Combination of Bacillus and Low Fertigation Input Promoted the Growth and Productivity of Chinese Cabbage and Enriched Beneficial Rhizosphere Bacteria Lechevalieria
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Growth Performance and Root Vitality of Chinese Cabbage
2.4. Occurrence of Soft Rot Disease
2.5. Determination of the Endogenous Hormones in Chinese Cabbage Roots
2.5.1. Standard Curves of ABA, IAA, SA, and ZT
2.5.2. Analyses of the Endogenous Hormones in Cabbage Roots
2.6. Analysis of the Rhizosphere Microbiota of Chinese Cabbage
2.6.1. Sample Preparation
2.6.2. DNA Extraction and Illumina Miseq Sequencing
2.6.3. Bioinformatic Analysis of the Soil Microbial Communities
3. Results
3.1. Growth Performance of Chinese Cabbage under Different Fertigation Conditions
3.2. Combined B006 and Low Fertigation Input Suppressed Cabbage Soft Rot Disease
3.3. Endogenous Hormones in Cabbage Roots under Different Fertigation Conditions
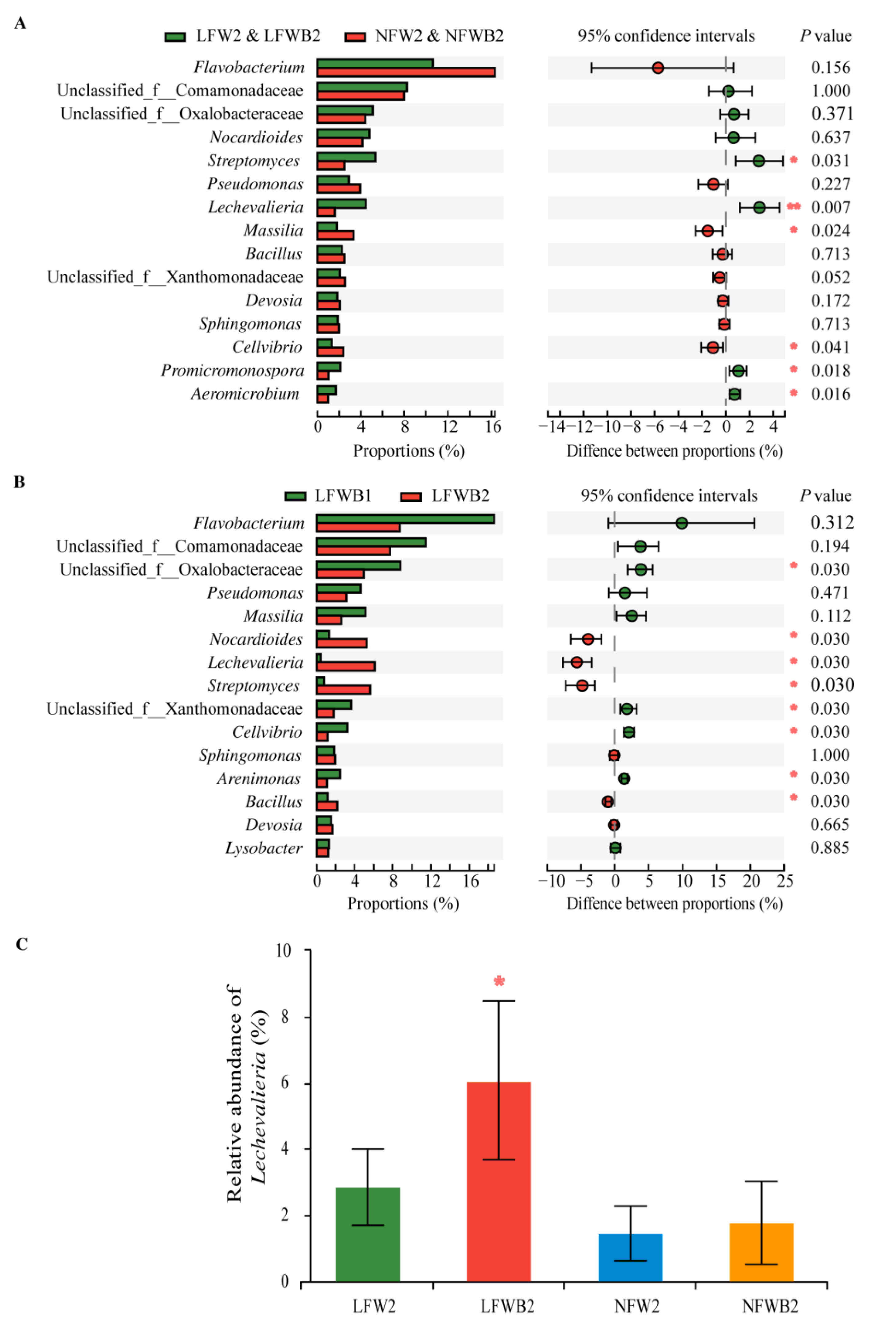
3.4. Rhizosphere Microbial Communities Reshaped by Fertigation and Bacillus Application
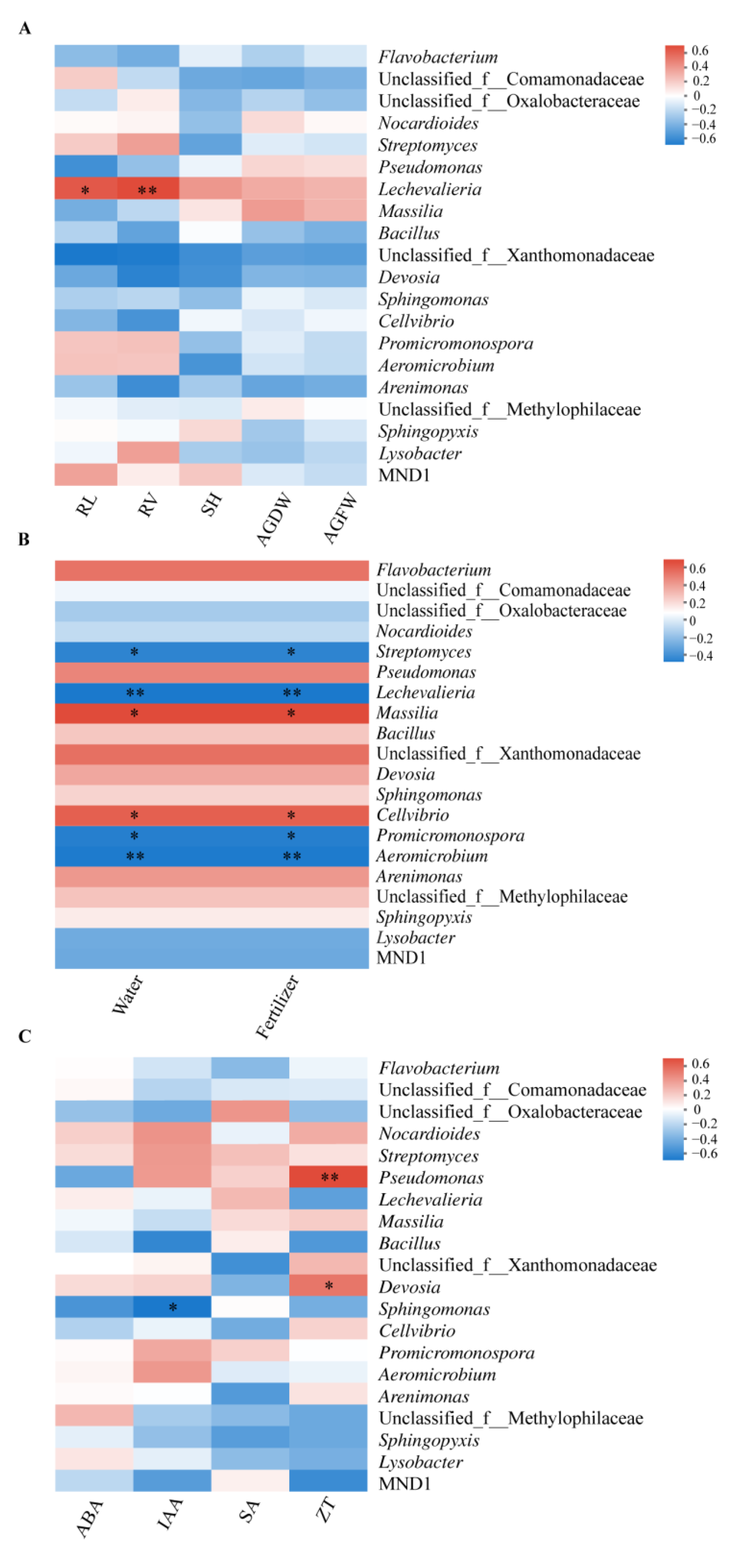
3.5. Correlation of the Rhizosphere Bacteria to Plant Growth, Fertigation Levels, and Hormones
4. Discussion
4.1. Healthy Cabbage Growth Promoted by Improved Bacillus Application
4.2. Specific Assembly of Bacterial Genera Shaped by Improved Bacillus Application
4.3. Enrichment of Specific Bacterial Genera and Hormone Production Are Positively Related to the Improved Bacillus Application and Healthy Cabbage Growth
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, G.F.; Wang, N.Q.; Zhang, Y.Y.; Wang, Z.; Zhang, Y.; Yu, J.; Zhang, Y.; Wei, Z.; Xu, Y.; Geisen, S.; et al. The relative importance of soil moisture in predicting bacterial wilt disease occurrence. Soil Ecol. Lett. 2021, 3, 356–366. [Google Scholar] [CrossRef]
- Kannaiyan, S.; Prasad, N.N. Effect of soil moisture on the influence of muskmelon wilt. Madras Agric. J. 1974, 61, 53–54. [Google Scholar]
- Li, Y.; Li, J.Q.; Gao, L.H.; Tian, Y.Q. Irrigation has more influence than fertilization on leaching water quality and the potential environmental risk in excessively fertilized vegetable soils. PLoS ONE 2018, 13, e0204570. [Google Scholar] [CrossRef] [PubMed]
- Talukder, Z.I.; McDonald, A.J.; Price, A.H. Loci controlling partial resistance to rice blast do not show marked QTL×environment interaction when plant nitrogen status alters disease severity. New Phytol. 2010, 168, 455–464. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Naylor, R.; Crews, T.; David, M.B.; Drinkwater, L.E.; Holland, E.; Johnes, P.J.; Katzenberger, J.; Martinelli, L.A.; Matson, P.A.; et al. Nutrient imbalances in agricultural development. Science 2009, 324, 1519–1520. [Google Scholar] [CrossRef]
