Corin Deficiency Diminishes Intestinal Sodium Excretion in Mice
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Models
2.2. Tissue Collection and Preparation
2.3. Immunostaining
2.4. Gene Expression Analysis by PCR and qRT-PCR
2.5. Aldosterone Treatment
2.6. Fecal Na+ and Cl− Measurements
2.7. Statistics
3. Results
3.1. Corin Protein Expression in Mouse Intestines
3.2. Intestinal Corin, Nppa, and Npra mRNA Expression in WT and Corin KO Mice
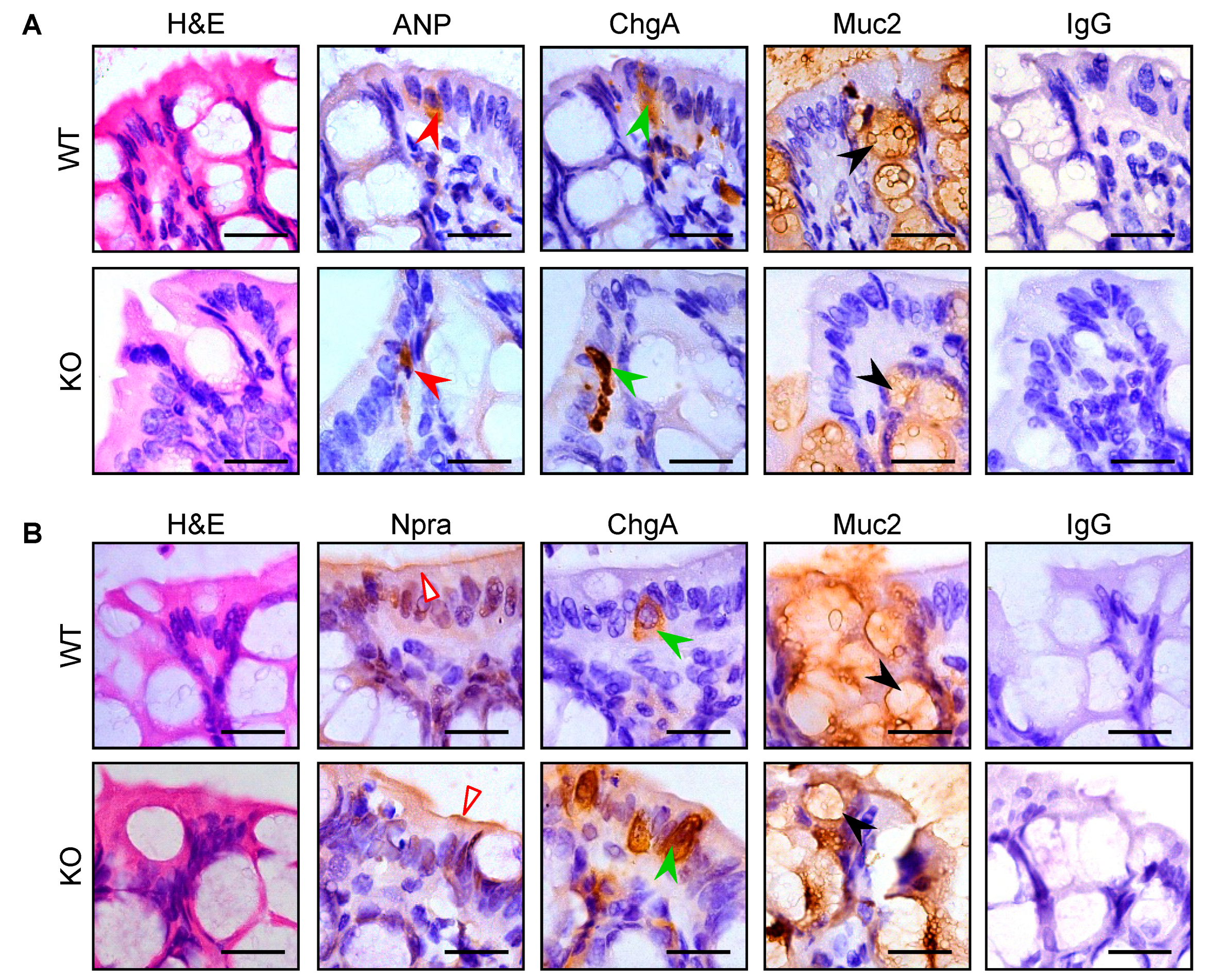
3.3. Intestinal Pro-ANP/ANP and Npra Protein Expression in WT and Corin KO Mice
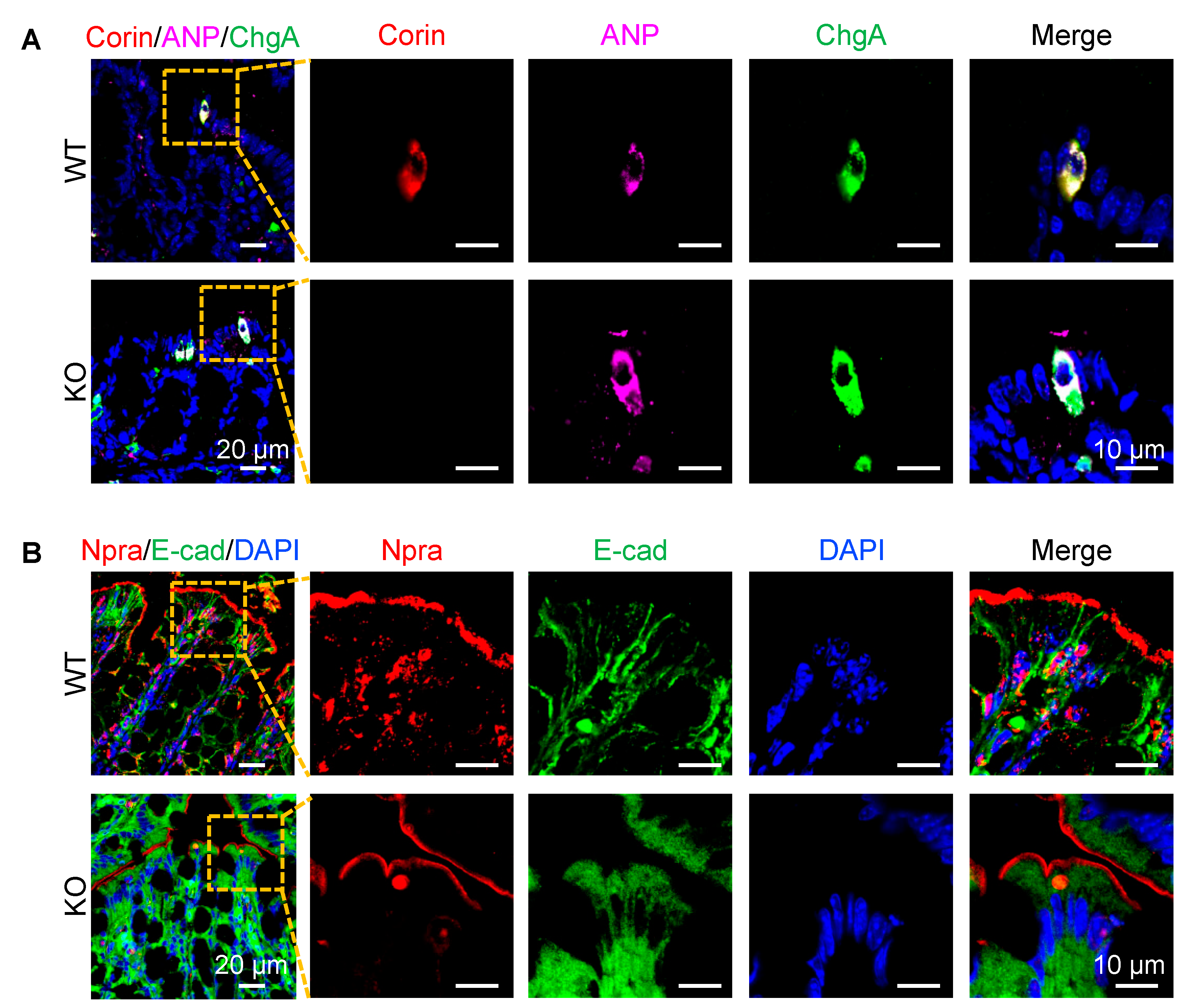
3.4. Co-Localization of Corin and ANP Proteins in Intestinal Enteroendocrine Cells
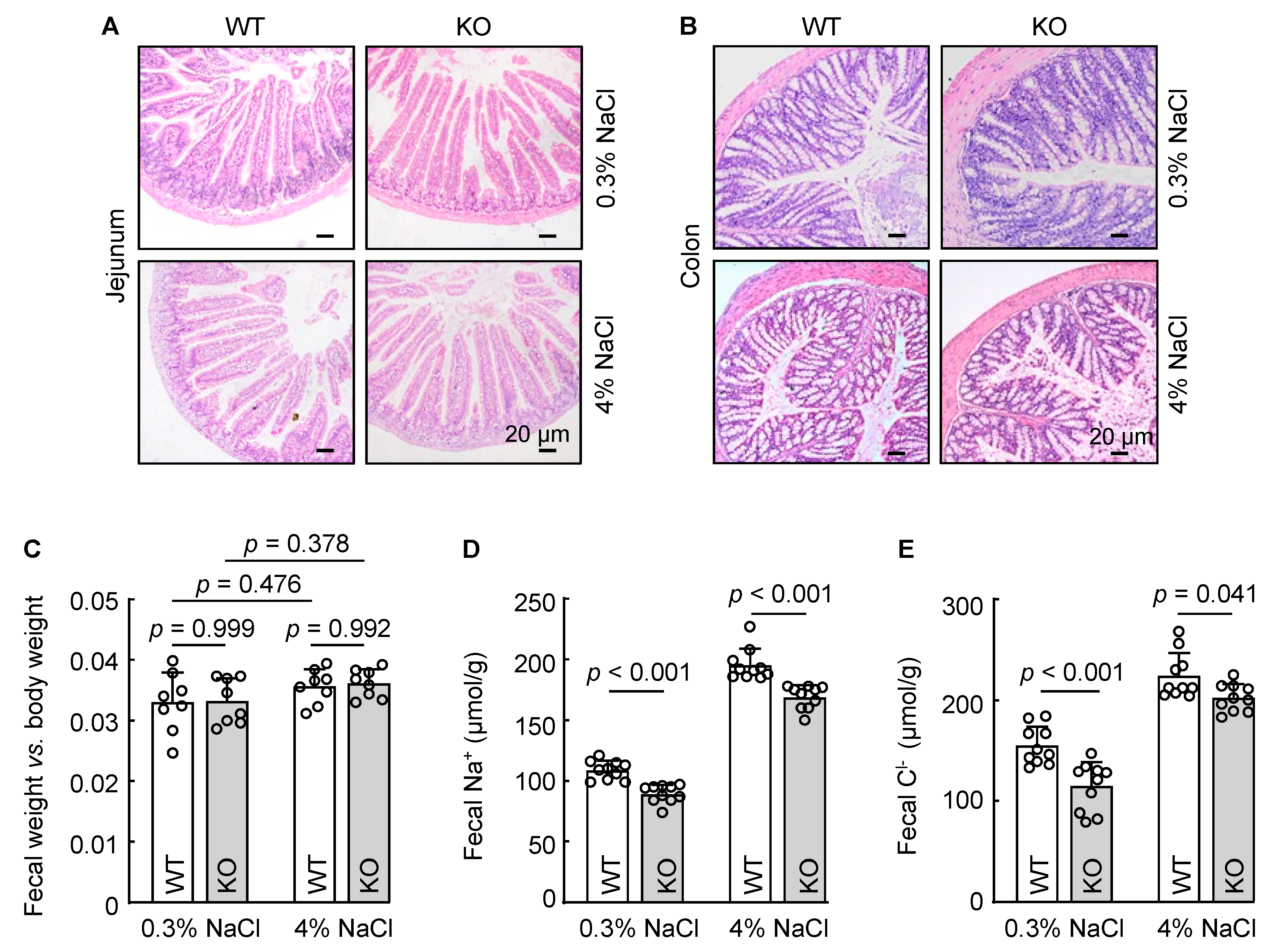
3.5. Reduced Fecal Na+ and Cl− Excretion in Corin KO Mice
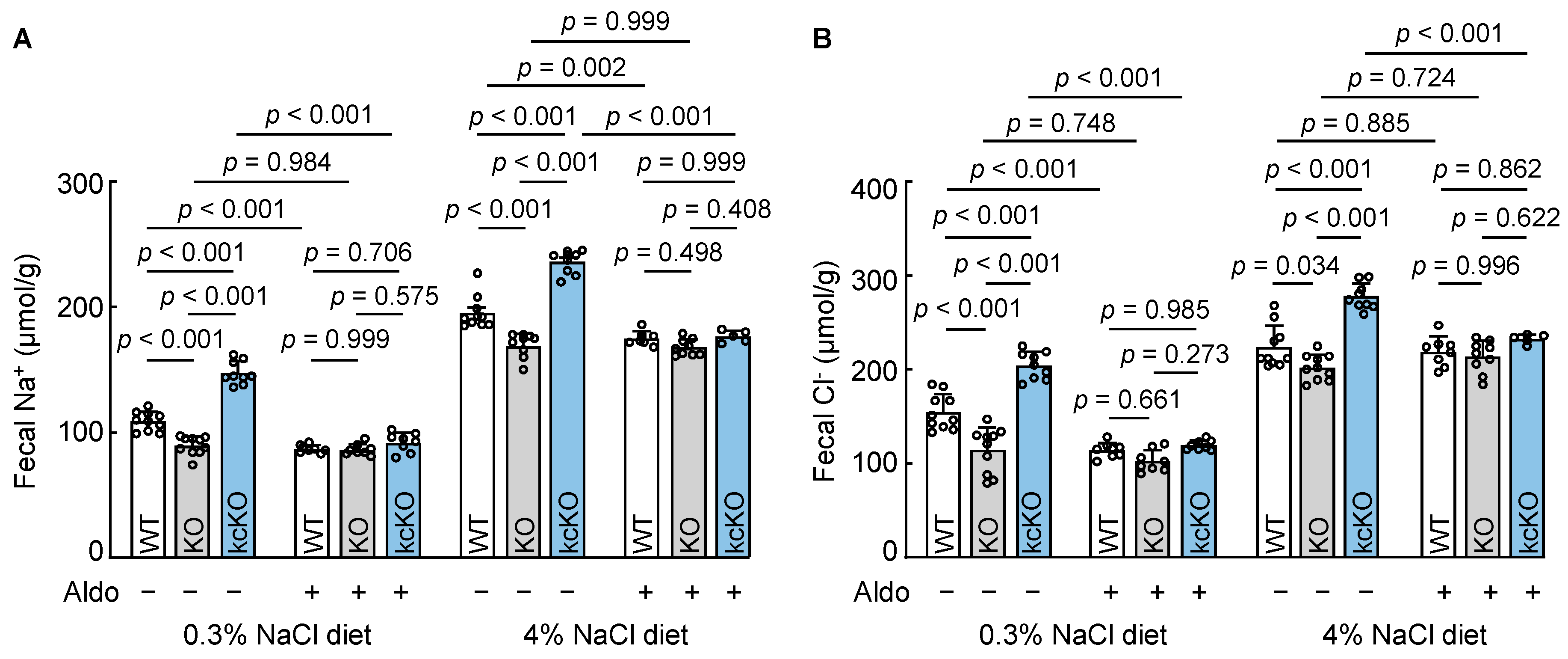
3.6. Cardiac Corin Is Dispensable for Intestinal Na+ and Cl− Excretion in Mice
3.7. Renal-Corin-Deficient Mice Exhibited Increased Fecal Na+ and Cl− Excretion
3.8. Effects of Aldosterone on Fecal Na+ and Cl− Excretion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bernal, A.; Zafra, M.A.; Simón, M.J.; Mahía, J. Sodium Homeostasis, a Balance Necessary for Life. Nutrients 2023, 15, 395. [Google Scholar] [CrossRef] [PubMed]
- Strazzullo, P.; D’Elia, L.; Kandala, N.B.; Cappuccio, F.P. Salt intake, stroke, and cardiovascular disease: Meta-analysis of prospective studies. BMJ 2009, 339, b4567. [Google Scholar] [CrossRef]
- Osanai, T.; Fujiwara, N.; Saitoh, M.; Sasaki, S.; Tomita, H.; Nakamura, M.; Osawa, H.; Yamabe, H.; Okumura, K. Relationship between salt intake, nitric oxide and asymmetric dimethylarginine and its relevance to patients with end-stage renal disease. Blood Purif. 2002, 20, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Rossier, B.C.; Bochud, M.; Devuyst, O. The Hypertension Pandemic: An Evolutionary Perspective. Physiology 2017, 32, 112–125. [Google Scholar] [CrossRef]
- Ellison, D.H.; Welling, P. Insights into Salt Handling and Blood Pressure. N. Engl. J. Med. 2021, 385, 1981–1993. [Google Scholar] [CrossRef]
- Bie, P. Mechanisms of sodium balance: Total body sodium, surrogate variables, and renal sodium excretion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R945–R962. [Google Scholar] [CrossRef]
