Bacillus velezensis Strain HN-Q-8 Induced Resistance to Alternaria solani and Stimulated Growth of Potato Plant
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Potato Cultivars, Early Blight Strain, and B. velezensis Strain
2.2. Preparation of B. velezensis and Pathogenic Fungi
2.3. Soil Pretreatment and Potato Planting Methods
2.4. Preinoculation with Strain HN-Q-8 and Inoculation of Pathogenic Fungus
2.5. Defensive Enzyme Activity Assays
2.6. Growth-Promotion Test
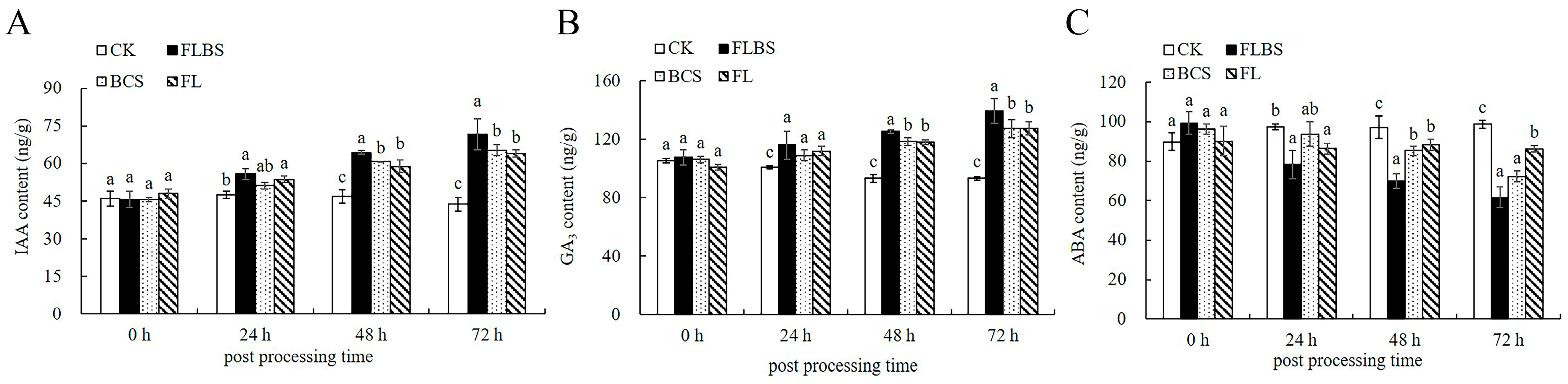
2.7. Determination of Potato Leaf IAA, GA3, and ABA Contents
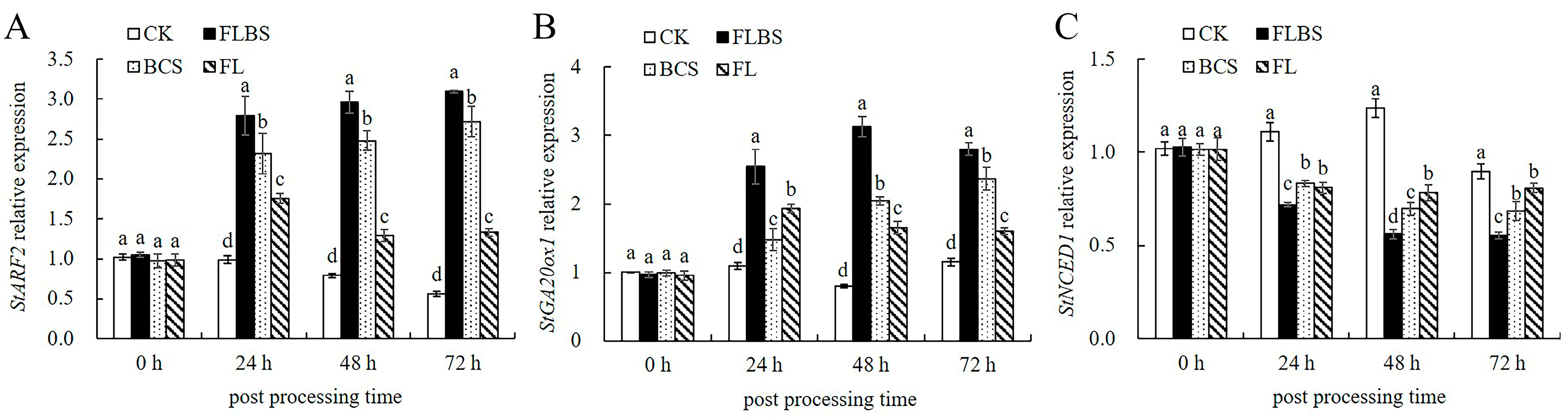
2.8. Gene Expression Analysis of the ISR Signaling Pathway and Growth-Related Factors
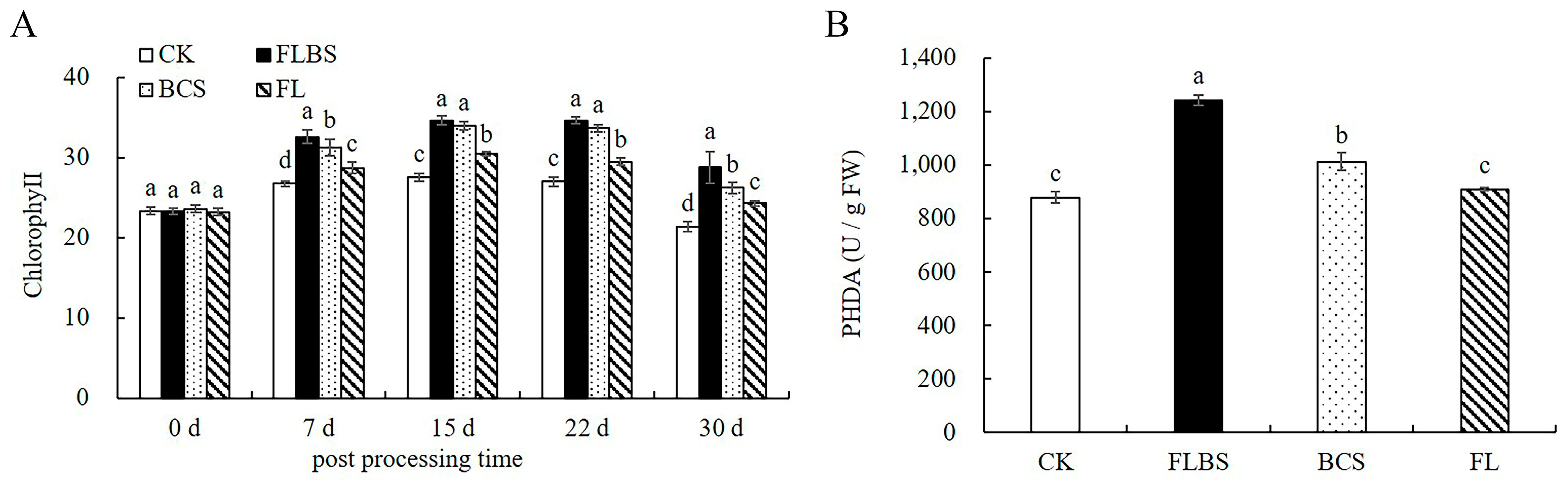
2.9. Determination of Chlorophyll Content, Biomass, and Root Activity
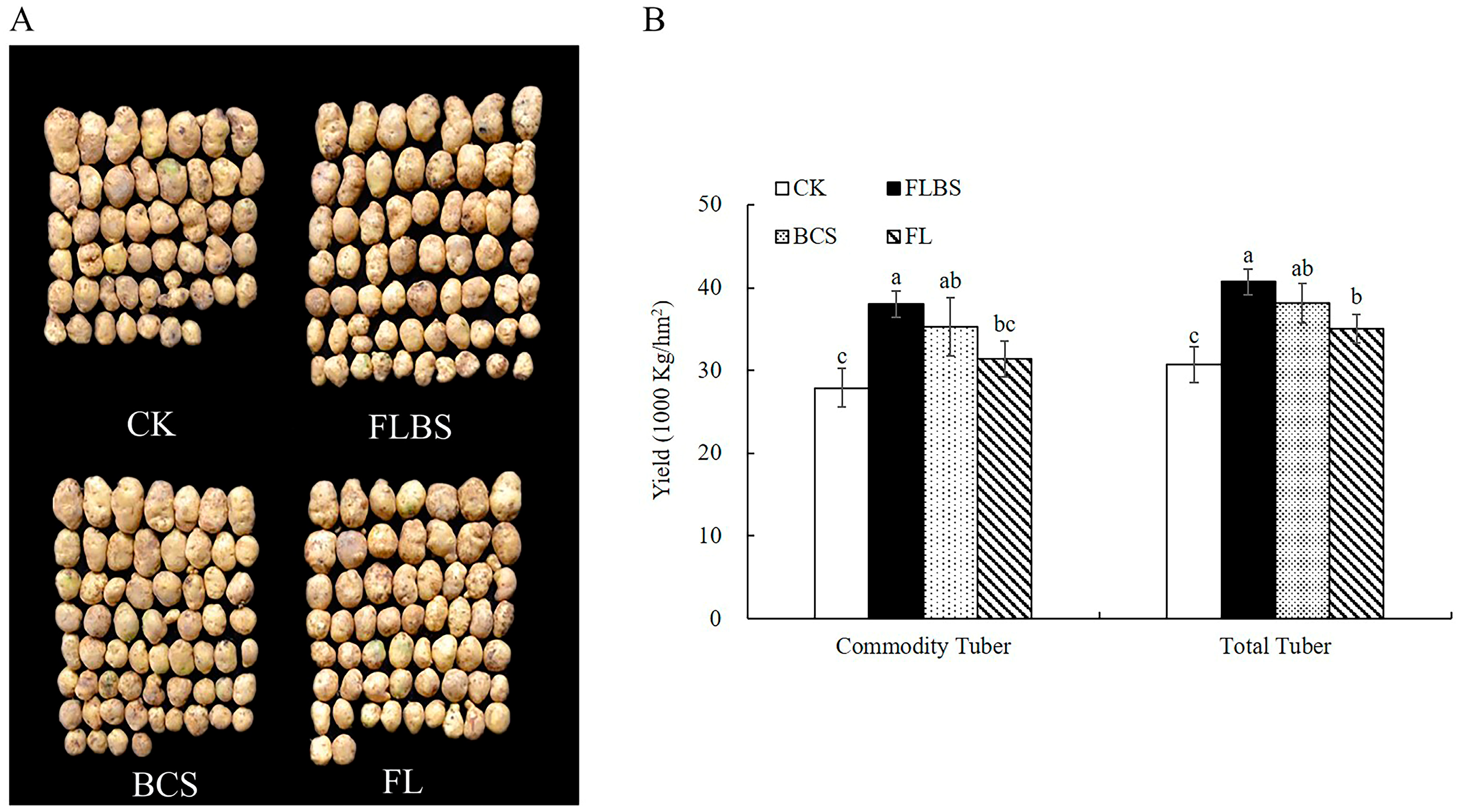
2.10. Effect of Strain HN-Q-8 on Potato Yield
- Total increase rate of tuber yield = ((total yield in the treatment area − total yield in the control area)/total yield in the control area) × 100%;
- Increase rate of commercial potato tuber yield = ((commercial potato tuber yield in the treatment area − commercial potato tuber yield in the control area)/commercial potato tuber yield output in the control area) × 100% (potato tubers of >200 g were considered as of commercial value).
2.11. Statistical Analysis
3. Results
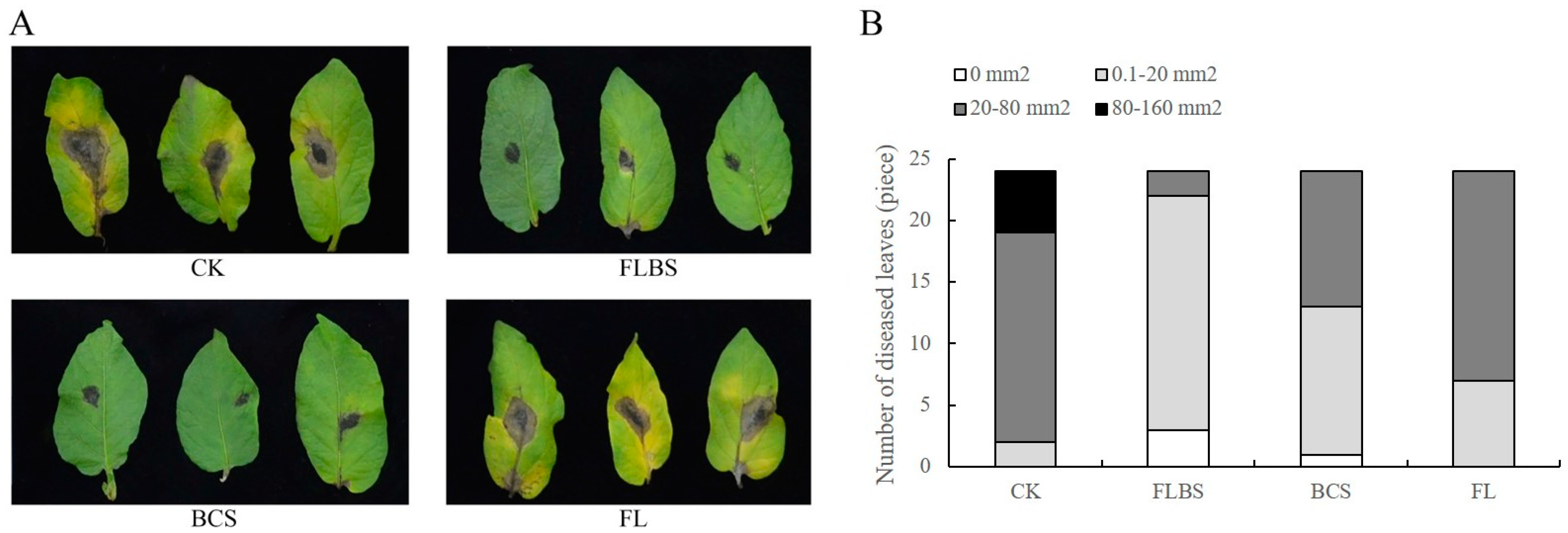
3.1. Strain HN-Q-8 Induced the Resistance of Potato Leaves to Early Blight
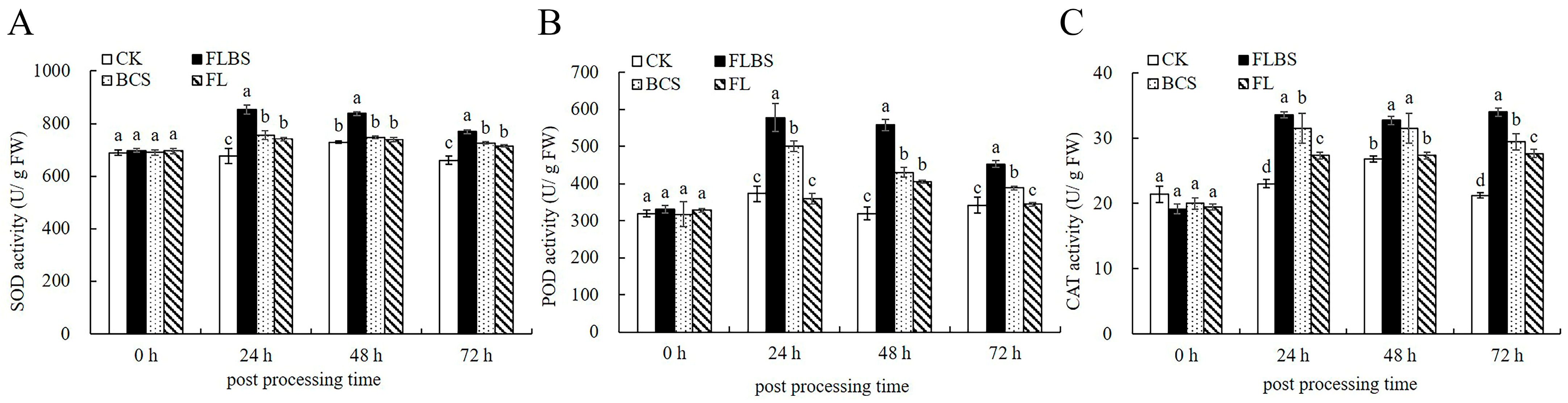
3.2. Strain HN-Q-8 Stimulates Potato Defensive Enzyme Activities
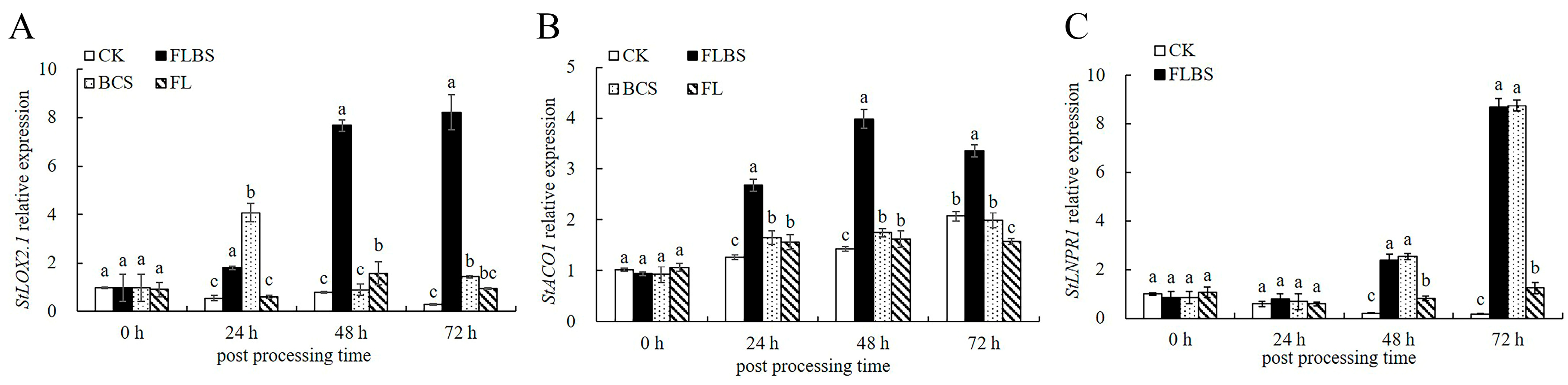
