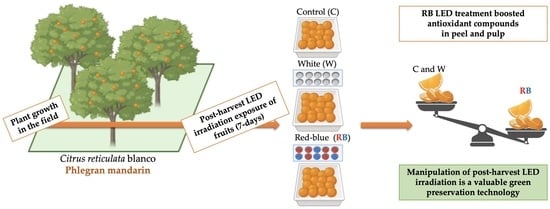
Modulation of Antioxidant Compounds in Fruits of Citrus reticulata Blanco Using Postharvest LED Irradiation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
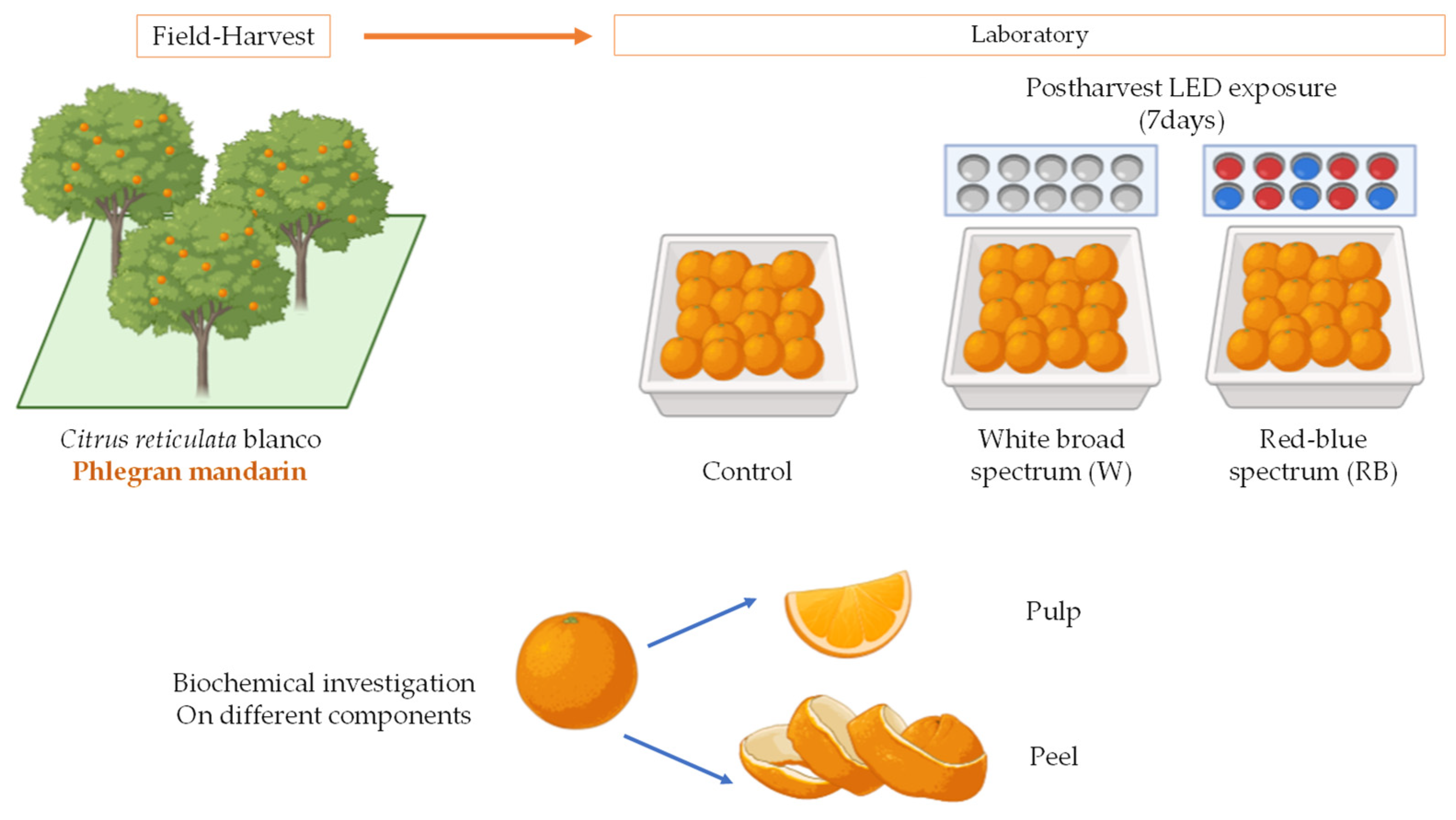
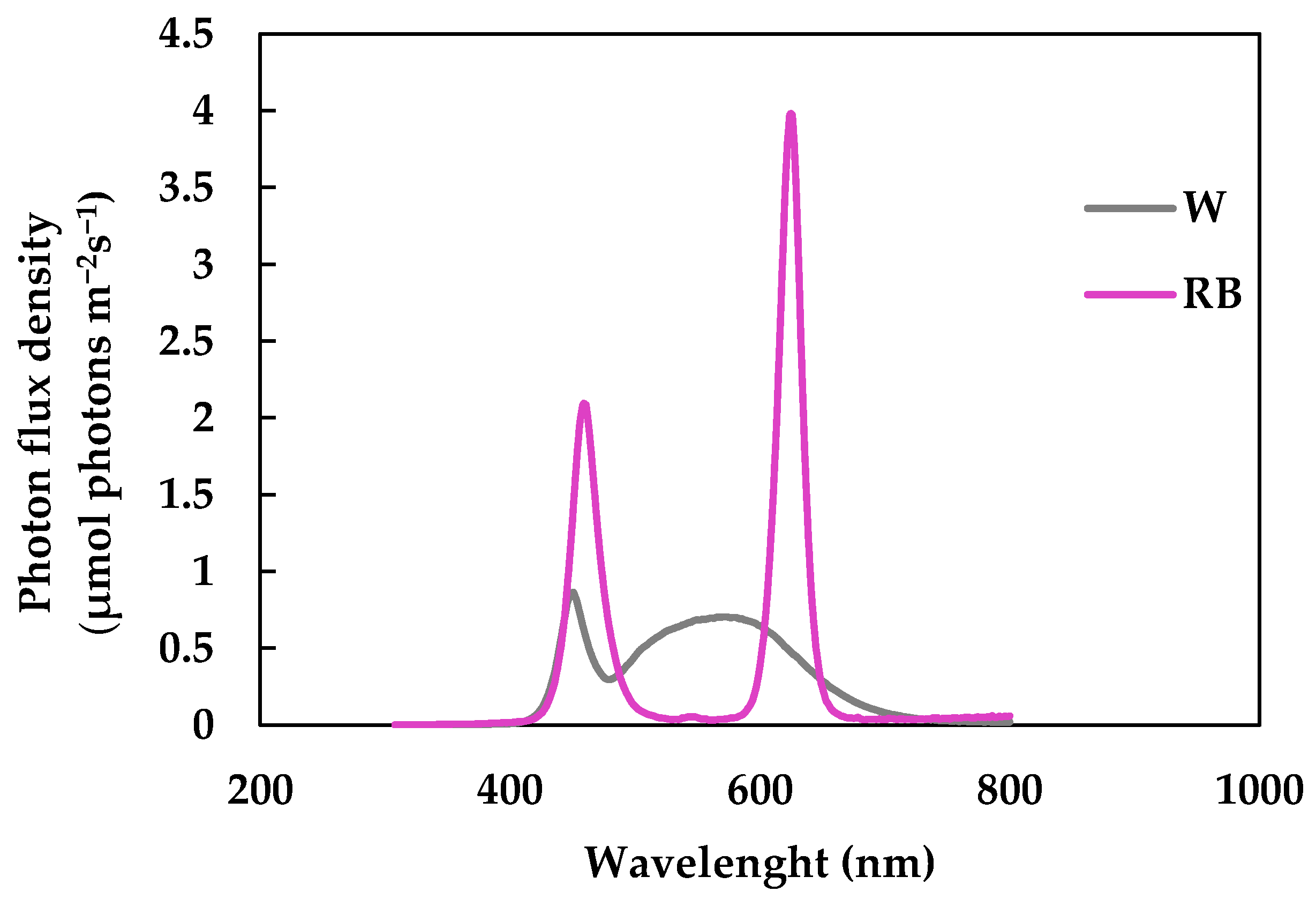
2.1. Plant Material, Sample Preparation, Storage Conditions, and Light Treatment
2.2. Preparation of Methanolic Extracts
2.3. Total Polyphenol Concentration
2.4. Flavonoids Content Determination
2.5. Anthocyanin Determination
2.6. Carotenoid and Ascorbic Acid Content Analysis
2.7. Sample Antioxidant Activity Determination
2.7.1. DPPH Method
2.7.2. FRAP Method
2.8. Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS)
2.9. Statistical Treatment of Data
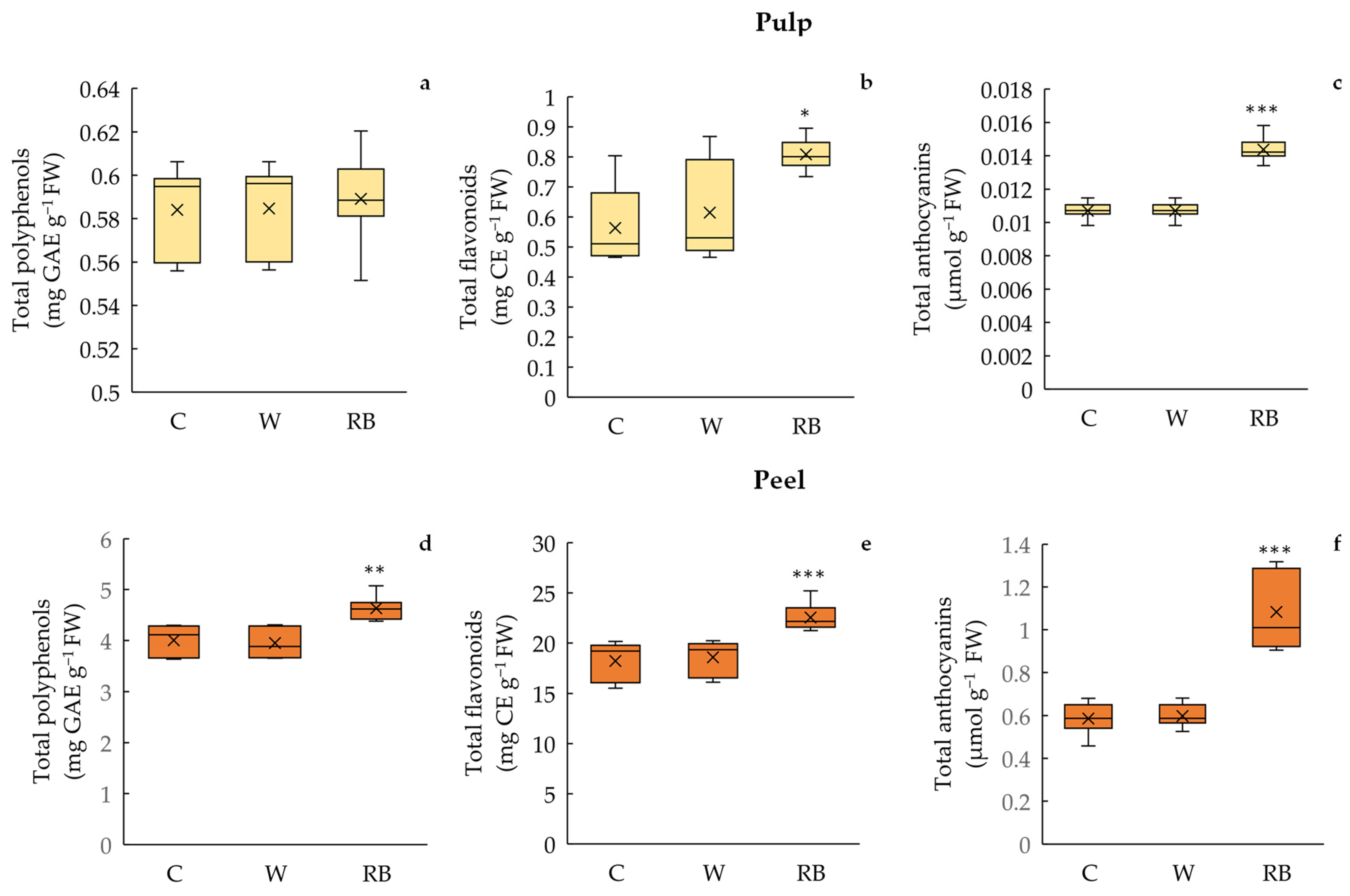
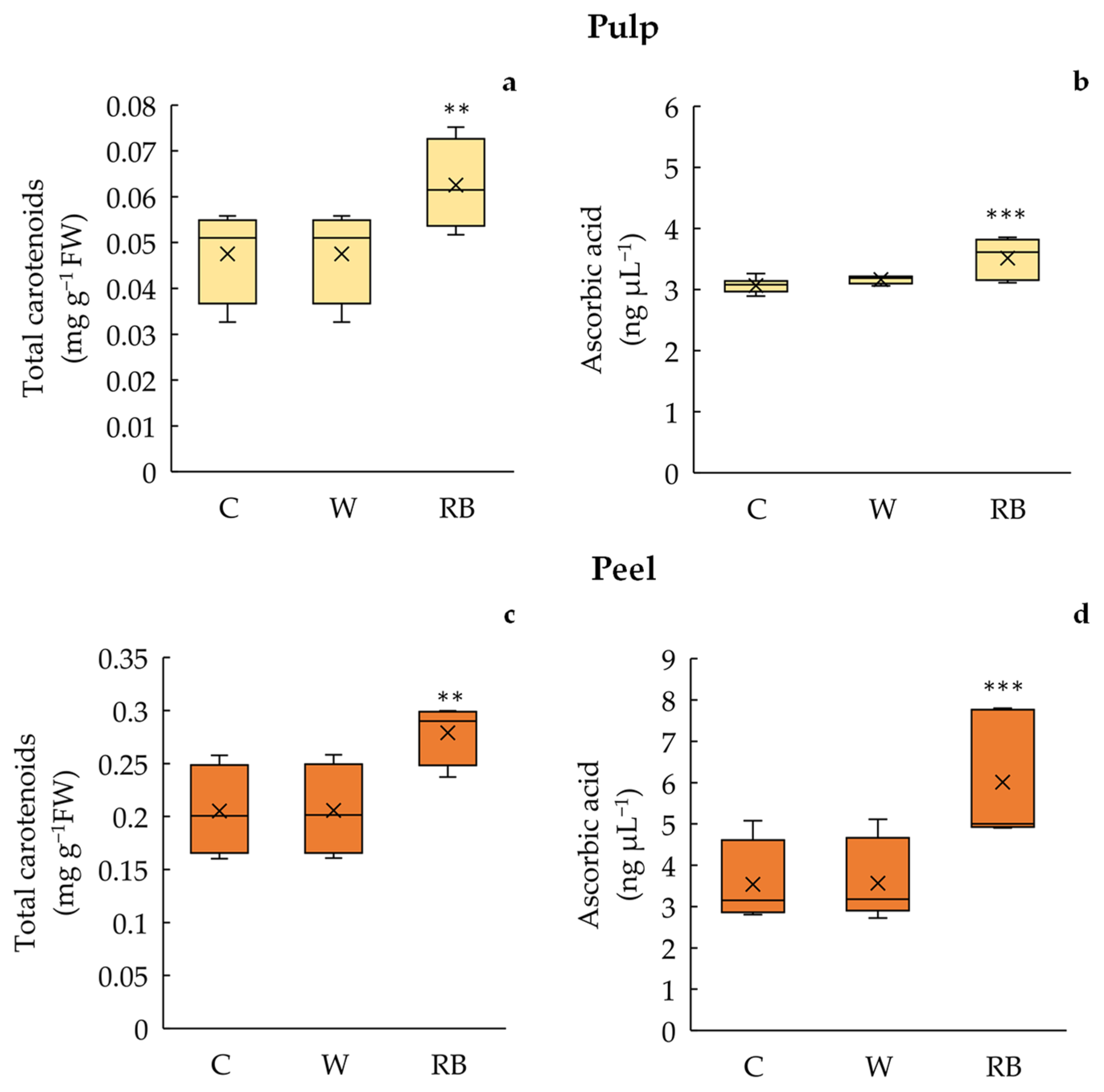
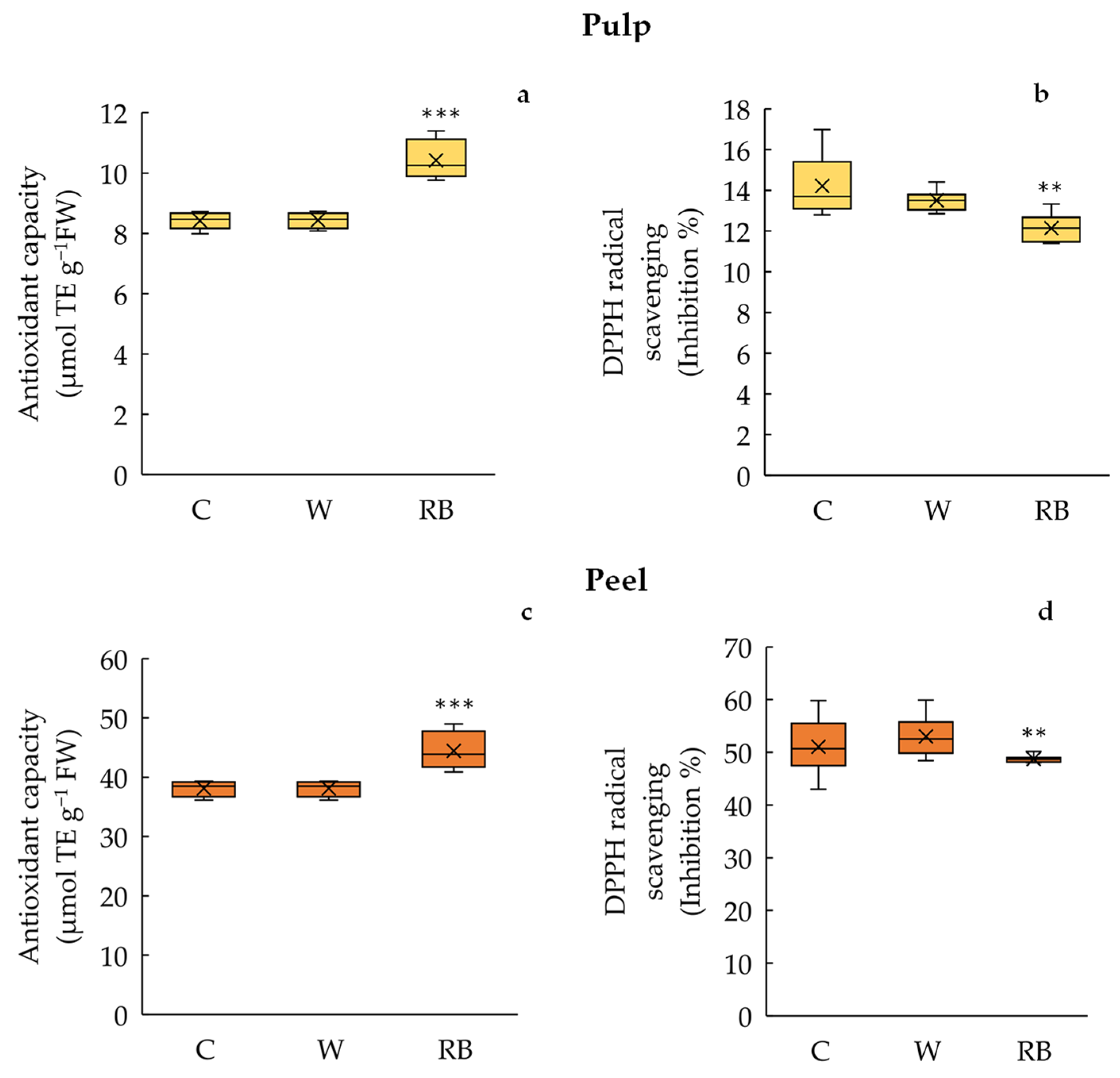
3. Results
3.1. Total Phenolic Compounds in Control, W, and RB Fruits
Total Polyphenols, Flavonoid, and Anthocyanin Content
3.2. Total Carotenoids and Ascorbic Acid Content
3.3. Antioxidant Capacity and DPPH Radical Scavenging Activity
3.4. Polyphenolic Profile of Pulp and Peel of W and RB Fruits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moulehi, I.; Bourgou, S.; Ourghemmi, I.; Tounsi, M.S. Variety and ripening impact on phenolic composition and Antioxidant activity of mandarin (Citrus reticulate Blanco) and bitter orange (Citrus aurantium L.) seeds extracts. Ind. Crop. Prod. 2012, 39, 74–80. [Google Scholar] [CrossRef]
- Ghasemi, K.; Ghasemi, Y.; Ebrahimzadeh, M.A. Antioxidant activity, phenol, and flavonoid contents of 13 citrus species peels and tissues. Pak. J. Pharm. Sci. 2009, 22, 277–281. [Google Scholar]
- Smith, M.; Smith, J.C. Repurposing therapeutics for COVID-19: Supercomputer-based docking to the SARS-CoV-2 viral spike protein and viral spike protein-human ACE2 interface. ChemRxiv 2020, 1–28. [Google Scholar] [CrossRef]
