Role of Muscle Ultrasound for the Study of Frailty in Elderly Patients with Diabetes: A Pilot Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
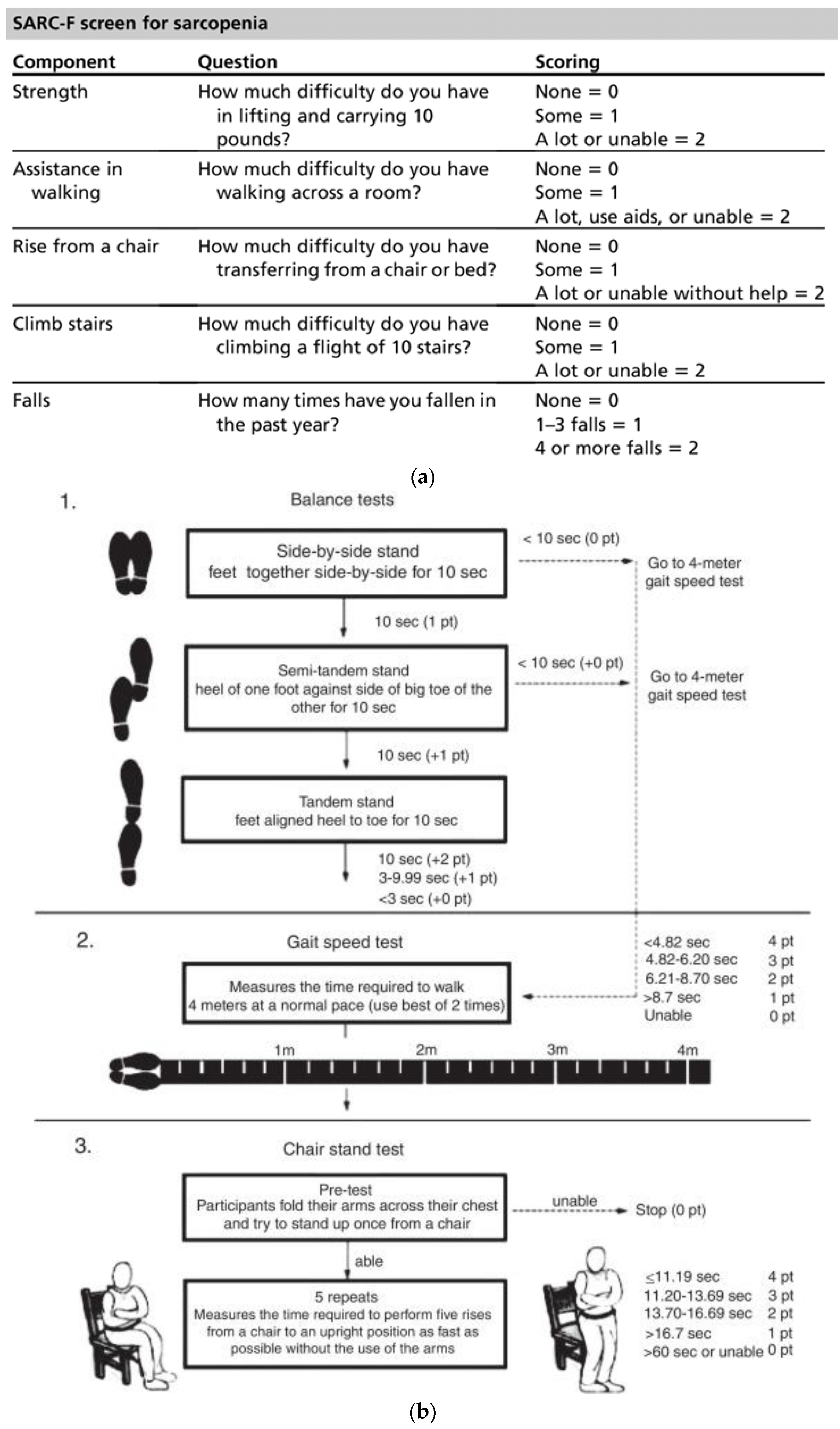
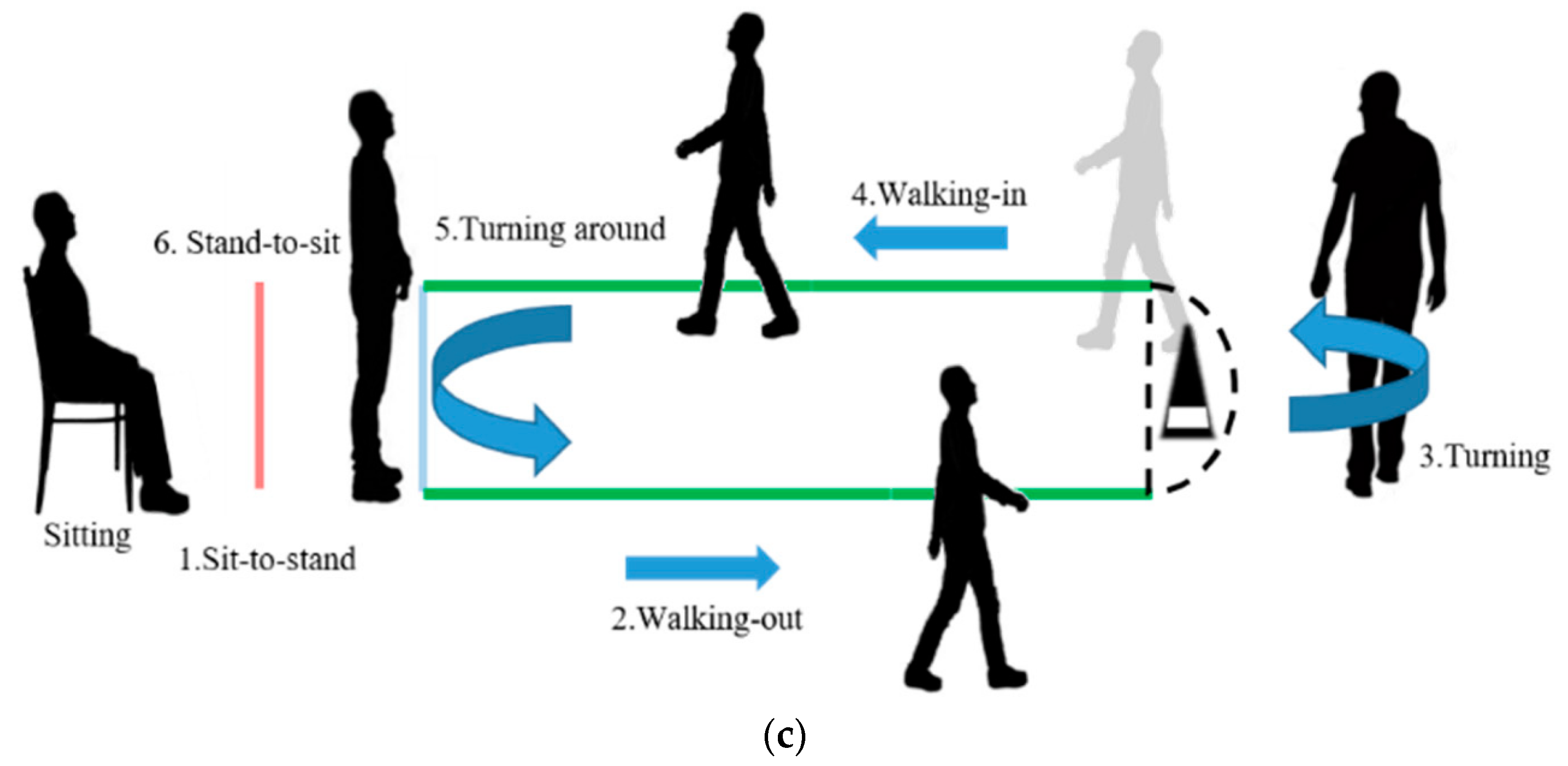
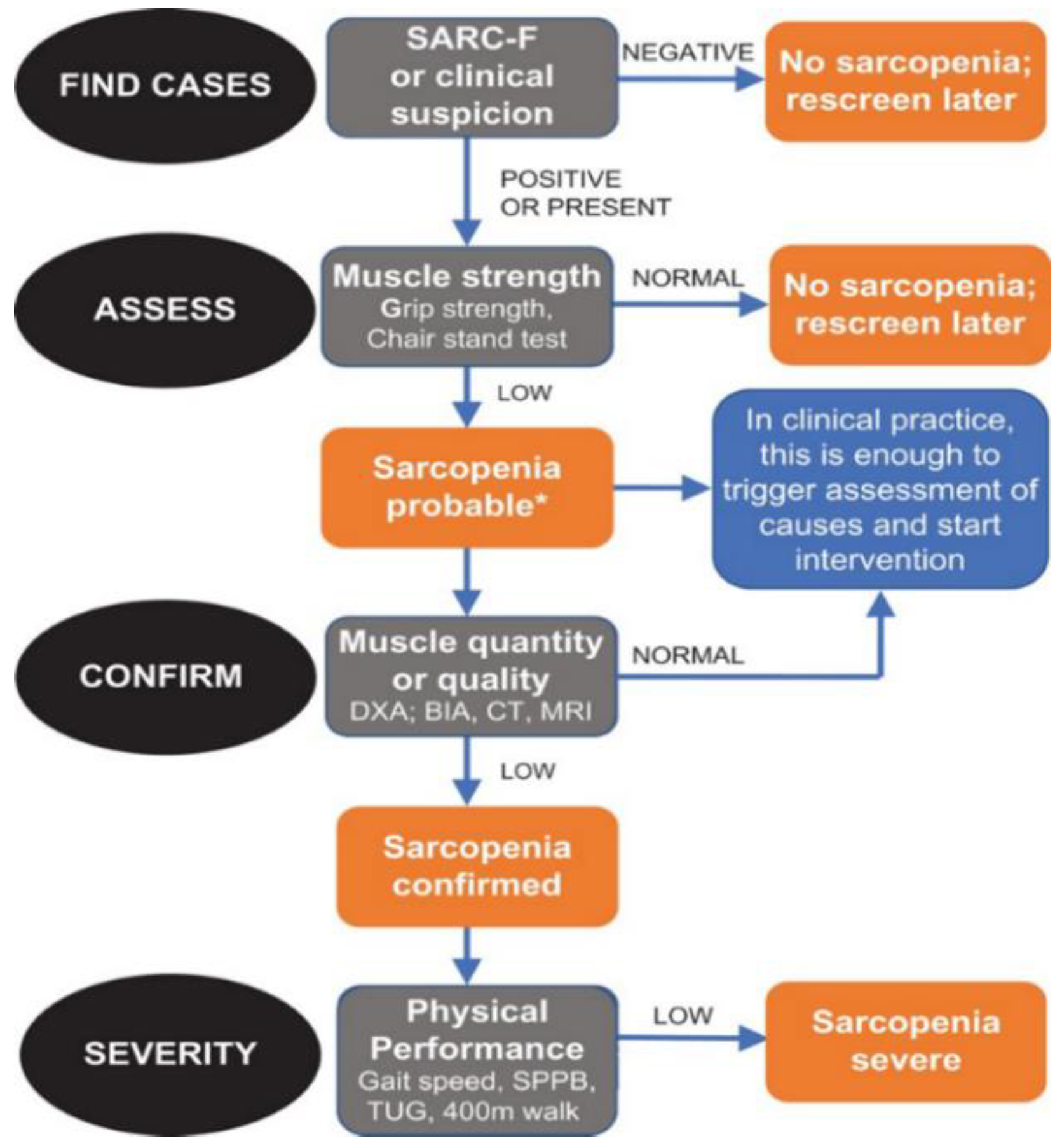
2.1. Evaluation of Sarcopenia
2.2. BIA
2.3. Dynamometer
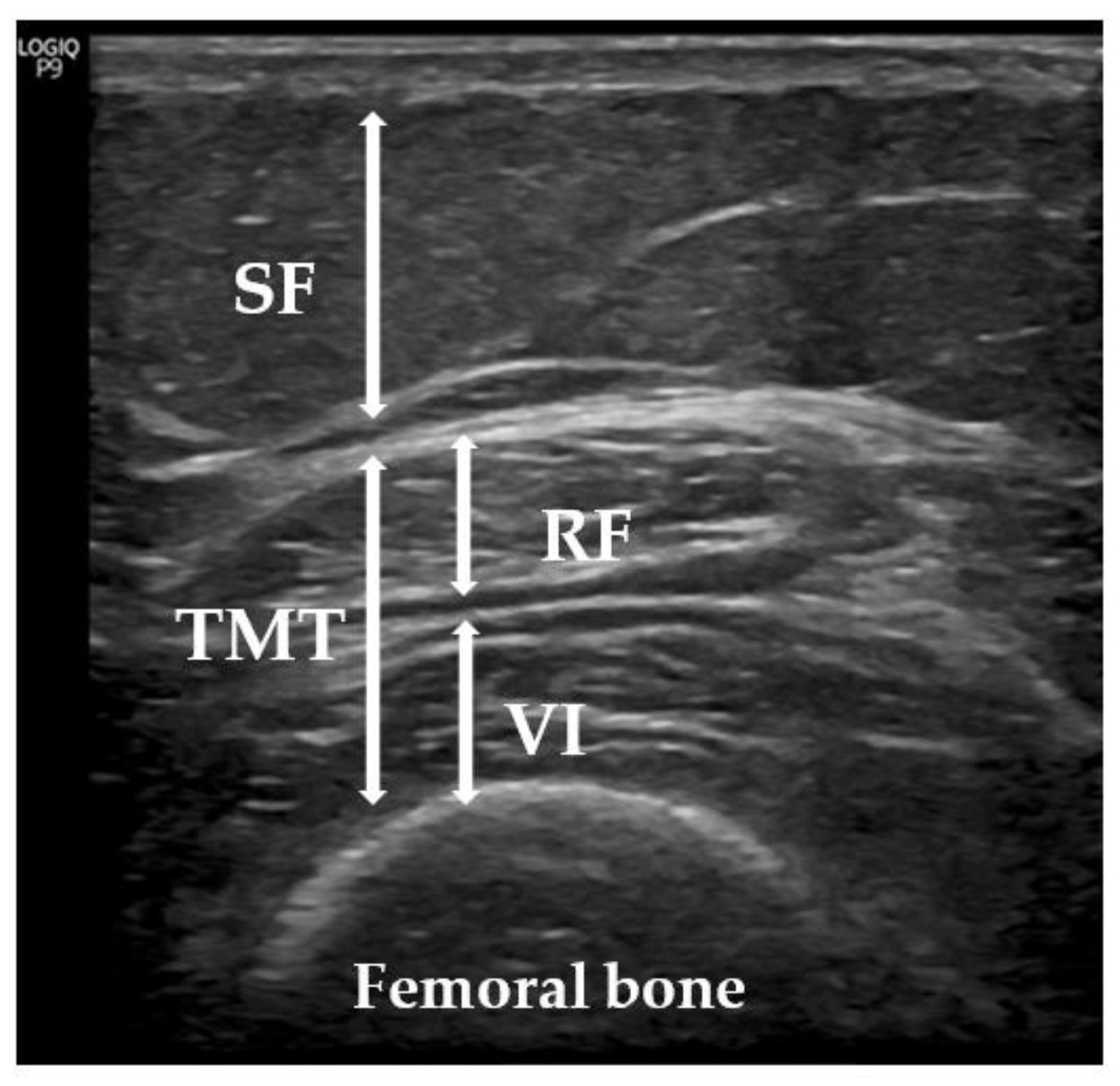
2.4. Ultrasonic Technique
2.5. Statistical Analysis
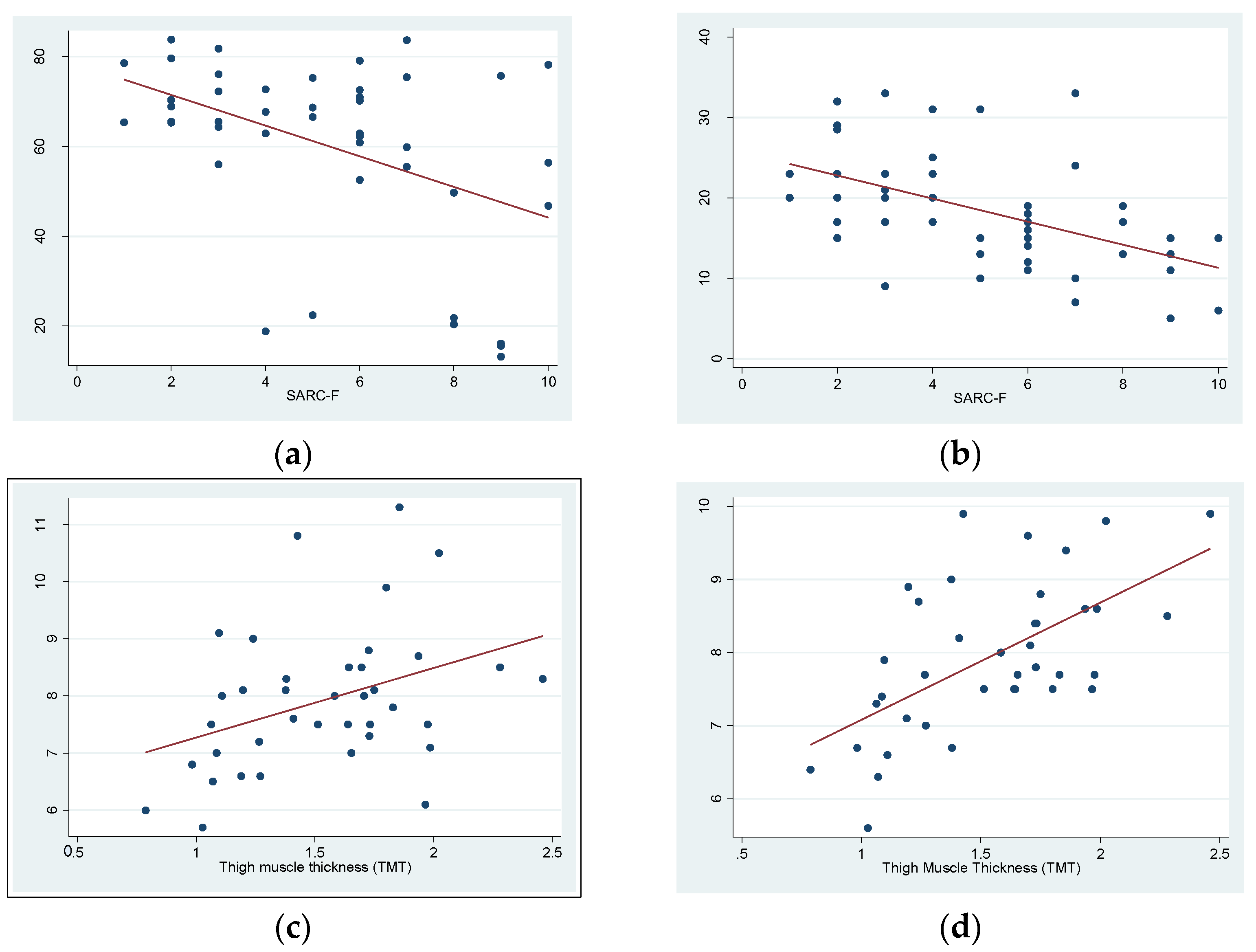
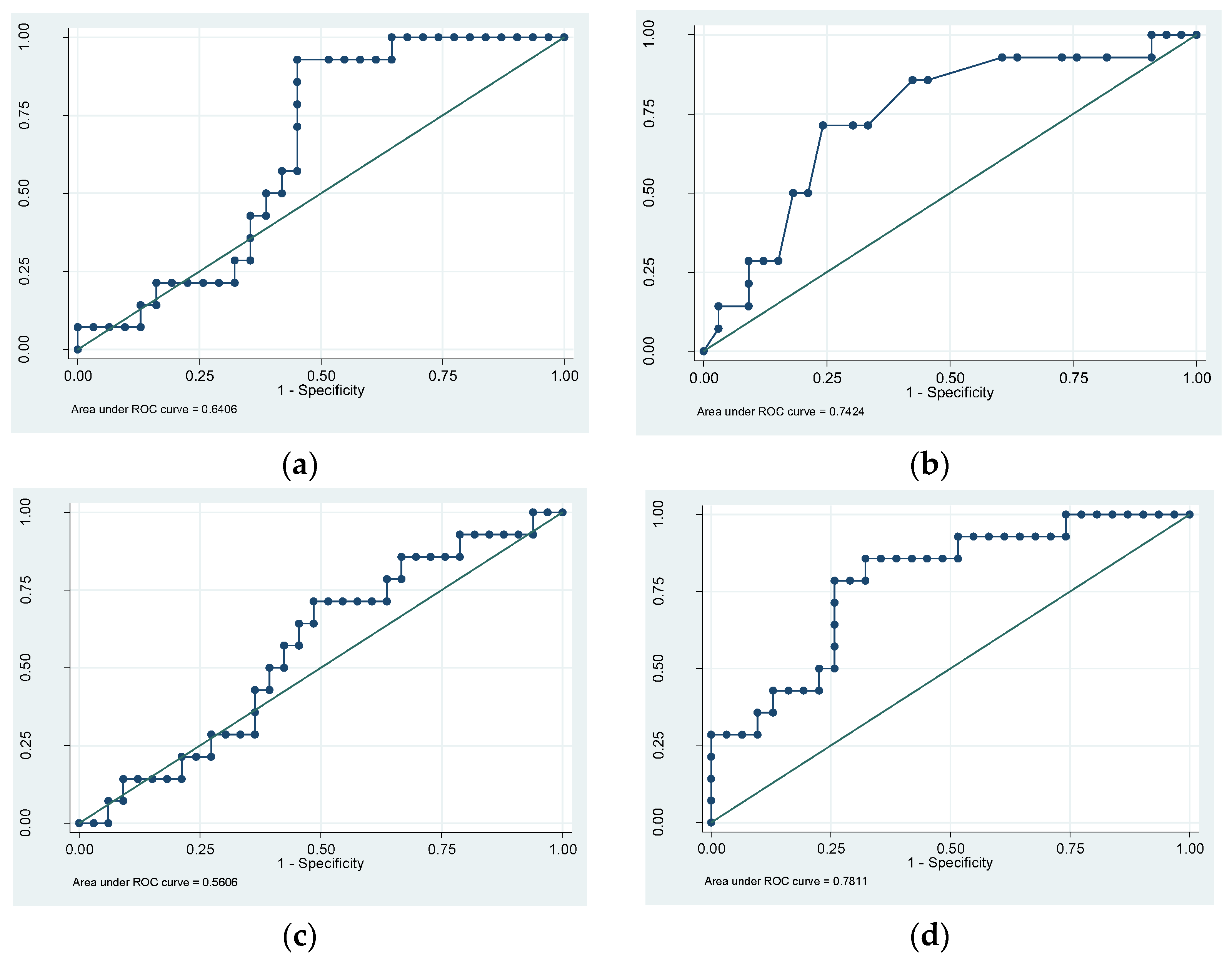
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jokar, T.O.; Rhee, P.M.; Zangbar, B.; Kulvatunyou, N.; Khalil, M.; O’keeffe, T.; Tang, A.L.; Friese, R.S.; Gries, L.M.; Joseph, B. Redefining the Association Between Old Age and Poor Outcomes after Trauma: The Impact of the Frailty Syndrome. J. Am. Coll. Surg. 2015, 221, S83–S84. [Google Scholar] [CrossRef]
- Ensrud, K.E.; Ewing, S.K.; Cawthon, P.M.; Fink, H.A.; Taylor, B.; Cauley, J.A.; Dam, T.-T.; Marshall, L.M.; Orwoll, E.; Cummings, S.R.; et al. A Comparison of Frailty Indexes for the Prediction of Falls, Disability, Fractures, and Mortality in Older Men. J. Am. Geriatr. Soc. 2009, 57, 492–498. [Google Scholar] [CrossRef]
- Zaslavsky, O.; Zelber-Sagi, S.; Gray, S.L.; LaCroix, A.Z.; Brunner, R.L.; Wallace, R.B.; O’Sullivan, M.J.; Cochrane, B.; Woods, N.F. Comparison of Frailty Phenotypes for Prediction of Mortality, Incident Falls, and Hip Fracture in Older Women. J. Am. Geriatr. Soc. 2016, 64, 1858–1862. [Google Scholar] [CrossRef]
