Antioxidant, Antidiabetic, and Anti-Obesity Properties of Apple Pulp Extracts (Malus domestica Bork): A Comparative Study of 15 Local and Commercial Cultivars from Spain
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Reagents, and Plant Samples
2.2. Total Phenolic Content (TPC) Using Folin-Ciocalteu Assay
2.3. Radical Scavenging Activity by 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
2.4. Antioxidant Activity by Xanthine/Xanthine Oxidase (X/XO) Assay
2.5. Alpha-Glucosidase (α-GLU) Inhibition Assay
2.6. Advance Glycation End Products (AGEs) Production Assay
2.7. Pancreatic Lipase Inhibition Assay
2.8. Statistical Analysis
3. Results
3.1. Determination of Total Polyphenol Content (TPC)
3.2. Determination of Antioxidant Capacity
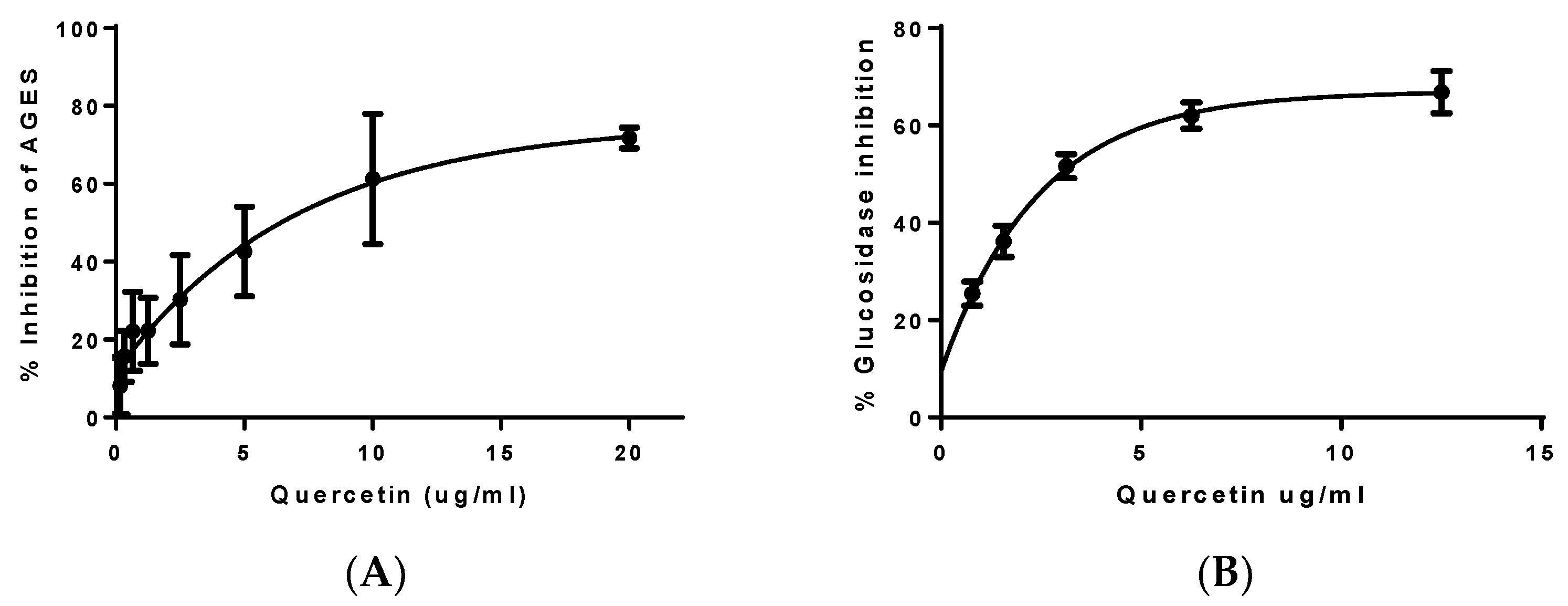
3.3. Determination of Antidiabetic Capacity