- Costanzo, G.; Vitale, E.; Iesce, M.R.; Naviglio, D.; Amoresano, A.; Fontanarosa, C.; Spinelli, M.; Ciaravolo, M.; Arena, C. Antioxidant Properties of Pulp, Peel and Seeds of Phlegrean Mandarin (Citrus reticulata Blanco) at Different Stages of Fruit Ripening. Antioxidants 2022, 11, 187. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; Florença, S.G.; Barroca, M.J.; Anjos, O. The link between the consumer and the innovations in food product development. Foods 2020, 9, 1317. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef]
- Xu, F.; Cao, S.; Shi, L.; Chen, W.; Su, X.; Yang, Z. Blue light irradiation affects anthocyanin content and enzyme activities involved in postharvest strawberry fruit. J. Agric. Food Chem. 2014, 62, 4778–4783. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Shi, L.; Chen, W.; Cao, S.; Su, X.; Yang, Z. Scientia Horticulturae Effect of blue light treatment on fruit quality, antioxidant enzymes and radical-scavenging activity in strawberry fruit. Sci. Hortic. 2014, 175, 181–186. [Google Scholar] [CrossRef]
- Dutta Gupta, S. Light emitting diodes for agriculture: Smart lighting. Light Emit. Diodes Agric. Smart Light. 2017, XIX, 1–334. [Google Scholar] [CrossRef]
- Vitale, E.; Velikova, V.; Tsonev, T.; Ferrandino, I.; Capriello, T. and Arena. C. The Interplay between Light Quality and Biostimulant Application Affects the Antioxidant Capacity and Photosynthetic Traits of Soybean (Glycine max L. Merrill). Plants 2021, 10, 861. [Google Scholar] [CrossRef]
- Vitale, E.; Velikova, V.; Tsonev, T.; Costanzo, G.; Paradiso, R.; Arena, C. Manipulation of light quality is an effective tool to regulate photosynthetic capacity and fruit antioxidant properties of Solanum lycopersicum L. cv. ‘Microtom’ in a controlled environment. PeerJ 2022, 10, e13677. [Google Scholar] [CrossRef]
- Hoffmann, A.M.; Noga, G.; Hunsche, M. High blue light improves acclimation and photosynthetic recovery of pepper plants exposed to UV stress. Environ. Exp. Bot. 2014, 109, 254–263. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, L.; Setiawan, C.K.; Yamawaki, K.; Asai, T.; Nishikawa, F.; Maezawa, S.; Sato, H.; Kanemitsu, N.; Kato, M. Effect of red and blue LED light irradiation on ascorbate content and expression of genes related to ascorbate metabolism in postharvest broccoli. Postharvest Biol. Technol. 2014, 94, 97–103. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, L.; Kato, M.; Yamawaki, K.; Kiriiwa, Y.; Yahata, M.; Ikoma, Y.; Matsumoto, H. Effect of the combination of ethylene and red LED light irradiation on carotenoid accumulation and carotenogenic gene expression in the flavedo of citrus fruit. J. Agric. Food Chem. 2012, 60, 197–201. [Google Scholar] [CrossRef]
- Costanzo, G.; Iesce, M.R.; Naviglio, D.; Ciaravolo, M.; Vitale, E.; Arena, C. Comparative Studies on Different Citrus Cultivars: A Revaluation of Waste Mandarin Components. Antioxidants 2020, 9, 517. [Google Scholar] [CrossRef]
- Nassarawa, S.S.; Abdelshafy, A.M.; Xu, Y.; Luo, Z. Effect of Light-Emitting Diodes (LEDs) on the Quality of Fruits and Vegetables During Postharvest Period: A Review. Food Bioprocess Technol. 2021, 14, 388–414. [Google Scholar] [CrossRef]
- Bantis, F.; Smirnakou, S.; Ouzounis, T.; Koukounaras, A.; Ntagkas, N.; Radoglou, K. Current status and recent achievements in the field of horticulture with the use of light-emitting diodes (LEDs). Sci. Hortic. 2018, 235, 437–451. [Google Scholar] [CrossRef]
- Branas, C.; Azcondo, F.J.; Alonso, J.M. Solid-state lighting: A system review. IEEE Ind. Electron. Mag. 2013, 7, 6–14. [Google Scholar] [CrossRef]
- Lee, Y.J.; Ha, J.Y.; Oh, J.E.; Cho, M.S. The effect of LED irradiation on the quality of cabbage stored at a low temperature. Food Sci. Biotechnol. 2014, 23, 1087–1093. [Google Scholar] [CrossRef]
- Baenas, N.; Iniesta, C.; González-Barrio, R.; Nuñez-Gómez, V.; Periago, M.J.; García-Alonso, F.J. Post-Harvest Use of Ultraviolet Light (UV) and Light Emitting Diode (LED) to Enhance Bioactive Compounds in Refrigerated Tomatoes. Molecules 2021, 26, 1847. [Google Scholar] [CrossRef]
- Sun, B.; Richardo-da-Silva, J.M.; Sprenger, I. Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Mancinelli, A.L.; Huang Yang, C.P.; Lindquist, P.; Anderson, R.; Rabino, I. Photocontrol of Anthocyanin Synthesis. Plant Physiol. 1975, 55, 251–257. [Google Scholar] [CrossRef]