- Martín-Sánchez, F.J.; Rodríguez-Adrada, E.; Mueller, C.; Vidán, M.T.; Christ, M.; Peacock, W.F.; Rizzi, M.A.; Alquezar, A.; Piñera, P.; Aragues, P.L.; et al. The Effect of Frailty on 30-day Mortality Risk in Older Patients With Acute Heart Failure Attended in the Emergency Department. Acad. Emerg. Med. 2017, 24, 298–307. [Google Scholar] [CrossRef]
- Sirois, M.-J.; Griffith, L.; Perry, J.; Daoust, R.; Veillette, N.; Lee, J.; Pelletier, M.; Wilding, L.; Émond, M. Measuring Frailty Can Help Emergency Departments Identify Independent Seniors at Risk of Functional Decline After Minor Injuries. J. Gerontol. Ser. A 2015, 72, 68–74. [Google Scholar] [CrossRef]
- Ozturk, Y.; Koca, M.; Burkuk, S.; Unsal, P.; Dikmeer, A.; Oytun, M.G.; Bas, A.O.; Kahyaoglu, Z.; Deniz, O.; Coteli, S.; et al. The role of muscle ultrasound to predict sarcopenia. Nutrition 2022, 101, 111692. [Google Scholar] [CrossRef]
- Khadra, D.; Itani, L.; Tannir, H.; Kreidieh, D.; El Masri, D.; El Ghoch, M. Association between sarcopenic obesity and higher risk of type 2 diabetes in adults: A systematic review and meta-analysis. World J. Diabetes 2019, 10, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Ciudin, A.; Simó-Servat, A.; Palmas, F.; Barahona, M.J. Sarcopenic obesity: A new challenge in the clinical practice. Endocrinol. Diabetes Nutr. 2020, 67, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Benton, E.; Liteplo, A.S.; Shokoohi, H.; Loesche, M.A.; Yacoub, S.; Thatphet, P.; Wongtangman, T.; Liu, S.W. A pilot study examining the use of ultrasound to measure sarcopenia, frailty and fall in older patients. Am. J. Emerg. Med. 2021, 46, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Cánovas, J.; López-Sampalo, A.; Cobos-Palacios, L.; Ricci, M.; Hernández-Negrín, H.; Mancebo-Sevilla, J.J.; Álvarez-Recio, E.; López-Carmona, M.D.; Pérez-Belmonte, L.M.; Gómez-Huelgas, R.; et al. Management of Type 2 Diabetes Mellitus in Elderly Patients with Frailty and/or Sarcopenia. Int. J. Environ. Res. Public Health 2022, 19, 8677. [Google Scholar] [CrossRef]