- Ajdary, K.L.; Singh, D.K.; Singh, A.K.; Khanna, M. Modelling of nitrogen leaching from experimental onion field under fertigation. Agric. Water Manag. 2007, 89, 15–28. [Google Scholar] [CrossRef]
- Lv, H.F.; Lin, S.; Wang, Y.F.; Lian, X.J.; Zhao, Y.M.; Li, Y.J.; Du, J.; Wang, Z.; Wang, J.; Butterbach-Bahl, K. Drip fertigation significantly reduces nitrogen leaching in solar greenhouse vegetable production system. Environ. Pollut. 2019, 245, 694–701. [Google Scholar] [CrossRef]
- Yang, H.; Du, T.; Qiu, R.; Chen, J.; Wang, F.; Li, Y.; Wang, C.; Gao, L.; Kang, S. Improved water use efciency and fruit quality of greenhouse crops under regulated deficit irrigation in northwest China. Agric. Water Manag. 2017, 179, 193–204. [Google Scholar] [CrossRef]
- Cai, M.; Zhang, R.; Lv, S.; Sun, X.J.; Wang, A.; Wang, X.R. Effects of fertigation with fertilizer reduction on growth, yield and quality of tomato in solar greenhouse. North. Horticul. 2020, 3, 48–53. (In Chinese) [Google Scholar] [CrossRef]
- Li, Y.M.; Ren, J.; Liu, H.T.; Wang, L.C. Introduction and enlightment of fertigation in Israel. J. Jilin Agric. Sci. 2014, 39, 91–93. (In Chinese) [Google Scholar] [CrossRef]
- Armengaud, P.; Zambaux, K.; Hills, A.; Sulpice, R.; Pattison, R.J.; Blatt, M.R.; Amtmann, A. EZ-Rhizo: Integrated software for the fast and accurate measurement of root system architecture. Plant J. 2009, 57, 945–956. [Google Scholar] [CrossRef]
- Chen, W.L.; Li, J.; Zhu, H.H.; Chen, J.Z.; Yao, Q. A review of the regulation of plant root system architecture by rhizosphere microorganisms. Acta Ecol. Sin. 2016, 36, 5285–5297. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Z.; Zhang, G.; Chen, H.; Wang, J. Physiological and biochemical responses of different order roots in Fraxinus mandshurica seedlings to drought stress. Sci. Silvae Sin. 2009, 45, 16–21. (In Chinese) [Google Scholar]
- Zhang, X.D.; Wang, Z.W.; Han, Q.F.; Wang, Z.Y.; Min, A.C.; Jia, Z.K.; Nie, J.F. Effects of water stress on the root structure and physiological characteristics of early-stage maize. Acta Ecol. Sin. 2016, 36, 2969–2977. [Google Scholar] [CrossRef]
- Xu, F.; Liao, H.; Zhang, Y.; Yao, M.; Liu, J.; Sun, L.; Zhang, X.; Yang, J.; Wang, K.; Wang, X.; et al. Coordination of root auxin with the fungus Piriformospora indica and bacterium Bacillus cereus enhances rice rhizosheath formation under soil drying. ISME J. 2021, 16, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; von Wiren, N. Signaling pathways underlying nitrogen-dependent changes in root system architecture: From model to crop species. J. Exp. Bot. 2020, 71, 4393–4404. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zeng, R.; Liao, H. Improving crop nutrient efficiency through root architecture modifications. J. Integr. Plant Biol. 2016, 58, 193–202. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, M.; Zhang, M.; Lan, J.; Wang, W.; Ta, X.; Liu, Y. Transcriptome analysis provides novel insights into high-soil-moisture-elevated susceptibility to Ralstonia solanacearum infection in ginger (Zingiber officinale Roscoe cv. Southwest). Plant Physiol. Biochem. 2018, 132, 547–556. [Google Scholar] [CrossRef]
