Somatic Growth and Maturity for Four Species of River Cooter Including Pseudemys concinna suwanniensis, P. nelsoni, P. peninsularis, and P. texana
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Sampling Protocol
2.3. Modeling Growth
2.3.1. Mark-Recapture von Bertalanffy Growth Model
2.3.2. Incorporating Site Covariates
2.3.3. Predicted Length at Age and Derived Age at Maturity
2.3.4. Bayesian Implementation
3. Results
3.1. Sampling
3.2. Growth
3.2.1. Species-Specific Growth Rates
3.2.2. Species-Specific Derived Age at Maturity and Longevity
3.2.3. Site-Specific Effects on Growth
3.2.4. Sexual Dimorphism
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turtle Taxonomy Working Group. Turtles of the world: Annotated checklist and atlas of taxonomy, synonymy, distribution, and conservation status. In Conservation Biology of Freshwater Turtles and Tortoises: A Compilation Project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group, 9th ed.; Rhodin, A., Iverson, J., van Dijk, P.P., Stanford, C.B., Goode, E.V., Buhlman, K.A., Bour, R., Eds.; Chelonian Research Foundation and Turtle Conservancy: Lunenburg, MA, USA, 2021; pp. 1–472. [Google Scholar]
- Spinks, P.Q.; Thomson, R.C.; Pauly, G.B.; Newman, C.E.; Mount, G.; Shaffer, H.B. Misleading phylogenetic inferences based on single-exemplar sampling in the turtle genus Pseudemys. Mol. Phylogenetics Evol. 2013, 68, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.R. Pseudemys nelsoni-Florida Red-Bellied Turtle. In Biology and Conservation of Florida Turtles; Meylan, P., Ed.; Chelonian Research Foundation: Lunenburg, MA, USA, 2006; pp. 314–323. [Google Scholar]
- Jackson, D.R. Pseudemys concinna-River Cooter. In Biology and Conservation of Florida Turtles; Meylan, P., Ed.; Chelonian Research Foundation: Lunenburg, MA, USA, 2006; pp. 326–337. [Google Scholar]
- Thomas, R.B.; Jansen, K.P. Pseudemys floridana—Florida Cooter. In Biology and Conservation of Florida Turtles; Meylan, P.A., Ed.; Chelonian Research Foundation: Lunenburg, MA, USA, 2006; pp. 338–347. [Google Scholar]
- Lindeman, P.V. Diet, growth, body size, and reproductive potential of the Texas River Cooter (Pseudemys texana) in the South Llano River, Texas. Southwest. Nat. 2007, 52, 586–594. [Google Scholar] [CrossRef]
- Bancroft, G.T.; Godley, J.S.; Gross, D.T.; Rojas, N.N.; Sutphen, D.A.; McDiarmid, R.W. Large-Scale Operations Management Test of Use of the White Amur for Control of Problem Aquatic Plants: The Herpetofauna of Lake Conway: Species Accounts; U.S. Army Engineer Waterways Experiment Station: Vicksburg, MS, USA, 1983; Report No.: A-83-5. [Google Scholar]
- Meylan, P.A.; Stevens, C.A.; Barnwell, M.E.; Dohm, E.D. Observations on the turtle community of Rainbow Run, Marion Co., Florida. Fla. Sci. 1992, 55, 219–228. [Google Scholar]
- Pearson, R.G.; Stanton, J.; Shoemaker, K.T.; Aiello-Lammens, M.; Ersts, P.J.; Horning, N.; Fordham, D.; Raxworthy, C.J.; Ryu, H.Y.; McNees, J.; et al. Life history and spatial traits predict extinction risk due to climate change. Nat. Clim. Chang. 2014, 4, 217–221. [Google Scholar] [CrossRef]
- Liu, C.; Comte, L.; Olden, J.D. Heads you win, tails you lose: Life-history traits predict invasion and extinction risk of the world’s freshwater fishes. Aquatic. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 773–779. [Google Scholar] [CrossRef]
- Lindeman, P.V. Growth curves for Graptemys, with a comparison to other Emydid Turtles. Am. Midl. Nat. 1999, 142, 141–151. [Google Scholar] [CrossRef]
- Munscher, E.C.; Walde, A.D.; Riedle, J.D.; Hootman, T.; Weber, A.S.; Osborne, W.; Brown, J.; Ross, S.G.; Butterfield, B.P.; Hauge, J.B. Population metrics for the perceived common and abundant Peninsula Cooter and Florida Red-bellied Cooter in a Florida, USA, spring ecosystem. Herpetol. Conserv. Biol. 2022, 17, 51–66. [Google Scholar]
- Aresco, M.J.; Dobie, J.L. Variation in shell arching and sexual size dimorphism of River Cooters, Pseudemys concinna, from two river systems in Alabama. J. Herpetol. 2000, 34, 313. [Google Scholar] [CrossRef]
- Johnston, G.R.; Mitchell, J.C.; Munscher, E.C.; Shemitz, G.A.; Butt, P.L.; Cole, S.; Hootman, T. Temporal variation in a turtle assemblage inhabiting a Florida spring-fed river. Southeast. Nat. 2020, 19, 271–282. [Google Scholar] [CrossRef]
- Kramer, M. Home range of the Florida red-bellied turtle (Pseudemys nelsoni) in a Florida spring run. Copeia 1995, 1995, 883–890. [Google Scholar] [CrossRef]