- Wagner, C.A. Effect of mineralocorticoids on acid-base balance. Nephron Physiol. 2014, 128, 26–34. [Google Scholar] [CrossRef]
- Theilig, F.; Wu, Q. ANP-induced signaling cascade and its implications in renal pathophysiology. Am. J. Physiol. Renal. Physiol. 2015, 308, F1047–F1055. [Google Scholar] [CrossRef]
- Goetze, J.P.; Bruneau, B.G.; Ramos, H.R.; Ogawa, T.; de Bold, M.K.; de Bold, A.J. Cardiac natriuretic peptides. Nat. Rev. Cardiol. 2020, 17, 698–717. [Google Scholar] [CrossRef]
- Kuhn, M. Molecular Physiology of Membrane Guanylyl Cyclase Receptors. Physiol. Rev. 2016, 96, 751–804. [Google Scholar] [CrossRef]
- Yan, W.; Wu, F.; Morser, J.; Wu, Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc. Natl. Acad. Sci. USA 2000, 97, 8525–8529. [Google Scholar] [CrossRef]
- Dong, N.; Niu, Y.; Chen, Y.; Sun, S.; Wu, Q. Function and regulation of corin in physiology and disease. Biochem. Soc. Trans. 2020, 48, 1905–1916. [Google Scholar] [CrossRef]
- Yan, W.; Sheng, N.; Seto, M.; Morser, J.; Wu, Q. Corin, a mosaic transmembrane serine protease encoded by a novel cDNA from human heart. J. Biol. Chem. 1999, 274, 14926–14935. [Google Scholar] [CrossRef] [PubMed]
- Hooper, J.D.; Clements, J.A.; Quigley, J.P.; Antalis, T.M. Type II transmembrane serine proteases. Insights into an emerging class of cell surface proteolytic enzymes. J. Biol. Chem. 2001, 276, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Szabo, R.; Bugge, T.H. Membrane-anchored serine proteases as regulators of epithelial function. Biochem. Soc. Trans. 2020, 48, 517–528. [Google Scholar] [CrossRef]
- Martin, C.E.; List, K. Cell surface-anchored serine proteases in cancer progression and metastasis. Cancer Metastasis Rev. 2019, 38, 357–387. [Google Scholar] [CrossRef]
- Hooper, J.D.; Scarman, A.L.; Clarke, B.E.; Normyle, J.F.; Antalis, T.M. Localization of the mosaic transmembrane serine protease corin to heart myocytes. Eur. J. Biochem. 2000, 267, 6931–6937. [Google Scholar] [CrossRef] [PubMed]