- Hu, F.; Kong, C.H.; Chen, X.H.; Zhang, Z.X. Effects of different water‚ fertility‚ and light conditions on allelopathic traits of rice. Chin. J. Appl. Ecol. 2003, 14, 2265–2268. (In Chinese) [Google Scholar] [CrossRef]
- Yuan, Y.; Long, R.H.; Tao, J.; Wang, Q.; Li, S.K. Effect of amount and method of irrigation and fertilization on yield and quality of spinach. J. Irrig. Drain. 2023, 42, 19–24. (In Chinese) [Google Scholar] [CrossRef]
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant-pathogen warfare under changing climate conditions. Curr. Biol. 2018, 28, R619–R634. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.J.; Kong, H.G.; Choi, K.; Kwon, S.K.; Song, J.Y.; Lee, J.; Lee, P.A.; Choi, S.Y.; Seo, M.; Lee, H.J.; et al. Rhizosphere microbiome structure alters to enable wilt resistance in tomato. Nat. Biotechnol. 2018, 36, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wang, X.; Yang, T.; Friman, V.P.; Geisen, S.; Wei, Z.; Xu, Y.; Jousset, A.; Shen, Q. Chemical structure predicts the effect of plant-derived low-molecular weight compounds on soil microbiome structure and pathogen suppression. Funct. Ecol. 2020, 34, 2158–2169. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, T.; Friman, V.P.; Xu, Y.; Shen, Q.; Jousset, A. Trophic network architecture of root-associated bacterial communities determines pathogen invasion and plant health. Nat. Commun. 2015, 6, 8413. [Google Scholar] [CrossRef]
- Raza, W.; Wang, J.; Wu, Y.; Ling, N.; Wei, Z.; Huang, Q.; Shen, Q. Effects of volatile organic compounds produced by Bacillus amyloliquefaciens on the growth and virulence traits of tomato bacterial wilt pathogen Ralstonia solanacearum. Appl. Microbiol. Biotechnol. 2016, 100, 7639–7650. [Google Scholar] [CrossRef]
- Na, X.F.; Yu, H.L.; Wang, P.; Zhu, W.; Niu, Y.; Huang, J. Vegetation biomass and soil moisture coregulate bacterial community succession under altered precipitation regimes in a desert steppe in northwestern China. Soil Biol. Biochem. 2019, 136, 107520. [Google Scholar] [CrossRef]
- Naylor, D.; DeGraaf, S.; Purdom, E.; Coleman-Derr, D. Drought and host selection influence bacterial community dynamics in the grass root microbiome. ISME J. 2017, 11, 2691–2704. [Google Scholar] [CrossRef]
- Santos-Medellín, C.; Edwards, J.; Liechty, Z.; Nguyen, B.; Sundaresan, V. Drought stress results in a compartment-specific restructuring of the rice root-associated microbiomes. mBio 2017, 8, e00764-17. [Google Scholar] [CrossRef]
- Wang, Z.H.; Song, Y. Toward understanding the genetic bases underlying plant-mediated “cry for help” to the microbiota. IMeta 2022, 1, e8. [Google Scholar] [CrossRef]