- Munscher, E.; Walde, A.D.; Stratmann, T.; Butterfield, B.P. Exceptional growth rates observed in immature Pseudemys from a protected spring system in Florida. Herpetol. Notes 2015, 8, 133–140. [Google Scholar]
- Riedle, J.D.; Kuhns, E.H.; Munscher, E.C.; Walde, A.D.; Salvatico, N.; Keserauskis, M.; Butterfield, B.P.; Hauge, J.B. The freshwater turtle community at Blue Spring State Park, Volusia County, Florida, USA. Herpetol. Conserv. Biol. 2016, 11, 362–372. [Google Scholar]
- Munscher, E.C.; Walde, A.D.; Riedle, J.D.; Ross, S.G.; Salvatico, N.; Collins, C.; Farris, M.; Butterfield, B.P.; Hauge, J.B. Turtle assemblage in a highly altered spring system: Comal Springs, Texas. Southwest. Nat. 2020, 64, 109. [Google Scholar] [CrossRef]
- Knight, R. Silenced Springs: From Tragedy to Hope; FSI Press Gainesville: High Springs, FL, USA, 2015. [Google Scholar]
- Cantonati, M.; Fensham, R.J.; Stevens, L.E.; Gerecke, R.; Glazier, D.S.; Goldscheider, N.; Knight, R.L.; Richardson, J.S.; Springer, A.E.; Tockner, K. Urgent plea for global protection of springs. Conserv. Biol. 2021, 35, 378–382. [Google Scholar] [CrossRef]
- Wilson, D.S.; Tracy, C.R.; Tracy, C.R. Estimating age of turtles from growth rings: A critical evaluation of the technique. Herpetologica 2003, 59, 178–194. [Google Scholar] [CrossRef]
- Fabens, A.J. Properties and fitting of the von Bertalanffy growth curve. Growth 1965, 29, 265–289. [Google Scholar]
- Armstrong, D.P.; Brooks, R.J. Estimating ages of turtles from growth data. Chelonian Conserv. Biol. 2014, 13, 9. [Google Scholar] [CrossRef]
- von Bertalanffy, L. Untersuchungen Über die Gesetzlichkeit des Wachstums: I. Teil: Allgemeine Grundlagen der Theorie; Mathematische und physiologische Gesetzlichkeiten des Wachstums bei Wassertieren. Wilhelm Roux Arch. Entwickl. Mech. Org. 1934, 131, 613–652. [Google Scholar]
- Francis, R.I.C.C. Maximum likelihood estimation of growth and growth variability from tagging data. N. Z. J. Mar. Freshw. Res. 1988, 22, 43–51. [Google Scholar] [CrossRef]
- Taylor, N.G.; Walters, C.J.; Martell, S.J.D. A new likelihood for simultaneously estimating von Bertalanffy growth parameters, gear selectivity, and natural and fishing mortality. Can. J. Fish. Aquat. Sci. 2005, 62, 215–223. [Google Scholar] [CrossRef]
- Day, T.; Taylor, P.D. Von Bertalanffy’s growth equation should not be used to model age and size at maturity. Am. Nat. 1997, 149, 381–393. [Google Scholar] [CrossRef]
- Lester, N.P.; Shuter, B.J.; Abrams, P.A. Interpreting the von Bertalanffy model of somatic growth in fishes: The cost of reproduction. Proc. R. Soc. Lond. B 2004, 271, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.P.; Brooks, R.J. Application of hierarchical biphasic growth models to long-term data for snapping turtles. Ecol. Model. 2013, 250, 119–125. [Google Scholar] [CrossRef]
- Jensen, A.L. Beverton and Holt life history invariants result from optimal trade-off of reproduction and survival. Can. J. Fish. Aquat. Sci. 1996, 53, 820–822. [Google Scholar] [CrossRef]
- Charnov, E.L.; Gislason, H.; Pope, J.G. Evolutionary assembly rules for fish life histories. Fish Fish. 2013, 14, 213–224. [Google Scholar] [CrossRef]
- Prince, J.; Hordyk, A.; Valencia, S.R.; Loneragan, N.; Sainsbury, K. Revisiting the concept of Beverton –Holt life-history invariants with the aim of informing data-poor fisheries assessment. ICES J. Mar. Sci. 2015, 72, 194–203. [Google Scholar] [CrossRef]
- Mangel, M. The inverse life-history problem, size-dependent mortality and two extensions of results of Holt and Beverton. Fish Fish. 2017, 18, 1192–1200. [Google Scholar] [CrossRef]
- Rolim, F.A.; Siders, Z.A.; Caltabellotta, F.P.; Rotundo, M.M.; Vaske-Júnior, T. Growth and derived life-history characteristics of the Brazilian electric ray Narcine brasiliensis. J. Fish Biol. 2020, 97, 396–408. [Google Scholar] [CrossRef]
- Caltabellotta, F.P.; Siders, Z.A.; Cailliet, G.M.; Motta, F.S.; Gadig, O.B.F. Preliminary age and growth of the deep-water goblin shark Mitsukurina owstoni (Jordan, 1898). Mar. Freshw. Res. 2021, 72, 432–438. [Google Scholar] [CrossRef]
- Walsh, S.J.; Knowles, L.; Katz, B.G.; Strom, D.G. Hydrology, Water Quality, and Aquatic Communities of Selected Springs in the St. Johns River Water Management District, Florida; U. S. Geological Survey: Reston, VA, USA, 2009; Report No.: 2009–0546.
