The Diversity of Small Mammals along a Large River Valley Revealed from Pellets of Tawny Owl Strix aluco
Abstract
Simple Summary
Abstract
1. Introduction
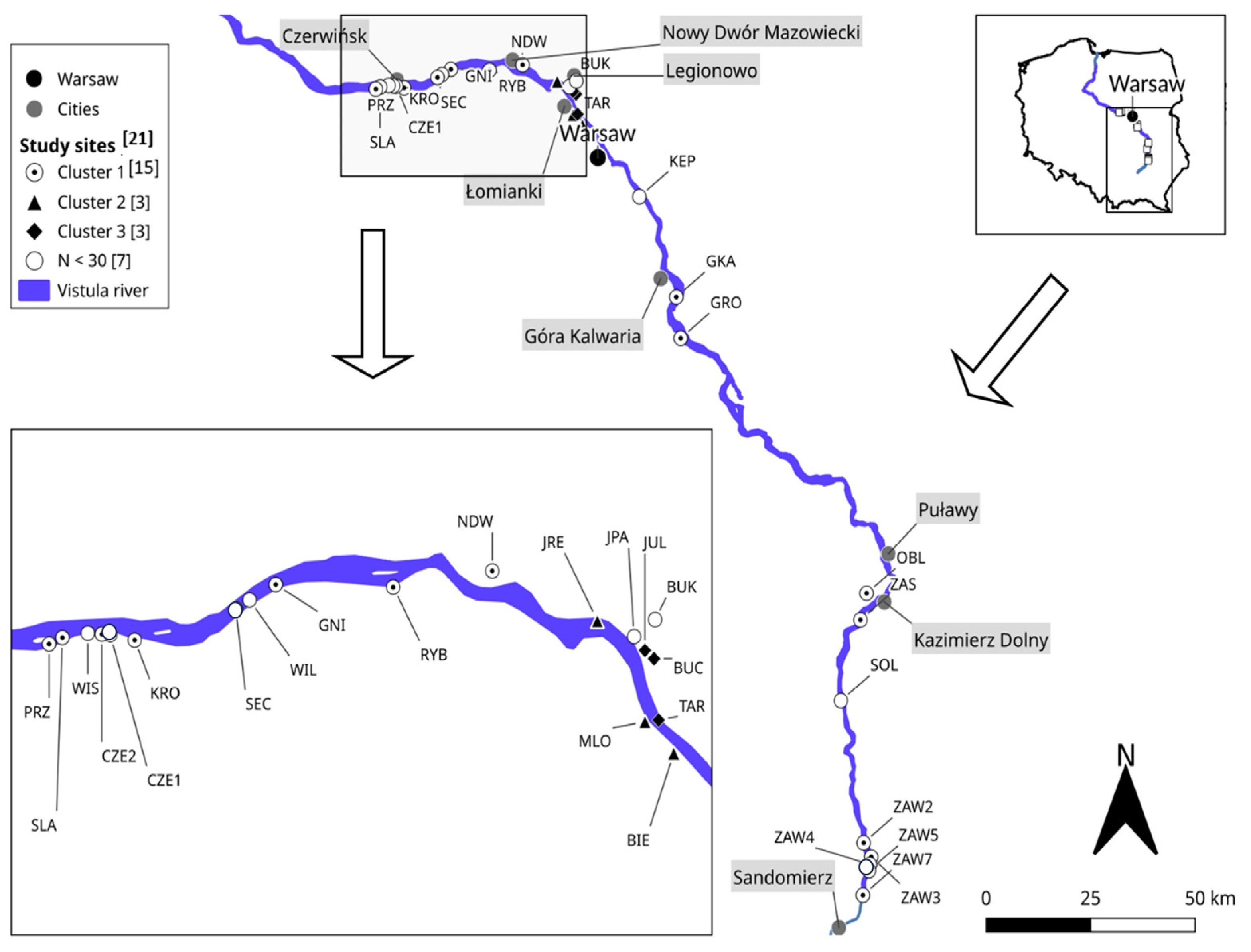
2. Materials and Methods
Small Mammal Community Estimates
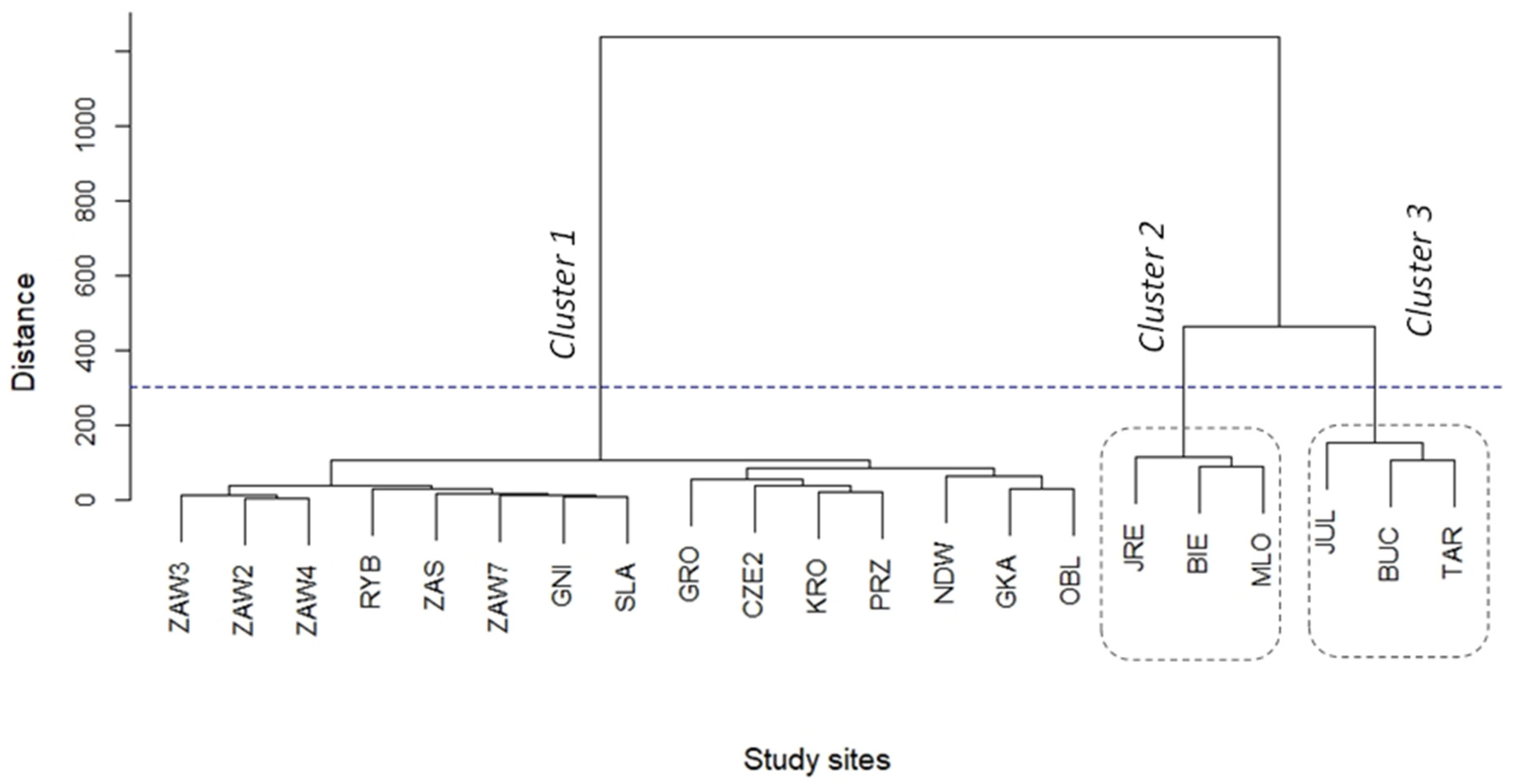
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pedroli, B.; de Blust, G.; van Looy, K.; van Rooij, S. Setting targets in strategies for river restoration. Landsc. Ecol. 2002, 17 (Suppl. S1), 5–18. [Google Scholar] [CrossRef]
- Ward, J.V.; Tockner, K.; Schiemer, F. Biodiversity of floodplain river ecosystems: Ecotones and connectivity. Regul. Rivers Res. Manag. 1999, 15, 125–139. [Google Scholar] [CrossRef]
- Décamps, H.; Pinay, G.; Naiman, R.; Petts, G.E.; McLain, M.E.; Hillbricht-Ilkowska, A.; Hanley, T.A.; Holmes, R.M.; Quinn, J.; Gibert, J.; et al. Riparian zones: Where biogeochemistry meets biodiversity in management practice. Pol. J. Ecol. 2004, 52, 3–18. [Google Scholar]
- Decher, J.; Norris, R.W.; Fahr, J. Small mammal survey in the upper Seli River valley, Sierra Leone. Mammalia 2010, 74, 163–176. [Google Scholar] [CrossRef]
- Tockner, K.; Stanford, J.A. Riverine floodplains: Present state and future trends. Environ. Conserv. 2002, 29, 308–330. [Google Scholar] [CrossRef]
- Castellano, C.; Bruno, D.; Comín, F.A.; Rey Benayas, J.M.; Masip, A.; Jimėnez, J.J. Environmental drivers for riparian restoration success and ecosystem services supply in Mediterranean agricultural landscapes. Agric. Ecosyst. Environ. 2022, 337, 108048. [Google Scholar] [CrossRef]
- Forman, R.T.T. Land Mosaics: The Ecology of Landscapes and Regions; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Junk, W.J.; Bayley, P.B.; Sparks, R.E. The flood pulse concept in river-floodplain systems. Can. Spec. Publ. Fish. Aquat. Sci. 1989, 106, 110–127. [Google Scholar]
- Jongman, R.H.G. Homogenisation and fragmentation of the European landscape: Ecological consequences and solutions. Landsc. Urban Plan. 2002, 58, 211–221. [Google Scholar] [CrossRef]
- Gliwicz, J. Competitive interactions within a forest rodent community in Central Poland. Oikos 1981, 37, 353–362. [Google Scholar] [CrossRef]