- Sommermann, L.; Babin, D.; Behr, J.H.; Chowdhury, S.P.; Sandmann, M.; Windisch, S.; Neumann, G.; Nesme, J.; Sørensen, S.J.; Schellenberg, I.; et al. Long-term fertilization strategy impacts Rhizoctonia solani-microbe interactions in soil and rhizosphere and defense responses in lettuce. Microorganisms 2022, 10, 1717. [Google Scholar] [CrossRef]
- Chen, C.L.; Li, X.Y.; Bo, Z.J.; Du, W.X.; Fu, L.; Tian, Y.; Cui, S.; Shi, Y.; Xie, H. Occurrence, characteristics, and PCR-based detection of Pectobacterium polaris causing soft rot of Chinese cabbage in China. Plant Dis. 2021, 105, 2880–2887. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Song, L.P. Integrated management of Chinese cabbage soft rot disease at Tianjin district. Sci. Technol. Tianjin Agric. Forest. 2018, 6, 27–28. [Google Scholar]
- Yang, X.L. Occurrence and integrated management technique of Chinese cabbage soft rot disease. Vegetables 2010, 8, 16–17. (In Chinese) [Google Scholar]
- Wei, L.; Liang, Z.H.; Zhang, Y. Integrated management methods on the management of cabbage soft rot disease in heavy rain season. J. Chang. Veg. 2016, 15, 52–53. (In Chinese) [Google Scholar]
- Zeng, X.P.; Xiao, M.; Wang, H.F.; Fu, M.Y.; He, S.; Wang, S.Y. Disease investigation and epidemic pattern of cabbage soft rot in Hainan Province. Agric. Develop. Equip. 2018, 7, 177,181. (In Chinese) [Google Scholar]
- Dalsing, B.L.; Truchon, A.N.; Gonzalez-Orta, E.T.; Milling, A.S.; Allen, C. Ralstonia solanacearum uses inorganic nitrogen metabolism for virulence, ATP production, and detoxifification in the oxygen-limited host xylem environment. mBio 2015, 6, e02471-14. [Google Scholar] [CrossRef]
- Geng, Y.; Guo, R.; Zhang, A.; Govrin, E.; Li, S.D. Growth and yield of eggplant promoted by the application of Bacillus velezensis B006 agent under different soil water potential conditions. J. Agric. Resour. Environ. 2020, 37, 398–406. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, S.; Guo, R.; Zhang, A.; Govrin, E.M.; Li, S.D.; Shen, R.; Luo, M. Plant growth promotion and soft rot disease control of Chinese cabbage affected by application of Bacillus velezensis B006 under different fertigation conditions. Chin. J. Biol. Control 2021, 37, 531–537. [Google Scholar] [CrossRef]
- Jia, K.; Gao, Y.H.; Huang, X.Q.; Guo, R.J.; Li, S.D. Rhizosphere inhibition of cucumber fusarium wilt by different surfactin-excreting strains of Bacillus subtilis. Plant Pathol. J. 2015, 31, 140–151. [Google Scholar] [CrossRef]
- Gao, Y.H.; Guo, R.J.; Li, S.D. Draft genome sequence of Bacillus velezensis B6, a rhizobacterium that can control plant diseases. Genome Announ. 2018, 6, e00182-18. [Google Scholar] [CrossRef]