- Chung, Y.C.; Chen, S.J.; Hsu, C.K.; Chang, C.T.; Chou, S.T. Studies on the antioxidative activity of Graptopetalum paraguayense E. Walther. Food Chem. 2005, 91, 419–424. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays et al. Determination of Antioxidant Capacity. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- George, B.; Kaur, C.; Khurdiya, D.S.; Kapoor, H.C. Antioxidants in tomato (Lycopersium esculentum) as a function of genotype. Food Chem. 2004, 84, 45–51. [Google Scholar] [CrossRef]
- Taulavuori, E.; Taulavuori, K.; Holopainen, J.K.; Julkunen-Tiitto, R.; Acar, C.; Dincer, I. Targeted use of LEDs in improvement of production efficiency through phytochemical enrichment. J. Sci. Food Agric. 2017, 97, 5059–5064. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Xu, Y.; Sang, Y.; Mei, S.; Xu, C.; Yu, X.; Pan, T.; Cheng, C.; Zhang, J.; et al. The Effects of Storage Temperature, Light Illumination, and Low-Temperature Plasma on Fruit Rot and Change in Quality of Postharvest Gannan Navel Oranges. Foods 2022, 11, 3707. [Google Scholar] [CrossRef]
- Connor, A.M.; Stephens, M.J.; Hall, H.K.; Alspach, P.A. Variation and heritabilities of antioxidant activity and total phenolic concentration estimated from a red raspberry factorial experiment. J. Am. Soc. Hortic. Sci. 2005, 130, 403–411. [Google Scholar] [CrossRef]
- Lugasi, A.; Hóvári, J. Antioxidant properties of commercial alcoholic and nonalcoholic beverages. Mol. Nutr. Food Res. 2003, 47, 79–86. [Google Scholar] [CrossRef]
- Lee, S.U.; Lee, J.H.; Choi, S.H.; Lee, J.S.; Kameyama, M.O.; Kozukue, N.; Friedman, M. Flavonoid content in fresh, home-processed, and light-exposed onions and in dehydrated commercial onion products. J. Agric. Food Chem. 2008, 56, 8541–8548. [Google Scholar] [CrossRef]
- Ballester, A.R.; Lafuente, M.T. LED blue light-induced changes in phenolics and ethylene in citrus fruit: Implication in elicited resistance against Penicillium digitatum infection. Food Chem. 2017, 218, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Chen, C.T.; Wang, C.Y. The influence of light and maturity on fruit quality and flavonoid content of red raspberries. Food Chem. 2009, 112, 676–684. [Google Scholar] [CrossRef]