- Munscher, E.C.; Campbell, J.B.; Hauge, J.B.; Hootman, T.; Osborne, W.; Butterfield, B.P.; Hauge, J.B. Natural history note: Pseudemys floridana peninsularis and Pseudemys nelsoni: Longetivity in the wild. Herpetol. Rev. 2020, 51, 111–113. [Google Scholar]
- Munscher, E.C.; Butterfield, B.P.; Munscher, J.S.; Barrett, E.A.; Hauge, J.B. The North American Freshwater Turtle Research Group (NAFTRG): An undergraduate research experience (URE) and citizen scientist project. Reptiles Amphib. 2013, 20, 119–129. [Google Scholar] [CrossRef]
- Ernst, C.H.; Lovich, J.E. Turtles of the United States and Canada; JHU Press: Baltimore, MD, USA, 2009. [Google Scholar]
- Cagle, F.R. A system of marking turtles for future identification. Copeia 1939, 1939, 170–173. [Google Scholar] [CrossRef]
- Runyan, A.; Meylan, P. PIT tag retention in Trachemys and Pseudemys. Herpetol. Rev. 2005, 36, 45–46. [Google Scholar]
- Gwinn, D.C.; Allen, M.S.; Rogers, M.W. Evaluation of procedures to reduce bias in fish growth parameter estimates resulting from size-selective sampling. Fish. Res. 2010, 105, 75–79. [Google Scholar] [CrossRef]
- Lorenzen, K. Toward a new paradigm for growth modeling in fisheries stock assessments: Embracing plasticity and its consequences. Fish. Res. 2016, 180, 4–22. [Google Scholar] [CrossRef]
- Matthias, B.G.; Ahrens, R.N.M.; Allen, M.S.; Tuten, T.; Siders, Z.A.; Wilson, K.L. Understanding the effects of density and environmental variability on the process of fish growth. Fish. Res. 2018, 198, 209–219. [Google Scholar] [CrossRef]
- Lewandowski, D.; Kurowicka, D.; Joe, H. Generating random correlation matrices based on vines and extended onion method. J. Multivar. Anal. 2009, 100, 1989–2001. [Google Scholar] [CrossRef]
- Thorson, J.T.; Munch, S.B.; Cope, J.M.; Gao, J. Predicting life history parameters for all fishes worldwide. Ecol. Appl. 2017, 27, 2262–2276. [Google Scholar] [CrossRef]
- Jackson, D.R. Pseudemys nelsoni (Carr 1938)—Florida Red-Bellied Turtle. In Conservation Biology of Freshwater Turtles and Tortoises; Rhodin, A., Pritchard, P., van Dijk, P.P., Saumure, R., Buhlmann, K., Iverson, J., Mittermeier, R.A., Eds.; Chelonian Research Foundation: Lunenburg, MA, USA, 2010; pp. 041.1–041.8. Available online: http://www.iucn-tftsg.org/cbftt/ (accessed on 10 August 2022).
- Heinrich, G.L.; Jackson, D.R.; Walsh, T.J.; Lee, D.S. Southernmost occurrence of the Suwannee Cooter, Pseudemys concinna suwanniensis (Testudines: Emydidae). J. North Am. Herpetol. 2015, 1, 53–59. [Google Scholar] [CrossRef]
- Aresco, M.J. Reproductive ecology of Pseudemys floridana and Trachemys scripta (Testudines: Emydidae) in northwestern Florida. J. Herpetol. 2004, 38, 249–256. [Google Scholar] [CrossRef]
- Beverton, R.J.H.; Holt, S.J. A review of the lifespans and mortality rates of fish in nature, and their relation to growth and other physiological characteristics. In Ciba Foundation Symposium—The Lifespan of Animals (Colloquia on Ageing); John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1959; pp. 142–180. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9780470715253.ch10 (accessed on 13 November 2022).