- Jędrzejewska, B.; Jędrzejewski, W. Predation in Vertebrate Communities: The Bialowieza Primeval Forest as a Case Study; Ecological Studies Series; Springer: New York, NY, USA, 1998; Volume 135, 450p. [Google Scholar]
- Balčiauskas, L.; Balčiauskienė, L. Small Mammal Diversity Changes in a Baltic Country, 1975–2021: A Review. Life 2022, 12, 1887. [Google Scholar] [CrossRef] [PubMed]
- Pardini, R.; Marques de Souza, S.; Braga-Neto, R.; Metzger, J.P. The role of forest structure, fragment size and corridors in maintaining small mammal abundance and diversity in an Atlantic Forest landscape. Biol. Conserv. 2005, 124, 253–266. [Google Scholar] [CrossRef]
- Silva, M.; Hartling, L.; Opps, S.B. Small mammals in agricultural landscapes of Prince Edward Island (Canada): Effects of habitat characteristics at three different spatial scales. Biol. Conserv. 2005, 126, 556–568. [Google Scholar] [CrossRef]
- Fischer, C.; Thies, C.; Tscharntke, T. Small mammals in agricultural landscapes: Opposing responses to farming practices and landscape complexity. Biol. Conserv. 2011, 144, 1130–1136. [Google Scholar] [CrossRef]
- Benton, T.G.; Vickery, J.A.; Wilson, J.D. Farmland biodiversity: Is habitat heterogeneity the key? TREE 2003, 18, 182–188. [Google Scholar] [CrossRef]
- Bentley, J.M.; Catterall, C.P.; Smith, G.C. Effects of fragmentation of araucarian vine forest on small mammal communities. Conserv. Biol. 2000, 14, 1075–1087. [Google Scholar] [CrossRef]
- Fialho, M.Y.G.; Cerboncini, R.A.S.; Passamani, M. Linear forest patches and the conservation of small mammals in human-altered landscapes. Mamm. Biol. 2019, 96, 87–92. [Google Scholar] [CrossRef]
- Ward, J.V.; Malard, F.; Tockner, K. Landscape ecology: A framework for integrating pattern and process in river corridors. Landsc. Ecol. 2002, 17 (Suppl. S1), 35–45. [Google Scholar] [CrossRef]
- Xu, H.; Plieninger, T.; Primdahl, J. A Systematic Comparison of Cultural and Ecological Landscape Corridors in Europe. Land 2019, 8, 41. [Google Scholar] [CrossRef]
- Romanowski, J. Vistula river valley as the ecological corridor for mammals. Pol. J. Ecol. 2007, 55, 805–819. [Google Scholar]
- Romanowski, J.; Dudek, D.; Kowalczyk, K. The role of islands in maintaining the connectivity of habitat for mammals in Vistula river valley. Ecohydrol. Hydrobiol. 2008, 8, 411–418. [Google Scholar] [CrossRef]