- Wang, J.-C.; Wu, W.-T.; Chang, K.-V.; Chen, L.-R.; Chi, S.-Y.; Kara, M.; Özçakar, L. Ultrasound Imaging for the Diagnosis and Evaluation of Sarcopenia: An Umbrella Review. Life 2021, 12, 9. [Google Scholar] [CrossRef]
- Ticinesi, A.; Meschi, T.; Narici, M.V.; Lauretani, F.; Maggio, M. Muscle Ultrasound and Sarcopenia in Older Individuals: A Clinical Perspective. J. Am. Med. Dir. Assoc. 2017, 18, 290–300. [Google Scholar] [CrossRef]
- Donini, L.M.; Busetto, L.; Bauer, J.M.; Bischoff, S.; Boirie, Y.; Cederholm, T.; Cruz-Jentoft, A.J.; Dicker, D.; Frühbeck, G.; Giustina, A.; et al. Critical appraisal of definitions and diagnostic criteria for sarcopenic obesity based on a systematic review. Clin. Nutr. 2020, 39, 2368–2388. [Google Scholar] [CrossRef]
- Sengul Aycicek, G.; Ozsurekci, C.; Caliskan, H.; Kizilarslanoglu, M.C.; Tuna Dogrul, R.; Balci, C.; Unsal, P.; Esme, M.; Yavuz, B.B.; Cankurtaran, M.; et al. Ultrasonography versus bioelectrical impedance analysis: Which predicts muscle strength better? Acta Clin. Belg. 2021, 76, 204–208. [Google Scholar] [CrossRef]
- Rustani, K.; Kundisova, L.; Capecchi, P.L.; Nante, N.; Bicchi, M. Ultrasound measurement of rectus femoris muscle thickness as a quick screening test for sarcopenia assessment. Arch. Gerontol. Geriatr. 