- Gibbons, J.W.; Semlitsch, R.D.; Greene, J.L.; Schubauer, J.P. Variation in age and size at maturity of the Slider Turtle (Pseudemys scripta). Am. Nat. 1981, 117, 841–845. [Google Scholar] [CrossRef]
- Thomson, R.C.; Spinks, P.Q.; Shaffer, H.B. A global phylogeny of turtles reveals a burst of climate-associated diversification on continental margins. Proc. Natl. Acad. Sci. USA 2021, 118, e2012215118. [Google Scholar] [CrossRef] [PubMed]
- Gabry, J.; Češnovar, R. Cmdstanr: R interface to “CmdStan”. 2022. Available online: https://mc-stan.org/cmdstanr/ (accessed on 13 November 2022).
- Caltabellotta, F.P.; Siders, Z.A.; Murie, D.J.; Motta, F.S.; Cailliet, G.M.; Gadig, O.B.F. Age and growth of three endemic threatened guitarfishes Pseudobatos horkelii, P. percellens and Zapteryx brevirostris in the western South Atlantic Ocean. J. Fish Biol. 2019, 95, 1236–1248. [Google Scholar] [CrossRef] [PubMed]
- STAN Development Team. Stan Modeling Language: User’s Guide and Reference Manual; Version 2.29. Available online: https://mc-stan.org/users/documentation/ (accessed on 13 November 2022).
- Gelman, A.; Rubin, D.B. Inference from iterative simulation using multiple sequences. Stat. Sci. 1992, 7, 457–472. [Google Scholar] [CrossRef]