- Adamczyk, K.; Chełkowska, H.; Walkowa, W. The community of rodents in environments of the suburban zone. Pol. Ecol. Stud. 1988, 14, 171–195. [Google Scholar]
- Rajska-Jurgiel, E.; Mazurkiewicz, M. The effect of spatial structure of environment on density of rodents in suburban zone. Pol. Ecol. Stud. 1988, 14, 145–169. [Google Scholar]
- Bajkiewicz-Grabowska, E. Hydrological problems of the Vistula river valley near Płock. Hydrobiologia 1993, 251, 159–165. [Google Scholar] [CrossRef]
- Raczyński, J.; Ruprecht, A.L. The effect of digestion on the osteological composition of owl pellets. Acta Ornithol. 1974, 14, 25–38. [Google Scholar]
- Romanowski, J.; Żmihorski, M. Seasonal and habitat variation in the diet of the Tawny owl (Strix aluco L.) in Central Poland during unusually warm years. Biologia 2009, 64, 365–369. [Google Scholar] [CrossRef]
- Żmihorski, M.; Balčiauskienė, L.; Romanowski, J. Small mammals in the diet of the Tawny Owl (Strix aluco L.) in Central European lowland. Pol. J. Ecol. 2008, 56, 693–700. [Google Scholar]
- Heisler, L.M.; Somers, C.M.; Poulin, R.G. Owl pellets: A more effective alternative to conventional trapping for broad-scale studies of small mammal communities. Methods Ecol. Evol. 2016, 7, 96–103. [Google Scholar] [CrossRef]
- Balčiauskienė, L. Analysis of Tawny Owl (Strix aluco) food remains as a tool for long-term monitoring of small mammals. Acta Zool. Litu. 2005, 15, 85–89. [Google Scholar] [CrossRef]
- Corine Guideline. 2019. Available online: https://land.copernicus.eu/user-corner/technical-library/corine-land-cover-nomenclature-guidelines/html/index.html (accessed on 17 December 2019).
- Sanders, H.L. Marine benthic diversity: A comparative study. Am. Nat. 1968, 102, 243–282. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Entsminger, G.L. EcoSim Null Models Software for Ecology, version 7; Acquired Intelligenc Inc. & Kesey-Bear: Jericho, VT, USA, 2006; Available online: http://www.garyentsminger.com/ecosim/ (accessed on 10 May 2023).
- Ward, J.H., Jr. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Murtagh, F.; Legendre, P. Ward’s hierarchical agglomerative clustering method: Which algorithms implement Ward’s criterion? J. Classif. 2014, 31, 274–295. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.r-project.org/ (accessed on 10 May 2023).
