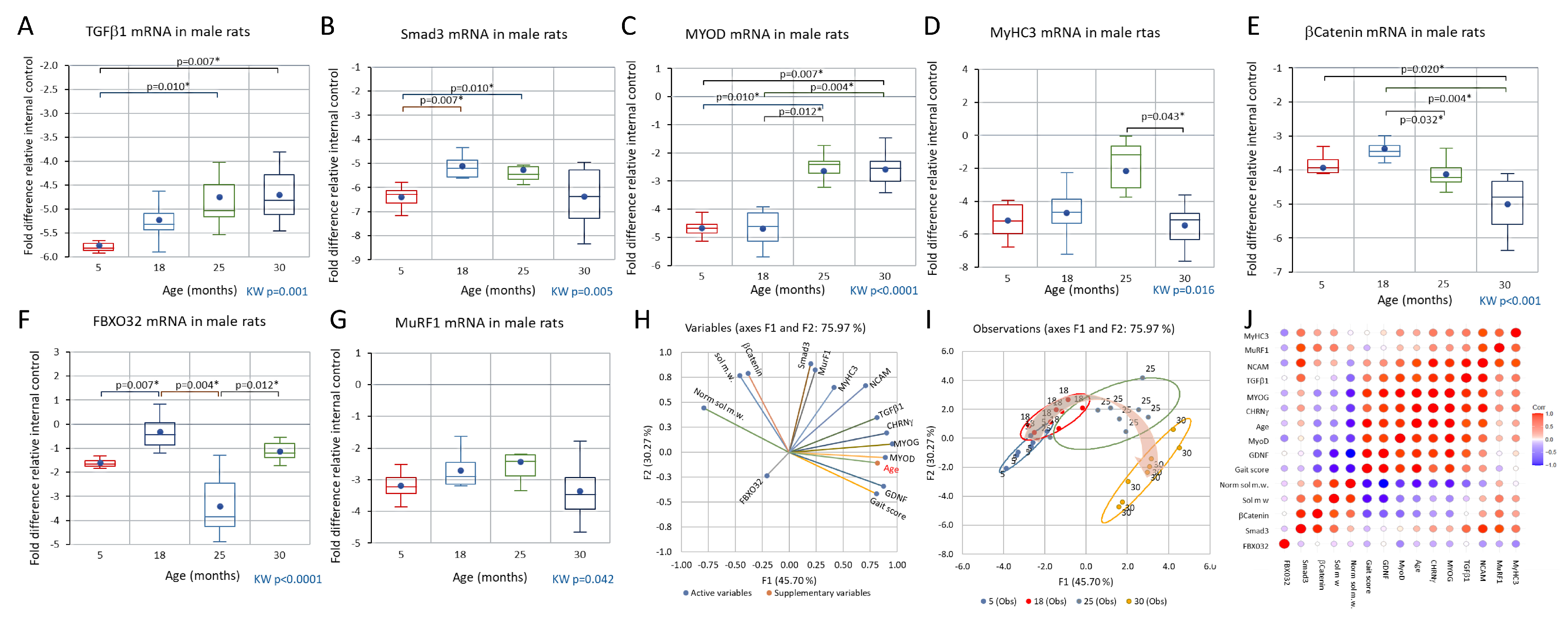
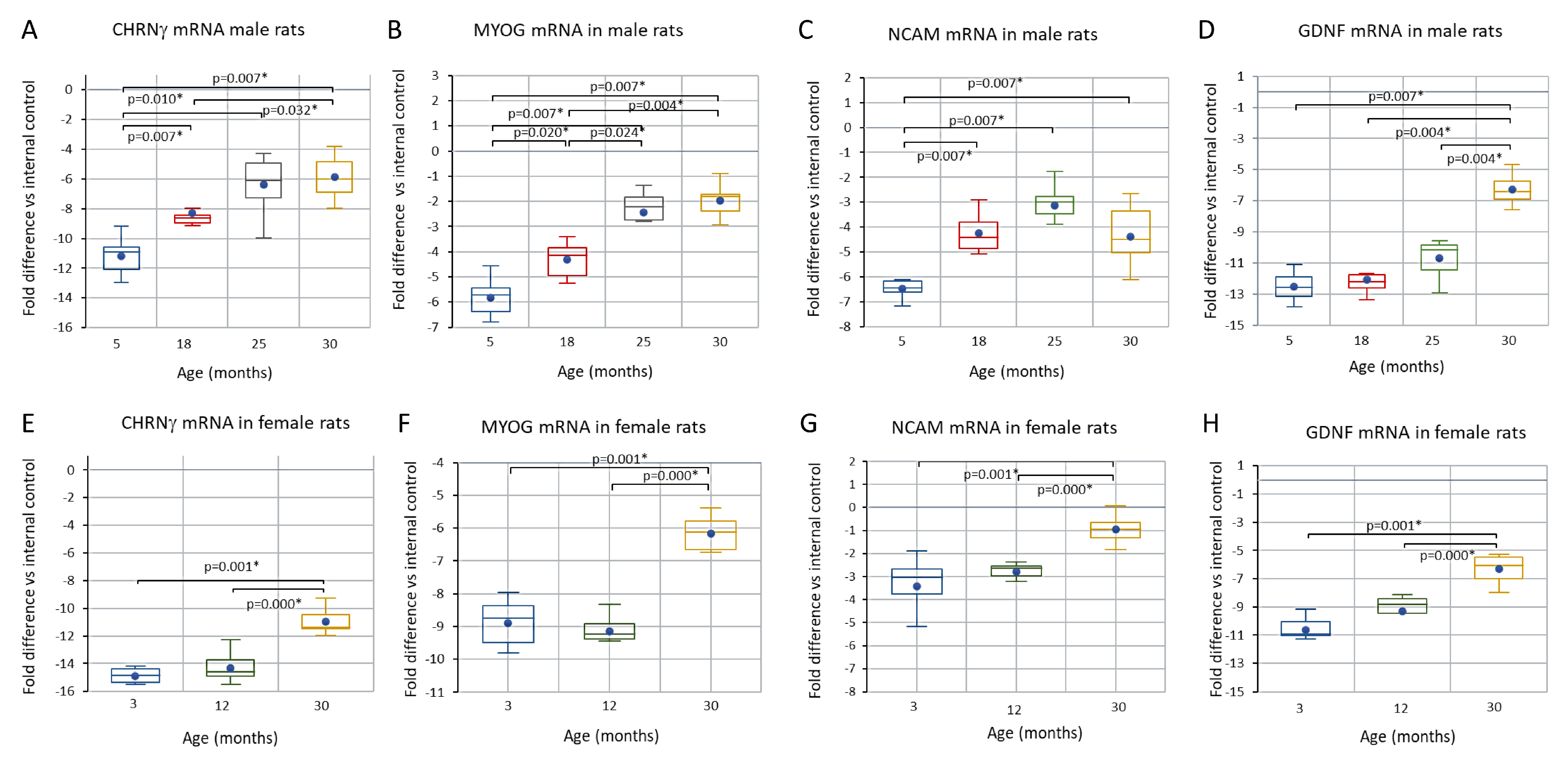
3.4. Comparison of CHRNγ, MYOG, NCAM, and GDNF mRNAs in Aging Male and Female Rats and in the Response to Axotomy in Female Rats
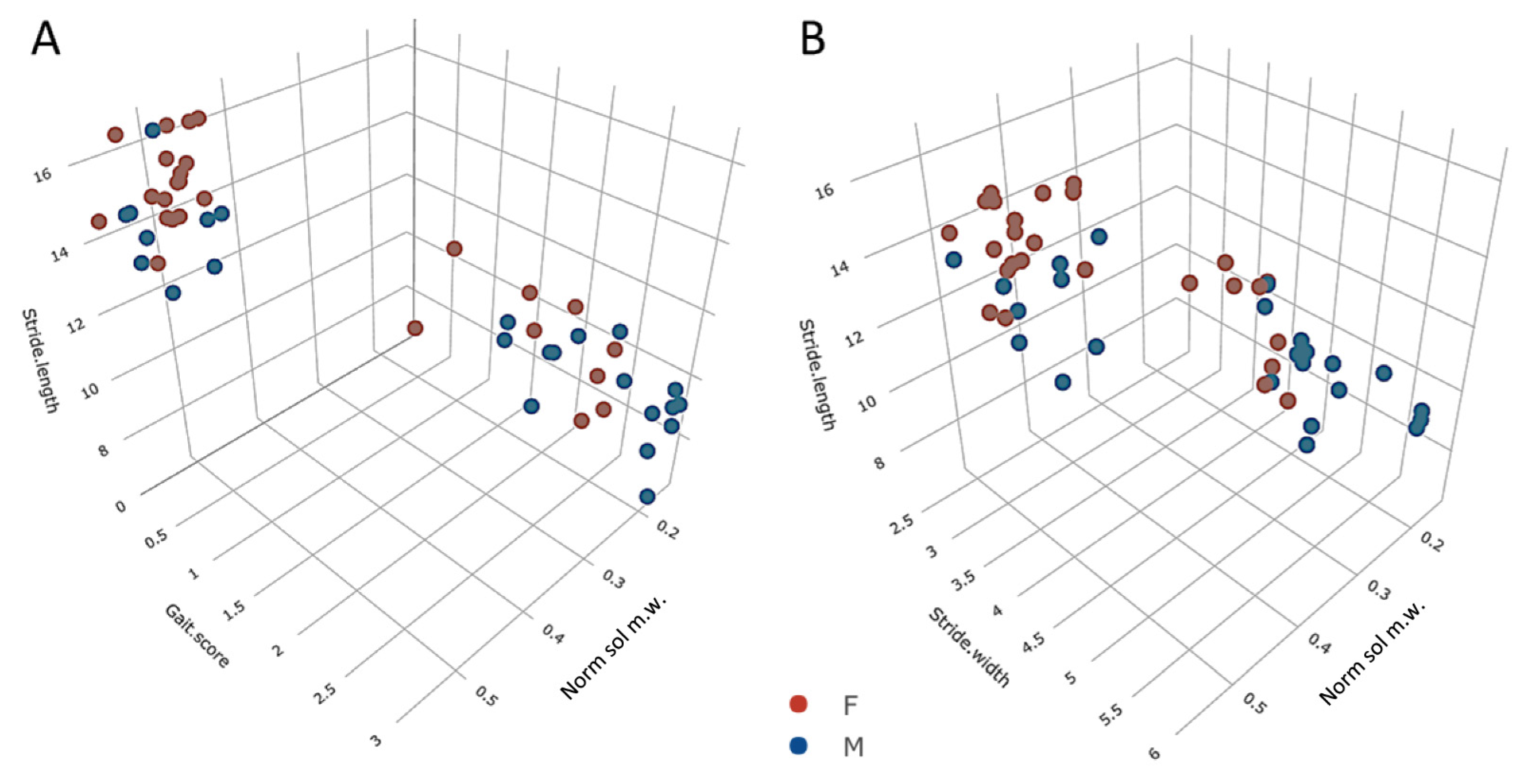
3.5. Trends across the Adult Lifespan
4. Discussion
4.1. General Comments and Limitations of This Study
4.2. Natural History of Sarcopenia in the Rat
4.2.1. Preclinical Phase of Sarcopenia
4.2.2. Clinical Phase of Sarcopenia
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. A Lifecourse Approach to Health; WHO/NMH/HPS/00.2; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH Sarcopenia Project: Rationale, Study Description, Conference Recommendations, and Final Estimates. J. Gerontol. Ser. A 2014, 69, 547–558. [Google Scholar] [CrossRef]
- Cao, L.; Morley, J.E. Sarcopenia Is Recognized as an Independent Condition by an International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) Code. J. Am. Med. Dir. Assoc. 2016, 17, 675–677. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Tieland, M.; Verdijk, L.B.; Leenders, M.; Dirks, M.L.; de Groot, L.C.; van Loon, L.J. There Are No Nonresponders to Resistance-Type Exercise Training in Older Men and Women. J. Am. Med. Dir. Assoc. 2015, 16, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, T.; Ulfhake, B. Sarcopenia: What Is the Origin of This Aging-Induced Disorder? Front. Genet. 2021, 12, 688526. [Google Scholar] [CrossRef]
- Phu, S.; Boersma, D.; Duque, G. Exercise and Sarcopenia. J. Clin. Densitom. 2015, 18, 488–492. [Google Scholar] [CrossRef]
- Mckendry, J.; Breen, L.; Shad, B.J.; Greig, C.A. Muscle morphology and performance in master athletes: A systematic review and meta-analyses. Ageing Res. Rev. 2018, 45, 62–82. [Google Scholar] [CrossRef] [PubMed]
- Kirkendall, D.T.; Garrett, W.E., Jr. The Effects of Aging and Training on Skeletal Muscle. Am. J. Sports Med. 1998, 26, 598–602. [Google Scholar] [CrossRef]
- Trappe, S. Master athletes. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, S196–S207. [Google Scholar] [CrossRef]
- Ganse, B.; Kleerekoper, A.; Knobe, M.; Hildebrand, F.; Degens, H. Longitudinal trends in master track and field performance throughout the aging process: 83,209 results from Sweden in 16 athletics disciplines. Geroscience 2020, 42, 1609–1620. [Google Scholar] [CrossRef]
- Edström, E.; Altun, M.; Bergman, E.; Johnson, H.; Kullberg, S.; Ramírez-León, V.; Ulfhake, B. Factors contributing to neuromuscular impairment and sarcopenia during aging. Physiol. Behav. 2007, 92, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Ballak, S.B.; Degens, H.; de Haan, A.; Jaspers, R.T. Aging related changes in determinants of muscle force generating capacity: A comparison of muscle aging in men and male rodents. Ageing Res. Rev. 2014, 14, 43–55. [Google Scholar] [CrossRef]
- Hepple, R.T.; Rice, C.L. Innervation and neuromuscular control in ageing skeletal muscle. J. Physiol. 2016, 594, 1965–1978. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Gutmann, E. Age Changes in the Neuromuscular System; Gutmann, E., Hanzlikova, V., Eds.; Scientechnica: Bristol, UK, 1972. [Google Scholar]
- Brown, M.; Hasser, E.M. Complexity of Age-Related Change in Skeletal Muscle. J. Gerontol. Ser. A 1996, 51, B117–B123. [Google Scholar] [CrossRef] [PubMed]
- Caccia, M.R.; Harris, J.B.; Johnson, M.A. Morphology and physiology of skeletal muscle in aging rodents. Muscle Nerve 1979, 2, 202–212. [Google Scholar] [CrossRef]
- Rosenheimer, J.L.; Smith, D.O. Differential changes in the end-plate architecture of functionally diverse muscles during aging. J. Neurophysiol. 1985, 53, 1567–1581. [Google Scholar] [CrossRef]
- Fujisawa, K. Some observations on the skeletal musculature of aged rats: Part 1. Histological aspects. J. Neurol. Sci. 1974, 22, 353–366. [Google Scholar] [CrossRef]
- Fujisawa, K. Some observations on the skeletal musculature of aged rats: Part 2. Fine morphology of diseased muscle fibres. J. Neurol. Sci. 1975, 24, 447–469. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, E.; HanzlÍKovÁ, V. Motor Unit in Old Age. Nature 1966, 209, 921–922. [Google Scholar] [CrossRef]
- Gutmann, E.; Hanzlíková, V.; Vyskočil, F. Age changes in cross striated muscle of the rat. J. Physiol. 1971, 216, 331–343. [Google Scholar] [CrossRef]