- Maroga, G.M.; Soundy, P.; Sivakumar, D. Different Postharvest Responses of Fresh-Cut Sweet Peppers Related to Quality and Antioxidant and Phenylalanine Ammonia Lyase Activities during Exposure to Light-Emitting Diode Treatments. Foods 2019, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Darko, E.; Hamow, K.A.; Marcek, T.; Dernovics, M.; Ahres, M.; Galiba, G. Modulated Light Dependence of Growth, Flowering, and the Accumulation of Secondary Metabolites in Chilli. Front. Plant Sci. 2022, 13, 801656. [Google Scholar] [CrossRef]
- Jie, L.H.; Jantan, I.; Yusoff, S.D.; Jalil, J.; Husain, K. Sinensetin: An Insight on Its Pharmacological Activities, Mechanisms of Action and Toxicity. Front. Pharmacol. 2021, 11, 553404. [Google Scholar] [CrossRef]
- Mottaghipisheh, J.; Taghrir, H.; Boveiri Dehsheikh, A.; Zomorodian, K.; Irajie, C.; Mahmoodi Sourestani, M.; Iraji, A. Linarin, a Glycosylated Flavonoid, with Potential Therapeutic Attributes: A Comprehensive Review. Pharmaceuticals 2021, 29, 1104. [Google Scholar] [CrossRef]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic Acid Derivatives and Their Biological Efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef]
- Yalameha, B.; Nejabati, H.R.; Nouri, M. Cardioprotective potential of vanillic acid. Clin. Exp. Pharmacol. Physiol. 2023, 50, 193–204. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Imaizumi, T.; Kanegae, T.; Wada, M. Cryptochrome nucleocytoplasmic distribution and gene expression are regulated by light quality in the fern Adiantum capillus-veneris. Plant Cell. 2000, 12, 81–96. [Google Scholar] [CrossRef]
- Loi, M.; Villani, A.; Paciolla, F.; Mulè, G.; Paciolla, C. Challenges and Opportunities of Light-Emitting Diode (LED) as Key to Modulate Antioxidant Compounds in Plants. A Review. Antioxidants 2021, 10, 42. [Google Scholar] [CrossRef]
- Li, H.; Lin, Y.; Chen, X.; Bai, Y.; Wang, C.; Xu, X.; Lai, Z. Effects of blue light on flavonoid accumulation linked to the expression of miR393, miR394 and miR395 in longan embryogenic calli. PLoS ONE 2018, 13, e0191444. [Google Scholar] [CrossRef]
- Ramalingayya, G.V.; Nampoothiri, M.; Nayak, P.G.; Kishore, A.; Shenoy, R.R.; Rao, C.M.; Nandakumar, K. Naringin and Rutin Alleviates Episodic Memory Deficits in Two Differentially Challenged Object Recognition Tasks. Pharmacogn. Mag. 2016, 12, 63–70. [Google Scholar]