- Lesiński, G.; Romanowski, J.; Gryz, J.; Olszewski, A.; Kowalski, M.; Krauze-Gryz, D.; Olech, B.; Pepłowska-Marczak, D.; Tarłowski, A. Small mammals of Kampinos National Park and its protection zone, as revealed by analyses of the diet of tawny owls Strix aluco. Fragm. Faun. 2013, 56, 65–81. [Google Scholar] [CrossRef]
- Fischer, C.; Schröder, B. Predicting spatial and temporal habitat use of rodents in a highly intensive agricultural area. Agric. Ecosyst. Environ. 2014, 189, 145–153. [Google Scholar] [CrossRef]
- Serafini, V.N.; Priotto, J.W.; Gomez, M.D. Effects of agroecosystem landscape complexity on small mammals: A multi-species approach at different spatial scales. Landsc. Ecol. 2019, 34, 1117–1129. [Google Scholar] [CrossRef]
- Heroldová, M.; Bryja, J.; Zejda, J.; Tkadlec, E. Structure and diversity of small mammal communities in agriculture landscape. Agric. Ecosyst. Environ. 2007, 120, 206–210. [Google Scholar] [CrossRef]
- Montgomery, W.I. The behaviour of Apodemus. Symp. Zool. Soc. Lond. 1985, 55, 89–115. [Google Scholar]
- Newson, R. Differences in numbers, reproduction and survival between two neighbouring populations of bank voles (Clethrionomys glareolus). Ecology 1963, 44, 110–120. [Google Scholar] [CrossRef]
- Kozakiewicz, M. Habitat isolation and ecological barriers—The effect on small mammal populations and communities. Acta Theriol. 1993, 38, 1–38. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Gortat, T. Abundance and seasonal dynamics of bank voles in a patchy agricultural landscape. Pol. Ecol. Stud. 1994, 20, 209–214. [Google Scholar]
- Babińska-Werka, J.; Malinowska, B. Synurbization of the yellow-necked mouse A. flavicollis in Warsaw. In Fauna of Cities: Preservation of Biodiversity in Cities; Indykiewicz, P., Jerzak, L., Barczak, T., Eds.; ATR: Bydgoszcz, Poland, 2008; pp. 144–150, (In Polish with English Abstract). [Google Scholar]
- Gortat, T.; Barkowska, M.; Gryczyńska-Siemiątkowska, A.; Pieniążek, A.; Kozakiewicz, A.; Kozakiewicz, M. The effects of urbanization—Small mammal communities in a gradient of human pressure in Warsaw city, Poland. Pol. J. Ecol. 2014, 62, 163–172. [Google Scholar] [CrossRef]