- Ichiki, T.; Huntley, B.K.; Heublein, D.M.; Sandberg, S.M.; McKie, P.M.; Martin, F.L.; Jougasaki, M.; Burnett, J.C., Jr. Corin is present in the normal human heart, kidney, and blood, with pro-B-type natriuretic peptide processing in the circulation. Clin. Chem. 2011, 57, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.; Knudson, O.; Wu, F.; Morser, J.; Dole, W.P.; Wu, Q. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc. Natl. Acad. Sci. USA 2005, 102, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cui, Y.; Shen, J.; Jiang, J.; Chen, S.; Peng, J.; Wu, Q. Salt-sensitive hypertension and cardiac hypertrophy in transgenic mice expressing a corin variant identified in blacks. Hypertension 2012, 60, 1352–1358. [Google Scholar] [CrossRef]
- Nigrovic, P.A.; Gray, D.H.; Jones, T.; Hallgren, J.; Kuo, F.C.; Chaletzky, B.; Gurish, M.; Mathis, D.; Benoist, C.; Lee, D.M. Genetic inversion in mast cell-deficient (Wsh) mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. Am. J. Pathol. 2008, 173, 1693–1701. [Google Scholar] [CrossRef]
- Buckley, C.L.; Stokes, A.J. Corin-deficient W-sh mice poorly tolerate increased cardiac afterload. Regul. Pept. 2011, 172, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shen, J.; Cui, Y.; Jiang, J.; Chen, S.; Peng, J.; Wu, Q. Impaired sodium excretion and salt-sensitive hypertension in corin-deficient mice. Kidney Int. 2012, 82, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Yao, S.; Du, M.F.; Mu, J.J.; Chu, C.; Hu, G.L.; Liao, Y.Y.; Chen, C.; Wang, D.; Ma, Q.; et al. Associations of corin genetic polymorphisms with salt sensitivity, blood pressure changes, and hypertension incidence in Chinese adults. J. Clin. Hypertens. 2021, 23, 2115–2123. [Google Scholar] [CrossRef] [PubMed]
- Dries, D.L.; Victor, R.G.; Rame, J.E.; Cooper, R.S.; Wu, X.; Zhu, X.; Leonard, D.; Ho, S.I.; Wu, Q.; Post, W.; et al. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation 2005, 112, 2403–2410. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, T.; Niu, Y.; He, M.; Wang, C.; Liu, M.; Yang, J.; Zhang, Y.; Zhou, J.; Fukuda, K.; et al. Identification and functional analysis of CORIN variants in hypertensive patients. Hum. Mutat. 2017, 38, 1700–1710. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yuan, X.; Zhong, Y.; Zhang, Y.; Zhang, S.; Li, S.; Zhao, Y.; Zheng, W.; Liu, J.; Xia, Y.; et al. Single-Nucleotide Polymorphisms in the 3’ Untranslated Region of CORIN Associated with Cardiovascular Diseases in a Chinese Han Population: A Case-Control Study. Front. Cardiovasc. Med. 2021, 8, 625072. [Google Scholar] [CrossRef]
