Berberine and Its Study as an Antidiabetic Compound
Abstract
Simple Summary
Abstract
1. Introduction
2. Berberine as a Therapeutic Natural Compound
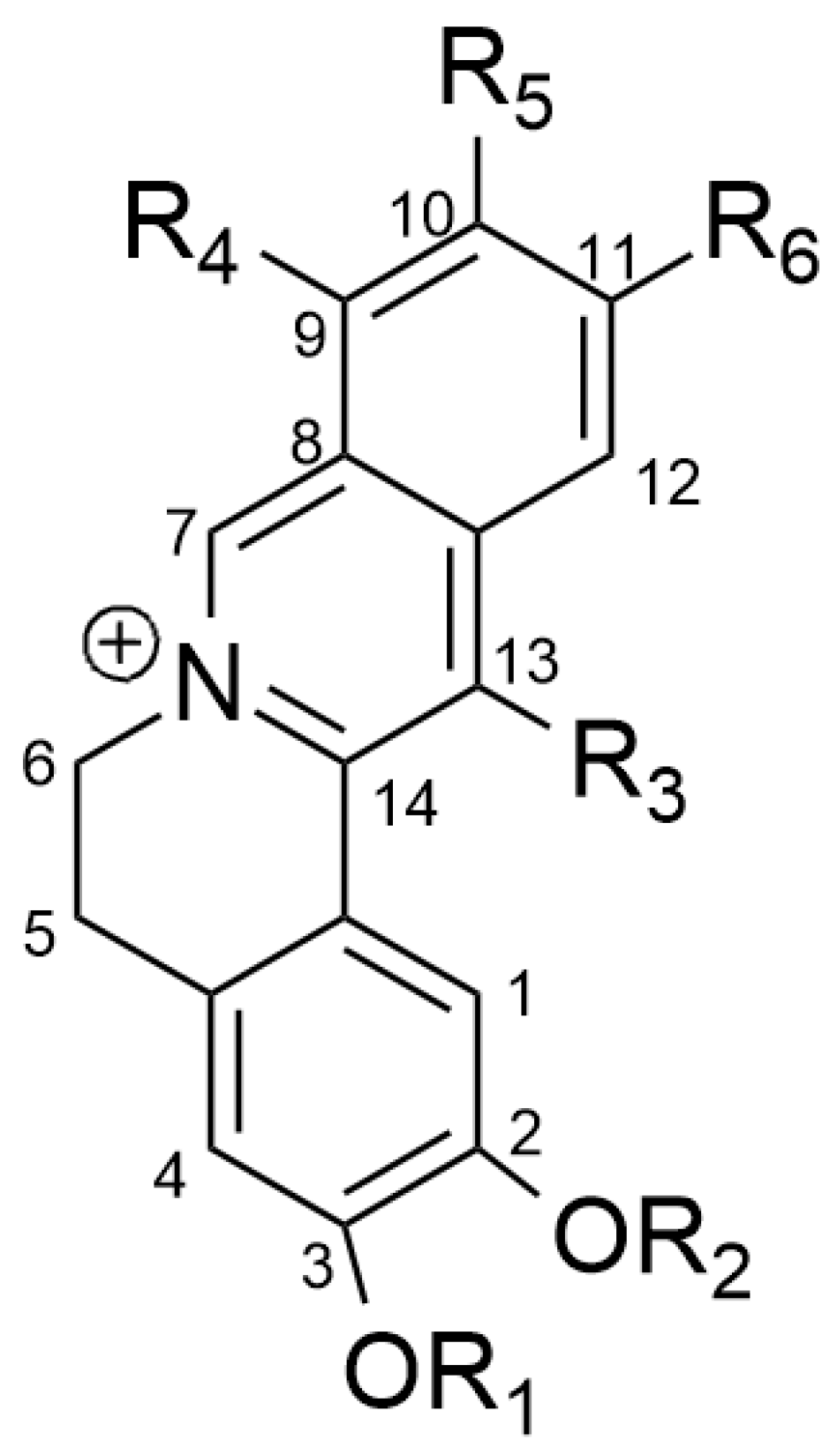
3. Berberine and Structure–Activity Relationship (SAR)
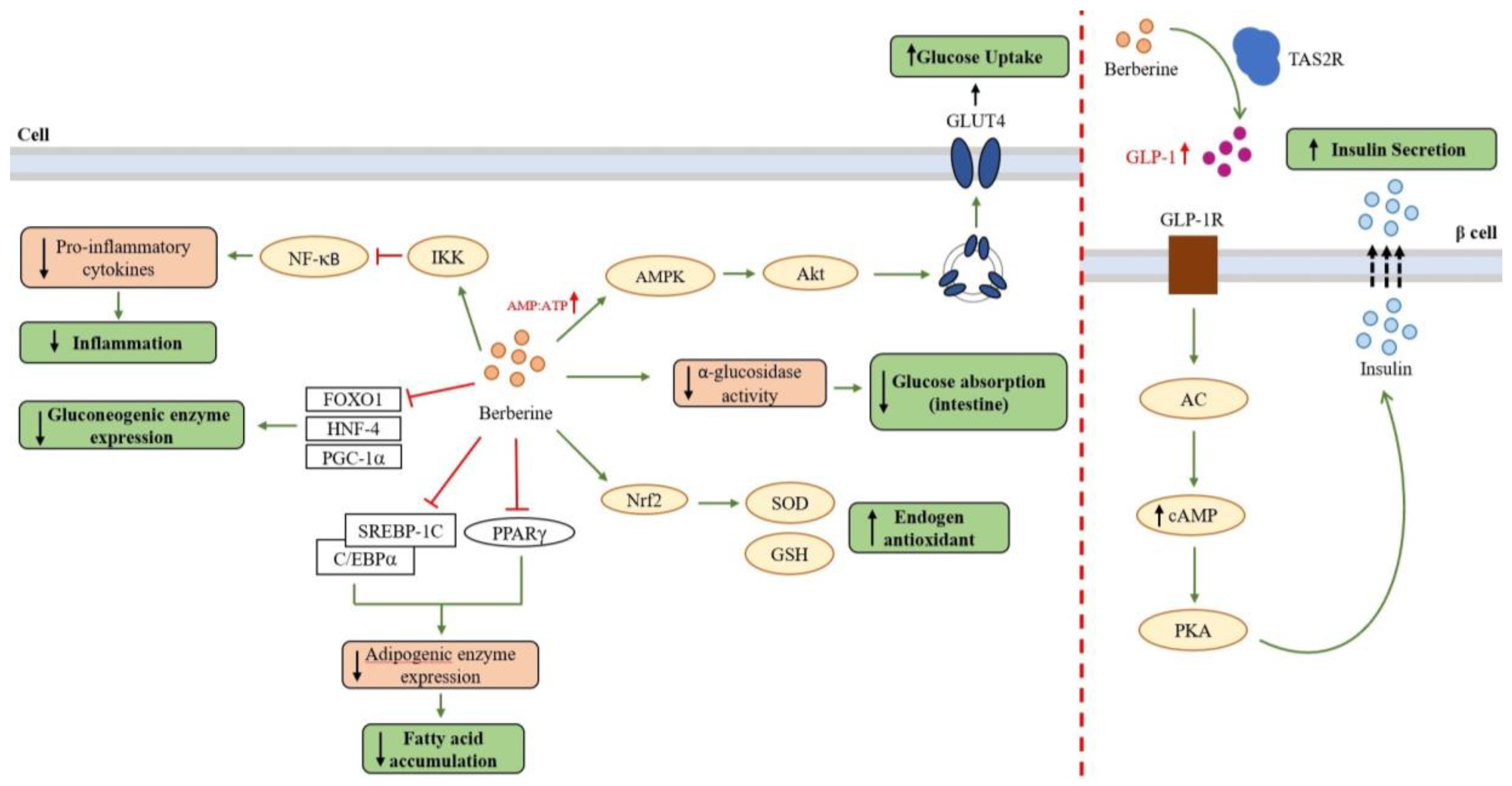
4. Berberine as an Antidiabetic Compound
4.1. Berberine as Anti-Diabetic Agent via the AMPK Pathway
4.2. Berberine as an Anti-Hyperglycemic Agent
4.3. Berberine as Anti-Adipogenic and Anti-Hyperlipidemic Agent
4.4. Berberine as Anti-Inflammatory and Antioxidant Agent
5. Development of Berberine Usage
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Widowati, W. Potensi Antioksidan Sebagai Antidiabetes. Maranatha J. Med. Health 2018, 7, 1–11. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; IDF: Brussels, Belgium, 2021; ISBN 978-2-930229-98-0. [Google Scholar]
- Prawitasari, D.S. Diabetes Melitus Dan Antioksidan. KELUWIH J. Kesehat. dan Kedokt. 2019, 1, 48–52. [Google Scholar] [CrossRef]
- Gutiérrez-Rodelo, C.; Roura-Guiberna, A.; Alberto Olivares-Reyes, J. Molecular Mechanisms of Insulin Resistance: An Update. Gac. Media Mex. 2017, 153, 197–209. [Google Scholar]
- Bare, Y.; Maulidi, A.; Sari, D.R.T.; Tiring, S.S.N.D. Studi in Silico Prediksi Potensi 6-Gingerol Sebagai Inhibitor c-Jun N-Terminal Kinases (JNK). J. Jejaring Mat. dan Sains 2019, 1, 59–63. [Google Scholar] [CrossRef]
- Maksum, I.P.; Maulana, A.F.; Yusuf, M.; Mulyani, R.; Destiarani, W.; Rustaman, R. Molecular Dynamics Simulation of a TRNA-Leucine Dimer with an A3243G Heteroplasmy Mutation in Human Mitochondria Using a Secondary Structure Prediction Approach. Indones. J. Chem. 2022, 22, 1043–1051. [Google Scholar] [CrossRef]
- Destiarani, W.; Mulyani, R.; Yusuf, M.; Maksum, I.P. Molecular Dynamics Simulation of T10609C and C10676G Mutations of Mitochondrial ND4L Gene Associated With Proton Translocation in Type 2 Diabetes Mellitus and Cataract Patients. Bioinform. Biol. Insights 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- Puspita, S.R.; Fariz, M.A.; Muhammad, Y.; Maksum Iman, P. Simulation Modeling of A3243g Mutations on TRNALeu (UUR) against Type 2 Diabetes Mellitus Using In Silico Method. Res. J. Chem. Environ. 2023, 27, 65–71. [Google Scholar] [CrossRef]
- Maksum, I.P.; Farhani, A.; Rachman, S.D.; Ngili, Y. Making of the A3243g Mutant Template through Site Directed Mutagenesis as Positive Control in PASA-Mismatch Three Bases. Int. J. PharmTech Res. 2013, 5, 441–450. [Google Scholar]
- Akita, Y.; Koga, Y.; Iwanaga, R.; Wada, N.; Tsubone, J.; Fukuda, S.; Nakamura, Y.; Kato, H. Fatal Hypertrophic Cardiomyopathy Associated with an A8296G Mutation in the Mitochondrial TRNA(Lys) Gene. Hum. Mutat. 2000, 15, 382. [Google Scholar] [CrossRef]