- Roff, D. Evolution of Life Histories: Theory and Analysis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Stanford, C.B.; Iverson, J.B.; Rhodin, A.G.; van Dijk, P.P.; Mittermeier, R.A.; Kuchling, G.; Berry, K.H.; Bertolero, A.; Bjorndal, K.A.; Blanck, T.E.; et al. Turtles and tortoises are in trouble. Curr. Biol. 2020, 30, R721–R735. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef]
- Dreslik, M.J. Ecology of the river cooter, Pseudemys concinna, in a southern Illinois floodplain lake. Herpetol. Nat. Hist. 1997, 5, 135–145. [Google Scholar]
- Huestis, D.L.; Meylan, P.A. The turtles of Rainbow Run (Marion County, Florida): Observations on the genus Pseudemys. Southeast. Nat. 2004, 3, 595–612. [Google Scholar] [CrossRef]
- Gibbons, J.W.; Coker, J.W. Ecological and life history aspects of the cooter, Chrysemys floridana (Le Conte). Herpetologica 1977, 33, 29–33. [Google Scholar]
- Jackson, D.R. Reproductive strategies of sympatric freshwater emydid turtles in northern peninsular Florida. Bull. Fla. State Mus. Biol. Sci. 1988, 33, 113–158. [Google Scholar]
- Lovich, J.E.; Gibbons, J.W. A review of techniques for quantifying sexual size dimorphism. Growth Dev. Aging 1992, 56, 269–282. [Google Scholar] [PubMed]
- Jackson, D.R.; Walker, R.N. Reproduction in the Suwannee cooter, Pseudemys concinna suwanniensis. Bull. Fla. State Mus. Biol. Sci. 1997, 41, 69–167. [Google Scholar]