- Dong, N.; Fang, C.; Jiang, Y.; Zhou, T.; Liu, M.; Zhou, J.; Shen, J.; Fukuda, K.; Qin, J.; Wu, Q. Corin mutation R539C from hypertensive patients impairs zymogen activation and generates an inactive alternative ectodomain fragment. J. Biol. Chem. 2013, 288, 7867–7874. [Google Scholar] [CrossRef]
- Dong, N.; Zhou, T.; Zhang, Y.; Liu, M.; Li, H.; Huang, X.; Liu, Z.; Wu, Y.; Fukuda, K.; Qin, J.; et al. Corin mutations K317E and S472G from preeclamptic patients alter zymogen activation and cell surface targeting. J. Biol. Chem. 2014, 289, 17909–17916. [Google Scholar] [CrossRef]
- Ibebuogu, U.N.; Gladysheva, I.P.; Houng, A.K.; Reed, G.L. Decompensated heart failure is associated with reduced corin levels and decreased cleavage of pro-atrial natriuretic peptide. Circ. Heart Fail. 2011, 4, 114–120. [Google Scholar] [CrossRef]
- Peng, H.; Zhu, F.; Shi, J.; Han, X.; Zhou, D.; Liu, Y.; Zhi, Z.; Zhang, F.; Shen, Y.; Ma, J.; et al. Serum Soluble Corin is Decreased in Stroke. Stroke 2015, 46, 1758–1763. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Q.; Zhang, M.; Yu, J.; Ren, L.; Li, J.; Ma, S.; He, Y.; Hu, W.; Peng, H. Soluble Corin Predicts the Risk of Cardiovascular Disease: A 10-Year Follow-Up Study. JACC Asia 2022, 2, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Chen, S.; Wang, W.; Zhou, Y.; Wu, Q. Corin in clinical laboratory diagnostics. Clin. Chim. Acta 2012, 413, 378–383. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Q.; Peng, H.; Zhong, C.; Guo, D.; Huangfu, X.; Chao, X.; Wang, A.; Jin, J.; Zhang, Y. Predictive value of serum soluble corin in the risk of hyperglycemia: A population-based prospective cohort study in China. Clin. Chim. Acta 2018, 479, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Han, X.; Zhang, X.; Wang, Y.; Wang, T. Circulating soluble corin as a potential biomarker for cardiovascular diseases: A translational review. Clin. Chim. Acta 2018, 485, 106–112. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; He, M.; Zhou, T.; Niu, Y.; Sun, S.; Li, H.; Zhang, C.; Zhang, S.; Liu, M.; et al. Krüppel-like factor 17 upregulates uterine corin expression and promotes spiral artery remodeling in pregnancy. Proc. Natl. Acad. Sci. USA 2020, 117, 19425–19434. [Google Scholar] [CrossRef] [PubMed]