- Wilson, F.H.; Hariri, A.; Farhi, A.; Zhao, H.; Petersen, K.F.; Toka, H.R.; Nelson-Williams, C.; Raja, K.M.; Kashgarian, M.; Shulman, G.I.; et al. A Cluster of Metabolic Defects Caused by Mutation in a MitF. Stevens RBenjamin, Sunochondrial TRNA. Science 2004, 306, 1190–1194. [Google Scholar] [CrossRef]
- Azizah, M.I.; Mulyani, R.; Maksum, I.P. Design and Optimization of PCR-RFLP Assay for Detection of G9053A and T15663C Mutation in Mitochondrial DNA. Res. J. Chem. Environ. 2023, 27, 1–5. [Google Scholar] [CrossRef]
- Maksum, I.P.; Saputra, S.R.; Indrayati, N.; Yusuf, M.; Subroto, T. Bioinformatics Study of m.9053G>A Mutation at the ATP6 Gene in Relation to Type 2 Diabetes Mellitus and Cataract Diseases. Bioinform. Biol. Insights 2017, 11, 1177932217728515. [Google Scholar] [CrossRef]
- Maksum, I.P. Patogenetika, Investigasi & Terapi Penyakit Mitokondria; Bitread Publishing: Jakarta, Indonesia, 2018. [Google Scholar]
- American Diabetes Association Standards of Medical Care in Diabetes-2014. Diabetes Care 2014, 37, 14–80. [CrossRef]
- Maulana, A.F.; Sriwidodo, S.; Rukayadi, Y.; Maksum, I.P. In Silico Study of Mangostin Compounds and Its Derivatives as Inhibitors of α-Glucosidase Enzymes for Anti-Diabetic Studies. Biology 2022, 11, 1837. [Google Scholar] [CrossRef]
- Pulgaron, E.R.; Delamater, A.M. Obesity and Type 2 Diabetes in Children: Epidemiology and Treatment. Curr. Diab. Rep. 2014, 14. [Google Scholar] [CrossRef]
- Narasimhan, S.; Weinstock, R.S. Youth-Onset Type 2 Diabetes Mellitus: Lessons Learned from the TODAY Study. Mayo Clin. Proc. 2014, 89, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Chen, L.; Hatch, G.M. Berberine as a Therapy for Type 2 Diabetes and Its Complications: From Mechanism of Action to Clinical Studies. Biochem. Cell Biol. 2015, 93, 479–486. [Google Scholar] [CrossRef]
- Tseng, P.S.; Ande, C.; Moremen, K.W.; Crich, D. Influence of Side Chain Conformation on the Activity of Glycosidase Inhibitors. Angew. Chemie-Int. Ed. 2023, 62, 2–6. [Google Scholar] [CrossRef]
- Chennaiah, A.; Bhowmick, S.; Vankar, Y.D. Conversion of Glycals into Vicinal-1,2-Diazides and 1,2-(or 2,1)-Azidoacetates Using Hypervalent Iodine Reagents and Me3SiN3. Application in the Synthesis of: N -Glycopeptides, Pseudo-Trisaccharides and an Iminosugar. RSC Adv. 2017, 7, 41755–41762. [Google Scholar] [CrossRef]
- Rajasekaran, P.; Ande, C.; Vankar, Y.D. Synthesis of (5,6 & 6,6)-Oxa-Oxa Annulated Sugars as Glycosidase Inhibitors from 2-Formyl Galactal Using Iodocyclization as a Key Step. Arkivoc 2022, 2022, 5–23. [Google Scholar] [CrossRef]
- Kesuma, D.; Siswandono, S.; Purwanto, B.T.; Hardjono, S. Uji in Silico Aktivitas Sitotoksik Dan Toksisitas Senyawa Turunan N-(Benzoil)-N’-Feniltiourea Sebagai Calon Obat Antikanker. J. Pharm. Sci. Clin. Res. 2018, 3, 1–11. [Google Scholar] [CrossRef]