- Ansved, T.; Larsson, L. Quantitative and qualitative morphological properties of the soleus motor nerve and the L5 ventral root in young and old rats: Relation to the number of soleus muscle fibres. J. Neurol. Sci. 1990, 96, 269–282. [Google Scholar] [CrossRef]
- Larsson, L. Motor units: Remodeling in aged animals. J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50, 91–95. [Google Scholar]
- Larsson, L. Morphological and functional characteristics of the ageing skeletal muscle in man. A cross-sectional study. Acta Physiol. Scand. Suppl. 1978, 457, 1–36. [Google Scholar] [PubMed]
- Edström, E.; Ulfhake, B. Sarcopenia is not due to lack of regenerative drive in senescent skeletal muscle. Aging Cell 2005, 4, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Orssatto, L.B.R.; Borg, D.N.; Blazevich, A.J.; Sakugawa, R.L.; Shield, A.J.; Trajano, G.S. Intrinsic motoneuron excitability is reduced in soleus and tibialis anterior of older adults. Geroscience 2021, 43, 2719–2735. [Google Scholar] [CrossRef] [PubMed]
- Padilla, C.J.; Harrigan, M.E.; Harris, H.; Schwab, J.M.; Rutkove, S.B.; Rich, M.M.; Clark, B.C.; Arnold, W.D. Profiling age-related muscle weakness and wasting: Neuromuscular junction transmission as a driver of age-related physical decline. Geroscience 2021, 43, 1265–1281. [Google Scholar] [CrossRef]
- Aagaard, P.; Suetta, C.; Caserotti, P.; Magnusson, S.P.; Kjaer, M. Role of the nervous system in sarcopenia and muscle atrophy with aging: Strength training as a countermeasure. Scand. J. Med. Sci. Sports 2010, 20, 49–64. [Google Scholar] [CrossRef]
- Rudrappa, S.S.; Wilkinson, D.J.; Greenhaff, P.L.; Smith, K.; Idris, I.; Atherton, P.J. Human Skeletal Muscle Disuse Atrophy: Effects on Muscle Protein Synthesis, Breakdown, and Insulin Resistance—A Qualitative Review. Front. Physiol. 2016, 7, 361. [Google Scholar] [CrossRef] [PubMed]
- Tomonaga, M. Histochemical and Ultrastructural Changes in Senile Human Skeletal Muscle. J. Am. Geriatr. Soc. 1977, 25, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Jennekens, F.; Tomlinson, B.; Walton, J. Histochemical aspects of five limb muscles in old age an autopsy study. J. Neurol. Sci. 1971, 14, 259–276. [Google Scholar] [CrossRef]
- Oertel, G. Changes in human skeletal muscles due to ageing. Acta Neuropathol. 1986, 69, 309–313. [Google Scholar] [CrossRef]
- Scelsi, R.; Marchetti, C.; Poggi, P. Histochemical and ultrastructural aspects of m. vastus lateralis in sedentary old people (age 65–89 years). Acta Neuropathol. 1980, 51, 99–105. [Google Scholar] [CrossRef]
- Lexell, J.; Downham, D. What is the effect of ageing on type 2 muscle fibres? J. Neurol. Sci. 1992, 107, 250–251. [Google Scholar] [CrossRef]
- Lexell, J.; Downham, D.; Sjöström, M. Distribution of different fibre types in human skeletal muscles: A statistical and computational model for the study of fibre type grouping and early diagnosis of skeletal muscle fibre denervation and reinnervation. J. Neurol. Sci. 1983, 61, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Lexell, J.; Henriksson-Larsén, K.; Winblad, B.; Sjöström, M. Distribution of different fiber types in human skeletal muscles: Effects of aging studied in whole muscle cross sections. Muscle Nerve 1983, 6, 588–595. [Google Scholar] [CrossRef]
- Lexell, J.; Taylor, C.C.; Sjöström, M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J. Neurol. Sci. 1988, 84, 275–294. [Google Scholar] [CrossRef]
- Venturelli, M.; Reggiani, C.; Richardson, R.S.; Schena, F. Skeletal Muscle Function in the Oldest-Old: The Role of Intrinsic and Extrinsic Factors. Exerc. Sport Sci. Rev. 2018, 46, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Rowan, S.L.; Purves-Smith, F.M.; Solbak, N.M.; Hepple, R.T. Accumulation of severely atrophic myofibers marks the acceleration of sarcopenia in slow and fast twitch muscles. Exp. Gerontol. 2011, 46, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Kadi, F.; Charifi, N.; Denis, C.; Lexell, J. Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve 2004, 29, 120–127. [Google Scholar] [CrossRef]
- Franco, I.; Fernandez-Gonzalo, R.; Vrtačnik, P.; Lundberg, T.R.; Eriksson, M.; Gustafsson, T. Healthy skeletal muscle aging: The role of satellite cells, somatic mutations and exercise. Int. Rev. Cell Mol. Biol. 2019, 346, 157–200. [Google Scholar] [PubMed]