- Kaitu’u-Lino, T.J.; Ye, L.; Tuohey, L.; Dimitriadis, E.; Bulmer, J.; Rogers, P.; Menkhorst, E.; Van Sinderen, M.; Girling, J.E.; Hannan, N.; et al. Corin, an enzyme with a putative role in spiral artery remodeling, is up-regulated in late secretory endometrium and first trimester decidua. Hum. Reprod. 2013, 28, 1172–1180. [Google Scholar] [CrossRef]
- Mika, K.; Marinić, M.; Singh, M.; Muter, J.; Brosens, J.J.; Lynch, V.J. Evolutionary transcriptomics implicates new genes and pathways in human pregnancy and adverse pregnancy outcomes. eLife 2021, 10, e69584. [Google Scholar] [CrossRef]
- Fang, C.; Shen, L.; Dong, L.; Liu, M.; Shi, S.; Dong, N.; Wu, Q. Reduced urinary corin levels in patients with chronic kidney disease. Clin. Sci. 2013, 124, 709–717. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, Y.; Chen, L.; Chu, C.; Wang, Y.; Lv, Y.; He, M.; Martin, M.; Huang, P.H.; Mu, J.J.; et al. Short-Term High-Salt Diet Increases Corin Level to Regulate the Salt-Water Balance in Humans and Rodents. Am. J. Hypertens. 2018, 31, 253–260. [Google Scholar] [CrossRef]
- Avigad Laron, E.; Aamar, E.; Enshell-Seijffers, D. The Serine Protease Activity of Corin Is Required for Normal Pigment Type Switching. J. Investig. Dermatol. 2019, 139, 257–259. [Google Scholar] [CrossRef]
- He, M.; Zhou, T.; Niu, Y.; Feng, W.; Gu, X.; Xu, W.; Zhang, S.; Wang, Z.; Zhang, Y.; Wang, C.; et al. The protease corin regulates electrolyte homeostasis in eccrine sweat glands. PLoS Biol. 2021, 19, e3001090. [Google Scholar] [CrossRef]
- Nordberg, R.C.; Wang, H.; Wu, Q.; Loboa, E.G. Corin is a key regulator of endochondral ossification and bone development via modulation of vascular endothelial growth factor A expression. J. Tissue Eng. Regen. Med. 2018, 12, 2277–2286. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, J.; Liu, M.; Wu, Q.; Dong, N. Role of the protease corin in chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2018, 12, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, W.; Dong, N.; Lou, J.; Srinivasan, D.K.; Cheng, W.; Huang, X.; Liu, M.; Fang, C.; Peng, J.; et al. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature 2012, 484, 246–250. [Google Scholar] [CrossRef]