- Yin, J.; Xing, H.; Ye, J. Efficacy of Berberine in Patients with Type 2 Diabetes. Metabolism. 2008, 57, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Wang, N.; Zhao, L.; Lu, F. Berberine in The Treatment of Type 2 Diabetes Mellitus: A Systemic Review and Meta-Analysis. Evid.-Based Complement. Altern. Med. 2012, 2012, 591654. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ehli, E.A.; Kittelsrud, J.; Ronan, P.J.; Munger, K.; Downey, T.; Bohlen, K.; Callahan, L.; Munson, V.; Jahnke, M.; et al. Lipid-Lowering Effect of Berberine in Human Subjects and Rats. Phytomedicine 2012, 19, 861–867. [Google Scholar] [CrossRef]
- Yin, J.; Ye, J.; Jia, W. Effects and Mechanisms of Berberine in Diabetes Treatment. Acta Pharm. Sin. B 2012, 2, 327–334. [Google Scholar] [CrossRef]
- Purwaningsih, I.; Maksum, I.P.; Sumiarsa, D.; Sriwidodo, S. A Review of Fibraurea Tinctoria and Its Component, Berberine, as an Antidiabetic and Antioxidant. Molecules 2023, 28, 1294. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural Products for the Treatment of Type 2 Diabetes Mellitus: Pharmacology and Mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef]
- Di, S.; Han, L.; An, X.; Kong, R.; Gao, Z.; Yang, Y.; Wang, X.; Zhang, P.; Ding, Q.; Wu, H.; et al. In Silico Network Pharmacology and in Vivo Analysis of Berberine-Related Mechanisms against Type 2 Diabetes Mellitus and Its Complications. J. Ethnopharmacol. 2021, 276, 114180. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, I.; Kumar, S.; Rajesh, S. Dipeptidyl Peptidase IV Inhibitory Activity of Berberine and Mangiferin: An In Silico Approach. Int. J. Clin. Endocrinol. Metab. 2017, 3, 018–022. [Google Scholar] [CrossRef]
- Mandar, B.K.; Khanal, P.; Patil, B.M.; Dey, Y.N.; Pasha, I. In Silico Analysis of Phytoconstituents from Tinospora Cordifolia with Targets Related to Diabetes and Obesity. Silico Pharmacol. 2021, 9, 3. [Google Scholar] [CrossRef]
- Shang, X.F.; Yang, C.J.; Morris-Natschke, S.L.; Li, J.C.; Yin, X.D.; Liu, Y.Q.; Guo, X.; Peng, J.W.; Goto, M.; Zhang, J.Y.; et al. Biologically Active Isoquinoline Alkaloids Covering 2014–2018. Med. Res. Rev. 2020, 40, 2212–2289. [Google Scholar] [CrossRef] [PubMed]
- Laborda, P.; Wang, S.Y.; Voglmeir, J. Influenza Neuraminidase Inhibitors: Synthetic Approaches, Derivatives and Biological Activity. Molecules 2016, 21, 1513. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Chung, S.M.; Enkhtaivan, G.; Patel, R.V.; Shin, H.S.; Mistry, B.M. Molecular Docking Studies and Biological Evaluation of Berberine–Benzothiazole Derivatives as an Anti-Influenza Agent via Blocking of Neuraminidase. Int. J. Mol. Sci. 2021, 22, 2368. [Google Scholar] [CrossRef]
