Effect of Head-Up/-Down Tilt on ECG Segments and Myocardial Temporal Dispersion in Healthy Subjects
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Statistical Analysis
3. Results
4. Discussion
4.1. PP and RR Variability and Coherences
4.2. P Wave and Atrioventricular Conduction Variability
4.3. Intrinsicoid Deflection, Intraventricular Conduction, and Repolarization Variability
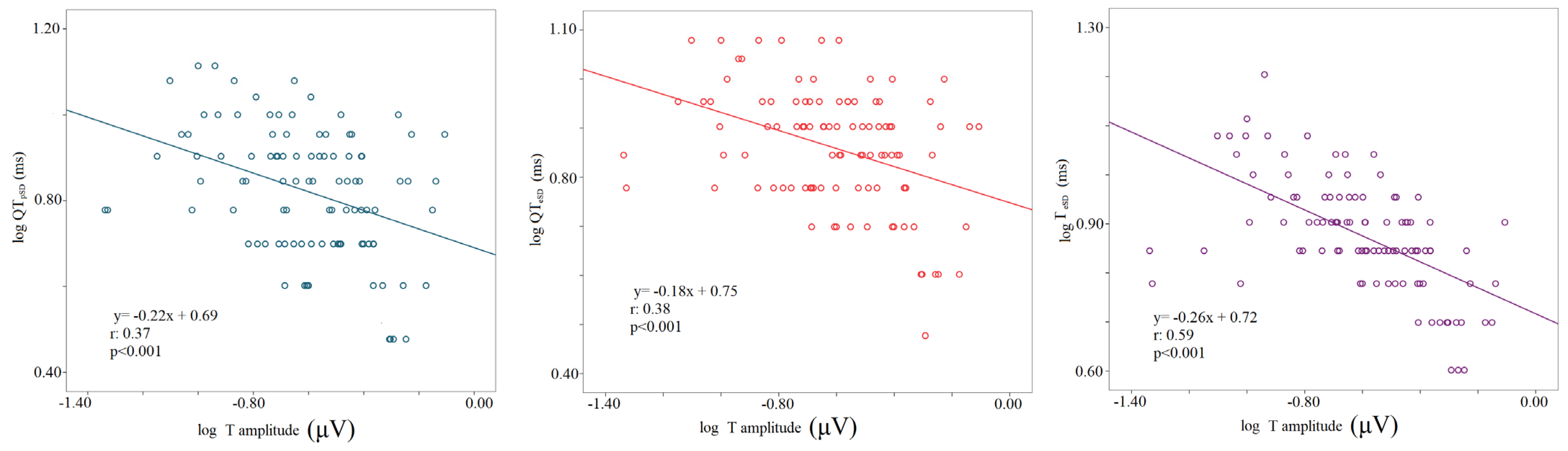
4.4. T Wave Amplitude
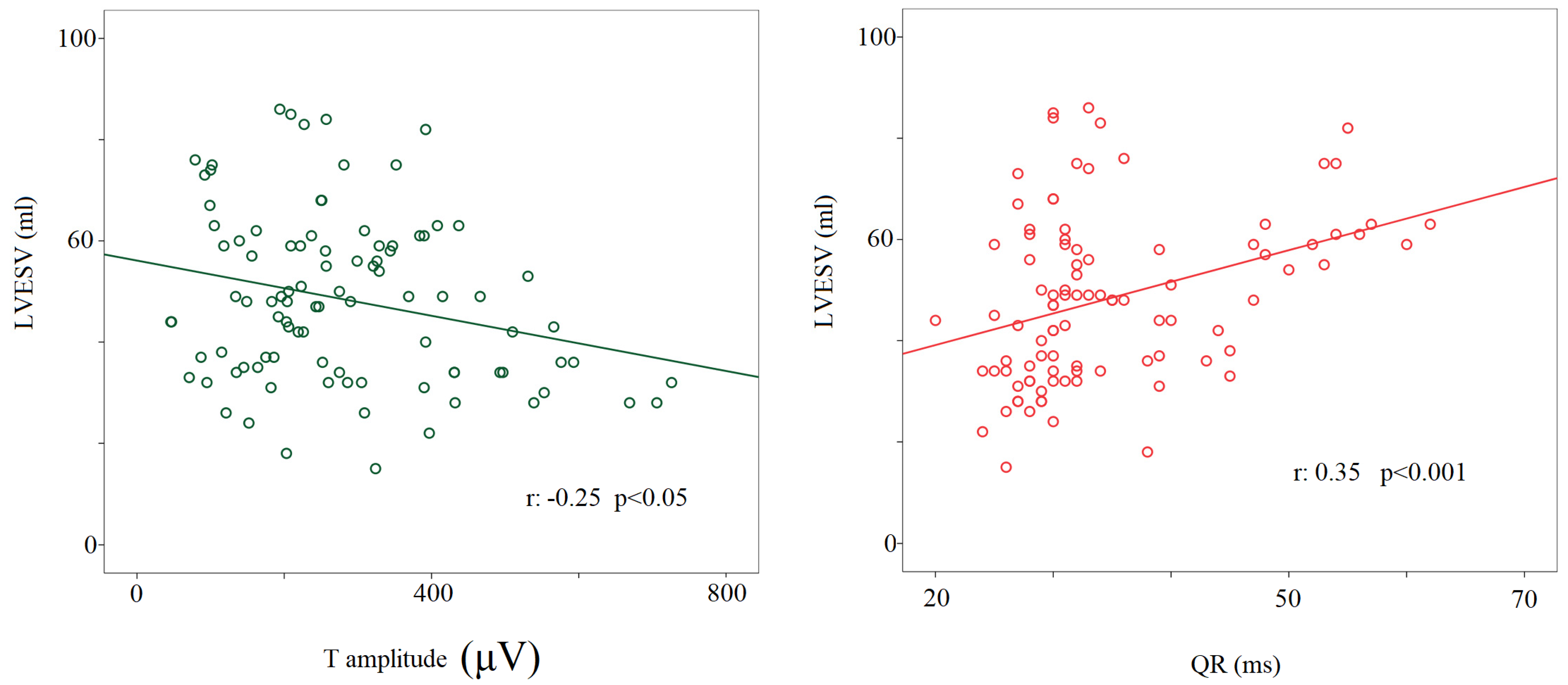
4.5. Clinical Applications
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plappert, F.; Wallman, M.; Abdollahpur, M.; Platonov, P.G.; Östenson, S.; Sandberg, F. An atrioventricular node model incorporating autonomic tone. Front. Physiol. 2022, 13, 976468. [Google Scholar] [CrossRef] [PubMed]
- Koskela, J.K.; Tahvanainen, A.; Tikkakoski, A.J.; Kangas, P.; Uitto, M.; Viik, J.; Kähönen, M.; Mustonen, J.; Pörsti, I. Resting heart rate predicts cardiac autonomic modulation during passive head-up tilt in subjects without cardiovascular diseases. Scand. Cardiovasc. J. 2022, 56, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, G.; Moscucci, F.; Fiorucci, C.; D’Alessandro, G.; Pascucci, M.; Magrì, D. P wave analysis and left ventricular systolic function in chronic heart failure. Possible insights form the P wave—PP interval spectral coherence. Minerva Cardioangiol. 2016, 64, 525–533. [Google Scholar] [PubMed]
- Piccirillo, G.; Magrì, D.; D’Alessandro, G.; Fiorucci, C.; Moscucci, F.; Di Iorio, C.; Mastropietri, F.; Parrotta, I.; Ogawa, M.; Lin, S.F.; et al. Oscillatory behavior of P wave duration and PR interval in experimental congestive heart failure: A preliminary study. Physiol. Meas. 2018, 39, 035010. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, G.; Moscucci, F.; Fiorucci, C.; Di Iorio, C.; Mastropietri, F.; Magrì, D. Time- and frequency-domain analysis of beat to beat P-wave duration, PR interval and RR interval can predict asystole as form of syncope during head-up tilt. Physiol. Meas. 2016, 37, 1910–1924. [Google Scholar] [CrossRef]