- Lesiński, G.; Gryz, J.; Krauze-Gryz, D.; Stolarz, P. Population increase and synurbization of the yellow-necked mouse Apodemus flavicollis in some wooded areas of Warsaw agglomeration, Poland, in the years 1983–2018. Urban Ecosyst. 2021, 24, 481–489. [Google Scholar] [CrossRef]
- Andrzejewski, R.; Babińska-Werka, J.; Gliwicz, J.; Goszczyński, J. Synurbization processes in population of Apodemus agrarius. I. Characteristics of populations in an urbanization gradient. Acta Theriol. 1978, 23, 341–358. [Google Scholar] [CrossRef]
- Adamczewska-Andrzejewska, K.; Mackin-Rogalska, R.; Nabagło, L. The effect of urbanization on density and population structure of Apodemus agrarius (Pallas, 1771). Pol. Ecol. Stud. 1988, 14, 197–211. [Google Scholar]
- Kowalski, M.; Lesiński, G. Small mammal fauna in Janowo (Warsaw voivodship) based on the analysis of Barn owl (Tyto alba Scop.) pellets. Prz. Zool. 1986, 30, 327–331, (In Polish with English Summary). [Google Scholar]
- Wilson, A.; Fenton, B.; Malloch, G.; Boag, B.; Hubbard, S.; Begg, G. Urbanisation versus agriculture: A comparison of local genetic diversity and gene flow between wood mouse Apodemus sylvaticus populations in human-modified landscapes. Ecography 2016, 39, 87–97. [Google Scholar] [CrossRef]
- Morris, P. Dormice; Whittet Books Ltd.: Suffolk, VA, USA, 2004. [Google Scholar]
- Lesiński, G.; Romanowski, J.; Budek, S. Winter diet of the long-eared owl Asio otus in various habitats of central and north-eastern Poland. Anim. Sci. 2016, 55, 81–88. [Google Scholar]
- Balčiauskas, L.; Balčiauskienė, L.; Baltrūnaitė, L. Root vole, Microtus oeconomus, in Lithuania: Changes in the distribution range. Folia Zool. 2010, 59, 267–277. [Google Scholar] [CrossRef]
- Raczyński, J.; Fedyk, S.; Gębczyńska, Z.; Pucek, M. Distribution of Micromammalia against natural differentiation of the Biebrza Valley habitats. Pol. Ecol. Stud. 1984, 10, 425–445. [Google Scholar]
- Rachwald, A. Habitat preference and activity of the noctule bat Nyctalus noctula in the Białowieża Primeval Forest. Acta Theriol. 1992, 37, 413–422. [Google Scholar] [CrossRef]
- Jones, G. Flight performance, echolocation and foraging behavior in noctule bat Nyctalus noctula. J. Zool. 1995, 237, 303–312. [Google Scholar] [CrossRef]