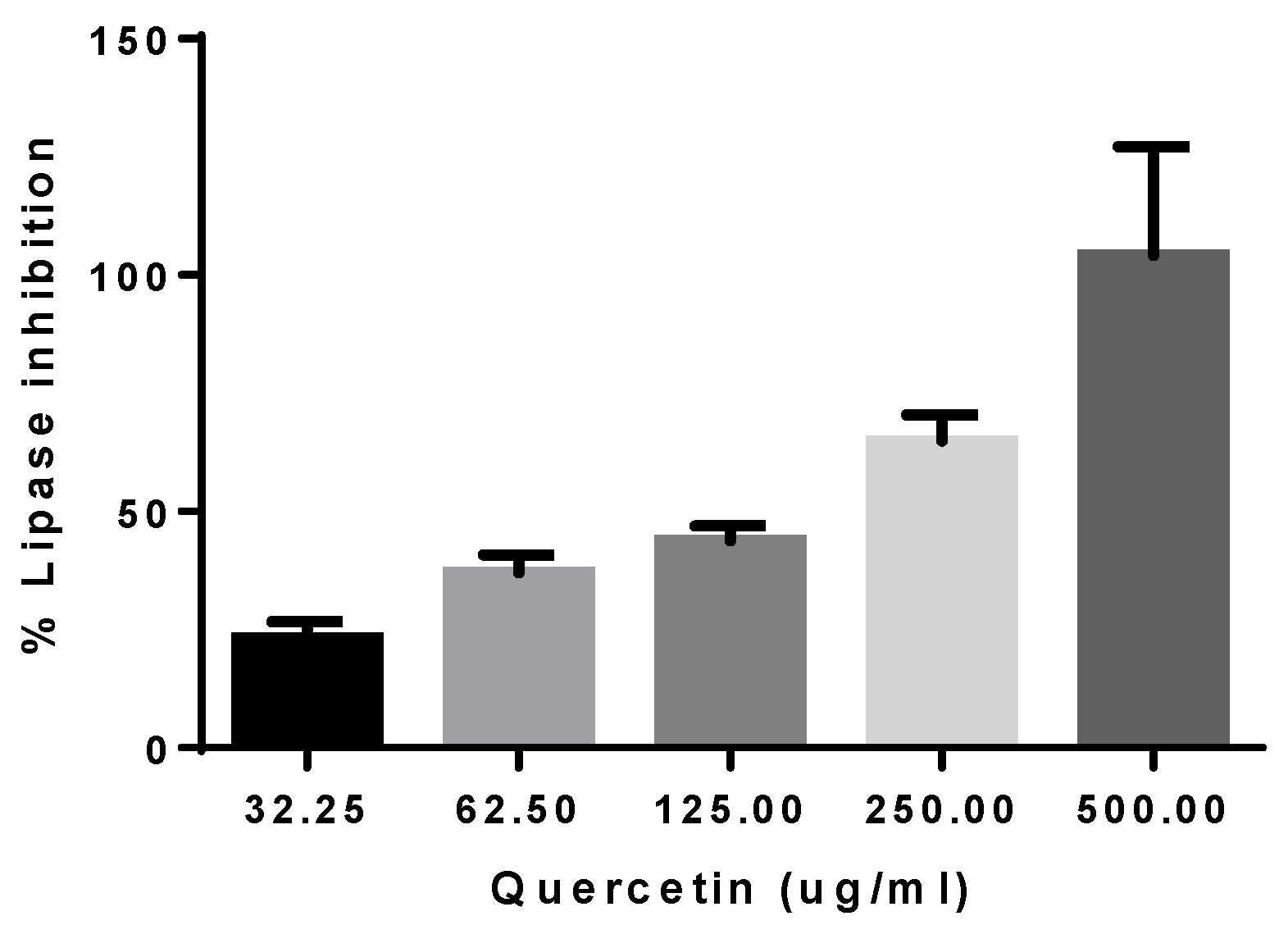
3.4. Determination of Anti-Obesity Capacity
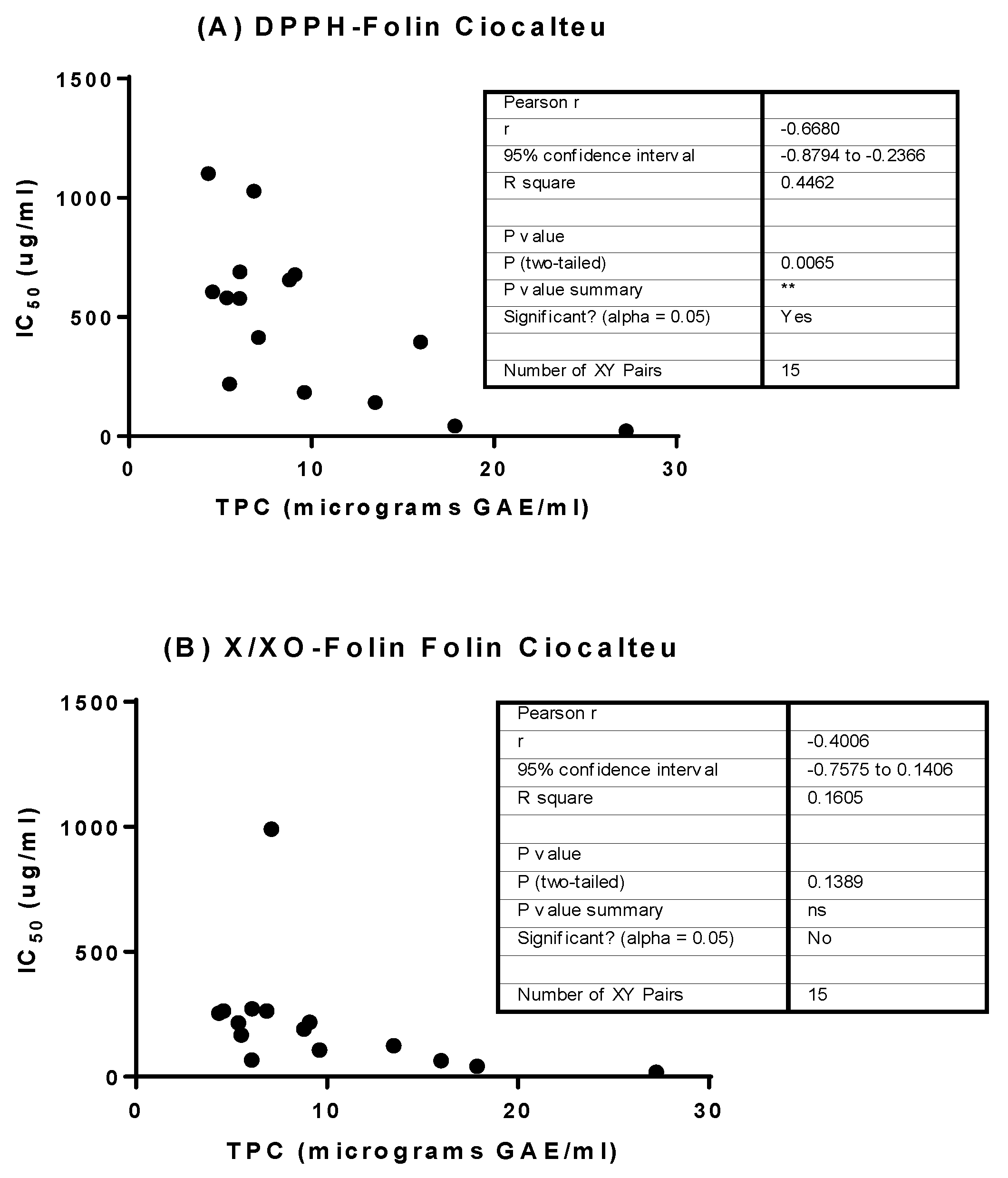
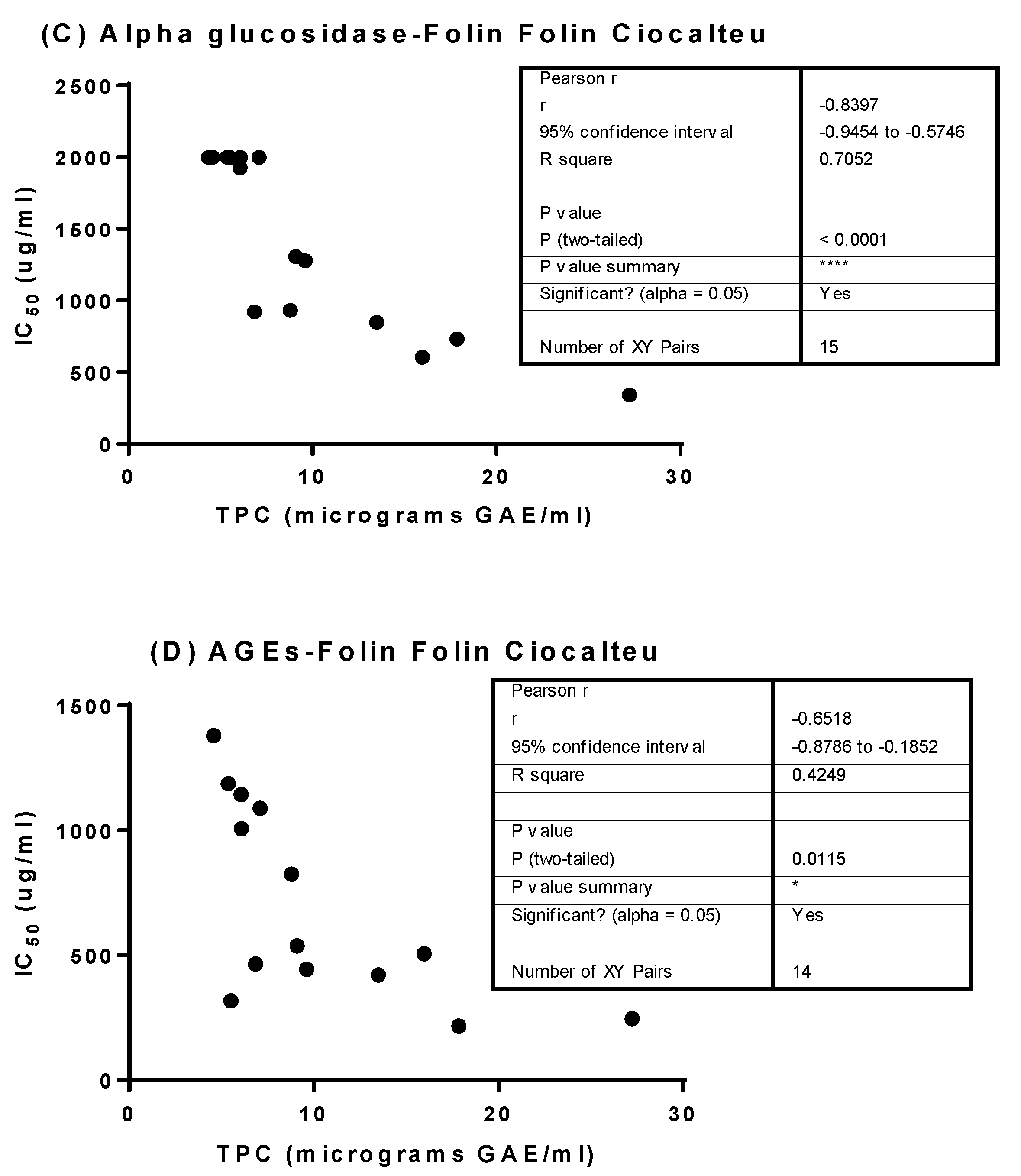
3.5. Determination of Linear Correlation between Polyphenol Content and Bioactivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dinda, B.; Dinda, M.; Roy, A.; Dinda, S. Dietary plant flavonoids in prevention of obesity and diabetes. Adv. Protein Chem. Struct. Biol. 2019, 120, 159–235. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.-M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G.; Kalle-Uhlmann, T.; Arregui, M.; Buijsse, B.; Boeing, H. Fruit and Vegetable Consumption and Changes in Anthropometric Variables in Adult Populations: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. PLoS ONE 2015, 10, e0140846. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. Int. Rev. J. 2012, 3, 506–516. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef]
- Butkeviciute, A.; Viskelis, J.; Liaudanskas, M.; Viskelis, P.; Janulis, V. Impact of Storage Controlled Atmosphere on the Apple Phenolic Acids, Flavonoids, and Anthocyanins and Antioxidant Activity In Vitro. Plants 2022, 11, 201. [Google Scholar] [CrossRef]
- Preti, R.; Tarola, A.M. Study of polyphenols, antioxidant capacity and minerals for the valorisation of ancient apple cultivars from Northeast Italy. Eur. Food Res. Technol. 2020, 247, 273–283. [Google Scholar] [CrossRef]
- Egbuna, C.; Awuchi, C.G.; Kushwaha, G.; Rudrapal, M.; Patrick-Iwuanyanwu, K.C.; Singh, O.; Odoh, U.E.; Khan, J.; Jeevanandam, J.; Kumarasamy, S.; et al. Bioactive Compounds Effective Against Type 2 Diabetes Mellitus: A Systematic Review. Curr. Top. Med. Chem. 2021, 21, 1067–1095. [Google Scholar] [CrossRef]
- Bule, M.; Abdurahman, A.; Nikfar, S.; Abdollahi, M.; Amini, M. Antidiabetic effect of quercetin: A systematic review and meta-analysis of animal studies. Food Chem. Toxicol. 2019, 125, 494–502. [Google Scholar] [CrossRef]
- Cao, H.; Chen, X. Structures required of flavonoids for inhibiting digestive enzymes. Anti-Cancer Agents Med. Chem. 2012, 12, 929–939. [Google Scholar] [CrossRef]
- Snyder, S.M.; Zhao, B.; Luo, T.; Kaiser, C.; Cavender, G.; Hamilton-Reeves, J.; Sullivan, D.K.; Shay, N.F. Consumption of Quercetin and Quercetin-Containing Apple and Cherry Extracts Affects Blood Glucose Concentration, Hepatic Metabolism, and Gene Expression Patterns in Obese C57BL/6J High Fat–Fed Mice. J. Nutr. 2016, 146, 1001–1007. [Google Scholar] [CrossRef]
- Gil Lee, S.; Parks, J.S.; Kang, H.W. Quercetin, a functional compound of onion peel, remodels white adipocytes to brown-like adipocytes. J. Nutr. Biochem. 2017, 42, 62–71. [Google Scholar] [CrossRef]
- Shahidi, F.; Dissanayaka, C.S. Phenolic-protein interactions: Insight from in-silico analyses—A review. Food Prod. Process. Nutr. 2023, 5, 2. [Google Scholar] [CrossRef]
- Muraki, I.; Imamura, F.; Manson, J.E.; Hu, F.B.; Willett, W.C.; van Dam, R.; Sun, Q. Fruit consumption and risk of type 2 diabetes: Results from three prospective longitudinal cohort studies. BMJ 2013, 347, f5001. [Google Scholar] [CrossRef]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Home|Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/home/en/ (accessed on 26 February 2023).