2019, 83, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Radaelli, R.; Bottaro, M.; Wilhelm, E.N.; Wagner, D.R.; Pinto, R.S. Time Course of Strength and Echo Intensity Recovery After Resistance Exercise in Women. J. Strength Cond. Res. 2012, 26, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Yamada, Y.; Fukumoto, Y.; Yokoyama, K.; Yoshida, T.; Miyake, M.; Yamagata, E.; Kimura, M.; Ishihara, T. Echo intensity obtained from ultrasonography images reflecting muscle strength in elderly men. Clin. Interv. Aging 2013, 8, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Minetto, M.A.; Caresio, C.; Menapace, T.; Hajdarevic, A.; Marchini, A.; Molinari, F.; Maffiuletti, N.A. Ultrasound-Based Detection of Low Muscle Mass for Diagnosis of Sarcopenia in Older Adults. PM&R 2016, 8, 453–462. [Google Scholar]
- Fu, H.; Wang, L.; Zhang, W.; Lu, J.; Yang, M. Diagnostic test accuracy of ultrasound for sarcopenia diagnosis: A systematic review and meta-analysis. J. Cachex Sarcopenia Muscle 2022, 14, 57–70. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 335, 806–808. [Google Scholar]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Malmstrom, K.M.; Morley, J.E. SARC-F: A simple questionnaire to rapidly diagnose sarcopenia. J. Am. Med. Dir. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Riskowski, J.; Hagedorn, T.; Dufour, A.; Hannan, M. Functional foot symmetry and its relation to lower extremity physical performance in older adults: The Framingham Foot Study. J. Biomech. 2012, 45, 1796–1802. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-Y.; Huang, H.-Y.; Liu, K.-C.; Chen, K.-H.; Hsu, S.J.-P.; Chan, C.-T. Subtask Segmentation of Timed Up and Go Test for Mobility Assessment of Perioperative Total Knee Arthroplasty. Sensors 2020, 20, 6302. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros-Pomar, M.D.; González-Arnáiz, E.; Maza, B.P.