- Zhang, W.; Li, S.; Lou, J.; Li, H.; Liu, M.; Dong, N.; Wu, Q. Atrial natriuretic peptide promotes uterine decidualization and a TRAIL-dependent mechanism in spiral artery remodeling. J. Clin. Investig. 2021, 131, e151053. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, S.; Du, C.; Wang, K.; Gu, X.; Sun, S.; Zhang, X.; Niu, Y.; Wang, C.; Liu, M.; et al. Renal Corin Is Essential for Normal Blood Pressure and Sodium Homeostasis. Int. J. Mol. Sci. 2022, 23, 11251. [Google Scholar] [CrossRef]
- Polzin, D.; Kaminski, H.J.; Kastner, C.; Wang, W.; Krämer, S.; Gambaryan, S.; Russwurm, M.; Peters, H.; Wu, Q.; Vandewalle, A.; et al. Decreased renal corin expression contributes to sodium retention in proteinuric kidney diseases. Kidney Int. 2010, 78, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Khoury, E.E.; Fokra, A.; Kinaneh, S.; Knaney, Y.; Aronson, D.; Abassi, Z. Distribution of Cardiac and Renal Corin and Proprotein Convertase Subtilisin/Kexin-6 in the Experimental Model of Cardio-Renal Syndrome of Various Severities. Front Physiol. 2021, 12, 673497. [Google Scholar] [CrossRef]
- Hu, X.; Su, M.; Lin, J.; Zhang, L.; Sun, W.; Zhang, J.; Sun, W.; Zhang, J.; Tian, Y.; Qiu, W. Corin Is Downregulated in Renal Ischemia/Reperfusion Injury and Is Associated with Delayed Graft Function after Kidney Transplantation. Dis. Markers 2019, 2019, 9429323. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Romero, M.F. Regulation of electroneutral NaCl absorption by the small intestine. Annu. Rev. Physiol. 2011, 73, 261–281. [Google Scholar] [CrossRef]
- Rao, M.C. Physiology of Electrolyte Transport in the Gut: Implications for Disease. Compr. Physiol. 2019, 9, 947–1023. [Google Scholar] [PubMed]
- Michell, A.R. The gut: The unobtrusive regulator of sodium balance. Perspect. Biol. Med. 1986, 29, 203–213. [Google Scholar] [CrossRef]
- Dong, L.; Wang, H.; Dong, N.; Zhang, C.; Xue, B.; Wu, Q. Localization of corin and atrial natriuretic peptide expression in human renal segments. Clin. Sci. 2016, 130, 1655–1664. [Google Scholar] [CrossRef]
- Engelstoft, M.S.; Lund, M.L.; Grunddal, K.V.; Egerod, K.L.; Osborne-Lawrence, S.; Poulsen, S.S.; Zigman, J.M.; Schwartz, T.W. Research Resource: A Chromogranin A Reporter for Serotonin and Histamine Secreting Enteroendocrine Cells. Mol. Endocrinol. 2015, 29, 1658–1671. [Google Scholar] [CrossRef]