- Kumar, P.; Sethi, S.; Sharma, R.R.; Singh, S.; Saha, S.; Sharma, V.K.; Verma, M.K.; Sharma, S.K. Nutritional characterization of apple as a function of genotype. J. Food Sci. Technol. 2018, 55, 2729–2738. [Google Scholar] [CrossRef] [PubMed]
- Podsędek, A.; Wilska-Jeszka, J.; Anders, B.; Markowski, J. Compositional characterisation of some apple varieties. Eur. Food Res. Technol. 2000, 210, 268–272. [Google Scholar] [CrossRef]
- Vegro, M.; Eccher, G.; Populin, F.; Sorgato, C.; Savazzini, F.; Pagliarani, G.; Tartarini, S.; Pasini, G.; Curioni, A.; Antico, A.; et al. Old Apple (Malus domestica L. Borkh) Varieties with Hypoallergenic Properties: An Integrated Approach for Studying Apple Allergenicity. J. Agric. Food Chem. 2016, 64, 9224–9236. [Google Scholar] [CrossRef]
- Jakobek, L.; García-Villalba, R.; Tomás-Barberán, F.A. Polyphenolic characterisation of old local apple varieties from Southeastern European region. J. Food Compos. Anal. 2013, 31, 199–211. [Google Scholar] [CrossRef]
- López, V.; Les, F.; Mevi, S.; Wandjou, J.G.N.; Cásedas, G.; Caprioli, G.; Maggi, F. Phytochemicals and Enzyme Inhibitory Capacities of the Methanolic Extracts from the Italian Apple Cultivar Mela Rosa dei Monti Sibillini. Pharmaceuticals 2020, 13, 127. [Google Scholar] [CrossRef]
- Mignard, P.; Font i Forcada, C.; Giménez, R.; Moreno, M.Á. Population Structure and Association Mapping for Agronomical and Biochemical Traits of a Large Spanish Apple Germplasm. Plants 2023, 12, 1249. [Google Scholar] [CrossRef] [PubMed]
- Drogoudi, P.D.; Pantelidis, G. Effects of position on canopy and harvest time on fruit physico-chemical and antioxidant properties in different apple cultivars. Sci. Hortic. 2011, 129, 752–760. [Google Scholar] [CrossRef]
- López, V.; Akerreta, S.; Casanova, E.; García-Mina, J.M.; Cavero, R.Y.; Calvo, M.I. In vitro antioxidant and anti-rhizopus activities of lamiaceae herbal extracts. Plant Foods Hum. Nutr. 2007, 62, 151–155. [Google Scholar] [CrossRef]
- Rodríguez-Chávez, J.L.; Coballase-Urrutia, E.; Nieto-Camacho, A.; Delgado-Lamas, G. Antioxidant Capacity of “Mexican Arnica” Heterotheca inuloides Cass Natural Products and Some Derivatives: Their Anti-Inflammatory Evaluation and Effect on C. elegans Life Span. Oxidative Med. Cell. Longev. 2015, 2015, 843237. [Google Scholar] [CrossRef]
- Cásedas, G.; Les, F.; Gómez-Serranillos, M.P.; Smith, C.; López, V. Bioactive and functional properties of sour cherry juice (Prunus cerasus). Food Funct. 2016, 7, 4675–4682. [Google Scholar] [CrossRef]