-D.; Barajas-Galindo, D.; Ariadel-Cobo, D.; González-Roza, L.; Cano-Rodríguez, I. Bioelectrical impedance analysis as an alternative to dual-energy x-ray absorptiometry in the assessment of fat mass and appendicular lean mass in patients with obesity. Nutrition 2021, 93, 111442. [Google Scholar] [CrossRef]
- Hida, T.; Ando, K.; Kobayashi, K.; Ito, K.; Tsushima, M.; Kobayakawa, T.; Morozumi, M.; Tanaka, S.; Machino, M.; Ota, K.; et al. Ultrasound measurement of thigh muscle thickness for assessment of sarcopenia. Nagoya J. Med. Sci. 2018, 80, 519–527. [Google Scholar]
- Kawai, H.; Kera, T.; Hirayama, R.; Hirano, H.; Fujiwara, Y.; Ihara, K.; Kojima, M.; Obuchi, S. Morphological and qualitative characteristics of the quadriceps muscle of community-dwelling older adults based on ultrasound imaging: Classification using latent class analysis. Aging Clin. Exp. Res. 2017, 30, 283–291. [Google Scholar] [CrossRef]
- Wilson, D.V.; Moorey, H.; Stringer, H.; Sahbudin, I.; Filer, A.; Lord, J.M.; Sapey, E. Bilateral Anterior Thigh Thickness: A New Diagnostic Tool for the Identification of Low Muscle Mass? J. Am. Med. Dir. Assoc. 2019, 20, 1247–1253. [Google Scholar] [CrossRef]
- de Souza, V.A.; Oliveira, D.; Cupolilo, E.N.; Miranda, C.S.; Colugnati, F.A.B.; Mansur, H.N.; Fernandes, N.M.D.S.; Bastos, M.G. Rectus femoris muscle mass evaluation by ultrasound: Facilitating sarcopenia diagnosis in pre-dialysis chronic kidney disease stages. Clinics 2018, 73, e392. [Google Scholar] [CrossRef]
- Welch, D.; Ndanyo, L.S.; Brown, S.; Agyapong-Badu, S.; Warner, M.; Stokes, M.; Samuel, D. Thigh muscle and subcutaneous tissue thickness measured using ultrasound imaging in older females living in extended care: A preliminary study. Aging Clin. Exp. Res. 2018, 30, 463–469. [Google Scholar] [CrossRef]