- Wang, J.Q.; Guo, R.J.; Wan, W.C.; Ma, G.Z.; Li, S.D. Insight into the surfactin production of Bacillus velezensis B006 through metabolomics analysis. J. Ind. Microbiol. Biot. 2018, 45, 1033–1044. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, X.W.; Zhou, S.M.; Wang, Y.J.; Yang, R.; Xu, F.D.; Mei, J.; Shen, G.; Li, Q.; He, D. Activities of key enzymes in root NADP-dehydrogenase system and their relationships with root vigor and grain yield formation in wheat. Sci. Agric. Sin. 2018, 51, 2060–2071. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Tang, N.; Tand, D.C.; Zhang, J.A. Optimized HPLC procedure for analyzing three endogenous hormones in Tulip bulbs. Bull. Bot. Res. 2021, 41, 130–137. (In Chinese) [Google Scholar] [CrossRef]
- Gao, Y.H.; Lu, X.H.; Guo, R.J.; Hao, J.J.; Miao, Z.Q.; Yang, L.; Li, S.D. Responses of soil abiotic properties and microbial community structure to 25-Year cucumber monoculture in commercial greenhouses. Agriculture 2021, 11, 341. [Google Scholar] [CrossRef]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; Del Rio, T.G.; et al. Defining the core Arabidopsis thaliana root microbiome. Nature 2012, 488, 86–90. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; van Themaat, E.V.L.; Ahmadinejad, N.; Assenza, F.; Rauf, P.; Huettel, B.; Reinhardt, R.; Schmelzer, E.; et al. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 2012, 488, 91–95. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Garrido-Oter, R.; Münch, P.C.; Weiman, A.; Dröge, J.; Pan, Y.; McHardy, A.C.; Schulze-Lefert, P. Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 2015, 17, 392–403. [Google Scholar] [CrossRef]
- Adams, R.I.; Miletto, M.; Taylor, J.W.; Bruns, T.D. Dispersal in microbes: Fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. ISME J. 2013, 7, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Callahan, B.; McMurdie, P.; Rosen, M.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Jiang, X.T.; Peng, X.; Deng, G.H.; Sheng, H.F.; Wang, Y.; Zhou, H.W.; Tam, N.F.Y. Illumina sequencing of 16S rRNA tag revealed spatial variations of bacterial communities in a mangrove wetland. Microbiol. Ecol. 2013, 66, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance in ecology. Mathematics 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Liu, C.; Cui, Y.M.; Li, X.Z.; Yao, M.J. microeco: An R package for data mining in microbial community ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar] [CrossRef]
- Adesemoye, A.; Torbert, H.; Kloepper, J. Plant growth-promoting rhizobacteria allow reduced application rates of chemical fertilizers. Microb. Ecol. 2009, 58, 921–929. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Leong, J.; Teintze, M.; Schroth, M.N. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 1980, 286, 885–886. [Google Scholar] [CrossRef]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial interactions in the rhizosphere: Benefcial infuences of plant growth-promoting rhizobacteria on nutrient acquisition process. Rev. Biol. Fert. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Song, Y.; Haney, C.H. Drought dampens microbiome development. Nat. Plants 2021, 7, 994–995. [Google Scholar] [CrossRef]