- Ling, Z.; Liu, X.; Cheng, Y.; Yan, X.; Wu, S. Gut microbiota and aging. Crit. Rev. Food Sci. Nutr. 2020, 1–56. [Google Scholar] [CrossRef] [PubMed]
- Baran, S.W.; Lim, M.A.; Do, J.P.; Stolyar, P.; Rabe, M.D.; Schaevitz, L.R.; Cadena, S.M. Digital Biomarkers Enable Automated, Longitudinal Monitoring in a Mouse Model of Aging. J. Gerontol. Ser. A 2021, 76, 1206–1213. [Google Scholar] [CrossRef]
- Pernold, K.; Rullman, E.; Ulfhake, B. Major oscillations in spontaneous home-cage activity in C57BL/6 mice housed under constant conditions. Sci. Rep. 2021, 11, 4961. [Google Scholar] [CrossRef] [PubMed]
- Dohnalová, L.; Lundgren, P.; Carty, J.R.E.; Goldstein, N.; Wenski, S.L.; Nanudorn, P.; Thiengmag, S.; Huang, K.-P.; Litichevskiy, L.; Descamps, H.C.; et al. A microbiome-dependent gut–brain pathway regulates motivation for exercise. Nature 2022, 612, 739–747. [Google Scholar] [CrossRef]
- Taetzsch, T.; Valdez, G. NMJ maintenance and repair in aging. Curr. Opin. Physiol. 2018, 4, 57–64. [Google Scholar] [CrossRef]
- Johnson, H.; Mossberg, K.; Arvidsson, U.; Piehl, F.; Hökfelt, T.; Ulfhake, B. Increase in α-CGRP and GAP-43 in aged motoneurons: A study of peptides, growth factors, and ChAT mRNA in the lumbar spinal cord of senescent rats with symptoms of hindlimb incapacities. J. Comp. Neurol. 1995, 359, 69–89. [Google Scholar] [CrossRef]
- Graber, T.G.; Kim, J.-H.; Grange, R.W.; McLoon, L.K.; Thompson, L.V. C57BL/6 life span study: Age-related declines in muscle power production and contractile velocity. Age 2015, 37, 9773. [Google Scholar] [CrossRef]
- Power, G.A.; Allen, M.D.; Gilmore, K.J.; Stashuk, D.W.; Doherty, T.J.; Hepple, R.T.; Taivassalo, T.; Rice, C.L. Motor unit number and transmission stability in octogenarian world class athletes: Can age-related deficits be outrun? J. Appl. Physiol. 2016, 121, 1013–1020. [Google Scholar] [CrossRef]
- Aare, S.; Spendiff, S.; Vuda, M.; Elkrief, D.; Perez, A.; Wu, Q.; Mayaki, D.; Hussain, S.N.A.; Hettwer, S.; Hepple, R.T. Failed reinnervation in aging skeletal muscle. Skelet. Muscle 2016, 6, 29. [Google Scholar] [CrossRef]
- Piasecki, M.; Ireland, A.; Jones, D.A.; McPhee, J.S. Age-dependent motor unit remodelling in human limb muscles. Biogerontology 2016, 17, 485–496. [Google Scholar] [CrossRef]
- Piasecki, M.; Ireland, A.; Coulson, J.; Stashuk, D.W.; Hamilton-Wright, A.; Swiecicka, A.; Rutter, M.K.; McPhee, J.S.; Jones, D.A. Motor unit number estimates and neuromuscular transmission in the tibialis anterior of master athletes: Evidence that athletic older people are not spared from age-related motor unit remodeling. Physiol. Rep. 2016, 4, e12987. [Google Scholar] [CrossRef] [PubMed]
- Piasecki, M.; Ireland, A.; Piasecki, J.; Stashuk, D.W.; Swiecicka, A.; Rutter, M.K.; Jones, D.A.; McPhee, J.S. Failure to expand the motor unit size to compensate for declining motor unit numbers distinguishes sarcopenic from non-sarcopenic older men. J. Physiol. 2018, 596, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Nunes, E.A.; Stokes, T.; McKendry, J.; Currier, B.S.; Phillips, S.M. Disuse-induced skeletal muscle atrophy in disease and nondisease states in humans: Mechanisms, prevention, and recovery strategies. Am. J. Physiol. Physiol. 2022, 322, C1068–C1084. [Google Scholar] [CrossRef]
- Moiseeva, V.; Cisneros, A.; Sica, V.; Deryagin, O.; Lai, Y.; Jung, S.; Andrés, E.; An, J.; Segalés, J.; Ortet, L.; et al. Senescence atlas reveals an aged-like inflamed niche that blunts muscle regeneration. Nature 2022, 613, 169–178. [Google Scholar] [CrossRef]
- Hunt, L.C.; Graca, F.A.; Pagala, V.; Wang, Y.-D.; Li, Y.; Yuan, Z.-F.; Fan, Y.; Labelle, M.; Peng, J.; Demontis, F. Integrated genomic and proteomic analyses identify stimulus-dependent molecular changes associated with distinct modes of skeletal muscle atrophy. Cell Rep. 2021, 37, 109971. [Google Scholar] [CrossRef] [PubMed]
- Tintignac, L.A.; Brenner, H.-R.; Rüegg, M.A. Mechanisms Regulating Neuromuscular Junction Development and Function and Causes of Muscle Wasting. Physiol. Rev. 2015, 95, 809–852. [Google Scholar] [CrossRef] [PubMed]
- Pannerec, A.; Springer, M.; Migliavacca, E.; Ireland, A.; Piasecki, M.; Karaz, S.; Jacot, G.; Metairon, S.; Danenberg, E.; Raymond, F.; et al. A robust neuromuscular system protects rat and human skeletal muscle from sarcopenia. Aging 2016, 8, 712–729. [Google Scholar] [CrossRef]