- Piccirillo, G.; Magrì, D.; Ogawa, M.; Song, J.; Chong, V.J.; Han, S.; Joung, B.; Choi, E.-K.; Hwang, S.; Chen, L.-S.; et al. Autonomic nervous system activity measured directly and QT interval variability in normal and pacing-induced tachycardia heart failure dogs. J. Am. Coll. Cardiol. 2009, 54, 840–850. [Google Scholar] [CrossRef]
- Piccirillo, G.; Magrì, D.; Pappadà, M.A.; Maruotti, A.; Ogawa, M.; Han, S.; Joung, B.; Rossi, P.; Nguyen, B.L.; Lin, S.-F.; et al. Autonomic nerve activity and the short-term variability of the Tpeak-Tend interval in dogs with pacing-induced heart failure. Heart Rhythm 2012, 9, 2044–2050. [Google Scholar] [CrossRef]
- Piccirillo, G.; Moscucci, F.; D’Alessandro, G.; Pascucci, M.; Rossi, P.; Han, S.; Chen, L.S.; Lin, S.-F.; Chen, P.-S.; Magrì, D. Myocardial repolarization dispersion and autonomic nerve activity in a canine experimental acute myocardial infarction model. Heart Rhythm 2014, 11, 110–118. [Google Scholar] [CrossRef]
- Piccirillo, G.; Moscucci, F.; Di Iorio, C.; Fabietti, M.; Mastropietri, F.; Crapanzano, D.; Bertani, G.; Sabatino, T.; Zaccagnini, G.; Crapanzano, D.; et al. Time- and frequency-domain analysis of repolarization phase during recovery from exercise in healthy subjects. Pacing Clin. Electrophysiol. 2020, 43, 1096–1103. [Google Scholar] [CrossRef]
- Porta, A.; Tobaldini, E.; Gnecchi-Ruscone, T.; Montano, N. RT variability unrelated to heart period and respiration progressively increases during graded head-up tilt. Am. J. Physiology. Heart Circ. Physiol. 2010, 298, H1406–H1414. [Google Scholar] [CrossRef]
- Baumert, M.; Porta, A.; Vos, M.A.; Malik, M.; Couderc, J.P.; Laguna, P.; Piccirillo, G.; Smith, G.L.; Tereshchenko, L.G.; Volders, P.G. QT interval variability in body surface ECG: Measurement, physiological basis, and clinical value: Position statement and consensus guidance endorsed by the European Heart Rhythm Association jointly with the ESC Working Group on Cardiac Cellular Electrophysiology. EP Eur. 2016, 18, 925–944. [Google Scholar]