3.3. ISR-Related Gene Expression Analysis
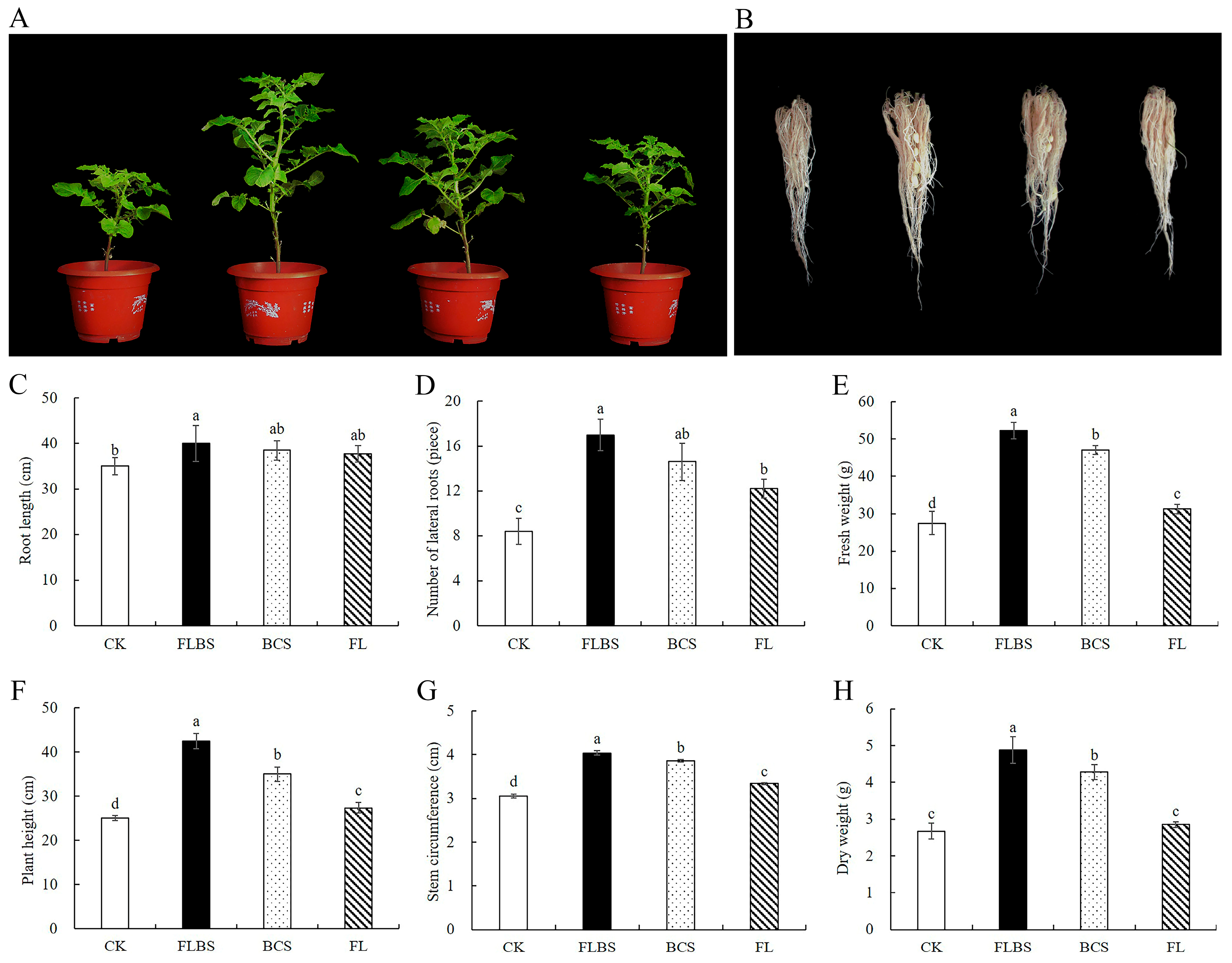
3.4. Effect of Strain HN-Q-8 on the Growth of Potato Seedlings
3.5. Effect of Strain HN-Q-8 on Potato Tuber Yield
3.6. Effects of Strain HN-Q-8 on Plant Hormones in Potato
3.7. Growth-Related Gene Expression Analysis
3.8. Strain HN-Q-8 Increases the Chlorophyll Content and Root Activity of Potato
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehmood, T.; Li, G.; Anjum, T.; Akram, W. Azospirillum lipoferum strain AL-3 reduces early blight disease of potato and enhance yield. Crop. Prot. 2021, 139, 105349. [Google Scholar] [CrossRef]
- Xue, W.Y.; Haynes, K.G.; Qu, X.S. Characterization of early blight resistance in potato cultivars. Plant. Dis. 2019, 103, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Borriss, R. Use of Plant-Associated Bacillus Strains as Biofertilizers and Biocontrol Agents in Agriculture; Springer: Berlin/Heidelberg, Germany, 2011; pp. 41–76. [Google Scholar] [CrossRef]
- Chowdhury, S.P.; Hartmann, A.; Gao, X.W.; Borriss, R. Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42-a review. Front. Microbiol. 2015, 6, 780. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhang, B.; Liu, H.; Han, J.; Zhang, Y. Identification of endophytic Bacillus velezensis ZSY-1 strain and antifungal activity of its volatile compounds against Alternaria solani and Botrytis cinerea. Biol. Control. 2017, 105, 27–39. [Google Scholar] [CrossRef]
- Chen, M.C.; Wang, J.P.; Zhu, Y.J.; Liu, B.; Yang, W.J.; Ruan, C.Q. Antibacterial activity against Ralstonia solanacearum of the lipopeptides secreted from the Bacillus amyloliquefaciens strain FJAT-2349. J. Appl. Microbiol. 2019, 126, 1519–1529. [Google Scholar] [CrossRef]