- Wang, Y.; Song, W.; Wang, J.; Wang, T.; Xiong, X.; Qi, Z.; Fu, W.; Yang, X.; Chen, Y.G. Single-cell transcriptome analysis reveals differential nutrient absorption functions in human intestine. J. Exp. Med. 2020, 217, e20191130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gu, X.; Zhang, Y.; Dong, N.; Wu, Q. Corin: A Key Mediator in Sodium Homeostasis, Vascular Remodeling, and Heart Failure. Biology 2022, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Armaly, Z.; Assady, S.; Abassi, Z. Corin: A new player in the regulation of salt-water balance and blood pressure. Curr. Opin. Nephrol. Hypertens. 2013, 22, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Gladysheva, I.P.; Robinson, B.R.; Houng, A.K.; Kováts, T.; King, S.M. Corin is co-expressed with pro-ANP and localized on the cardiomyocyte surface in both zymogen and catalytically active forms. J. Mol. Cell. Cardiol. 2008, 44, 131–142. [Google Scholar] [CrossRef]
- Tran, K.L.; Lu, X.; Lei, M.; Feng, Q.; Wu, Q. Upregulation of corin gene expression in hypertrophic cardiomyocytes and failing myocardium. Am. J. Physiol.-Heart Circ. Physiol. 2004, 287, H1625–H1631. [Google Scholar] [CrossRef]
- Wu, F.; Yan, W.; Pan, J.; Morser, J.; Wu, Q. Processing of pro-atrial natriuretic peptide by corin in cardiac myocytes. J. Biol. Chem. 2002, 277, 16900–16905. [Google Scholar] [CrossRef]
- Tanaka, M.; Itoh, H. Hypertension as a Metabolic Disorder and the Novel Role of the Gut. Curr. Hypertens. Rep. 2019, 21, 63. [Google Scholar] [CrossRef]
- Giordano, L.; Mihaila, S.M.; Eslami Amirabadi, H.; Masereeuw, R. Microphysiological Systems to Recapitulate the Gut-Kidney Axis. Trends Biotechnol. 2021, 39, 811–823. [Google Scholar] [CrossRef]
- Michell, A.R.; Debnam, E.S.; Unwin, R.J. Regulation of renal function by the gastrointestinal tract: Potential role of gut-derived peptides and hormones. Annu. Rev. Physiol. 2008, 70, 379–403. [Google Scholar] [CrossRef]
- Hatch, M. Intestinal adaptations in chronic kidney disease and the influence of gastric bypass surgery. Exp. Physiol. 2014, 99, 1163–1167. [Google Scholar] [CrossRef]
- Marmol, F.; Badaruddin, M.; Baig, A.; Ye, M.; Wysocki, J.; Tahaei, E.; Welling, P.; Bamberg, K.; Batlle, D. Fecal ammonium in mice with CKD: Gastrointestinal sequestration by sodium zirconium cyclosilicate. Am. J. Physiol.-Ren. Physiol. 2023, 324, F464–F471. [Google Scholar] [CrossRef] [PubMed]