- Piccirillo, G.; Moscucci, F.; Magrì, D. Air Pollution Role as Risk Factor of Cardioinhibitory Carotid Hypersensitivity. Atmosphere 2022, 13, 123. [Google Scholar] [CrossRef]
- Anonymous. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Piccirillo, G.; Moscucci, F.; Pascucci, M.; Pappadà, M.A.; D’Alessandro, G.; Rossi, P.; Quaglione, R.; Di Barba, D.; Barillà, F.; Magrì, D.; et al. Influence of aging and chronic heart failure on temporal dispersion of myocardial repolarization. Clin. Interv. Aging 2013, 8, 293–300. [Google Scholar] [CrossRef]
- Piccirillo, G.; Ogawa, M.; Song, J.; Chong, V.J.; Joung, B.; Han, S.; Magrì, D.; Chen, L.S.; Lin, S.F.; Chen, P.-S. Power spectral analysis of heart rate variability and autonomic nervous system activity measured directly in healthy dogs and dogs with tachycardia-induced heart failure. Heart Rhythm 2009, 6, 546–552. [Google Scholar] [CrossRef]
- Piccirillo, G.; Rossi, P.; Mitra, M.; Quaglione, R.; Dell’Armi, A.; Di Barba, D.; Maisto, D.; Lizio, A.; Barillà, F.; Magrì, D. Indexes of temporal myocardial repolarization dispersion and sudden cardiac death in heart failure: Any difference? Ann. Noninvasive Electrocardiol. 2013, 18, 130–139. [Google Scholar] [CrossRef]
- Berger, R.D.; Kasper, E.K.; Baughman, K.L.; Marban, E.; Calkins, H.; Tomaselli, G.F. Beat-to-beat QT interval variability: Novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopathy. Circulation 1997, 96, 1557–1565. [Google Scholar] [CrossRef]
- Charloux, A.; Lonsdorfer-Wolf, E.; Richard, R.; Lampert, E.; Oswald-Mammosser, M.; Mettauer, B.; Geny, B.; Lonsdorfer, J. A new impedance cardiograph device for the non-invasive evaluation of cardiac output at rest and during exercise: Comparison with the “direct” Fick method. Eur. J. Appl. Physiol. 2000, 82, 313–320. [Google Scholar] [CrossRef]
- Hsu, A.R.; Barnholt, K.E.; Grundmann, N.K.; Lin, J.H.; McCallum, S.W.; Friedlander, A.L. Sildenafil improves cardiac output and exercise performance during acute hypoxia, but not normoxia. J. Appl. Physiol. 2006, 100, 2031–2040. [Google Scholar] [CrossRef]