- Jones, T.T.; Hastings, M.D.; Bostrom, B.L.; Pauly, D.; Jones, D.R. Growth of captive leatherback turtles, Dermochelys coriacea, with inferences on growth in the wild: Implications for population decline and recovery. J. Exp. Mar. Biol. Ecol. 2011, 399, 84–92. [Google Scholar] [CrossRef]
- Congdon, J.D.; Gibbons, J.W. Biomass productivity of turtles in freshwater wetlands: A geographic comparison. Freshw. Wetl. Wildl. 1989, 61, 583–592. [Google Scholar]
- von Bertalanffy, L. Problems of organic growth. Nature 1949, 163, 156–158. [Google Scholar] [CrossRef]
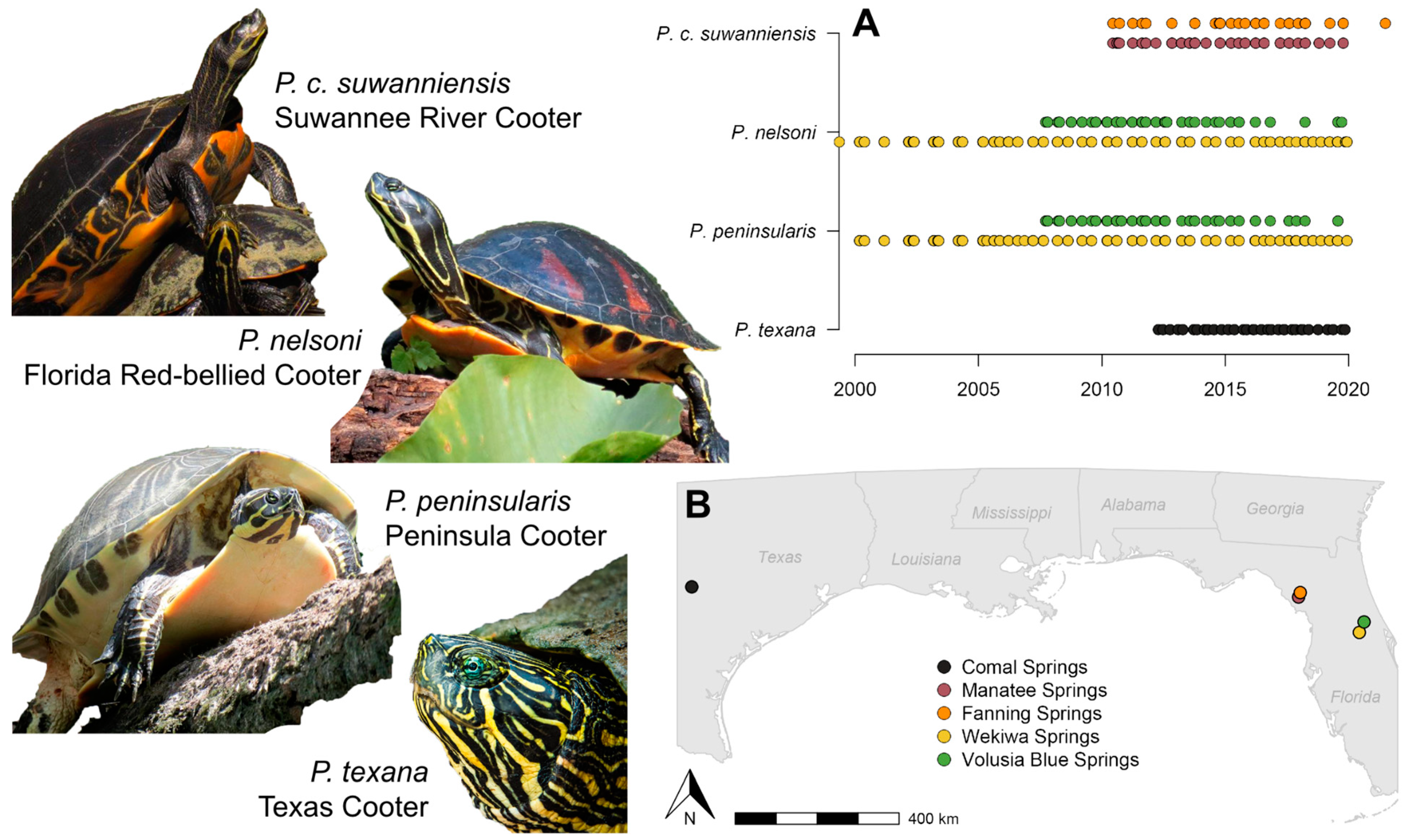
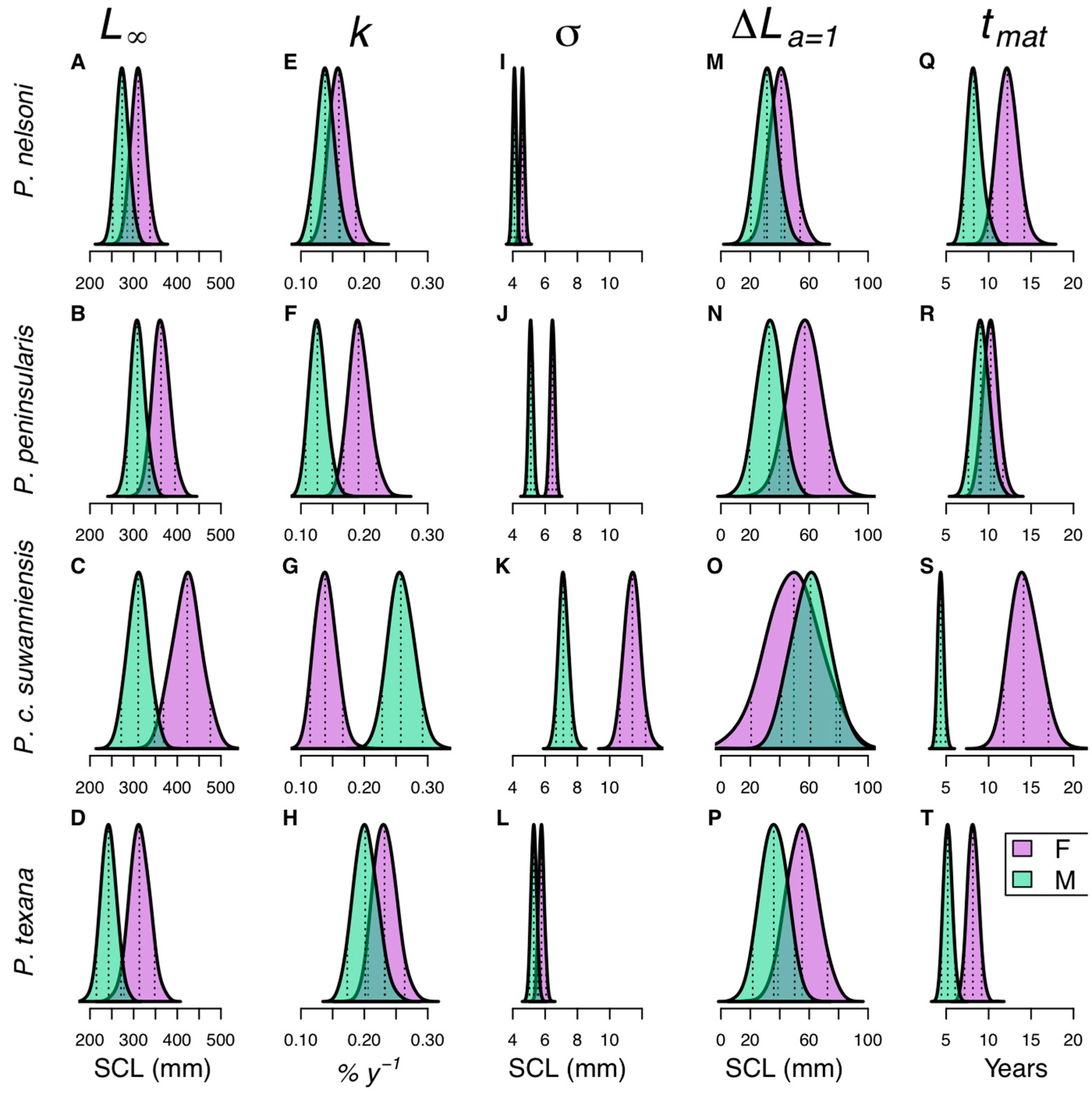
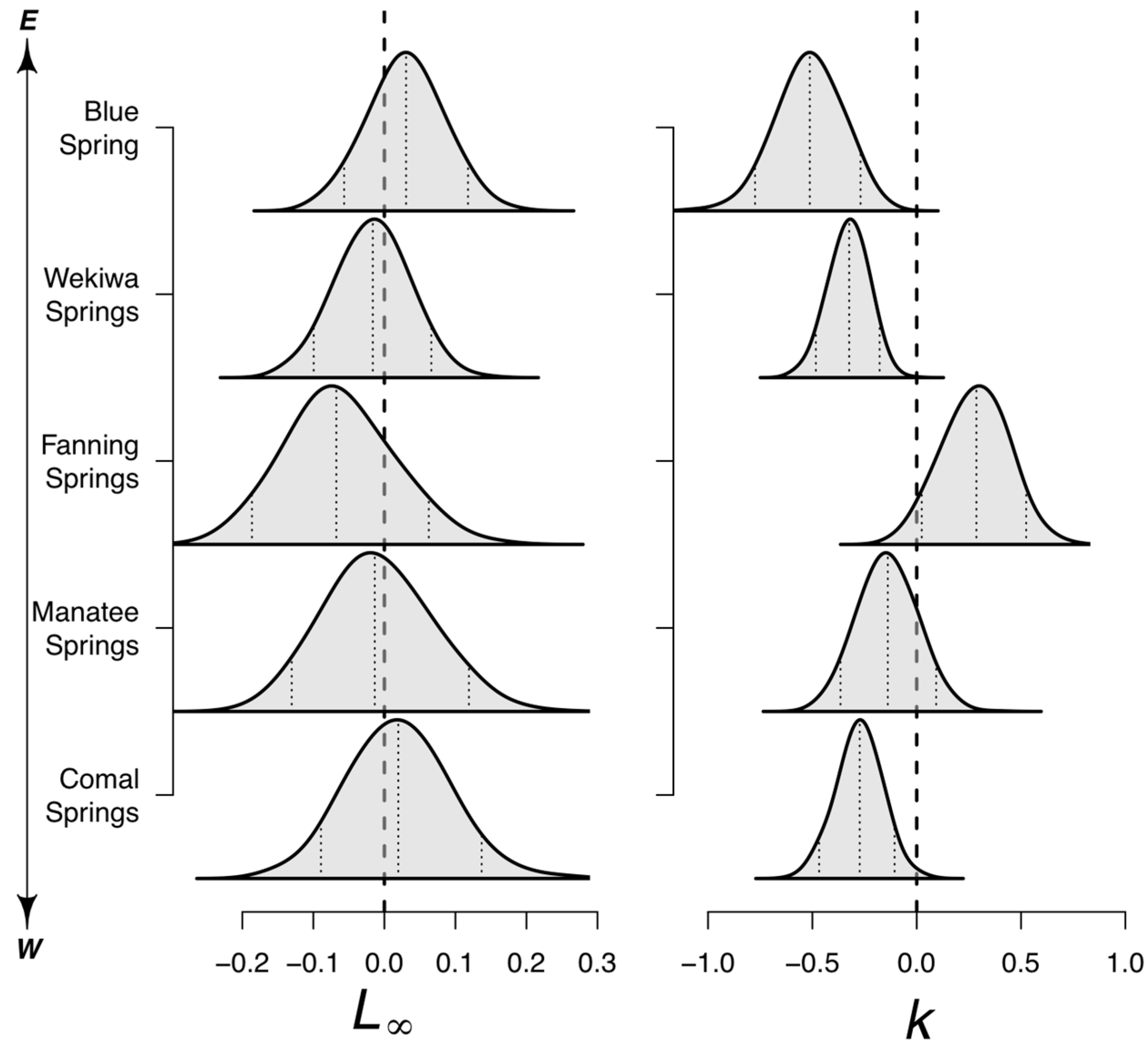
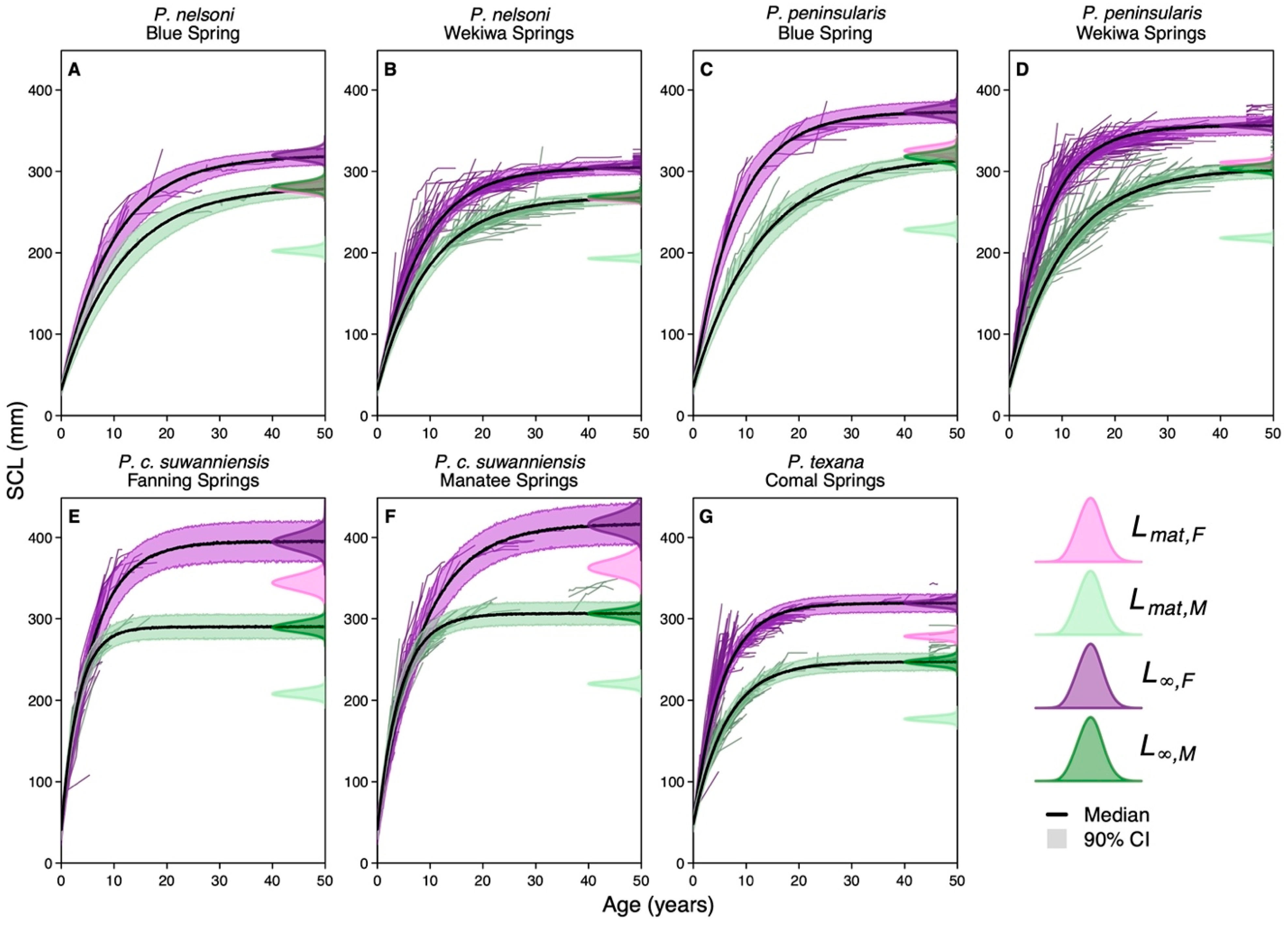
| Site | Size | Flow | Temp. | Nitrate | Vegetation | Bloom | Visits |
|---|---|---|---|---|---|---|---|
| Wekiwa Springs State Park | 2.67 | 164 | 22 | 1.2 | Moderate | High | 325,000 |
| Blue Springs State Park | 1.9 | 394 | 23 | 0.7 | Low | Low | 485,000 |
| Manatee Springs State Park | 1.53 | 380 | 22 | 2 | Moderate | High | 174,000 |
| Fanning Springs State Park | 0.7 | 300 | 22 | 6 | Low | High | 180,000 |
| Comal Springs–Landa Lake | 8.4 | 726.8 | 21 | 1.8 | High | High | 1,000,000 |
| Species | μ | σ | Range | Source |
|---|---|---|---|---|
| P. nelsoni | 32.1 | 1.84 * | 29–35 | Jackson [47] |
| P. peninsularis | 35.6 | 2.50 | — | Aresco [49] |
| P. c. suwanniensis | 41.3 | 1.02 * | 40–43 | Heinrich et al. [48] |