- Simó-Servat, A.; Ibarra, M.; Libran, M.; Rodríguez, S.; Perea, V.; Quirós, C.; Orois, A.; Pérez, N.; Simó, R.; Barahona, M.-J. Usefulness of Muscle Ultrasound to Study Sarcopenic Obesity: A Pilot Case-Control Study. J. Clin. Med. 2022, 11, 2886. [Google Scholar] [CrossRef]
- Sinclair, A.J.; Abdelhafiz, A.H.; Rodríguez-Mañas, L. Frailty and sarcopenia—Newly emerging and high impact complications of diabetes. J. Diabetes Its Complicat. 2017, 31, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Sbrignadello, S.; Göbl, C.; Tura, A. Bioelectrical Impedance Analysis for the Assessment of Body Composition in Sarcopenia and Type 2 Diabetes. Nutrients 2022, 14, 1864. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.; Murthy, S.; Tainter, C.; Lee, J.; Riddell, K.; Fintelmann, F.J.; Grabitz, S.D.; Timm, F.P.; Levi, B.; Kurth, T.; et al. Can Sarcopenia Quantified by Ultrasound of the Rectus Femoris Muscle Predict Adverse Outcome of Surgical Intensive Care Unit Patients as well as Frailty? A Prospective, Observational Cohort Study. Ann. Surg. 2016, 264, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-V.; Wu, W.-T.; Huang, K.-C.; Jan, W.H.; Han, D.-S. Limb muscle quality and quantity in elderly adults with dynapenia but not sarcopenia: An ultrasound imaging study. Exp. Gerontol. 2018, 108, 54–61. [Google Scholar] [CrossRef]
- Ikai, M.; Fukunaga, T. The size and strength per unit cross-sectional area of human muscle by means of ultrasonic measurement. Int. Z Angew. Physiol. 1968, 26, 26–32. [Google Scholar]