- Altun, M.; Besche, H.C.; Overkleeft, H.S.; Piccirillo, R.; Edelmann, M.J.; Kessler, B.M.; Goldberg, A.L.; Ulfhake, B. Muscle Wasting in Aged, Sarcopenic Rats Is Associated with Enhanced Activity of the Ubiquitin Proteasome Pathway. J. Biol. Chem. 2010, 285, 39597–39608. [Google Scholar] [CrossRef]
- Edstrom, E.; Altun, M.; Hagglund, M.; Ulfhake, B. Atrogin-1/MAFbx and MuRF1 are downregulated in aging-related loss of skeletal muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Grönholdt-Klein, M.; Altun, M.; Becklén, M.; Kahm, E.D.; Fahlström, A.; Rullman, E.; Ulfhake, B. Muscle atrophy and regeneration associated with behavioural loss and recovery of function after sciatic nerve crush. Acta Physiol. 2019, 227, e13335. [Google Scholar] [CrossRef]
- Skoglund, E.; Grönholdt-Klein, M.; Rullman, E.; Thornell, L.E.; Strömberg, A.; Hedman, A.; Cederholm, T.; Ulfhake, B.; Gustafsson, T. Longitudinal Muscle and Myocellular Changes in Community-Dwelling Men Over Two Decades of Successful Aging—The ULSAM Cohort Revisited. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2019, 75, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Hennig, R.; Lømo, T. Firing patterns of motor units in normal rats. Nature 1985, 314, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Altun, M.; Bergman, E.; Edström, E.; Johnson, H.; Ulfhake, B. Behavioral impairments of the aging rat. Physiol. Behav. 2007, 92, 911–923. [Google Scholar] [CrossRef]
- Jennekens, F.; Tomlinson, B.; Walton, J. Data on the distribution of fibre types in five human limb muscles An autopsy study. J. Neurol. Sci. 1971, 14, 245–257. [Google Scholar] [CrossRef]
- Li, L.T.; Jiang, G.; Chen, Q.; Zheng, J.N. Ki67 is a promising molecular target in the diagnosis of cancer (Review). Mol. Med. Rep. 2015, 11, 1566–1572. [Google Scholar] [CrossRef]
- Altun, M.; Edström, E.; Spooner, E.; Flores-Moralez, A.; Bergman, E.; Tollet-Egnell, P.; Norstedt, G.; Kessler, B.M.; Ulfhake, B. Iron load and redox stress in skeletal muscle of aged rats. Muscle Nerve 2007, 36, 223–233. [Google Scholar] [CrossRef]
- Ishihara, A.; Itoh, K.; Itoh, M.; Hirofuji, C.; Hayashi, H. Effects of hypophysectomy on soleus muscle fibers and spinal motoneurons in rats. Acta Neuropathol. 1995, 89, 204–208. [Google Scholar] [CrossRef]
- Fahlström, A.; Zeberg, H.; Ulfhake, B. Changes in behaviors of male C57BL/6J mice across adult life span and effects of dietary restriction. Age 2011, 34, 1435–1452. [Google Scholar] [CrossRef] [PubMed]
- Bair, W.-N.; Petr, M.; Alfaras, I.; Mitchell, S.J.; Bernier, M.; Ferrucci, L.; Studenski, S.A.; De Cabo, R. Of Aging Mice and Men: Gait Speed Decline Is a Translatable Trait, With Species-Specific Underlying Properties. J. Gerontol. Ser. A 2019, 74, 1413–1416. [Google Scholar] [CrossRef]
- Egerman, M.A.; Glass, D.J. Signaling pathways controlling skeletal muscle mass. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 59–68. [Google Scholar] [CrossRef]
- Ibebunjo, C.; Chick, J.M.; Kendall, T.; Eash, J.K.; Li, C.; Zhang, Y.; Vickers, C.; Wu, Z.; Clarke, B.A.; Shi, J.; et al. Genomic and Proteomic Profiling Reveals Reduced Mitochondrial Function and Disruption of the Neuromuscular Junction Driving Rat Sarcopenia. Mol. Cell Biol. 2013, 33, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Broom, L.; Stephen, J.; Nayar, V.; VanderHorst, V.G. Shifts in Gait Signatures Mark the End of Lifespan in Mice, with Sex Differences in Timing. Front. Aging Neurosci. 2021, 13, 716993. [Google Scholar] [CrossRef] [PubMed]
- Wigston, D.J.; English, A.W. Fiber-type proportions in mammalian soleus muscle during postnatal development. J. Neurobiol. 1992, 23, 61–70. [Google Scholar] [CrossRef]
- Naruse, M.; Trappe, S.; Trappe, T.A. Human skeletal muscle-specific atrophy with aging: A comprehensive review. J. Appl. Physiol. 2023, 134, 900–914. [Google Scholar] [CrossRef]
- Schiaffino, S.; Rossi, A.C.; Smerdu, V.; Leinwand, L.A.; Reggiani, C. Developmental myosins: Expression patterns and functional significance. Skelet. Muscle 2015, 5, 22. [Google Scholar] [CrossRef]
- Soendenbroe, C.; Andersen, J.L.; Mackey, A.L. Muscle-nerve communication and the molecular assessment of human skeletal muscle denervation with aging. Am. J. Physiol. Physiol. 2021, 321, C317–C329. [Google Scholar] [CrossRef]