| P. texana | 48.0 | 3.61 | 41–53 | Lindeman [6] |
| Species | Females | Males | |
|---|---|---|---|
| P. nelsoni | 311 (286–338) | 274 (252–298) | |
| 0.16 (0.137–0.186) | 0.138 (0.116–0.161) | ||
| 4.6 (4.41–4.81) | 4.1 (3.92–4.28) | ||
| 41 (29.5–53.9) | 31.3 (20.3–43.3) | ||
| 271 (249–294) | 197 (181–214) | ||
| 12.2 (10.46–14.21) | 8.26 (7.04–9.89) | ||
| −0.681 (−0.842–−0.55) | −0.902 (−1.12–−0.733) | ||
| P. peninsularis | 363 (334–395) | 309 (284–337) | |
| 0.191 (0.167–0.219) | 0.126 (0.108–0.149) | ||
| 6.45 (6.21–6.68) | 5.12 (4.91–5.34) | ||
| 56.9 (39.8–73.7) | 32.8 (19.4–45.7) | ||
| 316 (291–344) | 222 (204–242) | ||
| 10.25 (8.86–11.69) | 9.07 (7.63–10.64) | ||
| −0.538 (−0.657–−0.444) | −0.969 (−1.21–−0.777) | ||
| P. c. suwanniensis | 423 (368–476) | 311 (272–348) | |
| 0.138 (0.115–0.166) | 0.258 (0.228–0.292) | ||
| 11.4 (10.6–12.2) | 7.13 (6.68–7.65) | ||
| 49.5 (20.5–78.3) | 61 (41.2–81) | ||
| 369 (321–415) | 223 (195–250) | ||
| 14.12 (11.78–17.04) | 4.36 (3.85–4.92) | ||
| −0.746 (−0.949–−0.594) | −0.555 (−0.675–−0.463) | ||
| P. texana | 313 (278–348) | 243 (215–271) | |
| 0.232 (0.206–0.264) | 0.201 (0.173–0.233) | ||
| 5.78 (5.53–6.06) | 5.3 (5.04–5.58) | ||
| 55.2 (38.9–72.5) | 36 (21.6–50.2) | ||
| 272 (242–304) | 174 (154–195) | ||
| 8.14 (7.16–9.18) | 5.19 (4.44–6.06) | ||