- Kim, J.H.; Ryu, Y.B.; Lee, W.S.; Kim, Y.H. Neuraminidase Inhibitory Activities of Quaternary Isoquinoline Alkaloids from Corydalis Turtschaninovii Rhizome. Bioorganic Med. Chem. 2014, 22, 6047–6052. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kapoor, A.; Bhatnagar, A. Physiological and Pathological Roles of Aldose Reductase. Metabolites 2021, 11, 655. [Google Scholar] [CrossRef]
- Tang, W.H.; Martin, K.A.; Hwa, J. Aldose Reductase, Oxidative Stress, and Diabetic Mellitus. Front. Pharmacol. 2012, 3, 87. [Google Scholar] [CrossRef]
- Kou, S.; Han, B.; Wang, Y.; Huang, T.; He, K.; Han, Y.; Zhou, X.; Ye, X.; Li, X. Synergetic Cholesterol-Lowering Effects of Main Alkaloids from Rhizoma Coptidis in HepG2 Cells and Hypercholesterolemia Hamsters. Life Sci. 2016, 151, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMP-Activated Protein Kinase: A Master Switch in Glucose and Lipid Metabolism. Rev. Endocr. Metab. Disord. 2004, 5, 119–125. [Google Scholar] [CrossRef]
- Kahn, B.B.; Alquier, T.; Carling, D.; Hardie, D.G. AMP-Activated Protein Kinase: Ancient Energy Gauge Provides Clues to Modern Understanding of Metabolism. Cell Metab. 2005, 1, 15–25. [Google Scholar] [CrossRef]
- Brusq, J.-M.; Ancellin, N.; Grondin, P.; Guillard, R.; Martin, S.; Saintillan, Y.; Issandou, M. Inhibition of Lipid Synthesis Activation of AMP Kinase: An Additional Mechanism for the Hypolipidemic Effects of Berberine. J. Lipid Res. 2006, 47, 1281–1288. [Google Scholar] [CrossRef]
- Kim, S.H.; Shin, E.-J.; Kim, E.-D.; Bayaraa, T.; Frost, S.C.; Hyun, C.-K. Berberin Activates GLUT1-Mediated Glucose Uptake in 3T3-L1 Adipocytes. Biol. Pharm. Bull. 2007, 30, 2120–2125. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, W.S.; Kim, K.H.; Yoon, M.J.; Cho, H.J.; Shen, Y.; Ye, J.-M.; Lee, C.H.; Oh, W.K.; Kim, C.T.; et al. Berberine, a Natural Plant Product, Activates AMP-Activated Protein Kinase With Beneficial Metabolic Effects in Diabetic and Insulin-Resistant States. Diabetes 2006, 55, 2256–2264. [Google Scholar] [CrossRef]
- Cheng, Z.; Pang, T.; Gu, M.; Gao, A.-H.; Xie, C.-M.; Li, J.-Y.; Nan, F.-J.; Li, J. Berberine-Stimulated Glucose Uptake in L6 Myotubes Involves Both AMPK and P38 MAPK. Biochim. Biophys. Acta (BBA)-General Subj. 2006, 1760, 1682–1689. [Google Scholar] [CrossRef]
- Yin, J.; Gao, Z.; Liu, D.; Liu, Z.; Ye, J. Berberine Improves Glucose Metabolism Through Induction of Glycolysis. Am. J. Physiol. Metab. 2008, 294, E148–E156. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.D.; Cheng, C.H.K.; Tan, R.X. Monoamine Oxidase Inhibitors from Rhizoma of Coptis Chinensis. Planta Med. 2001, 67, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Iagodina, O.V.; Nikol’skaia, E.B.; Faddeeva, M.D. Inhibiton of Liver Mitochondrial Monoamine Oxidase Activity by Alkaloids Isolated from Chelidonium and Macleaya and by Their Derivative Drugs. Tsitologiia 2003, 45, 1032–1037. [Google Scholar]