- Hendrickse, P.; Galinska, M.; Hodson-Tole, E.; Degens, H. An evaluation of common markers of muscle denervation in denervated young-adult and old rat gastrocnemius muscle. Exp. Gerontol. 2018, 106, 159–164. [Google Scholar] [CrossRef]
- Cashman, N.R.; Covault, J.; Wollman, R.L.; Sanes, J.R. Neural cell adhesion molecule in normal, denervated, and myopathic human muscle. Ann. Neurol. 1987, 21, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Missias, A.C.; Chu, G.C.; Klocke, B.J.; Sanes, J.R.; Merlie, J.P. Maturation of the acetylcholine receptor in skeletal muscle: Regulation of the AChR gamma-to-epsilon switch. Dev. Biol. 1996, 179, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Sarto, F.; Stashuk, D.W.; Franchi, M.V.; Monti, E.; Zampieri, S.; Valli, G.; Sirago, G.; Candia, J.; Hartnell, L.M.; Paganini, M.; et al. Effects of short-term unloading and active recovery on human motor unit properties, neuromuscular junction transmission and transcriptomic profile. J. Physiol. 2022, 600, 4731–4751. [Google Scholar] [CrossRef]
- Tesch, P.A.; Lundberg, T.R.; Alkner, B.A.; Rullman, E.; Gustafsson, T.; Fernandez-Gonzalo, R. Three months of bed rest induce a residual transcriptomic signature resilient to resistance exercise countermeasures. FASEB J. 2020, 34, 7958–7969. [Google Scholar]
- Stocksley, M.A.; Awad, S.S.; Young, C.; Lightowlers, R.N.; Brenner, H.-R.; Slater, C.R. Accumulation of NaV1 mRNAs at differentiating postsynaptic sites in rat soleus muscles. Mol. Cell Neurosci. 2005, 28, 694–702. [Google Scholar] [CrossRef]
- Rowan, S.L.; Rygiel, K.; Purves-Smith, F.M.; Solbak, N.M.; Turnbull, D.M.; Hepple, R.T. Denervation Causes Fiber Atrophy and Myosin Heavy Chain Co-Expression in Senescent Skeletal Muscle. PLoS ONE 2012, 7, e29082. [Google Scholar] [CrossRef]
- Rannou, F.; Pennec, J.-P.; Morel, J.; Guéret, G.; Leschiera, R.; Droguet, M.; Gioux, M.; Giroux-Metges, M.-A. Nav1.4 and Nav1.5 are modulated differently during muscle immobilization and contractile phenotype conversion. J. Appl. Physiol. 2011, 111, 495–507. [Google Scholar] [CrossRef]
- Desaphy, J.-F.; Pierno, S.; Léoty, C.; George, A.L.; De Luca, A.; Camerino, D.C. Skeletal muscle disuse induces fibre type-dependent enhancement of Na+ channel expression. Brain 2001, 124, 1100–1113. [Google Scholar] [CrossRef] [PubMed]
- Plikus, M.V.; Wang, X.; Sinha, S.; Forte, E.; Thompson, S.M.; Herzog, E.L.; Driskell, R.R.; Rosenthal, N.; Biernaskie, J.; Horsley, V. Fibroblasts: Origins, definitions, and functions in health and disease. Cell 2021, 184, 3852–3872. [Google Scholar] [CrossRef]
- Chapman, M.A.; Meza, R.; Lieber, R.L. Skeletal muscle fibroblasts in health and disease. Differentiation 2016, 92, 108–115. [Google Scholar] [CrossRef]
- McKee, T.J.; Perlman, G.; Morris, M.; Komarova, S.V. Extracellular matrix composition of connective tissues: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 10542. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sathe, A.A.; Smith, G.R.; Ruf-Zamojski, F.; Nair, V.; Lavine, K.J.; Xing, C.; Sealfon, S.C.; Zhou, L. Heterogeneous origins and functions of mouse skeletal muscle-resident macrophages. Proc. Natl. Acad. Sci. USA 2020, 117, 20729–20740. [Google Scholar] [CrossRef] [PubMed]
- Buvinic, S.; Balanta-Melo, J.; Kupczik, K.; Vásquez, W.; Beato, C.; Toro-Ibacache, V. Muscle-Bone Crosstalk in the Masticatory System: From Biomechanical to Molecular Interactions. Front. Endocrinol. 2020, 11, 606947. [Google Scholar] [CrossRef] [PubMed]
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, G. Muscle, Bone, and Fat Crosstalk: The Biological Role of Myokines, Osteokines, and Adipokines. Curr. Osteoporos. Rep. 2020, 18, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Oishi, Y.; Ogata, T.; Yamamoto, K.-I.; Terada, M.; Ohira, T.; Ohira, Y.; Taniguchi, K.; Roy, R.R. Cellular adaptations in soleus muscle during recovery after hindlimb unloading. Acta Physiol. 2008, 192, 381–395. [Google Scholar] [CrossRef]
- Buller, A.J.; Eccles, J.C.; Eccles, R.M. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J. Physiol. 1960, 150, 417–439. [Google Scholar] [CrossRef]
- Lexell, J.; Downham, D.Y. The occurrence of fibre-type grouping in healthy human muscle: A quantitative study of cross-sections of whole vastus lateralis from men between 15 and 83 years. Acta Neuropathol. 1991, 81, 377–381. [Google Scholar] [CrossRef]