- Lepretre, P.M.; Koralsztein, J.P.; Billat, V.L. Effect of exercise intensity on relationship between VO2max and cardiac output. Med. Sci. Sport. Exerc. 2004, 36, 1357–1363. [Google Scholar] [CrossRef]
- Tonelli, A.R.; Alnuaimat, H.; Li, N.; Carrie, R.; Mubarak, K.K. Value of impedance cardiography in patients studied for pulmonary hypertension. Lung 2011, 189, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Gordon, N.; Abbiss, C.R.; Maiorana, A.J.; Marston, K.J.; Peiffer, J.J. Intrarater reliability and agreement of the physioflow bioimpendance cardiography device during rest, moderate and High-intensity exercise. Kinesiology 2018, 50 (Suppl. 1), 140–149. [Google Scholar]
- Leão, R.N.; Silva, P.M.D. Impedance Cardiography in the Evaluation of Patients with Arterial Hypertension. Int. J. Cardiovasc. Sci. 2019, 32, 61–69. [Google Scholar] [CrossRef]
- Anand, G.; Yu, Y.; Lowe, A.; Kalra, A. Bioimpedance analysis as a tool for hemodynamic monitoring: Overview, methods and challenges. Physiol. Meas. 2021, 42, 03TR01. [Google Scholar] [CrossRef]
- Montano, N.; Ruscone, T.G.; Porta, A.; Lombardi, F.; Pagani, M.; Malliani, A. Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation 1994, 90, 1826–1831. [Google Scholar] [CrossRef]
- Naylor, J.M.; Chow, C.M.; McLean, A.S.; Heard, R.C.; Avolio, A. Cardiovascular responses to short-term head-down positioning in healthy young and older adults. Physiother. Res. Int. 2005, 10, 32–47. [Google Scholar] [CrossRef]
- Shen, M.J.; Zipes, D.P. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ. Res. 2014, 114, 1004–1021. [Google Scholar] [CrossRef]
- Toman, O.; Hnatkova, K.; Smetana, P.; Huster, K.M.; Šišáková, M.; Barthel, P.; Novotný, T.; Schmidt, G.; Malik, M. Physiologic heart rate dependency of the PQ interval and its sex differences. Sci. Rep. 2020, 10, 2551. [Google Scholar] [CrossRef]