- Chen, L.; Li, K.; Shang, J.; Wu, Y.; Chen, T.; Wanyan, Y.; Wang, E.; Tian, C.; Chen, W.; Chen, W.; et al. Plant growth–promoting bacteria improve maize growth through reshaping the rhizobacterial community in low-nitrogen and low-phosphorus soil. Biol. Fert. Soils 2021, 57, 1075–1088. [Google Scholar] [CrossRef]
- de Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, W.; Shi, L.; Wang, H.; Wang, Y.; Zhao, Y.; Xiang, W.; Wang, X. Lechevalieria rhizosphaerae sp. nov., a novel actinomycete isolated from rhizosphere soil of wheat (Triticum aestivum L.) and emended description of the genus Lechevalieria. Int. J. Syst. Evol. Microbiol. 2017, 67, 4655–4659. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yan, Y.; Liu, C.; Huang, J.; Xiang, W.; Huang, S. Secondary metabolites and genetic system of the rare actinobacteria Lechevalieria rhizosphaerae NEAU-A2. Microbiol. China 2021, 48, 2318–2328. [Google Scholar] [CrossRef]
- Kang, S.M.; Khan, A.L.; Hamayun, M.; Hussain, J.; Joo, G.J.; You, Y.H.; Kim, J.-G.; Lee, I.-J. Gibberellin-producing Promicromonospora sp. SE188 improves Solanum lycopersicum plant growth and influences endogenous plant hormones. J. Microbiol. 2012, 50, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Reeves, A.R.; Cernota, W.H.; Brikun, I.A.; Wesley, R.K.; Weber, J.M. Engineering precursor flow for increased erythromycin production in Aeromicrobium erythreum. Metab. Eng. 2004, 6, 300–312. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Tan, X.; Guo, R.; Li, S.; Luo, M. Screening of antagonistic Streptomyces 26B and its efficacy on the control of soft rot disease of Chinese cabbage in soil with different water content. Chin. J. Biol. Control 2022, 39, 157–166. [Google Scholar] [CrossRef]
- Xiong, W.; Guo, S.; Jousset, A.; Zhao, Q.; Wu, H.; Li, R.; Kowalchuk, G.A.; Shen, Q. Bio-fertilizer application induces soil suppressiveness against Fusarium wilt disease by reshaping the soil microbiome. Soil Biol. Biochem. 2017, 114, 238–247. [Google Scholar] [CrossRef]
- Hu, J.; Yang, T.J.; Friman, V.P.; Kowalchuk, G.A.; Hautier, Y.; Li, M.; Wei, Z.; Xu, Y.; Shen, Q.; Jousset, A. Introduction of probiotic bacterial consortia promotes plant growth via impacts on the resident rhizosphere microbiome. Proc. Res. Soc. B 2021, 288, 20211396. [Google Scholar] [CrossRef]