- Lexell, J.; Downham, D.; Sjöström, M. Distribution of different fibre types in human skeletal muscles: Fibre type arrangement in m. vastus lateralis from three groups of healthy men between 15 and 83 years. J. Neurol. Sci. 1986, 72, 211–222. [Google Scholar] [CrossRef]
- Messa, G.A.M.; Piasecki, M.; Rittweger, J.; McPhee, J.S.; Koltai, E.; Radak, Z.; Simunic, B.; Heinonen, A.; Suominen, H.; Korhonen, M.T.; et al. Absence of an aging-related increase in fiber type grouping in athletes and non-athletes. Scand. J. Med. Sci. Sports 2020, 30, 2057–2069. [Google Scholar] [CrossRef]
- Baur, J.A.; Chen, D.; Chini, E.N.; Chua, K.; Cohen, H.Y.; de Cabo, R.; Deng, C.; Dimmeler, S.; Gius, D.; Guarente, L.P.; et al. Dietary restriction: Standing up for sirtuins. Science 2010, 329, 1012–1013. [Google Scholar] [CrossRef]
- Desaphy, J.-F.; Pierno, S.; Liantonio, A.; De Luca, A.; Didonna, M.P.; Frigeri, A.; Nicchia, G.P.; Svelto, M.; Camerino, C.; Zallone, A.; et al. Recovery of the soleus muscle after short- and long-term disuse induced by hindlimb unloading: Effects on the electrical properties and myosin heavy chain profile. Neurobiol. Dis. 2005, 18, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Kelly, N.A.; Hammond, K.G.; Stec, M.J.; Bickel, C.S.; Windham, S.T.; Tuggle, S.C.; Bamman, M.M. Quantification and characterization of grouped type I myofibers in human aging. Muscle Nerve 2018, 57, E52–E59. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yao, Z.; Sarkar, D.; Lawrence, M.; Sanchez, G.J.; Parker, M.H.; MacQuarrie, K.L.; Davison, J.; Morgan, M.T.; Ruzzo, W.L.; et al. Genome-wide MyoD Binding in Skeletal Muscle Cells: A Potential for Broad Cellular Reprogramming. Dev. Cell 2010, 18, 662–674. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Kumar, A.; Makonchuk, D.Y.; Li, H.; Kumar, A. Transforming growth factor-beta-activated kinase 1 is an essential regulator of myogenic differentiation. J. Biol. Chem. 2010, 285, 6401–6411. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, J.L.; Lu, J.; Song, Y.; Kwak, K.S.; Jiao, Q.; Rosenfeld, R.; Chen, Q.; Boone, T.; Simonet, W.S.; et al. Reversal of Cancer Cachexia and Muscle Wasting by ActRIIB Antagonism Leads to Prolonged Survival. Cell 2010, 142, 531–543. [Google Scholar] [CrossRef]
- Zhao, J.; Brault, J.J.; Schild, A.; Goldberg, A.L. Coordinate activation of autophagy and the proteasome pathway by FoxO transcription factor. Autophagy 2008, 4, 378–380. [Google Scholar] [CrossRef]
- Sacheck, J.M.; Hyatt, J.-P.K.; Raffaello, A.; Jagoe, R.T.; Roy, R.R.; Edgerton, V.R.; Lecker, S.H.; Goldberg, A.L. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J. 2007, 21, 140–155. [Google Scholar] [CrossRef]
- Lecker, S.H.; Jagoe, R.T.; Gilbert, A.; Gomes, M.; Baracos, V.; Bailey, J.; Price, S.R.; Mitch, W.E.; Goldberg, A.L. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004, 18, 39–51. [Google Scholar] [CrossRef]
- Clarke, B.A.; Drujan, D.; Willis, M.S.; Murphy, L.O.; Corpina, R.A.; Burova, E.; Rakhilin, S.V.; Stitt, T.N.; Patterson, C.; Latres, E.; et al. The E3 Ligase MuRF1 Degrades Myosin Heavy Chain Protein in Dexamethasone-Treated Skeletal Muscle. Cell Metab. 2007, 6, 376–385. [Google Scholar] [CrossRef]
- Glass, D.J. Skeletal muscle hypertrophy and atrophy signaling pathways. Int. J. Biochem. Cell Biol. 2005, 37, 1974–1984. [Google Scholar] [CrossRef]
- Chen, H.-H.; Tsai, L.-K.; Liao, K.-Y.; Wu, T.-C.; Huang, Y.-H.; Huang, Y.-C.; Chang, S.-W.; Wang, P.-Y.; Tsao, Y.-P.; Chen, S.-L. Muscle-restricted nuclear receptor interaction protein knockout causes motor neuron degeneration through down-regulation of myogenin at the neuromuscular junction. J. Cachex- Sarcopenia Muscle 2018, 9, 771–785. [Google Scholar] [CrossRef] [PubMed]
- McNeil, C.J.; Doherty, T.J.; Stashuk, D.W.; Rice, C.L. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve 2005, 31, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Kawabuchi, M.; Tan, H.; Wang, S. Age affects reciprocal cellular interactions in neuromuscular synapses following peripheral nerve injury. Ageing Res. Rev. 2011, 10, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Kawabuchi, M.; Chongjian, Z.; Nakamura, K.; Hirata, K. Morphological features of collateral innervation and supernumerary innervation in the skeletal muscles of presenile rats. Anat. Anz. 1995, 177, 251–265. [Google Scholar] [CrossRef]