- Castillo, J.; Hung, J.; Rodriguez, M.; Bastidas, E.; Laboren, I.; Jaimes, A. LED Fluorescence Spectroscopy for Direct Determination of Monoamine Oxidase B Inactivation. Anal. Biochem. 2005, 343, 293–298. [Google Scholar] [CrossRef]
- Cheng, Z.; Chen, A.-F.; Wu, F.; Sheng, L.; Zhang, H.-K.; Gu, M.; Li, Y.-Y.; Zhang, L.-N.; Hu, L.-H.; Li, J.-Y.; et al. 8,8-Dimethyldihydroberberine with Improved Bioavailability and Oral Efficacy on Obese and Diabetic Mouse Models. Bioorg. Med. Chem. 2010, 18, 5915–5924. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Li, J.-Y.; Gosby, A.; To, S.W.C.; Cheng, Z.; Miyoshi, H.; Taketo, M.M.; Cooney, G.J.; Kraegen, E.W.; James, D.E.; et al. Berberine and Its More Biologically Available Derivative, Dihydroberberine, Inhibit Mitochondrial Respiratory Complex I: A Mechanism for the Action of Berberine to Activate AMP-Activated Protein Kinase and Improve Insulin Action. Diabetes 2008, 57, 1414–1418. [Google Scholar] [CrossRef]
- Ko, B.-S.; Choi, S.B.; Park, S.K.; Jang, J.S.; Kim, Y.E.; Park, S. Insulin Sensitizing and Insulinotropic Action of Berberine from Cortidis Rhizoma. Biol. Pharm. Bull. 2005, 28, 1431–1437. [Google Scholar] [CrossRef]
- Chang, W.; Zhang, M.; Li, J.; Meng, Z.; Wei, S.; Du, H.; Chen, L.; Hatch, G.M. Berberine Improves Insulin Resistance in Cardiomyocytes via Activation of 5′-Adenosine Monophosphate-Activated Protein Kinase. Metabolism 2013, 62, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Davies, G.E. Berberine Inhibitis Adipogenesis in High-Fat Diet-Induced Obesity Mice. Fitoterapia 2010, 81, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Kaboli, P.J.; Leong, M.P.; Ismail, P.; Ling, K. Antitumor Effects of Berberine against EGFR, ERK1/2, P38 and AKT in MDA-MB231 and MCF-7 Breast Cancer Cells Using Molecular Modelling and in Vitro Study. Pharmacol. Reports 2018, 71, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Huang, R.; Li, Q.; Liu, Y.; Li, Y.; Chen, H.; Ai, G.; Xie, J.; Zeng, H.; Chen, J.; et al. Oxyberberine, an Absorbed Metabolite of Berberine, Possess Superior Hypoglycemic Effect via Regulating the PI3K/Akt and Nrf2 Signaling Pathways. Biomed. Pharmacother. 2021, 137. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, X. Network Pharmacology and Molecular Docking Analysis on Targets and Mechanisms of Berberine in Atypical Antipsychotic-Induced Metabolic Syndrome. Phytochem. Pharmacol. Tradit. Chinese Med. 2022, 17, 1934578X221129106. [Google Scholar] [CrossRef]
- Li, Z.; Chen, M.; Wang, Z.; Fan, Q.; Lin, Z.; Tao, X.; Wu, J.; Liu, Z.; Lin, R.; Zhao, C. Berberine Inhibits RA-FLS Cell Proliferation and Adhesion by Regulating RAS/MAPK/FOXO/HIF-1 Signal Pathway in the Treatment of Rheumatoid Arthritis. Bone Joint Res. 2023, 12, 91–102. [Google Scholar] [CrossRef]