- Sun, X.; Xu, Z.; Xie, J.; Hesselberg-Thomsen, V.; Tan, T.; Zheng, D.; Strube, M.L.; Dragoš, A.; Shen, Q.; Zhang, R.; et al. Bacillus velezensis stimulates resident rhizosphere Pseudomonas stutzeri for plant health through metabolic interactions. ISME J. 2022, 16, 774–787. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, K.; Qiu, L.; Ding, S.; Wang, H.; Liu, Z.; Zhang, M.; Wei, Z. Soil microbial co-occurrence patterns under controlled-release urea and fulvic acid applications. Microorganisms 2022, 10, 1823. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, H.; Yang, Z.; Cheng, X.; Zhao, Y.; Qin, L.; Bisseling, T.; Cao, Q.; Willemsen, V. Plant growth-promoting rhizobacterium Pseudomonas sp. CM11 specifically induces lateral roots. New Phytol. 2022, 235, 1575–1588. [Google Scholar] [CrossRef] [PubMed]
- Eichmann, R.; Richards, L.; Schäfer, P. Hormones as go-betweens in plant microbiome assembly. Plant J. 2021, 105, 518–541. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Y.; Li, S.S.; Han, G.Z. Insights into the origin and evolution of the plant hormone signaling machinery. Plant Physiol. 2015, 167, 872–886. [Google Scholar] [CrossRef]
- Ikegami, K.; Okamoto, M.; Seo, M.; Koshiba, T. Activation of abscisic acid biosynthesis in the leaves of Arabidopsis thaliana in response to water deficit. J. Plant Res. 2009, 122, 235–243. [Google Scholar] [CrossRef]
- Schroeder, J.I.; Kwak, J.M.; Allen, G.J. Guard cell abscisic acid signaling and engineering drought hardiness in plants. Nature 2001, 410, 327–330. [Google Scholar] [CrossRef]
- Zhang, J.H.; Zhang, X.P.; Liang, J.S. Exudation rate and hydraulic conductivity of maize roots are enhanced by soil drying and abscisic acid treatment. New Phytol. 1995, 131, 329–336. [Google Scholar] [CrossRef]
- Saini, S.; Sharma, I.; Kaur, N.; Pati, P.K. Auxin: A master regulator in plant root development. Plant Cell Rep. 2013, 32, 741–757. [Google Scholar] [CrossRef]
- Yan, Z.L.; Xuan, C.X.; Niu, J.Y.; Xi, L.L.; Liu, J.H.; Zhao, T.W. Effect of drought stress and water recovery on endogenous hormone content in roots of pea. Chin. J. Eco-Agric. 2009, 17, 297–301. [Google Scholar] [CrossRef]
- Aloni, R.; Langhans, M.; Aloni, E.; Ullrich, C.I. Role of cytokinin in the regulation of root gravitropism. Planta 2004, 220, 177–182. [Google Scholar] [CrossRef]
- Lohar, D.P.; Schaff, J.E.; Laskey, J.G.; Kieber, J.J.; Bilyeu, K.D.; Bird, D.M. Cytokinins play opposite roles in lateral root formation, and nematode and Rhizobial symbioses. Plant J. 2004, 38, 203–214. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; Van Onckelen, H.; Schmülling, T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 2003, 15, 2532–2550. [Google Scholar] [CrossRef] [PubMed]
- Dodd, I.C.; Zinovkina, N.Y.; Safronova, V.I.; Belimov, A.A. Rhizobacterial mediation of plant hormone status. Ann. Appl. Biol. 2010, 157, 361–379. [Google Scholar] [CrossRef]
- French, E.; Ghaste, M.; Widhalm, J.R.; Iyer-Pascuzzi, A.S. Defense hormones modulate root microbiome diversity and composition in tomato. bioRxiv 2019, preprint. [Google Scholar] [CrossRef]