- Young, A.; Stokes, M.; Crowe, M. The size and strength of the quadriceps muscles of old and young woman. Eur. J. Clin. Investig. 1984, 14, 282–287. [Google Scholar] [CrossRef]
- Miyatani, M.; Kanehisa, H.; Kuno, S.; Nishijima, T.; Fukunaga, T. Validity of ultrasonograph muscle thickness measurements for estimating muscle volume of knee extensors in humans. Eur. J. Appl. Physiol. 2002, 86, 203–208. [Google Scholar] [CrossRef]
- Sanada, K.; Kearns, C.F.; Midorikawa, T.; Abe, T. Prediction and validation of total and regional skeletal muscle mass by ultrasound in Japanese adults. Eur. J. Appl. Physiol. 2005, 96, 24–31. [Google Scholar] [CrossRef]
- Morley, J.E.; Malmstrom, T.K.; Rodriguez-Mañas, L.; Sinclair, A.J. Frailty, Sarcopenia and Diabetes. J. Am. Med. Dir. Assoc. 2014, 15, 853–859. [Google Scholar] [CrossRef]
- Oguz, A.; Sahin, M.; Tuzun, D.; Kurutas, E.B.; Ulgen, C.; Bozkus, O.; Gul, K. Irisin Is a Predictor of Sarcopenic Obesity in Type 2 Diabetes Mellitus: A Cross-Sectional Study. Medicine 2021, 100, e26529. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Goodpaster, B.H.; Strotmeyer, E.S.; Kuller, L.H.; Broudeau, R.; Kammerer, C.; de Rekeneire, N.; Harris, T.B.; Schwartz, A.V.; Tylavsky, F.A.; et al. Health, Aging, and Body Composition Study. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: The health, aging, and body composition study. Diabetes Care 2007, 30, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Mirón Mombiela, R.; Vucetic, J.; Rossi, F.; Tagliafico, A.S. Ultrasound biomarkers for sarcopenia: ¿what can we tell so far? Semin. Musculoskelet. Radiol. 2020, 24, 181–193. [Google Scholar] [CrossRef] [PubMed]
| Mean ± SD | |
|---|---|