- Rossi, P.; Cauti, F.M.; Limite, L.R.; Iaia, L.; Allegretti, G.; Di Renzi, P.; Longa, G.D.; Quaglione, R.; Piccirillo, G.; Bianchi, S. Interatrial conduction times in paroxysmal atrial fibrillation patients with normal atrial volume and their correlation with areas of local prolonged bipolar electrograms. J. Electrocardiol. 2020, 58, 19–26. [Google Scholar] [CrossRef]
- Aiken, A.V.; Goldhaber, J.I.; Chugh, S.S. Delayed intrinsicoid deflection: Electrocardiographic harbinger of heart disease. Ann. Noninvasive Electrocardiol. 2022, 27, e12940. [Google Scholar] [CrossRef]
- O’Neal, W.T.; Qureshi, W.T.; Nazarian, S.; Kawel-Boehm, N.; Bluemke, D.A.; Lima, J.A.; Soliman, E.Z. Electrocardiographic Time to Intrinsicoid Deflection and Heart Failure: The Multi-Ethnic Study of Atherosclerosis. Clin. Cardiol. 2016, 39, 531–536. [Google Scholar] [CrossRef]
- Shattock, M.J.; Park, K.C.; Yang, H.Y.; Lee, A.W.C.; Niederer, S.; MacLeod, K.T.; Winter, J. Restitution slope is principally determined by steady-state action potential duration. Cardiovasc. Res. 2017, 113, 817–828. [Google Scholar] [CrossRef]
- Smetana, P.; Batchvarov, V.; Hnatkova, K.; John Camm, A.; Malik, M. Sex differences in the rate dependence of the T wave descending limb. Cardiovasc. Res. 2003, 58, 549–554. [Google Scholar] [CrossRef]
- Andersen, M.P.; Xue, J.Q.; Graff, C.; Kanters, J.K.; Toft, E.; Struijk, J.J. New descriptors of T wave morphology are independent of heart rate. J. Electrocardiol. 2008, 41, 557–561. [Google Scholar] [CrossRef]
- Hasan, M.A.; Abbott, D.; Baumert, M. Relation between beat-to-beat QT interval variability and T wave amplitude in healthy subjects. Ann. Noninvasive Electrocardiol. 2012, 17, 195–203. [Google Scholar] [CrossRef]
- Vrtovec, B.; Starc, V.; Starc, R. Beat-to-beat QT interval variability in coronary patients. J. Electrocardiol. 2000, 33, 119–125. [Google Scholar] [CrossRef]

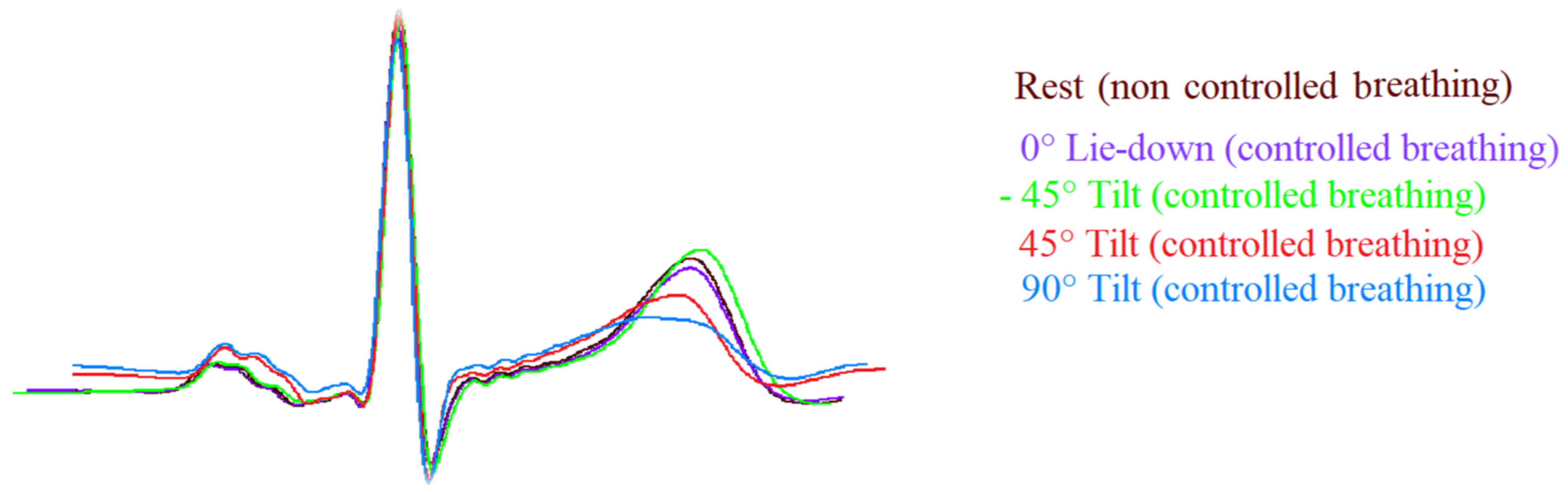
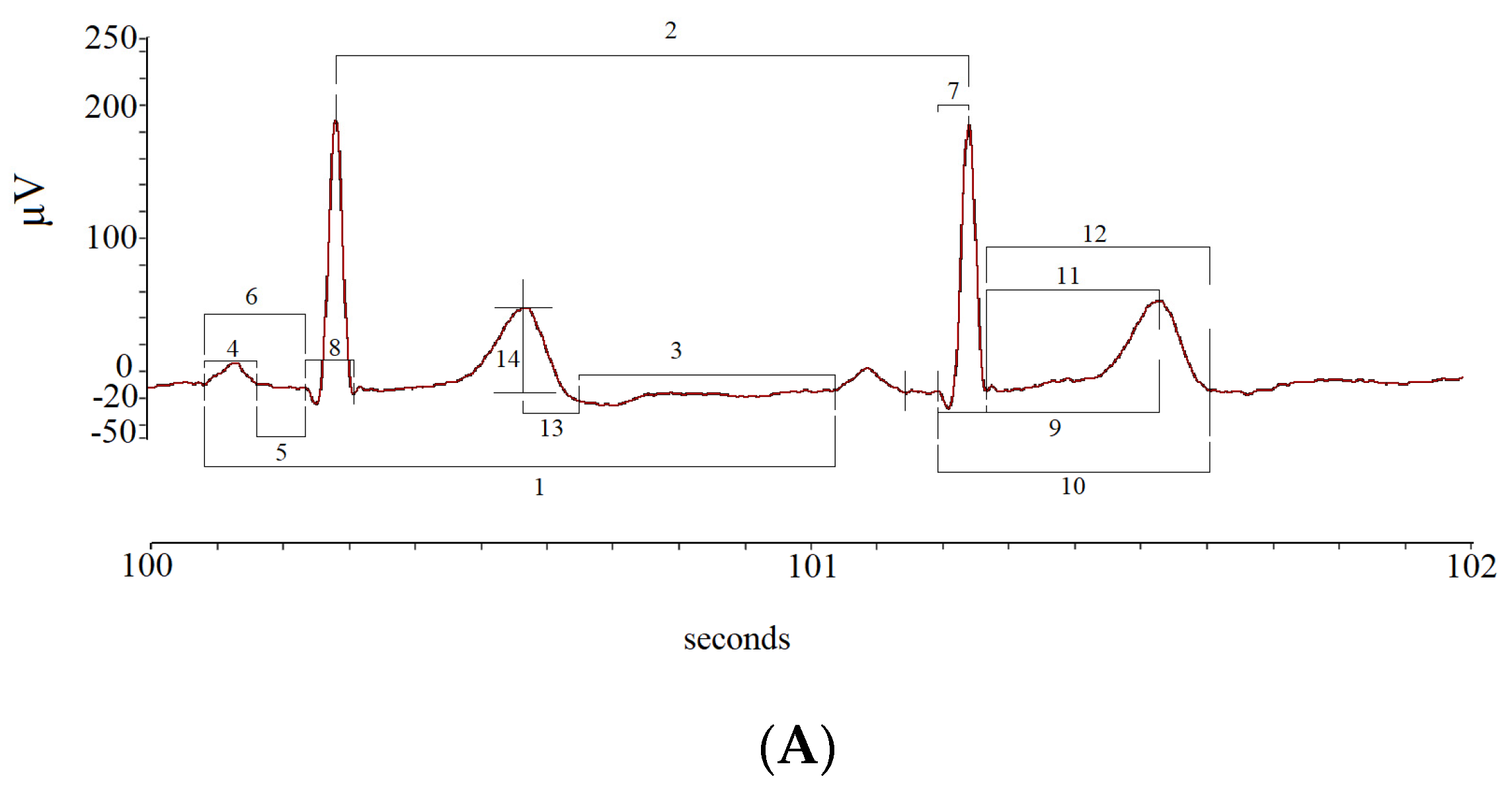
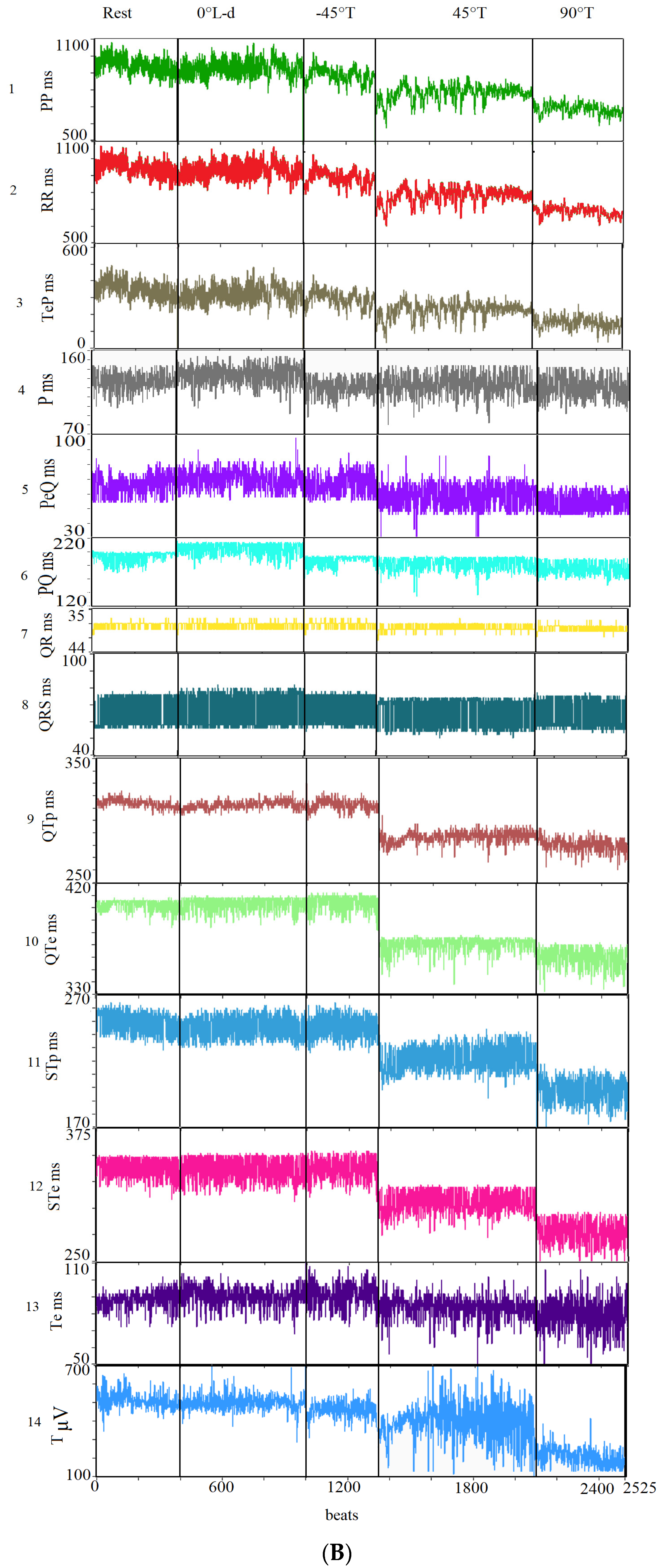
| Rest | 0° L-d | −45° Tilt | 45° Tilt | 90° Tilt | p Value | |