- Hatch, M.; Freel, R.W.; Vaziri, N.D. Local upregulation of colonic angiotensin II receptors enhances potassium excretion in chronic renal failure. Am. J. Physiol. 1998, 274, F275–F282. [Google Scholar] [CrossRef] [PubMed]
- Malsure, S.; Wang, Q.; Charles, R.P.; Sergi, C.; Perrier, R.; Christensen, B.M.; Maillard, M.; Rossier, B.C.; Hummler, E. Colon-specific deletion of epithelial sodium channel causes sodium loss and aldosterone resistance. J. Am. Soc. Nephrol. 2014, 25, 1453–1464. [Google Scholar] [CrossRef]
- Booth, R.E.; Johnson, J.P.; Stockand, J.D. Aldosterone. Adv. Physiol. Educ. 2002, 26, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Negussie, A.B.; Dell, A.C.; Davis, B.A.; Geibel, J.P. Colonic Fluid and Electrolyte Transport 2022: An Update. Cells 2022, 11, 1712. [Google Scholar] [CrossRef] [PubMed]
- Masilamani, S.; Kim, G.H.; Mitchell, C.; Wade, J.B.; Knepper, M.A. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J. Clin. Investig. 1999, 104, R19–R23. [Google Scholar] [CrossRef] [PubMed]
- Rotin, D.; Staub, O. Function and Regulation of the Epithelial Na(+) Channel ENaC. Compr. Physiol. 2021, 11, 2017–2045. [Google Scholar] [PubMed]
- Rossier, B.C.; Staub, O.; Hummler, E. Genetic dissection of sodium and potassium transport along the aldosterone-sensitive distal nephron: Importance in the control of blood pressure and hypertension. FEBS Lett. 2013, 587, 1929–1941. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, X.; Wang, K.; Li, W.; He, M.; Zhou, T.; Liu, M.; Wu, Q.; Dong, N. Corin Deficiency Diminishes Intestinal Sodium Excretion in Mice. Biology 2023, 12, 945. https://doi.org/10.3390/biology12070945
Gu X, Wang K, Li W, He M, Zhou T, Liu M, Wu Q, Dong N. Corin Deficiency Diminishes Intestinal Sodium Excretion in Mice. Biology. 2023; 12(7):945. https://doi.org/10.3390/biology12070945
Chicago/Turabian StyleGu, Xiabing, Kun Wang, Wenguo Li, Meiling He, Tiantian Zhou, Meng Liu, Qingyu Wu, and Ningzheng Dong. 2023. "Corin Deficiency Diminishes Intestinal Sodium Excretion in Mice" Biology 12, no. 7: 945. https://doi.org/10.3390/biology12070945
APA StyleGu, X., Wang, K., Li, W., He, M., Zhou, T., Liu, M., Wu, Q., & Dong, N. (2023). Corin Deficiency Diminishes Intestinal Sodium Excretion in Mice. Biology, 12(7), 945. https://doi.org/10.3390/biology12070945