- Mackie, I.; Racey, P.A. Habitat use varies with reproductive state in noctule bats (Nyctalus noctula): Implications for conservation. Biol. Conserv. 2007, 140, 70–77. [Google Scholar] [CrossRef]
- Walsh, A.L.; Harris, S. Foraging habitat preferences of vespertilionid bats in Britain. J. Appl. Ecol. 1996, 33, 508–518. [Google Scholar] [CrossRef]
- Lesiński, G.; Fuszara, E.; Kowalski, M. Foraging areas and relative density of bats (Chiroptera) in differently human transformed landscapes. Z. Säugetierkunde 2000, 65, 129–137. [Google Scholar]
- Smith, P.G.; Racey, P.A. Natterer’s bats prefer foraging in broad-leaved woodlands and river corridors. J. Zool. 2008, 275, 314–322. [Google Scholar] [CrossRef]
- Vindigni, M.A.; Morris, A.D.; Miller, D.A.; Kalcounis-Rüppell, M.C. Use of modified water sources by bats in a managed pine landscape. For. Ecol. Manag. 2009, 258, 2056–2061. [Google Scholar] [CrossRef]
- Scott, S.J.; McLaren, G.; Jones, G.; Harris, S. The impact of riparian habitat quality on the foraging and activity of pipistrelle bats (Pipistrellus spp.). J. Zool. 2010, 280, 371–378. [Google Scholar] [CrossRef]
- Monck-Whipp, L.; Martin, A.E.; Francis, C.M.; Fahring, L. Farmland heterogeneity benefits bats in agricultural landscapes. Agric. Ecosyst. Environ. 2018, 253, 131–139. [Google Scholar] [CrossRef]
- Lesiński, G.; Kowalski, M.; Stolarz, P.; Gryz, J.; Krauze-Gryz, D.; Romanowski, J. Distribution of the European water vole Arvicola amphibius (Linnaeus, 1758) in Mazowsze and southern Podlasie. Fragm. Faun. 2017, 60, 129–140. [Google Scholar] [CrossRef]
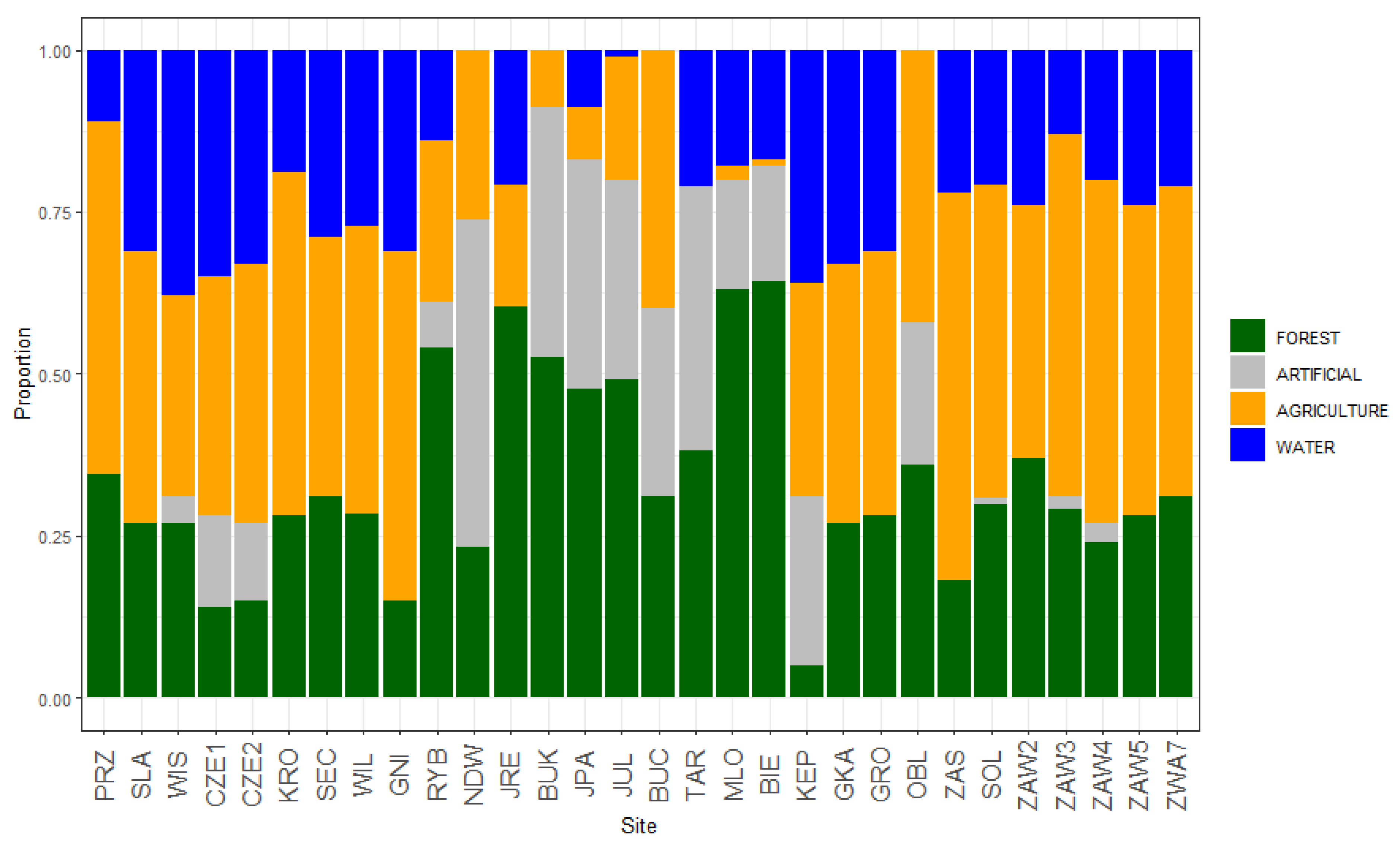
| Class | Description |
|---|---|
| Artificial surfaces | Discontinuous urban fabric, industrial or commercial units, |
| mineral extraction sites, dump sites, green urban areas, sport and leisure facilities | |
| Agricultural areas | Non-irrigated arable land, fruit trees and berry plantations, grasslands, complex cultivation patterns, land principally occupied by agriculture, with significant areas of natural vegetation |
| Forests and semi natural areas | Broad-leaved forest, coniferous forest, mixed forest, transitional woodland–shrubland |