|---|---|---|---|---|---|---|
| Controlled Breathing | ||||||
| TPPP, ms2 | 1166 (2217) | 1246 (637) | 1603 (3614) | 1739 (1221) | 1308 (1612) | ns |
| p value: TPPP vs. TPRR | 0.05 | 0.05 | 0.05 | 0.05 | 0.001 | |
| VLFPP, ms2 | 580 (845) | 377 (637) | 810 (2116) | 784 (796) | 780 (707) | ns |
| p value: VLFPP vs. VLFRR | 0.05 | ns | 0.05 | 0.05 | ns | |
| LFPP, ms2 | 251 (1252) @ | 285 (344) #@ | 366 (977) | 437 (534) | 487 (727) | <0.05 |
| p value: LFPP vs. LFRR | 0.05 | ns | ns | ns | ns | |
| LFPP CF, Hz | 0.10 ± 0.02 | 0.10 ± 0.02 | 0.10 ± 0.02 | 0.09 ± 0.02 | 0.09 ± 0.02 | ns |
| p value: LFPP vs. LFRRCF | ns | ns | ns | ns | ns | |
| HFPP, ms2 | 167 (484) *@ | 219 (661) § | 278 (32) ╪╫ | 164 (96) ● | 100 (110) | <0.001 |
| p value: HFPP vs. HFRR | <0.001 | <0.05 | <0.05 | <0.05 | <0.001 | |
| HFPP CF, Hz | 0.30 ± 0.09 @*@@ | 0.25 ± 0.00 | 0.25 ± 0.06 | 0.25 ± 0.06 | 0.25 ± 0.09 | <0.05 |
| p value: HFPP vs. HFRR CF | ns | ns | ns | 0.001 | ns | |
| LF/HFPP, | 2.30 (2.37) @*@@ | 0.98 (1.14) @@§§ | 1.33 (1.27) ╪╫ | 2.57 (2.02) ● | 4.43 (3.76) | <0.001 |
| p value: LF/HFPP vs. LF/HFRR | 0.001 | 0.05 | 0.001 | 0.05 | 0.05 | |
| LFPP, nu | 64 (25) @@ | 46 (27) @@§§ | 51 (29) ╪╪╪ | 64 (22) ● | 71 (26) | <0.001 |
| p value: LFPP vs. LFRR nu | 0.001 | 0.05 | 0.001 | 0.001 | 0.05 | |
| HFPP, nu | 31 (20) @@ | 45 (25) @@§ | 37 (19) ╪╫ | 24 (17) ● | 16 (8) | <0.001 |
| p value: HFPP vs. HFRR nu | <0.05 | <0.05 | <0.05 | <0.05 | <0.001 | |
| Rest | 0° L-d | −45° Tilt | 45° Tilt | 90° Tilt | p Value | |
|---|---|---|---|---|---|---|
| Controlled Breathing | ||||||
| RR, ms | 845 ± 114 **⸸⸸ | 840 ± 106 ##§§ | 912 ± 123 ╪╪╫╫ | 825 ± 130 ●● | 697 ± 94 | <0.001 |
| RRSD, ms | 34 (29) | 35 (25) | 40 (40) | 41 (17) | 36 (20) | ns |
| PP, ms | 845 ± 114 **⸸⸸ | 841 ± 106 ##§§ | 917 ± 120 ╪╪╫╫ | 825 ± 130 ●● | 697 ± 94 | <0.001 |
| PPSD, ms | 34 (30) | 35 (24) | 40 (41) | 42 (15) | 37 (20) | ns |
| P, ms | 123 ± 9 ⸸⸸ | 123 ± 12 #§ | 121 ± 11 ╫ | 121 ± 10 ● | 114 ± 10 | <0.001 |
| PSD, ms | 8 (2) | 8 (2) | 9 (4) | 9 (3) | 9 (2) | ns |