| FFM * total body (%) | 60.41 ± 20.19 |
| FFM right leg (%) | 7.92 ± 1.25 |
| SRI * | 7.94 ± 1.04 |
| TMT right quadriceps (cm) | 1.55 ± 0.4 |
| Dynamometer (kg) ** | 14.84 ± 5.02 (female) and 25.03 ± 6.42 (male) |
| SARC-F < 4 | SARC-F > 3 ** | SPPB * < 7 ** | SPPB > 6 | TUG < 20 | TUG * > 19 ** | |
|---|---|---|---|---|---|---|
| n | 15 | 32 | 33 | 14 | 20 | 18 |
| Mean ± SD (cm) | 1.59 ± 0.36 | 1.54 ± 0.42 | 1.49 ± 0.40 | 1.57 ± 0.40 | 1.54 ± 0.39 | 1.45 ± 0.23 |
| p | 0.2 | 0.09 | 0.6 | |||
| R | 0.2 | −0.25 | 0.1 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simó-Servat, A.; Guevara, E.; Perea, V.; Alonso, N.; Quirós, C.; Puig-Jové, C.; Barahona, M.-J. Role of Muscle Ultrasound for the Study of Frailty in Elderly Patients with Diabetes: A Pilot Study. Biology 2023, 12, 884. https://doi.org/10.3390/biology12060884
Simó-Servat A, Guevara E, Perea V, Alonso N, Quirós C, Puig-Jové C, Barahona M-J. Role of Muscle Ultrasound for the Study of Frailty in Elderly Patients with Diabetes: A Pilot Study. Biology. 2023; 12(6):884. https://doi.org/10.3390/biology12060884
Chicago/Turabian StyleSimó-Servat, Andreu, Ernesto Guevara, Verónica Perea, Núria Alonso, Carmen Quirós, Carlos Puig-Jové, and María-José Barahona. 2023. "Role of Muscle Ultrasound for the Study of Frailty in Elderly Patients with Diabetes: A Pilot Study" Biology 12, no. 6: 884. https://doi.org/10.3390/biology12060884
APA StyleSimó-Servat, A., Guevara, E., Perea, V., Alonso, N., Quirós, C., Puig-Jové, C., & Barahona, M.-J. (2023). Role of Muscle Ultrasound for the Study of Frailty in Elderly Patients with Diabetes: A Pilot Study. Biology, 12(6), 884. https://doi.org/10.3390/biology12060884