- Spínola, V.; Castilho, P.C. Evaluation of Asteraceae herbal extracts in the management of diabetes and obesity. Contribution of caffeoylquinic acids on the inhibition of digestive enzymes activity and formation of advanced glycation end-products (in vitro). Phytochemistry 2017, 143, 29–35. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, Y.M.; Kim, H.; Kim, J.; Jang, D.S.; Kim, J.H.; Kim, J.S. Anti-obesity effect of Morus bombycis root extract: Anti-lipase activity and lipolytic effect. J. Ethnopharmacol. 2010, 130, 621–624. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Rigo, A.; Tonon, D.; Mattivi, F. Quantitation of Polyphenols in Different Apple Varieties. J. Agric. Food Chem. 2004, 52, 6532–6538. [Google Scholar] [CrossRef]
- Zhou, X.; Iqbal, A.; Li, J.; Liu, C.; Murtaza, A.; Xu, X.; Pan, S.; Hu, W. Changes in Browning Degree and Reducibility of Polyphenols during Autoxidation and Enzymatic Oxidation. Antioxidants 2021, 10, 1809. [Google Scholar] [CrossRef]
- Salvamani, S.; Gunasekaran, B.; Shaharuddin, N.A.; Ahmad, S.A.; Shukor, M.Y. Antiartherosclerotic Effects of Plant Flavonoids. BioMed Res. Int. 2014, 2014, 480258. [Google Scholar] [CrossRef]
- Wilms, L.C.; Hollman, P.C.H.; Boots, A.W.; Kleinjans, J.C. Protection by quercetin and quercetin-rich fruit juice against induction of oxidative DNA damage and formation of BPDE-DNA adducts in human lymphocytes. Mutat. Res. Toxicol. Environ. Mutagen. 2005, 582, 155–162. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, Y.; Sang, S. Dietary Quercetin Reduces Plasma and Tissue Methylglyoxal and Advanced Glycation End Products in Healthy Mice Treated with Methylglyoxal. J. Nutr. 2021, 151, 2601–2609. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, T.; Sang, S.; Lv, L. Quercetin Inhibits Advanced Glycation End Product Formation by Trapping Methylglyoxal and Glyoxal. J. Agric. Food Chem. 2014, 62, 12152–12158. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.-J.; Hsia, S.-M.; Lee, W.-H.; Wu, C.-H. Polyphenols with antiglycation activity and mechanisms of action: A review of recent findings. J. Food Drug Anal. 2017, 25, 84–92. [Google Scholar] [CrossRef]
- Núñez, S.; Moliner, C.; Valero, M.S.; Mustafa, A.M.; Maggi, F.; Gómez-Rincón, C.; López, V. Antidiabetic and anti-obesity properties of a polyphenol-rich flower extract from Tagetes erecta L. and its effects on Caenorhabditis elegans fat storages. J. Physiol. Biochem. 2023. [Google Scholar] [CrossRef]
- Modanwal, S.; Maurya, A.K.; Mishra, S.K.; Mishra, N. Development of QSAR model using machine learning and molecular docking study of polyphenol derivatives against obesity as pancreatic lipase inhibitor. J. Biomol. Struct. Dyn. 2022, 1–12. [Google Scholar] [CrossRef]
- Bialecka-Florjanczyk, E.; Fabiszewska, A.U.; Krzyczkowska, J.; Kurylowicz, A. Synthetic and Natural Lipase Inhibitors. Mini Rev. Med. Chem. 2018, 18, 672–683. [Google Scholar] [CrossRef]



| Sample | IC50 Values (ug/mL) in the Bioassays | |
|---|---|---|
| DPPH | X/XO | |
| Arraiza | 655.38 ± 17.19 | 190.92 ± 20.18 |
| De Mine | 1027.94 ± 32.56 | 263.14 ± 12.72 |
| Gordoncha | 689.60 ± 21.39 | 272.48 ± 13.98 |
| M. Tomate | 678.33 ± 27.06 | 218.40 ± 11.99 |
| Ziordia | 395.91 ± 18.54 | 64.48 ± 3.24 |