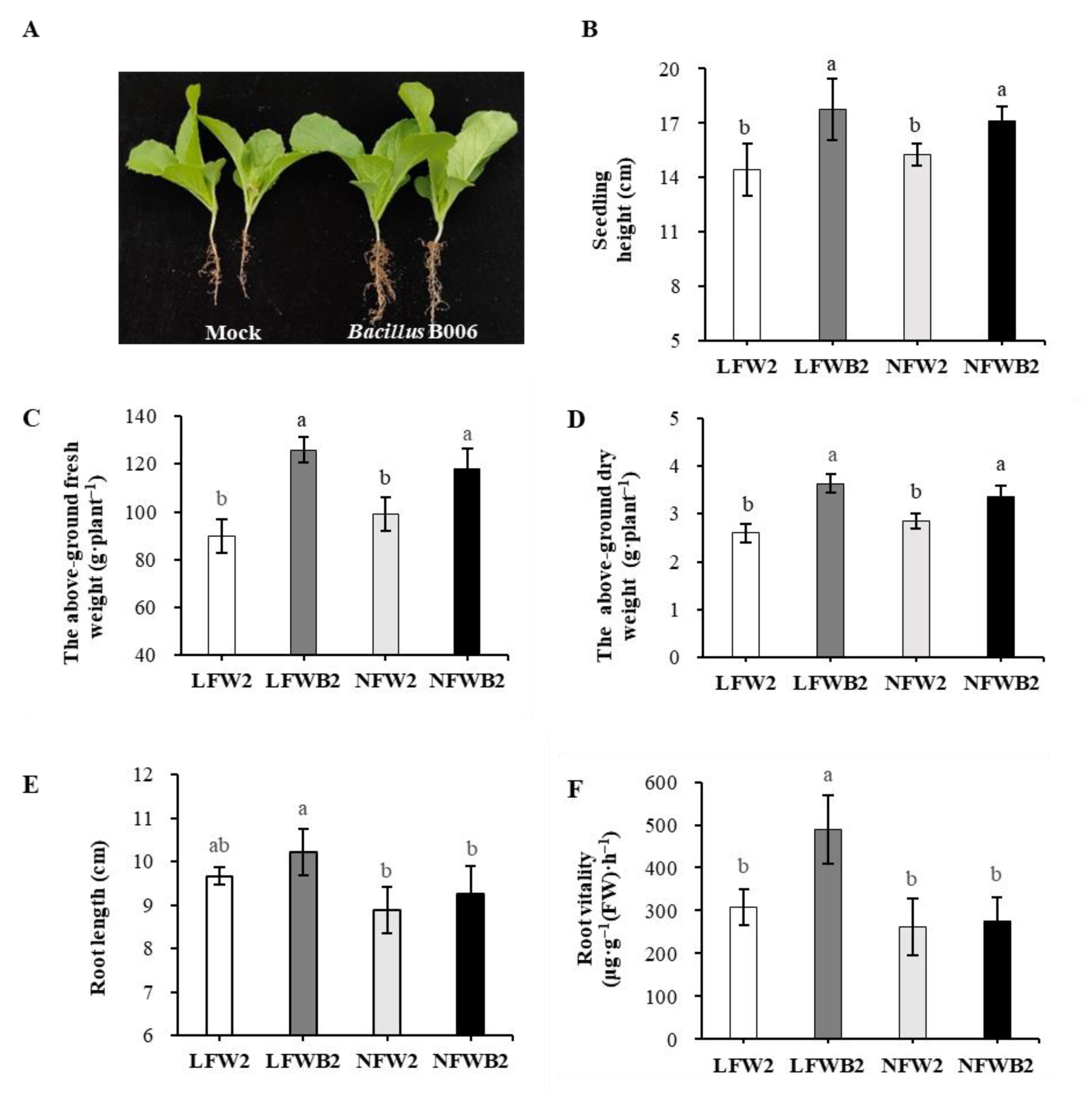
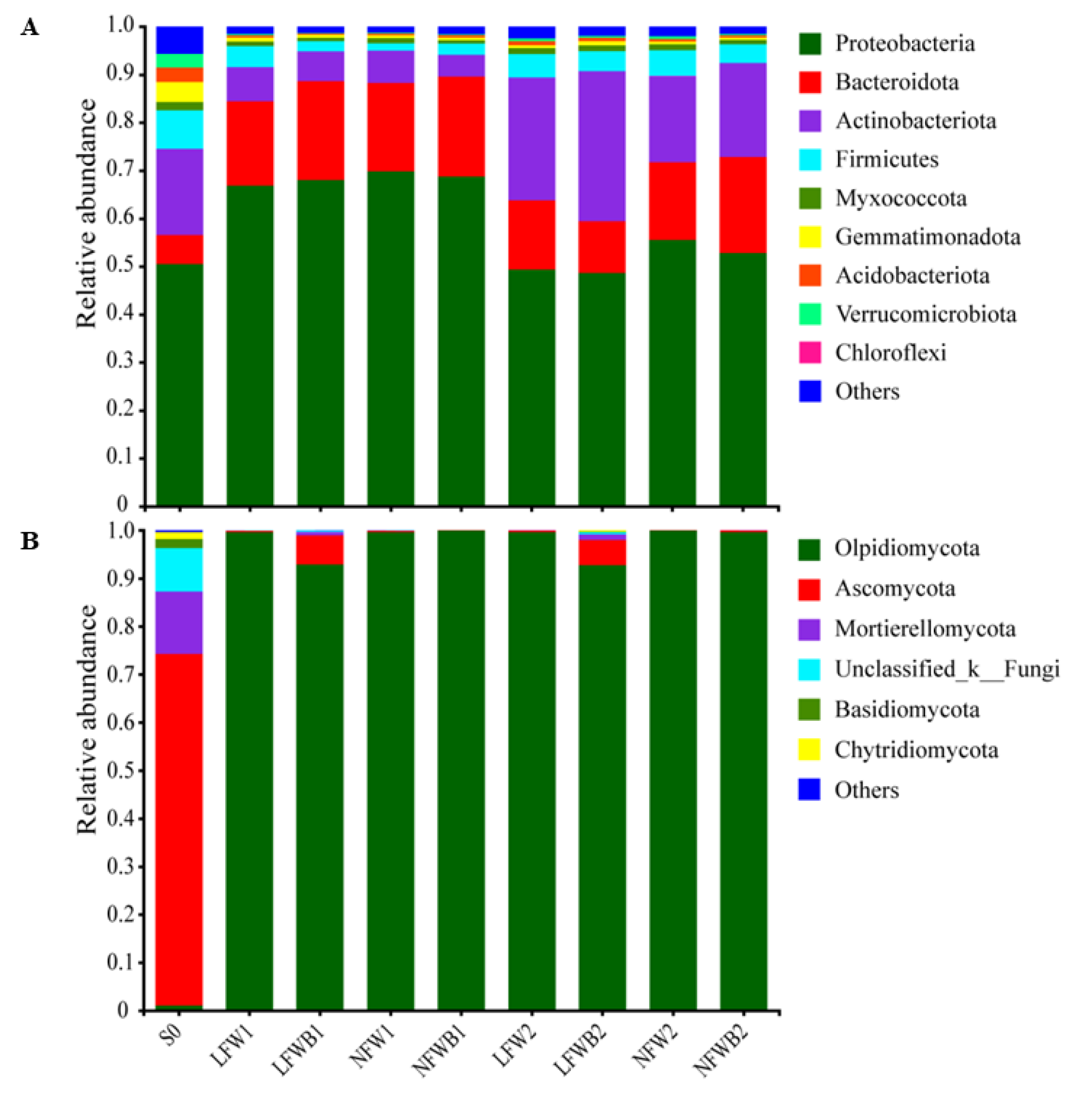
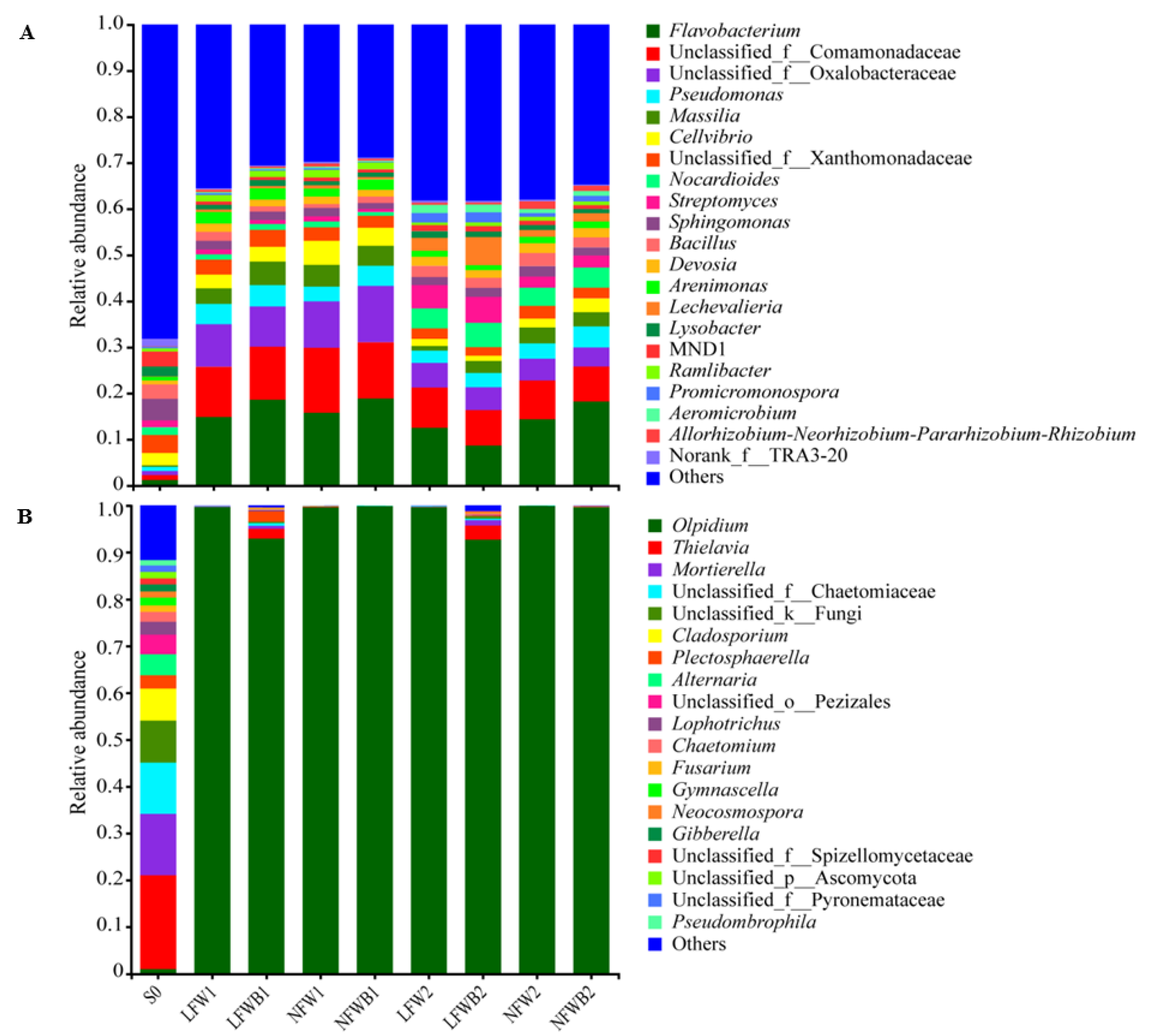
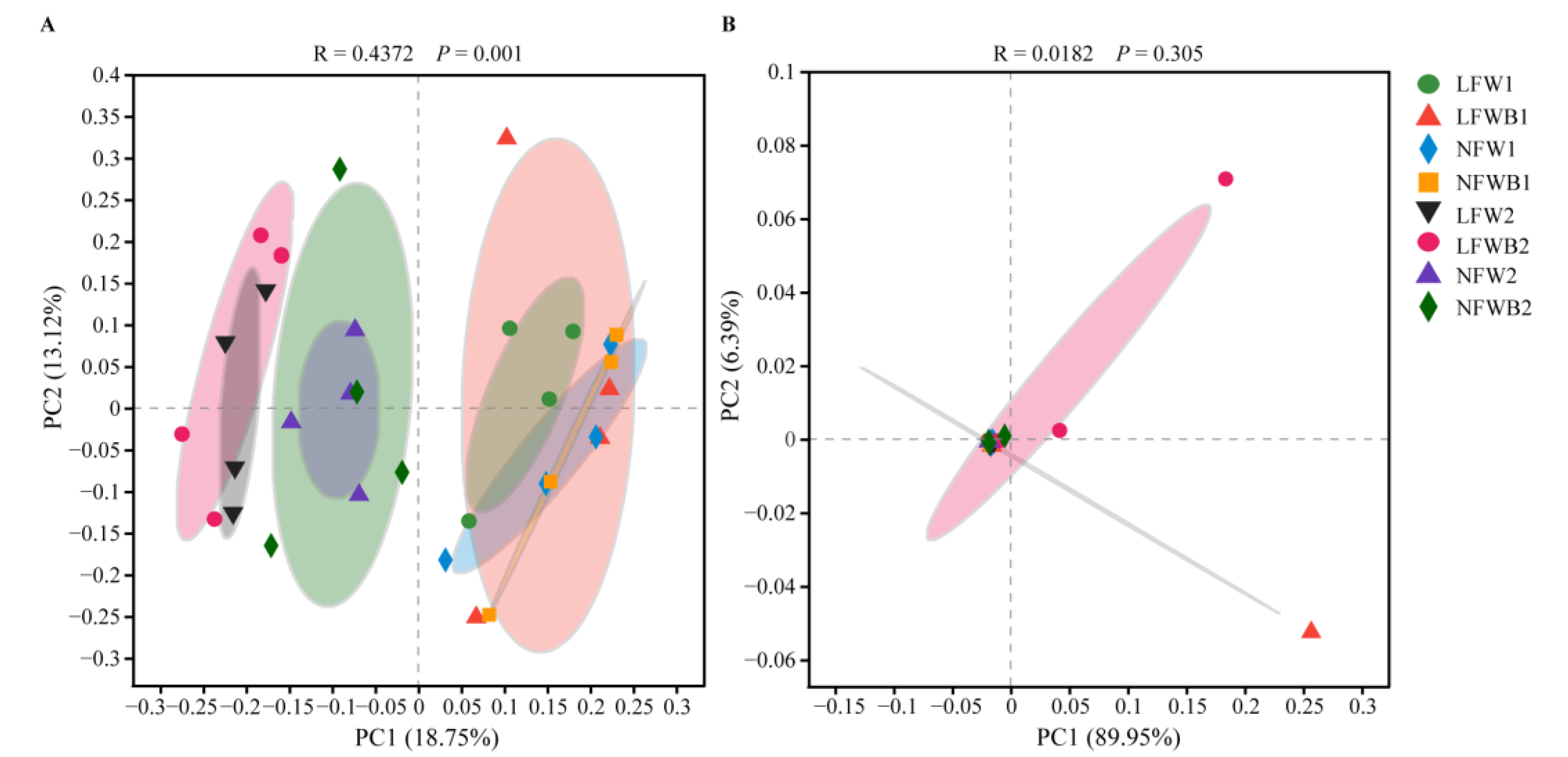
| Disease Level | Symptoms |
|---|---|
| 0 | No disease |
| 1 | Soft rot scab appeared on the lower 1–2 leaves |
| 2 | Soft rot scab appeared on the lower 3 leaves |
| 3 | Plants wilted, large areas of soft rot scab on outside leaves, the plant growth was seriously retarded |
| 4 | Stem soft rot, whole plant died |
| Treatment a | Disease Index b | Control Efficacy (%) | Seedling Survival Rate (%) | Yield (×104 Kg hm−2) |
|---|---|---|---|---|
| LFW | 6.1 ± 2.1 b | 56.7 | 86.6 ± 3.0 a | 4.1 ± 0.3 ab |
| LFWB | 2.7 ± 1.1 b | 80.9 | 89.2 ± 4.4 a | 4.3 ± 0.3 a |
| NFW | 14.1 ± 3.1 a | – | 67.1 ± 5.7 b | 3.5 ± 0.4 c |
| NFWB | 12.9 ± 2.2 a | 8.5 | 71.6 ± 7.4 b | 3.8 ± 0.2 bc |
| Samples b | ABA (µg g−1) | IAA (µg g−1) | SA (µg g−1) | ZT (µg g−1) |
|---|---|---|---|---|
| LFW1 | 0.60 ± 0.32 ab | – c | 14.33 ± 2.61 b | 5.46 ± 0.80 b |
| LFWB1 | 1.00 ± 0.15 a | 0.42 ± 0.25 bc | 18.43 ± 0.95 a | 7.08 ± 1.85 b |
| NFW1 | 0.88 ± 0.20 ab | – | 9.83 ± 2.70 bc | 6.32 ± 0.69 b |
| NFWB1 | 0.84 ± 0.27 ab | 0.34 ± 0.07 c | 17.05 ± 2.45 a | 6.19 ± 1.01 b |
| LFW2 | 0.65 ± 0.34 ab | 0.30 ± 0.01 c | 6.04 ± 0.67 d | 10.87 ± 1.40 a |
| LFWB2 | 0.72 ± 0.34 ab | 0.70 ± 0.28 ab | 5.08 ± 1.15 de | 11.50 ± 2.19 a |
| NFW2 | 0.63 ± 0.19 ab | – | 3.31 ± 1.00 e | 11.42 ± 0.89 a |
| NFWB2 | 0.50 ± 0.24 b | 1.07 ± 0.48 a | 7.55 ± 0.50 cd | 13.02 ± 1.77 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.-C.; Zhang, Y.-L.; Guo, X.-J.; Luo, M.; Li, S.-D.; Guo, R.-J. Combination of Bacillus and Low Fertigation Input Promoted the Growth and Productivity of Chinese Cabbage and Enriched Beneficial Rhizosphere Bacteria Lechevalieria. Biology 2023, 12, 1130. https://doi.org/10.3390/biology12081130
Zhang S-C, Zhang Y-L, Guo X-J, Luo M, Li S-D, Guo R-J. Combination of Bacillus and Low Fertigation Input Promoted the Growth and Productivity of Chinese Cabbage and Enriched Beneficial Rhizosphere Bacteria Lechevalieria. Biology. 2023; 12(8):1130. https://doi.org/10.3390/biology12081130
Chicago/Turabian StyleZhang, Shi-Chang, Yu-Lu Zhang, Xiao-Jing Guo, Ming Luo, Shi-Dong Li, and Rong-Jun Guo. 2023. "Combination of Bacillus and Low Fertigation Input Promoted the Growth and Productivity of Chinese Cabbage and Enriched Beneficial Rhizosphere Bacteria Lechevalieria" Biology 12, no. 8: 1130. https://doi.org/10.3390/biology12081130
APA StyleZhang, S.-C., Zhang, Y.-L., Guo, X.-J., Luo, M., Li, S.-D., & Guo, R.-J. (2023). Combination of Bacillus and Low Fertigation Input Promoted the Growth and Productivity of Chinese Cabbage and Enriched Beneficial Rhizosphere Bacteria Lechevalieria. Biology, 12(8), 1130. https://doi.org/10.3390/biology12081130