- Jemil, N.; Manresa, A.; Rabanal, F.; Ayed, H.B.; Hmidet, N.; Nasri, M. Structural characterization and identification of cyclic lipopeptides produced by Bacillus methylotrophicus DCS1 strain. J. Chromatogr. B-Biomed. Appl. 2017, 1060, 374–386. [Google Scholar] [CrossRef]
- Lim, S.M.; Yoon, M.Y.; Choi, G.J.; Choi, Y.H.; Jang, K.S.; Shin, T.S.; Park, H.W.; Yu, N.H.; Kim, J.C. Diffusible and volatile antifungal compounds produced by an antagonistic Bacillus velezensis G341 against various phytopathogenic fungi. Plant. Pathol. J. 2017, 33, 488. [Google Scholar] [CrossRef]
- Myo, E.M.; Liu, B.H.; Ma, J.J.; Shi, L.M.; Jiang, M.G.; Zhang, K.C.; Ge, B.B. Evaluation of Bacillus velezensis NKG-2 for bio-control activities against fungal diseases and potential plant growth promotion. Biol. Control. 2019, 134, 23–31. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant. Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotech. 2017, 33, 197. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Leon Reyes, H.A.; van der Does, D.; Verhage, A.; Koornneef, A.; van Pelt, J.A.; van Wees, S.C.M. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef]
- Wang, S.A.; Wu, H.J.; Zhan, J.A.; Xia, Y.F.; Gao, S.F.; Wang, W.D.; Xue, P.Q.; Gao, X.W. The role of synergistic action and molecular mechanism in the effect of genetically engineered strain Bacillus subtilis OKBHF in enhancing tomato growth and Cucumber mosaic virus resistance. Biol. Control. 2011, 56, 113–121. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.M.; Flors, V.; García-Agustín, P.; Jakab, G.; Mauch, F. Priming: Getting ready for battle. Mol. Plant. Microbe Interact. 2006, 19, 1062–1071. [Google Scholar] [CrossRef]