| PeQ, ms | 55 ± 14 ⸸⸸ | 59 ± 15 § | 59 ± 13 ╫ | 55 ± 14 ● | 47 ± 14 | <0.001 |
| PeQSD, ms | 7 (3) | 6 (3) | 8 (3) | 7 (4) | 7 (3) | ns |
| PQ, ms | 179 ± 17 ⸸⸸ | 186 ± 23 §§ | 182 ± 18 ╫╫ | 177 ± 18 ●● | 161 ± 16 | <0.001 |
| PQSD, | 7 (3) | 7 (3) | 7 (4) | 8 (4) | 8 (3) | ns |
| QR, ms | 34 ± 9 | 35 ± 9 | 35 ± 9 | 35 ± 10 | 35 ± 10 | ns |
| QRSD, ms | 3 (3) | 4 (4) | 4 (5) | 4 (4) | 4 (5) | ns |
| QRS, ms | 66 ± 17 | 66 ± 17 | 67 ± 17 | 65 ± 19 | 64 ± 19 | ns |
| QRSSD, ms | 5 (4) | 5 (4) | 6 (5) | 6 (4) | 6 (5) | ns |
| QTp, ms | 284 ± 20 *⸸ | 284 ± 18 #§§ | 298 ± 33 ╪╫╫ | 282 ± 22 ● | 268 ± 23 | <0.001 |
| QTpSD, | 6 (3) ⸋⸸ | 6 (4) ⸠§ | 7 (4) | 8 (5) | 8 (3) | <0.001 |
| QTe, ms | 370 ± 29 *⸸⸸ | 369 ± 32 #§ | 385 ± 26 ╪╫╫ | 369 ± 26 ●● | 343 ± 32 | <0.001 |
| QTeSD, ms | 7 (3) ⸡*⸋⸸ | 7 (2) #§ | 8 (3) | 8 (4) | 8 (5) | <0.001 |
| STp, ms | 218 ± 29 ⸸ | 219 ± 26 #§ | 226 ± 32 ╫ | 217 ± 28 ●● | 204 ± 28 | <0.001 |
| STpSD, ms | 6 (2) ⸸ | 6 (4) § | 7 (3) | 6 (3) ● | 7 (2) | <0.05 |
| STe, ms | 308 ± 33 *⸸⸸ | 308 ± 30 #§ | 318 ± 32 ╪╫╫ | 303 ± 32 ●● | 284 ± 29 | <0.001 |
| STeSD, ms | 6 (3) *⸸ | 6 (3) #§ | 7 (3) | 7 (3) ● | 8 (3) | <0.05 |
| Te, ms | 87 ± 12 | 90 ± 12 | 92 ± 10 | 86 ± 10 | 86 ± 25 | ns |
| TeSD, ms | 7 (2) *⸋⸸ | 7 (2) #§ | 8 (2) | 8 (3) ● | 9 (3) | <0.001 |
| TeP, ms | 301 ± 86 *⸸⸸ | 293 ± 74 ##§§ | 362 ± 89 ╪╪╫╫ | 291 ± 97 ●● | 196 ± 70 | <0.001 |
| TePSD, | 34 (29) | 36 (23) | 40 (39) | 41 (13) | 37 (17) | ns |
| T, μVolt | 297 (348) ⸋⸸⸸ | 313 (233)⸠§§ | 298 (202) ╪╫╫ | 266 (196) ● | 200 (127) | <0.001 |
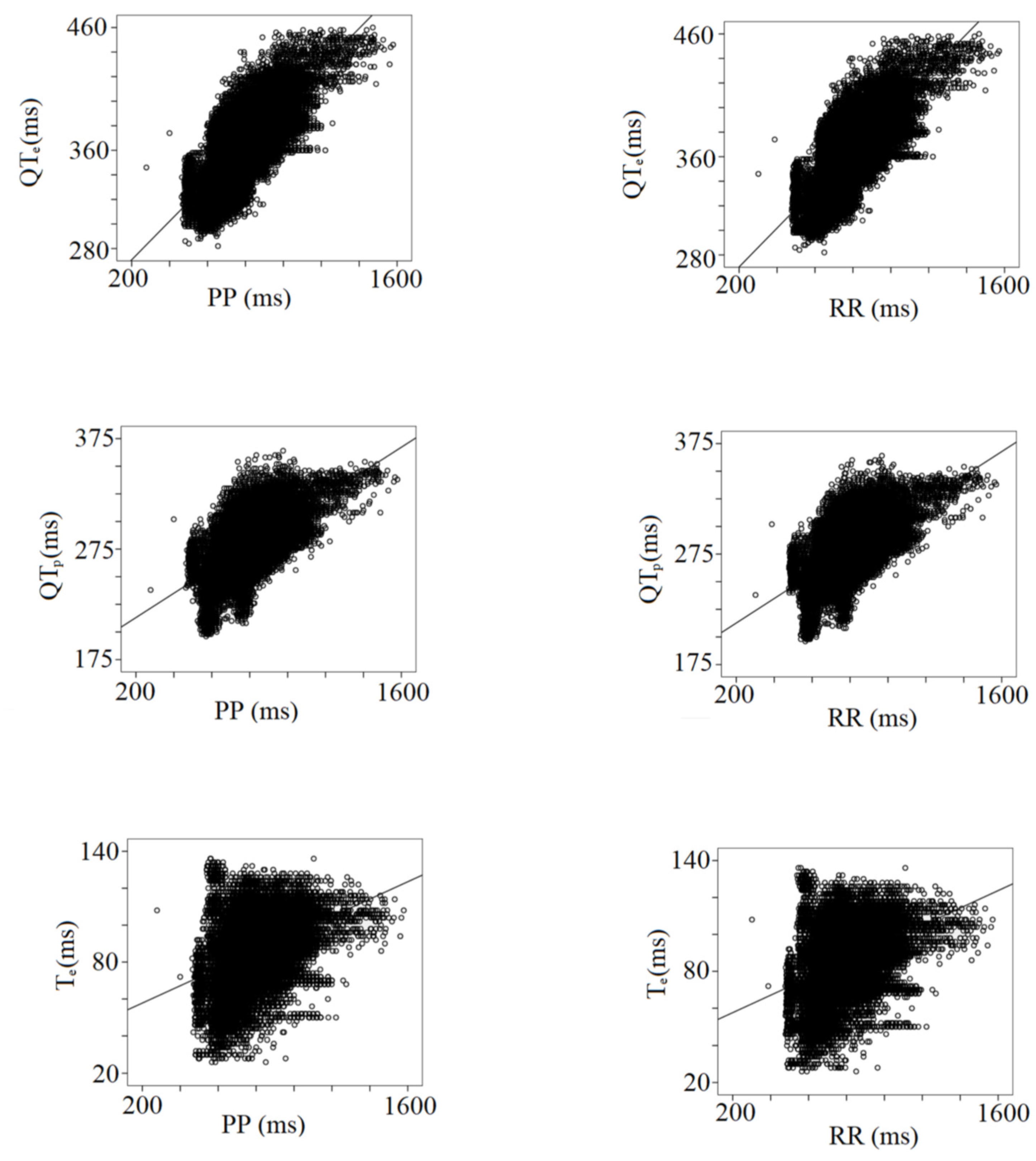
| r | Slope | Intercept | p Value | |
|---|---|---|---|---|
| PP | ||||
| P | 0.21 | 22.80 | 101.89 | <0.001 |
| PeQ | 0.13 | 0.02 | 41.97 | <0.001 |
| PQ | 0.24 | 0.04 | 143.78 | <0.001 |
| QR | 0.08 | 0.01 | 39.76 | <0.001 |
| QRS | 0.10 | 0.01 | 54.95 | <0.001 |
| QTp | 0.64 | 0.11 | 191.04 | <0.001 |
| QTe | 0.74 | 0.16 | 239.45 | <0.001 |
| STp | 0.48 | 0.10 | 136.08 | <0.001 |
| STe | 0.62 | 0.15 | 184.50 | <0.001 |