| −0.722 (−0.889–−0.58) | −1.1 (−1.38–−0.866) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siders, Z.A.; Stratmann, T.A.; Turner Tomaszewicz, C.N.; Walde, A.D.; Munscher, E.C. Somatic Growth and Maturity for Four Species of River Cooter Including Pseudemys concinna suwanniensis, P. nelsoni, P. peninsularis, and P. texana. Biology 2023, 12, 965. https://doi.org/10.3390/biology12070965
Siders ZA, Stratmann TA, Turner Tomaszewicz CN, Walde AD, Munscher EC. Somatic Growth and Maturity for Four Species of River Cooter Including Pseudemys concinna suwanniensis, P. nelsoni, P. peninsularis, and P. texana. Biology. 2023; 12(7):965. https://doi.org/10.3390/biology12070965
Chicago/Turabian StyleSiders, Zachary A., Theresa A. Stratmann, Calandra N. Turner Tomaszewicz, Andrew D. Walde, and Eric C. Munscher. 2023. "Somatic Growth and Maturity for Four Species of River Cooter Including Pseudemys concinna suwanniensis, P. nelsoni, P. peninsularis, and P. texana" Biology 12, no. 7: 965. https://doi.org/10.3390/biology12070965
APA StyleSiders, Z. A., Stratmann, T. A., Turner Tomaszewicz, C. N., Walde, A. D., & Munscher, E. C. (2023). Somatic Growth and Maturity for Four Species of River Cooter Including Pseudemys concinna suwanniensis, P. nelsoni, P. peninsularis, and P. texana. Biology, 12(7), 965. https://doi.org/10.3390/biology12070965