- Cawoy, H.; Mariutto, M.; Henry, G.; Fisher, C.; Vasilyeva, N.; Thonart, P.; Ongena, M. Plant defense stimulation by natural isolates of Bacillus depends on efficient surfactin production. Mol. Plant. Microbe Interact. 2014, 27, 87–100. [Google Scholar] [CrossRef]
- Pertot, I.; Puopolo, G.; Hosni, T.; Pedrotti, L.; Jourdan, E.; Ongena, M. Limited impact of abiotic stress on surfactin production in planta and on disease resistance induced by Bacillus amyloliquefaciens S499 in tomato and bean. FEMS Microbiol. Ecol. 2013, 86, 505–519. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant. Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Avdiushko, S.A.; Ye, X.S.; Kuc, J. Detection of several enzymatic activities in leaf prints of cucumber plants. Physiol. Mol. Plant. Pathol. 1993, 42, 441–454. [Google Scholar] [CrossRef]
- Felestrino, É.B.; Vieira, I.T.; Caneschi, W.L.; Cordeiro, I.F.; Assis, R.A.B.; Lemes, C.G.C.; Fonseca, N.P.; Sanchez, A.B.; Cepeda, J.C.C.; Ferro, J.A.; et al. Biotechnological potential of plant growth-promoting bacteria from the roots and rhizospheres of endemic plants in ironstone vegetation in southeastern Brazil. World J. Microbol. Biotech. 2018, 34, 156. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Hernández, A.G.; Glick, B.R.; Rossi, M.J. Plant growth-promoting activities and genomic analysis of the stress-resistant Bacillus megaterium STB1, a bacterium of agricultural and biotechnological interest. Biotechnol. Rep. 2020, 25, e00406. [Google Scholar] [CrossRef]