| Te | 0.40 | 0.05 | 48.44 | <0.001 |
| TeP | 0.97 | 0.80 | −383.24 | <0.001 |
| r | Slope | Intercept | p Value | |
| RR | ||||
| P | 0.24 | 0.03 | 99.15 | <0.001 |
| PeQ | 0.12 | 0.02 | 41.86 | <0.001 |
| PQ | 0.26 | 0.04 | 141.02 | <0.001 |
| QR | 0.08 | 0-.01 | 38.81 | <0.001 |
| QRS | 0.10 | 0.01 | 54.89 | <0.001 |
| QTp | 0.65 | 0.11 | 190.47 | <0.001 |
| QTe | 0.75 | 0.16 | 238.65 | <0.001 |
| STp | 0.48 | 0.10 | 135.59 | <0.001 |
| STe | 0.62 | 0.15 | 183.77 | <0.001 |
| Te | 0.40 | 0.05 | 48.21 | <0.001 |
| TeP | 0.96 | 0.80 | −379.78 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccirillo, G.; Moscucci, F.; Di Diego, I.; Mezzadri, M.; Caltabiano, C.; Carnovale, M.; Corrao, A.; Lospinuso, I.; Stefano, S.; Scinicariello, C.; et al. Effect of Head-Up/-Down Tilt on ECG Segments and Myocardial Temporal Dispersion in Healthy Subjects. Biology 2023, 12, 960. https://doi.org/10.3390/biology12070960
Piccirillo G, Moscucci F, Di Diego I, Mezzadri M, Caltabiano C, Carnovale M, Corrao A, Lospinuso I, Stefano S, Scinicariello C, et al. Effect of Head-Up/-Down Tilt on ECG Segments and Myocardial Temporal Dispersion in Healthy Subjects. Biology. 2023; 12(7):960. https://doi.org/10.3390/biology12070960
Chicago/Turabian StylePiccirillo, Gianfranco, Federica Moscucci, Ilaria Di Diego, Martina Mezzadri, Cristina Caltabiano, Myriam Carnovale, Andrea Corrao, Ilaria Lospinuso, Sara Stefano, Claudia Scinicariello, and et al. 2023. "Effect of Head-Up/-Down Tilt on ECG Segments and Myocardial Temporal Dispersion in Healthy Subjects" Biology 12, no. 7: 960. https://doi.org/10.3390/biology12070960
APA StylePiccirillo, G., Moscucci, F., Di Diego, I., Mezzadri, M., Caltabiano, C., Carnovale, M., Corrao, A., Lospinuso, I., Stefano, S., Scinicariello, C., Giuffrè, M., De Santis, V., Sciomer, S., Rossi, P., Fiori, E., & Magrì, D. (2023). Effect of Head-Up/-Down Tilt on ECG Segments and Myocardial Temporal Dispersion in Healthy Subjects. Biology, 12(7), 960. https://doi.org/10.3390/biology12070960