| Pomera de pomes agrias | 43.36 ± 2.92 | 42.29 ± 3.70 |
| Amarilla de Octubre | 23.50 ± 1.34 | 18.40 ± 1.29 |
| Doncella de San Martin | 141.38 ± 11.40 | 124.71 ± 7.10 |
| Pomera del pais | 1101.67 ± 65.40 | 254.55 ± 17.92 |
| Borau 01 | 414.03 ± 16.27 | 991.13 ± 145.80 |
| Esperiega de Ademuz | 218.76 ± 12.29 | 167.05 ± 32.92 |
| Manzana helada | 184.93 ± 10.49 | 107.20 ± 15.33 |
| Royal Gala | 580.73 ± 26.20 | 215.09 ± 28.13 |
| Verde Doncella | 578.04 ± 44.06 | 66.65 ± 6.71 |
| Pinova | 605.82 ± 26.06 | 263.25 ± 27.48 |
| Quercetin | 1.67 ± 0.06 | 13.48 ± 0.95 |
| Sample | IC50 Values (ug/mL) in the Bioassays | |
|---|---|---|
| α-Glucosidase | AGEs | |
| Arraiza | 933.24 ± 298.00 | 823.79 ± 124.50 |
| De Mine | 922.20 ± 199.10 | 464.84 ± 81.85 |
| Gordoncha | >2000 | 1006.47± 148.80 |
| M. Tomate | 1306.65 ± 211.00 | 536.78 ± 95.06 |
| Ziordia | 605.81 ± 161.40 | 506.10 ± 67.34 |
| Pomera de pomes agrias | 731.63 ± 42.49 | 215.08 ± 66.94 |
| Amarilla de Octubre | 341.87 ± 83.25 | 245.28 ± 36.73 |
| Doncella de San Martin | 848.45 ± 78.30 | 420.02 ± 61.64 |
| Pomera del pais | >2000 | - |
| Borau 01 | >2000 | 1087.58 ± 117.10 |
| Esperiega de Ademuz | >2000 | 316.71 ± 121.30 |
| Manzana helada | 1277.25 ± 135.40 | 443.09 ± 49.32 |
| Royal Gala | >2000 | 1186.47 ± 126.50 |
| Verde Doncella | 1926.88 ± 275.70 | 1143.54 ± 140.90 |
| Pinova | >2000 | 1379.56 ± 261.30 |
| Quercetin | 2.98 ± 0.40 | 6.42 ± 1.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millán-Laleona, A.; Bielsa, F.J.; Aranda-Cañada, E.; Gómez-Rincón, C.; Errea, P.; López, V. Antioxidant, Antidiabetic, and Anti-Obesity Properties of Apple Pulp Extracts (Malus domestica Bork): A Comparative Study of 15 Local and Commercial Cultivars from Spain. Biology 2023, 12, 891. https://doi.org/10.3390/biology12070891
Millán-Laleona A, Bielsa FJ, Aranda-Cañada E, Gómez-Rincón C, Errea P, López V. Antioxidant, Antidiabetic, and Anti-Obesity Properties of Apple Pulp Extracts (Malus domestica Bork): A Comparative Study of 15 Local and Commercial Cultivars from Spain. Biology. 2023; 12(7):891. https://doi.org/10.3390/biology12070891
Chicago/Turabian StyleMillán-Laleona, Adrián, Francisco Javier Bielsa, Eduardo Aranda-Cañada, Carlota Gómez-Rincón, Pilar Errea, and Víctor López. 2023. "Antioxidant, Antidiabetic, and Anti-Obesity Properties of Apple Pulp Extracts (Malus domestica Bork): A Comparative Study of 15 Local and Commercial Cultivars from Spain" Biology 12, no. 7: 891. https://doi.org/10.3390/biology12070891
APA StyleMillán-Laleona, A., Bielsa, F. J., Aranda-Cañada, E., Gómez-Rincón, C., Errea, P., & López, V. (2023). Antioxidant, Antidiabetic, and Anti-Obesity Properties of Apple Pulp Extracts (Malus domestica Bork): A Comparative Study of 15 Local and Commercial Cultivars from Spain. Biology, 12(7), 891. https://doi.org/10.3390/biology12070891