- Jain, V.; Viswanatha, G.L.; Manohar, D.; Shivaprasad, H.N. Isolation of Antidiabetic Principle from Fruit Rinds of Punica granatum. Evid. Based Complement. Altern. Med. 2012, 2012, 147202. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Kim, B.S.; Lee, H.O.; Kim, J.Y.; Kwon, K.H.; Cha, H.S.; Kim, J.H. An effect of light emitting diode (LED) irradiation treatment on the amplification of functional components of immature strawberry. Hortic. Environ. Biotechnol. 2011, 52, 35–39. [Google Scholar] [CrossRef]
- Liu, S.; Hu, L.; Jiang, D.; Xi, W. Effect of Post-Harvest LED and UV Light Irradiation on the Accumulation of Flavonoids and Limonoids in the Segments of Newhall Navel Oranges (Citrus sinensis Osbeck). Molecules 2019, 24, 1755. [Google Scholar] [CrossRef]
- Panjai, L.; Noga, G.; Hunsche, M.; Fiebig, A. Optimal red light irradiation time to increase health-promoting compounds in tomato fruit postharvest. Sci. Hortic. 2019, 251, 189–196. [Google Scholar] [CrossRef]
- Wang, S.; Jin, N.; Jin, L.; Xiao, X.; Hu, L.; Liu, Z.; Wu, Y.; Xie, Y.; Zhu, W.; Lyu, J.; et al. Response of Tomato Fruit Quality Depends on Period of LED Supplementary Light. Front Nutr. 2022, 9, 833723. [Google Scholar] [CrossRef] [PubMed]
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis, S.; Mastropasqua, L.; de Pinto, M.C. Vitamin C in Plants: From Functions to Biofortification. Antioxidants 2019, 8, 519. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Augmentation of leaf color parameters, pigments, vitamins, phenolic acids, flavonoids and antioxidant activity in selected Amaranthus tricolor under salinity stress. Sci. Rep. 2018, 8, 12349. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought stress effects on growth, ros markers, compatible solutes, phenolics, flavonoids, and antioxidant activity in Amaranthus tricolor. Appl. Biochem. Biotechnol. 2018, 186, 999–1016. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Bashir, T.; Ghosh, R.; Lee, S.K.; Bae, H. An overview of LEDs’ effects on the production of bioactive compounds and crop quality. Molecules 2017, 22, 1420. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.D.; Chang, J.W.; Ma, Q.L.; Chen, L.L.; Liu, S.; Jin, S.Z.; Han, J.W.; Xu, R.W.; Zhu, A.D.; Guo, J.; et al. Network analysis of postharvest senescence process in citrus revealed by transcriptomic and metabolomic profiling. Plant. Physiol. 2015, 168, 357–376. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, A.; Hussain, M.H.; Zaman, W.Q.; Mohsin, M.Z.; Zhan, J.; Liu, Z.; Tian, X.; Salim-Ur-Rehman; Khan, I.M.; Niazi, S.; et al. Advances in sustainable approaches utilizing orange peel waste to produce highly value-added bioproducts. Crit. Rev. Biotechnol. 2022, 42, 1284–1303. [Google Scholar] [CrossRef]
| Pulp | Peel | |||
|---|---|---|---|---|
| Compound | W | RB | W | RB |
| Naringin | 156.798 ns | 143.395 ns | 187.742 * | 63.560 |
| Quercetin | <0.1 | <0.1 | 0.740 *** | 0.173 |
| Vanillic acid | 2.465 ns | 2.884 ns | 16.320 | 29.024 *** |
| Caffeic acid | 4.442 | 4.766 ** | 15.166 | 22.893 *** |
| Delphinidin | <0.1 | <0.1 | 0.106 | 0.903 ** |
| Pelargonidin | <0.1 | <0.1 | <0.1 | 1.657 *** |
| Haesperetin | 0.166 | 0.360 ** | 2.714 * | 2.150 |
| Kaempferol 3-O-rutinoside | 29.931 | 33.867 * | 127.695 *** | 95.175 |
| Caffeoylquinic acid derivatives | 1.802 ** | 0.907 | 231.196*** | 35.454 |
| Quercetin rutinoside | 0.252 ** | 0.202 | 35.031 ns | 34.395 ns |
| Methyl gallate | <0.1 | <0.1 | 0.279 | 0.533 *** |
| EGC epicatechin dimer | <0.1 | <0.1 | 1.420 | 2.649 ** |
| EfisetinidolEC isomer 3 | <0.1 | <0.1 | <0.1 | 2.072 *** |
| Chlorogenic acid | 35.144 ** | 15.001 | 2311.387 ** | 2148.683 |
| Ferulic acid | 0.140 | 0.318 ** | 0.857 | 3.399 *** |
| Linarin | <0.1 | <0.1 | <0.1 | 0.130 *** |
| Puerarin | <0.1 | <0.1 | <0.1 | 0.192*** |
| Diosmetin | <0.1 | <0.1 | 0.105 ns | 0.108 ns |
| Astragalin | <0.1 | <0.1 | 0.246 | 1.354 ** |
| Coumaric acid | 1.719 ns | 1.400 ns | 3.869 | 9.582 ** |
| 3-p-coumaroylquinic acid | <0.1 | <0.1 | 3.050 ** | 1.736 |
| Orientin | <0.1 | <0.1 | 5.427 * | 3.770 |
| Isorhoifolin | 0.503 ** | 0.315 | 9.447 *** | 1.870 |
| Hyperoside | <0.1 | <0.1 | 0.771 *** | <0.1 |