- Zabidi, N.A.; Ishak, N.A.; Hamid, M.; Ashari, S.E.; Mohammad Latif, M.A. Inhibitory Evaluation of Curculigo Latifolia on α-Glucosidase, DPP (IV) and in Vitro Studies in Antidiabetic with Molecular Docking Relevance to Type 2 Diabetes Mellitus. J. Enzyme Inhib. Med. Chem. 2021, 36, 109–121. [Google Scholar] [CrossRef]
- Cao, J.; Li, L.; Xiong, L.; Wang, C.; Chen, Y.; Zhang, X. Research on the Mechanism of Berberine in the Treatment of COVID-19 Pneumonia Pulmonary Fibrosis Using Network Pharmacology and Molecular Docking. Phytomedicine Plus 2022, 2, 100252. [Google Scholar] [CrossRef]
- Lu, S.S.; Yu, Y.L.; Zhu, H.J.; Liu, X.D.; Liu, L.; Liu, Y.W.; Wang, P.; Xie, L.; Wang, G.J. Berberine Promotes Glucagon-like Peptide-1 (7–36) Amide Secretion in Streptozotocin-Induced Diabetic Rats. J. Endocrinol. 2009, 200, 159–165. [Google Scholar] [CrossRef]
- Tengholm, A.; Gylfe, E. CAMP Signalling in Insulin and Glucagon Secretion. Diabetes Obes. Metab. 2017, 19, 42–53. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, L.; Wang, X.; Liu, X.; Liu, X.; Xie, L.; Wang, G. Modulation of Glucagon-like Peptide-1 Release by Berberine: In Vivo and In Vitro Studies. Biochem. Pharmacol. 2010, 79, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Hao, G.; Zhang, Q.; Hua, W.; Wang, M.; Zhou, W.; Zong, S.; Huang, M.; Wen, X. Berberine Induces GLP-1 Secretion through Activation of Bitter Taste Receptor Pathways. Biochem. Pharmacol. 2015, 97, 173–177. [Google Scholar] [CrossRef]
- Kim, W.S.; Lee, Y.S.; Cha, S.H.; Jeong, H.W.; Cheo, S.S.; Lee, M.-R.; Oh, G.T.; Park, H.-S.; Lee, K.-U.; Lane, M.D.; et al. Berberine Improves Lipid Dysregulation in Obesity by Controlling Central and Peripheral AMPK Activity. Am. J. Physiol. Metab. 2009, 296, E812–E819. [Google Scholar] [CrossRef]
- Zhang, M.; Lv, X.; Li, J.; Meng, Z.; Wang, Q.; Chang, W.; Li, W.; Chen, L.; Liu, Y. Sodium Caprate Augments the Hypoglycemic Effect of Berberine via AMPK in Inhibiting Hepatic Gluconeogenesis. Mol. Cell. Endocrinol. 2012, 363, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Yan, J.; Shen, Y.; Tang, K.; Yin, J.; Zhang, Y.; Yang, D.; Liang, H.; Ye, J.; Weng, J. Berberine Improves Glucose Metabolism in Diabetic Rats by Inhibition of Hepatic Gluconeogenesis. PLoS One 2011, 6, e16556. [Google Scholar] [CrossRef]
- Paleva, R. Mekanisme Resistensi Insulin Terkait Obesitas. J. Ilm. Kesehat. Sandi Husada 2019, 10, 354–358. [Google Scholar]
- Rosen, E.D.; MacDougald, O.A. Adipocyte Differentiation from the inside Out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef]
- Jacobs, M.D.; Harrison, S.C. Structure of an IκBα/NF-ΚB Complex. Cell 1998, 95, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Khan, A. Antioxidants and Diabetes. Indian J. Endocrinol. Metab. 2012, 16, 267–271. [Google Scholar] [CrossRef]
- Ceriello, A.; Testa, R. Antioxidant Anti-Inflammatory Treatment in Type 2 Diabetes. Diabetes Care 2009, 32, 232–236. [Google Scholar] [CrossRef]