- Kawabuchi, M.; Zhou, C.J.; Wang, S.; Nakamura, K.; Liu, W.T.; Hirata, K. The spatiotemporal relationship among schwann cells, axons and postsynaptic acetylcholine receptor regions during muscle reinnervation in aged rats. Anat. Rec. 2001, 264, 183–202. [Google Scholar] [CrossRef]
- Pestronk, A.; Drachman, D.B.; Griffin, J.W. Effects of aging on nerve sprouting and regeneration. Exp. Neurol. 1980, 70, 65–82. [Google Scholar] [CrossRef]
- Klöppel, G.; Perren, A.; Heitz, P.U. The Gastroenteropancreatic Neuroendocrine Cell System and Its Tumors: The WHO Classification. Ann. New York Acad. Sci. 2004, 1014, 13–27. [Google Scholar] [CrossRef]
- Macpherson, P.C.D.; Wang, X.; Goldman, D. Myogenin regulates denervation-dependent muscle atrophy in mouse soleus muscle. J. Cell Biochem. 2011, 112, 2149–2159. [Google Scholar] [CrossRef]
- Moresi, V.; Williams, A.H.; Meadows, E.; Flynn, J.M.; Potthoff, M.J.; McAnally, J.; Shelton, J.M.; Backs, J.; Klein, W.H.; Richardson, J.A.; et al. Myogenin and Class II HDACs Control Neurogenic Muscle Atrophy by Inducing E3 Ubiquitin Ligases. Cell 2010, 143, 35–45. [Google Scholar] [CrossRef]
- Hu, P.; Geles, K.G.; Paik, J.H.; DePinho, R.A.; Tjian, R. Codependent activators direct myoblast-specific MyoD transcription. Dev. Cell 2008, 15, 534–546. [Google Scholar] [CrossRef]
- Wiberg, R.; Jonsson, S.; Novikova, L.N.; Kingham, P.J. Investigation of the Expression of Myogenic Transcription Factors, microRNAs and Muscle-Specific E3 Ubiquitin Ligases in the Medial Gastrocnemius and Soleus Muscles following Peripheral Nerve Injury. PLoS ONE 2015, 10, e0142699. [Google Scholar] [CrossRef] [PubMed]
- Purves-Smith, F.M.; Solbak, N.M.; Rowan, S.L.; Hepple, R.T. Severe atrophy of slow myofibers in aging muscle is concealed by myosin heavy chain co-expression. Exp. Gerontol. 2012, 47, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Altun, M.; Grönholdt-Klein, M.; Wang, L.; Ulfhake, B. Cellular Degradation Machineries in Age-Related Loss of Muscle Mass (Sarcopenia). In Senescence; Nagata, T., Ed.; InTech: Houston, TX, USA, 2012; pp. 269–286. [Google Scholar]
- Verdijk, L.B.; Koopman, R.; Schaart, G.; Meijer, K.; Savelberg, H.H.C.M.; van Loon, L.J.C. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am. J. Physiol. Metab. 2007, 292, E151–E157. [Google Scholar] [CrossRef] [PubMed]
- Verdijk, L.B.; Snijders, T.; Drost, M.; Delhaas, T.; Kadi, F.; van Loon, L.J.C. Satellite cells in human skeletal muscle; from birth to old age. Age 2014, 36, 545–557. [Google Scholar] [CrossRef]
- Ming, Y.; Bergman, E.; Edstöm, E.; Ulfhake, B. Evidence for increased GDNF signaling in aged sensory and motor neurons. Neuroreport 1999, 10, 1529–1535. [Google Scholar] [CrossRef][Green Version]
- Bergman, E.; Kullberg, S.; Ming, Y.; Ulfhake, B. Upregulation of GFRalpha-1 and c-ret in primary sensory neurons and spinal motoneurons of aged rats. J. Neurosci. Res. 1999, 57, 153–165. [Google Scholar] [CrossRef]
- Stanga, S.; Boido, M.; Kienlen-Campard, P. How to Build and to Protect the Neuromuscular Junction: The Role of the Glial Cell Line-Derived Neurotrophic Factor. Int. J. Mol. Sci. 2020, 22, 136. [Google Scholar] [CrossRef]
- Hollyday, M.; Hamburger, V. Reduction of the naturally occurring motor neuron loss by enlargement of the periphery. J. Comp. Neurol. 1976, 170, 311–320. [Google Scholar] [CrossRef]
- Oppenheim, R.W. Neurotrophic Survival Molecules for Motoneurons: An Embarrassment of Riches. Neuron 1996, 17, 195–197. [Google Scholar] [CrossRef]
- Rieger, F.; Nicolet, M.; Pinçon-Raymond, M.; Murawsky, M.; Levi, G.; Edelman, G.M. Distribution and role in regeneration of N-CAM in the basal laminae of muscle and Schwann cells. J. Cell Biol. 1988, 107, 707–719. [Google Scholar] [CrossRef]
- Csapo, R.; Gumpenberger, M.; Wessner, B. Skeletal Muscle Extracellular Matrix—What Do We Know About Its Composition, Regulation, and Physiological Roles? A Narrative Review. Front. Physiol. 2020, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Birch, H.L. Extracellular Matrix and Ageing. Subcell Biochem. 2018, 90, 169–190. [Google Scholar] [PubMed]