| Quercetin hexoside | <0.1 | <0.1 | 1.070 ** | 0.710 |
| Nicotinflorin | 22.905 ns | 20.713 ns | 133.682 ns | 121.503 ns |
| Naringenin-7-O-neohesperidoside | 79.538 ** | 41.186 | 150.450 *** | 27.212 |
| Isorhamnetin 3-neohesperidoside | 2.765 ns | 2.470 ns | 8.529 ** | 5.540 |
| Caffeine | <0.1 | <0.1 | 1.497 | 1.861 ** |
| Gallic acid | <0.1 | <0.1 | <0.1 | 0.269 *** |
| Delphinidin-3-O-glucoside | <0.1 | <0.1 | <0.001 | 0.669 ** |
| Cyanidin-3-O-glucoside | <0.1 | <0.1 | 0.066 | 0.104 ** |
| Peonidin-3-O-glucoside | <0.1 | <0.1 | 2.300 * | < 0.1 |
| Delphinidin rutinoside | 0.138 ** | <0.1 | 0.842 | 1.524 ** |
| Quercetin-3-glucoside | <0.1 | <0.1 | 1.894 ** | 0.2506 |
| Quercetin 3-O-rhamnoside | <0.1 | <0.1 | <0.1 ns | <0.1 ns |
| Valoneic acid dilactone | 0.519 *** | <0.1 | 7.839 ** | 0.171 |
| Sinensetin | 0.108 ** | <0.1 | 37.807 | 58.344 ** |
| Rutin | 0.141 *** | <0.1 | 1.613 ns | 1.639 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costanzo, G.; Vitale, E.; Iesce, M.R.; Spinelli, M.; Fontanarosa, C.; Paradiso, R.; Amoresano, A.; Arena, C. Modulation of Antioxidant Compounds in Fruits of Citrus reticulata Blanco Using Postharvest LED Irradiation. Biology 2023, 12, 1029. https://doi.org/10.3390/biology12071029
Costanzo G, Vitale E, Iesce MR, Spinelli M, Fontanarosa C, Paradiso R, Amoresano A, Arena C. Modulation of Antioxidant Compounds in Fruits of Citrus reticulata Blanco Using Postharvest LED Irradiation. Biology. 2023; 12(7):1029. https://doi.org/10.3390/biology12071029
Chicago/Turabian StyleCostanzo, Giulia, Ermenegilda Vitale, Maria Rosaria Iesce, Michele Spinelli, Carolina Fontanarosa, Roberta Paradiso, Angela Amoresano, and Carmen Arena. 2023. "Modulation of Antioxidant Compounds in Fruits of Citrus reticulata Blanco Using Postharvest LED Irradiation" Biology 12, no. 7: 1029. https://doi.org/10.3390/biology12071029
APA StyleCostanzo, G., Vitale, E., Iesce, M. R., Spinelli, M., Fontanarosa, C., Paradiso, R., Amoresano, A., & Arena, C. (2023). Modulation of Antioxidant Compounds in Fruits of Citrus reticulata Blanco Using Postharvest LED Irradiation. Biology, 12(7), 1029. https://doi.org/10.3390/biology12071029