- Murphy, M.P. How Mitochondria Produce Reactive Oxygen Species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Zhou, S.-W. Protective Effect of Berberine on Antioxidant Enzymes and Positive Transcription Elongation Factor b Expression in Diabetic Rat Liver. Fitoterapia 2011, 82, 184–189. [Google Scholar] [CrossRef]
- Tang, L.-Q.; Wei, W.; Chen, L.-M.; Liu, S. Effects of Berberine on Diabetes Induced by Alloxan and a High-Fat/High-Cholesterol Diet in Rats. J. Ethnopharmacol. 2006, 108, 109–115. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, X.; Wu, N.; Han, Y.; Wang, J.; Yu, Y.; Chen, Q. Efficacy and Safety of Berberine Alone for Several Metabolic Disorders: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Front. Pharmacol. 2021, 12, 885. [Google Scholar] [CrossRef] [PubMed]
- Ayun, A.Q.; Faridah, D.N.; Yuliana, N.D.; Andriyanto, A. Pengujian Toksisitas Akut LD50 Infusa Benalu Teh (Scurrula Sp.) Dengan Menggunakan Mencit (Mus Musculus). Acta Vet. Indones. 2021, 9, 53–63. [Google Scholar] [CrossRef]
- Labibah, L.; Rusdiana, T. Hubungan Jenis Kelamin Terhadap Eksipien Farmasi Dalam Mempengaruhi Bioavailabilitas Obat. Maj. Farmasetika 2022, 7, 176–188. [Google Scholar] [CrossRef]
- Kheir, M.M.; Wang, Y.; Hua, L.; Hu, J.; Li, L.; Lei, F.; Du, L. Acute Toxicity of Berberine and Its Correlation with the Blood Concentration in Mice. Food Chem. Toxicol. 2010, 48, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Ye, X.; Wang, D.; He, K.; Yang, Y.; Liu, X.; Li, X. Safety Evaluation of Main Alkaloids from Rhizoma Coptidis. J. Ethnopharmacol. 2013, 145, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Huang, Y.F.; Liang, J.; Zhou, H. Improved Up-and-down Procedure for Acute Toxicity Measurement with Reliable LD50 Verified by Typical Toxic Alkaloids and Modified Karber Method. BMC Pharmacol. Toxicol. 2022, 23, 3. [Google Scholar] [CrossRef]
- Singh, N.; Sharma, B. Toxicological Effects of Berberine and Sanguinarine. Front. Mol. Biosci. 2018, 5, 21. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zou, D.; Liu, W.; Yang, J.; Zhu, N.; Huo, L.; Wang, M.; Hong, J.; Wu, P.; et al. Treatment of Type 2 Diabetes and Dyslipidemia with The Natural Plant Alkaloid Berberine. J. Clin. Endocrinol. Metab. 2008, 93, 2559–2565. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, J.; Xue, R.; Wu, J.D.; Zhao, W.; Wang, Z.Z.; Wang, S.K.; Zhou, Z.X.; Song, D.Q.; Wang, Y.M.; et al. Berberine Lowers Blood Glucose in Type 2 Diabetes Mellitus Patients through Increasing Insulin Receptor Expression. Metabolism. 2010, 59, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Hao, H.P.; Xie, H.G.; Lai, L.; Wang, Q.; Liu, C.X.; Wang, G.J. Extensive Intestinal First-Pass Elimination and Predominant Hepatic Distribution of Berberine Explain Its Low Plasma Levels in Rats. Drug Metab. Dispos. 2010, 38, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Tan, L.H.; Wang, Y.F.; Luo, C.D.; Chen, H.B.; Lu, Q.; Li, Y.C.; Yang, X.B.; Chen, J.N.; Liu, Y.H.; et al. Comparison of Anti-Inflammatory Effects of Berberine, and Its Natural Oxidative and Reduced Derivatives from Rhizoma Coptidis in Vitro and in Vivo. Phytomedicine 2019, 52, 272–283. [Google Scholar] [CrossRef] [PubMed]