- Zhou, C.; Guo, J.S.; Zhu, L.; Xiao, X.; Xie, Y.; Zhu, J.; Ma, Z.Y.; Wang, J.F. PaeniBacillus polymyxa BFKC01 enhances plant iron absorption via improved root systems and activated iron acquisition mechanisms. Plant. Physiol. Bioch. 2016, 105, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Won, S.J.; Maung, C.E.H.; Choi, J.H.; Choi, S.I.; Ajuna, H.B.; Ahn, Y.S. Bacillus velezensis CE 100 inhibits root rot diseases (Phytophthora spp.) and promotes growth of Japanese cypress (Chamaecyparis obtusa Endlicher) seedlings. Microorganisms 2021, 9, 821. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Koumoutsia, A.; Scholza, R.; Schneider, K.; Vater, J.; Süssmuth, R.; Piel, J.; Borriss, R. Genome analysis of Bacillus amyloliquefaciens FZB42 reveals its potential for biochontrol of plant pathogens. J. Biotechnol. 2009, 40, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Qiu, Y.; Yang, S.; Wang, Y.; Wang, K.; Jiang, L.; Wang, H. Antagonistic activity of Bacillus velezensis SDTB038 against Phytophthora infestans in potato. Plant. Dis. 2021, 105, 1738–1747. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, Y.; Wang, D.; Yao, Z.; Wang, Y.; Liu, J.; Wang, A. A biocontrol strain of Bacillus subtilis WXCDD105 used to control tomato Botrytis cinerea and Cladosporium fulvum Cooke and promote the growth of seedlings. Int. J. Mol. Sci. 2018, 19, 1371. [Google Scholar] [CrossRef]
- Li, H.; Zhao, J.; Feng, H.; Huang, L.L.; Kang, Z.S. Biological control of wheat stripe rust by an endophytic Bacillus subtilis strain E1R-j in greenhouse and field trials. Crop. Prot. 2013, 43, 201–206. [Google Scholar] [CrossRef]