| Water bodies | Water courses, water bodies |
| Order or Suborder | Family | Species | N | RA (%) | RA (%) Order |
|---|---|---|---|---|---|
| Chiroptera | Vespertilionidae | Nyctalus noctula | 79 | 1.24 | |
| Chiroptera spp. | 48 | 0.76 | 2.00 | ||
| Soricomorpha | Talpidae | Talpa europaea | 79 | 1.24 | |
| Soricidae | Sorex araneus | 379 | 5.96 | ||
| Sorex minutus | 40 | 0.63 | |||
| Neomys fodiens | 6 | 0.09 | |||
| Crocidura leucodon | 2 | 0.03 | 7.95 | ||
| Rodentia | Cricetidae | Clethrionomys glareolus | 847 | 13.33 | |
| Microtus subterraneus | 59 | 0.93 | |||
| Microtus (Alexandromys) oeconomus | 324 | 5.10 | |||
| Microtus agrestis | 2 | 0.03 | |||
| Microtus arvalis | 736 | 11.58 | |||
| Microtus spp. | 103 | 1.62 | |||
| Muridae | Mus musculus | 99 | 1.56 | ||
| Rattus norvegicus | 75 | 1.18 | |||
| Micromys minutus | 422 | 6.64 | |||
| Apodemus agrarius | 858 | 13.50 | |||
| Apodemus sylvaticus | 190 | 2.99 | |||
| Apodemus flavicollis | 741 | 11.66 | |||
| Apodemus spp. | 1251 | 19.69 | |||
| Gliridae | Muscardinus avellanarius | 12 | 0.19 | 90.0 | |
| Carnivora | Mustelidae | Mustela nivalis | 3 | 0.05 | 0.05 |
| Total | 6355 | 100 | 100 |
| Study Site | Ni | Ns | Sr | Hr |
|---|---|---|---|---|
| PRZ | 63 | 7 | 6.1 | 1.44 |
| SLA | 32 | 12 | 12 | 2.21 |
| WIS | 11 | 4 | - | - |
| CZE1 | 22 | 6 | - | - |
| CZE2 | 100 | 13 | 9.2 | 1.82 |
| KRO | 112 | 13 | 8.8 | 1.87 |
| SEC | 24 | 6 | - | - |
| WIL | 10 | 6 | - | - |
| GNI | 32 | 11 | 11 | 2.16 |
| RYB | 39 | 6 | 5.6 | 1.30 |
| NDW | 162 | 13 | 8.6 | 1.84 |
| JRE | 412 | 12 | 7.6 | 1.62 |
| BUK | 6 | 2 | - | - |
| JPA | 7 | 4 | - | - |
| JUL | 785 | 13 | 8.9 | 1.98 |
| BUC | 854 | 13 | 9.3 | 1.98 |
| TAR | 773 | 13 | 8.1 | 1.82 |
| MLO | 526 | 16 | 9.9 | 2.04 |
| BIE | 406 | 13 | 7.5 | 1.57 |
| KEP | 9 | 5 | - | - |
| GKA | 74 | 10 | 7.2 | 1.53 |
| GRO | 183 | 14 | 8.5 | 1.90 |
| OBL | 110 | 13 | 8.3 | 1.74 |
| ZAS | 36 | 6 | 5.7 | 1.27 |
| SOL | 8 | 4 | - | - |
| ZAW2 | 38 | 5 | 4.7 | 1.21 |
| ZAW3 | 36 | 7 | 6.7 | 1.46 |
| ZAW4 | 39 | 8 | 7.3 | 1.44 |
| ZAW5 | 10 | 4 | - | - |
| ZAW7 | 34 | 10 | 9.9 | 2.03 |
| Species | Cluster 1 | Cluster 2 | Cluster 3 | |||
|---|---|---|---|---|---|---|
| Ni | RA (%) | Ni | RA (%) | Ni | RA (%) | |