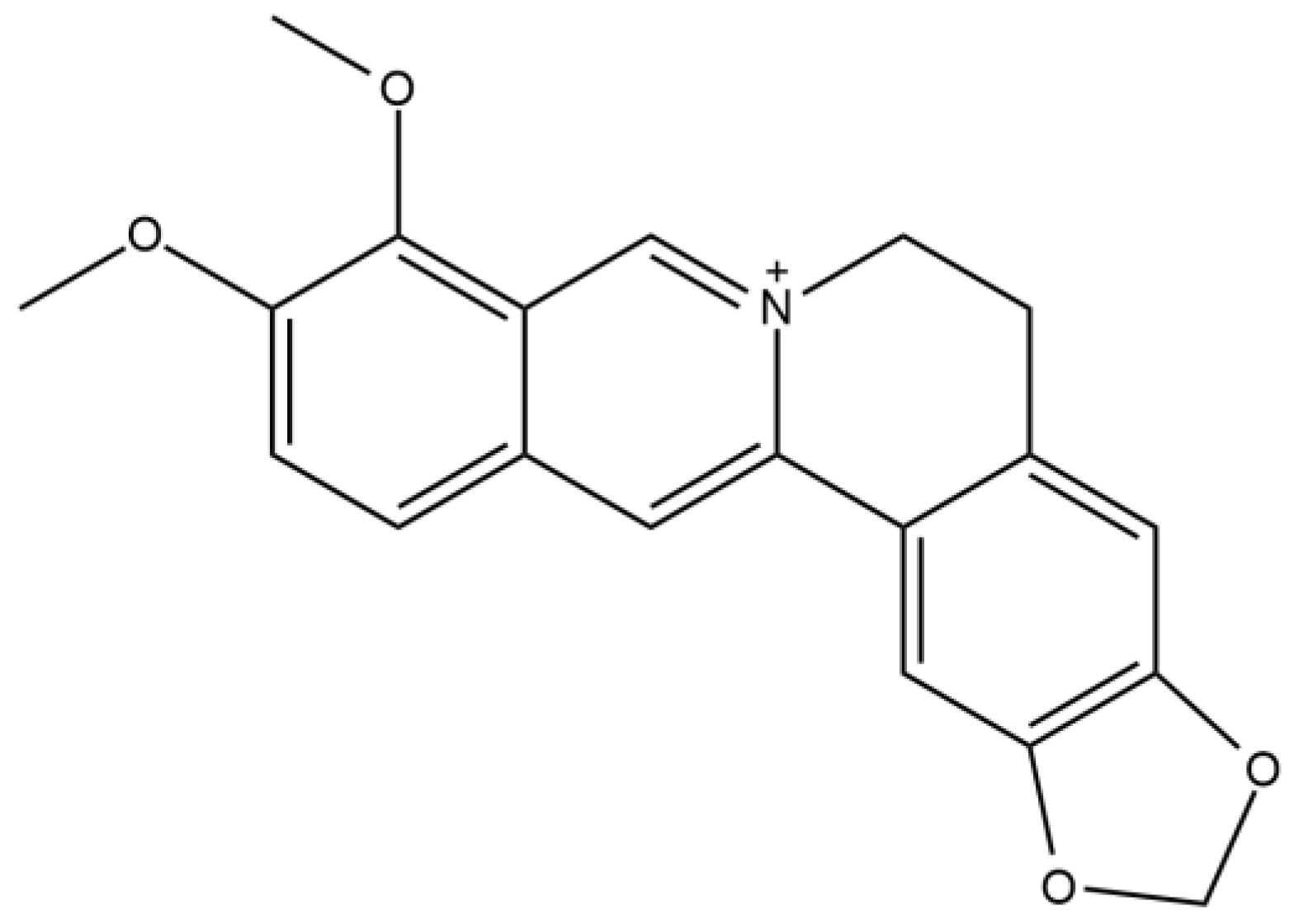
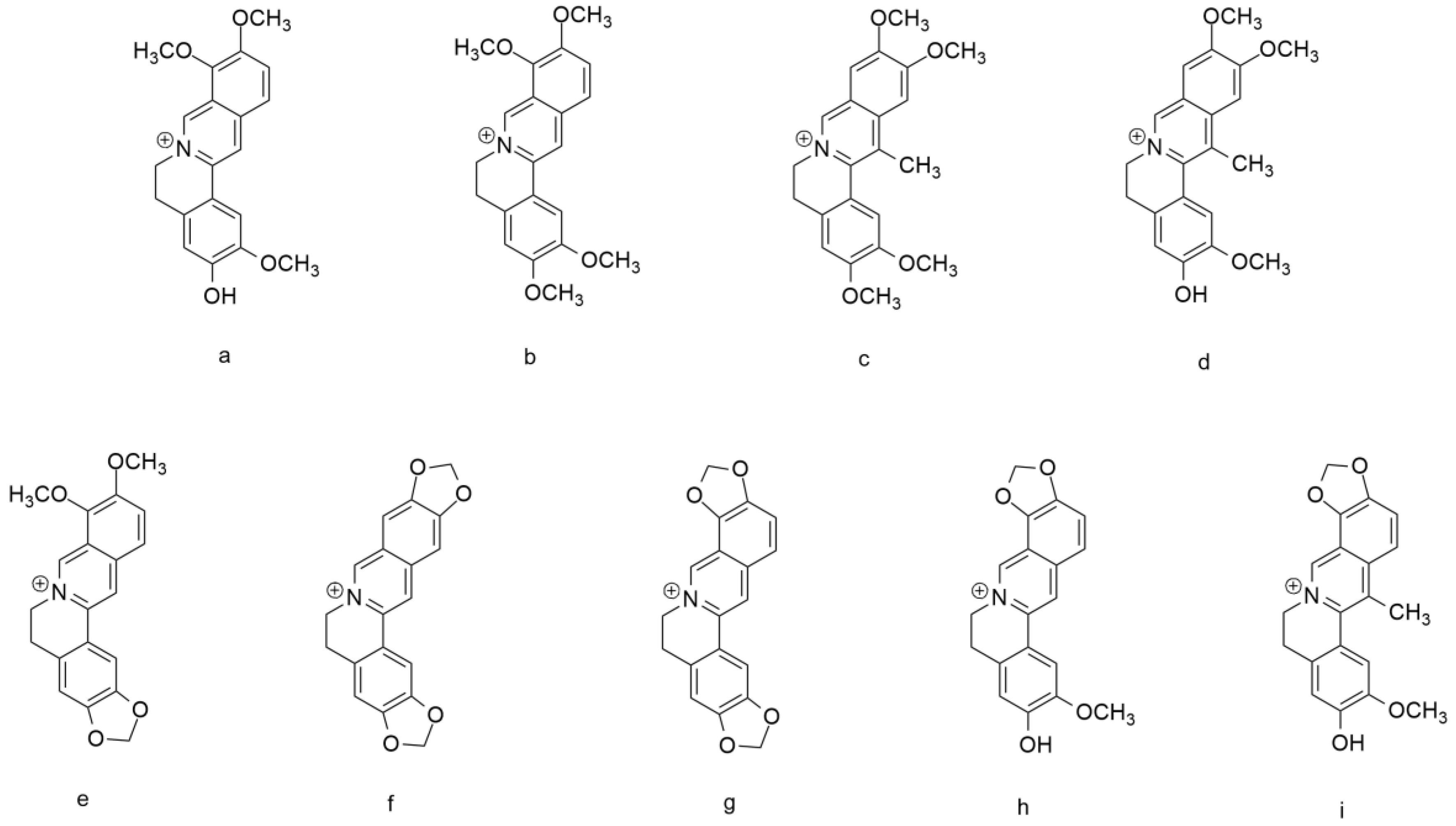
| Compounds | IC50 (μM) |
|---|---|
| Berberine | 13.5 ± 2.3 |
| Jatrorrhizine | 37.0 ± 1.8 |
| Pseudocoptisine | 65.2 ± 4.5 |
| Coptisine | 25.1 ± 0.8 |
| Palmatine | 12.8 ± 1.5 |
| Pseudodehydrocorydaline | 32.6 ± 2.1 |
| Dehydrocorylbulbine | 41.3 ± 3.5 |
| R1 | R2 | R3 | R4 | R5 | R6 | |
|---|---|---|---|---|---|---|
| 1 | -CH2- | H | OMe | OMe | H | |
| 2 | -CH2- | H | -OCH2O- | H | ||
| 3 | Me | Me | H | OMe | OMe | H |
| 4 | Me | Me | H | -OCH2O- | H | |
| 5 | Me | H | H | OMe | OMe | H |
| Protein Targets | Binding Energy (kcal/mol) | Interactions | References |
|---|---|---|---|
| PI3K Akt GSK-3β Keap-1 | −5.8 −8.3 −7.6 −8.6 | Gln8, Lys42 Lys179 Lys183 Val97, Ile236 | [56] |
| Akt | −7.83 | Arg4, Thr291, Val164, Met281, Ala177, Leu156, Met227, Asp292 | [55] |
| PPARG | −8.4 | Ile281, Arg288, Ile341 | [57] |
| FOXO3 FOXO4 IKK-β | −8.9 −9.0 −7.7 | *n/i *n/i *n/i | [58] |
| α-glucosidase DPP-IV Insulin receptor (IR) | −7.9 −8.9 −6.3 | Asp568, Tyr709 Tyr547, Gln553, Ser630 Gln1004 | [59] |
| TNF-α IL-6 | −6.24 −4.94 | Lys65, Phe144 Asn135 | [60] |
| Compound | LD50 | References | ||
|---|---|---|---|---|
| IV | IP | IG | ||
| Berberine hydrochloride | 9.04 ± 0.8 mg/kg | 57.61 ± 23.32 mg/kg | LD50 not found; 20.8 g/kg (safe dose) 41.6 g/kg (toxic dose) | [79] |
| - | - | 2.83 ± 1.21 g/kg | [81] | |
| Berberine from Rhizoma coptidis | - | - | 713.57 mg/kg (0.713 g/kg) | [80] |
| Powdered root B. vulgaris | - | - | 2.60 g/kg | [82] |
| Extract of B. vulgaris | - | - | 1.28 g/kg | [82] |
| Berberine sulphate | - | 205 mg/kg | - | [82] |
| Berberine | - | 23 mg/kg | - | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Utami, A.R.; Maksum, I.P.; Deawati, Y. Berberine and Its Study as an Antidiabetic Compound. Biology 2023, 12, 973. https://doi.org/10.3390/biology12070973
Utami AR, Maksum IP, Deawati Y. Berberine and Its Study as an Antidiabetic Compound. Biology. 2023; 12(7):973. https://doi.org/10.3390/biology12070973
Chicago/Turabian StyleUtami, Ayudiah Rizki, Iman Permana Maksum, and Yusi Deawati. 2023. "Berberine and Its Study as an Antidiabetic Compound" Biology 12, no. 7: 973. https://doi.org/10.3390/biology12070973
APA StyleUtami, A. R., Maksum, I. P., & Deawati, Y. (2023). Berberine and Its Study as an Antidiabetic Compound. Biology, 12(7), 973. https://doi.org/10.3390/biology12070973