- Martin, A.; Freyssenet, D. Phenotypic features of cancer cachexia-related loss of skeletal muscle mass and function: Lessons from human and animal studies. J. Cachex- Sarcopenia Muscle 2021, 12, 252–273. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, C.; Tiedemann, A.; Fairhall, N.J.; Hopewell, S.; Michaleff, Z.A.; Howard, K.; Clemson, L.; Lamb, S.E. Exercise for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 2019, 1, Cd012424. [Google Scholar] [CrossRef]
- Ferreira, L.F.; Scariot, E.L.; da Rosa, L.H.T. The effect of different exercise programs on sarcopenia criteria in older people: A systematic review of systematic reviews with meta-analysis. Arch. Gerontol. Geriatr. 2023, 105, 104868. [Google Scholar] [CrossRef]
- Fiatarone, M.A.; Marks, E.C.; Ryan, N.D.; Meredith, C.N.; Lipsitz, L.A.; Evans, W.J. High-Intensity Strength Training in Nonagenarians. JAMA 1990, 263, 3029–3034. [Google Scholar] [CrossRef]
- Whipple, R.H.; Wolfson, L.I.; Amerman, P.M. The Relationship of Knee and Ankle Weakness to Falls in Nursing Home Residents: An Isokinetic Study. J. Am. Geriatr. Soc. 1987, 35, 13–20. [Google Scholar] [CrossRef]
- Shi, T.J.; Tandrup, T.; Bergman, E.; Xu, Z.Q.; Ulfhake, B.; Hokfelt, T. Effect of peripheral nerve injury on dorsal root ganglion neurons in the C57 BL/6J mouse: Marked changes both in cell numbers and neuropeptide expression. Neuroscience 2001, 105, 249–263. [Google Scholar] [CrossRef]
- Zhang, M.D.; Tortoriello, G.; Hsueh, B.; Tomer, R.; Ye, L.; Mitsios, N.; Borgius, L.; Grant, G.; Kiehn, O.; Watanabe, M.; et al. Neuronal calcium-binding proteins 1/2 localize to dorsal root ganglia and excitatory spinal neurons and are regulated by nerve injury. Proc. Natl. Acad. Sci. USA 2014, 111, E1149–E1158. [Google Scholar] [CrossRef]

| Cohort | Sex | Age (Months) | Strain | Vendor | Cage Type | Temp °C | Humidity (%) | Feed | n |
|---|---|---|---|---|---|---|---|---|---|
| M5m | Male | 5 | CR-SD | CR | Type 4 Open | 21 ± 2 | 40–60 | Lactamin R70 | 10 |
| M12m | Male | 12 | CR-SD | CR | Type 4 Open | 21 ± 2 | 40–60 | Lactamin R70 | 9 |
| M18m | Male | 18 | CR-SD | CR | Type 4 Open | 21 ± 2 | 40–60 | Lactamin R70 | 11 |
| M25m | Male | 25 | CR-SD | CR | Type 4 Open | 21 ± 2 | 40–60 | Lactamin R70 | 8 |
| M30m | Male | 30 | Sprague Dawley | Scanbur | Type 4 Open | 21 ± 2 | 40–60 | Lactamin R70 | 16 |
| F3m&F3mA | Female | 3 | Sprague Dawley | Scanbur | Type 4 Open | 21 ± 2 | 40–60 | Lactamin R70 | 30 |
| F12m | Female | 12 | Sprague Dawley | Scanbur | Type 4 Open | 21 ± 2 | 40–60 | Lactamin R70 | 10 |
| F30m | Female | 30 | Sprague Dawley | Scanbur | Type 4 Open | 21 ± 2 | 40–60 | Lactamin R70 | 10 |
| Total | Males | 55 | |||||||
| Total | Females | 50 |
| Behavioral Tests | Gross Measures | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | Sex | Age (Months) | OF | Beam Balance | Mesh Crossing | Gait | Gait-Stage | BW | m. sol Weight | SI |
| M5m | Male | 5 | X | X | X | X | X | X | X | X |
| M12m | Male | 12 | X | X | X | X | X | X | X | X |
| M18m | Male | 18 | ND | ND | ND | ND | X | X | X | X |
| M25m | Male | 25 | X | X | X | ND | X | X | X | X |
| M30m | Male | 30 | X | X | X | X | X | X | X | X |
| F3m | Female | 3 | X | X | X | X | X | X * | X * | X * |
| F12m | Female | 12 | X | X | X | X | X | X * | X * | X * |
| F30m | Female | 30 | X | X | X | X | X | X * | X * | X * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
GrönholdtKlein, M.; Gorzi, A.; Wang, L.; Edström, E.; Rullman, E.; Altun, M.; Ulfhake, B. Emergence and Progression of Behavioral Motor Deficits and Skeletal Muscle Atrophy across the Adult Lifespan of the Rat. Biology 2023, 12, 1177. https://doi.org/10.3390/biology12091177
GrönholdtKlein M, Gorzi A, Wang L, Edström E, Rullman E, Altun M, Ulfhake B. Emergence and Progression of Behavioral Motor Deficits and Skeletal Muscle Atrophy across the Adult Lifespan of the Rat. Biology. 2023; 12(9):1177. https://doi.org/10.3390/biology12091177
Chicago/Turabian StyleGrönholdtKlein, Max, Ali Gorzi, Lingzhan Wang, Erik Edström, Eric Rullman, Mikael Altun, and Brun Ulfhake. 2023. "Emergence and Progression of Behavioral Motor Deficits and Skeletal Muscle Atrophy across the Adult Lifespan of the Rat" Biology 12, no. 9: 1177. https://doi.org/10.3390/biology12091177
APA StyleGrönholdtKlein, M., Gorzi, A., Wang, L., Edström, E., Rullman, E., Altun, M., & Ulfhake, B. (2023). Emergence and Progression of Behavioral Motor Deficits and Skeletal Muscle Atrophy across the Adult Lifespan of the Rat. Biology, 12(9), 1177. https://doi.org/10.3390/biology12091177