| Nyctalus noctula | 9 | 0.8 | 31 | 2.3 | 39 | 1.6 |
| Talpa europaea | 15 | 1.4 | 27 | 2.0 | 35 | 1.5 |
| Sorex araneus | 99 | 9.1 | 80 | 6.0 | 196 | 8.1 |
| Sorex minutus | 15 | 1.4 | 11 | 0.8 | 13 | 0.5 |
| Neomys fodiens | 5 | 0.5 | 1 | 0.1 | 0 | 0 |
| Crocidura leucodon | 2 | 0.2 | 0 | 0 | 0 | 0 |
| Clethrionomys glareolus | 198 | 18.2 | 318 | 23.7 | 306 | 12.7 |
| Microtus subterraneus | 7 | 0.6 | 51 | 3.8 | 1 | 0.0 |
| Microtus (Alexandromys) oeconomus | 165 | 15.1 | 47 | 3.5 | 98 | 4.1 |
| Microtus agrestis | 2 | 0.2 | 0 | 0 | 0 | 0 |
| Microtus arvalis | 223 | 20.5 | 94 | 7.0 | 403 | 16.7 |
| Mus musculus | 41 | 3.8 | 10 | 0.7 | 44 | 1.8 |
| Rattus norvegicus | 15 | 1.4 | 24 | 1.8 | 34 | 1.4 |
| Micromys minutus | 119 | 10.9 | 35 | 2.6 | 254 | 10.5 |
| Apodemus agrarius | 69 | 6.3 | 178 | 13.2 | 603 | 25.0 |
| Apodemus sylvaticus | 5 | 0.5 | 19 | 1.4 | 166 | 6.9 |
| Apodemus flavicollis | 87 | 8.0 | 417 | 31.0 | 220 | 9.1 |
| Muscardinus avellanarius | 12 | 1.1 | 0 | 0 | 0 | 0 |
| Mustela nivalis | 2 | 0.2 | 1 | 0.1 | 0 | 0 |
| Number of individuals Ni | 1090 | 100.0 | 1344 | 100.0 | 2412 | 100.0 |
| Number of species Ns | 19 | 16 | 14 | |||
| Rarefaction, Ni = 1090 | ||||||
| Species richness Sr | 19 | 15.7 | 13.5 | |||
| Diversity Hr | 2.26 | 2.05 | 2.17 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanowski, J.; Dudek-Godeau, D.; Lesiński, G. The Diversity of Small Mammals along a Large River Valley Revealed from Pellets of Tawny Owl Strix aluco. Biology 2023, 12, 1118. https://doi.org/10.3390/biology12081118
Romanowski J, Dudek-Godeau D, Lesiński G. The Diversity of Small Mammals along a Large River Valley Revealed from Pellets of Tawny Owl Strix aluco. Biology. 2023; 12(8):1118. https://doi.org/10.3390/biology12081118
Chicago/Turabian StyleRomanowski, Jerzy, Dorota Dudek-Godeau, and Grzegorz Lesiński. 2023. "The Diversity of Small Mammals along a Large River Valley Revealed from Pellets of Tawny Owl Strix aluco" Biology 12, no. 8: 1118. https://doi.org/10.3390/biology12081118
APA StyleRomanowski, J., Dudek-Godeau, D., & Lesiński, G. (2023). The Diversity of Small Mammals along a Large River Valley Revealed from Pellets of Tawny Owl Strix aluco. Biology, 12(8), 1118. https://doi.org/10.3390/biology12081118