- Zhou, L.; Song, C.X.; Muñoz, C.Y.; Kuipers, O.P. Bacillus cabrialesii BH5 protects tomato plants against Botrytis cinerea by production of specific antifungal compounds. Front. Microbiol. 2021, 12, 707609. [Google Scholar] [CrossRef]
- Zhang, D.; He, J.Y.; Haddadi, P.; Zhu, J.H.; Yang, Z.H.; Ma, L. Genome sequence of the potato pathogenic fungus Alternaria solani HWC-168 reveals clues for its conidiation and virulence. BMC Microbiol. 2018, 18, 176. [Google Scholar] [CrossRef]
- Silva, H.S.A.; Romeiro, R.D.; Macagnan, D.; Halfeld-Vieira, B.D.; Pereira, M.C.B.; Mounteer, A. Rhizobacterial induction of systemic resistance in tomato plants: Non-specific protection and increase in enzyme activities. Biol. Control. 2004, 29, 288–295. [Google Scholar] [CrossRef]
- Jiang, C.H.; Wu, F.; Yu, Z.Y.; Xie, P.; Ke, H.J.; Li, H.W.; Yu, Y.Y.; Guo, J.H. Study on screening and antagonistic mechanisms of Bacillus amyloliquefaciens 54 against bacterial fruit blotch (BFB) caused by Acidovorax avenae subsp. citrulli. Microbiol. Res. 2015, 170, 95–104. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, H.; Ren, Z.; Li, X.; Zhong, J.; Liu, E. Efficacy of Bacillus tequilensis strain JN-369 to biocontrol of rice blast and enhance rice growth. Bio. Control. 2021, 160, 104652. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, C.; Zhang, S.S.; Yu, H.L.; Pan, H.Y.; Zhang, H. Klebsiella jilinsis 2N3 promotes maize growth and induces resistance to northern corn leaf blight. Bio. Control. 2021, 156, 104554. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Wees, S.C.M.; Bakker, P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Trusov, Y.; Sewelam, N.; Rookes, J.E.; Kunkel, M.; Nowak, E.; Schenk, P.M.; Botella, J.M. Heterotrimeric G proteins-mediated resistance to necrotrophic pathogens includes mechanisms independent of salicylic acid-, jasmonic acid/ethylene-and abscisic acid-mediated defense signaling. Plant. J. 2009, 58, 69–81. [Google Scholar] [CrossRef]
- Nie, P.; Li, X.; Wang, S.; Guo, J.; Zhao, H.; Niu, D. Induced systemic resistance against Botrytis cinerea by Bacillus cereus AR156 through a JA/ET- and NPR1-dependent signaling pathway and activates PAMP-triggered immunity in Arabidopsis. Front. Plant. Sci. 2017, 8, 238. [Google Scholar] [CrossRef]
- Wang, D.; Weaver, N.D.; Kesarwani, M.; Dong, X. Induction of protein secretory pathway is required for systemic acquired resistance. Science 2005, 308, 1036–1104. [Google Scholar] [CrossRef]
- Wang, F.F.; Cui, X.K.; Sun, Y.D.; Chun, H. Ethylene signaling and regulation in plant growth and stress responses. Plant. Cell Rep. 2013, 32, 1099–1109. [Google Scholar] [CrossRef]
- Light, K.M.; Wisniewski, J.A.; Vinyard, W.A.; Kieber-Emmons, M.T. Perception of the plant hormone ethylene: Known-knowns and known-unknowns. J. Biol. Chem. 2016, 21, 715–728. [Google Scholar] [CrossRef]
- Ongena, M.; Jourdan, E.; Adam, A.; Paquot, M.; Brans, A.; Joris, B.; Thonart, P. Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ. Microbiol. 2007, 9, 1084–1090. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, S.Q.; Yang, Y.; Zhang, J.; Zhao, D.M.; Pan, Y.; Zhu, J.H. Antifungal effects of volatiles produced by Bacillus subtilis against Alternaria solani in potato. Front. Microbiol. 2020, 11, 1196. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ai, C.; Xin, L.; Zhou, G. The siderophore-producing bacterium, Bacillus subtilis CAS15, has a biocontrol effect on Fusarium wilt and promotes the growth of pepper. European J. Soil. Biol. 2011, 47, 138–145. [Google Scholar] [CrossRef]
- Ye, M.; Tang, X.; Yang, R.; Zhang, H.; Li, F.; Tao, F.; Li, F.; Wang, Z. Characteristics and application of a novel species of Bacillus: Bacillus velezensis. ACS Chem. Biol. 2018, 13, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Yan, H.H.; Sun, S.M.; Wang, Y.Q.; Zhao, X.R.; Wang, H.Y. Use of Bacillus velezensis SDTB022 against tobacco black shank (TBS) and the biochemical mechanism involved. Biol. Control. 2022, 165, 104785. [Google Scholar] [CrossRef]
- Jiang, C.H.; Liao, M.J.; Wang, H.K.; Zheng, M.Z.; Xu, J.J.; Guo, J.H. Bacillus velezensis, a potential and efficient biocontrol agent in conrol of pepper gray mold caused by Botrytis cinerea. Biol. Control. 2018, 126, 147–157. [Google Scholar] [CrossRef]
- Nisha, S.; Revathi, K.; Chandrasekaran, R.; Kirubakaran, S.A.; Sathish-Narayanan, S.; Stout, M.J.; Senthil-Nathan, S. Effect of plant compounds on induced activities of defense-related enzymes and pathogenesis related protein in bacterial blight disease susceptible rice plant. Physiol. Mol. Plant. Pathol. 2012, 80, 1–9. [Google Scholar] [CrossRef]
- Fallahi, H.; Scofield, G.N.; Badger, M.R.; Chow, W.S.; Furbank, R.T.; Ruan, Y.L. Localization of sucrose synthase in developing seed and siliques of Arabidopsis thaliana reveals diverse roles for SUS during development. J. Exp. Bot. 2008, 59, 3283–3295. [Google Scholar] [CrossRef]
- Park, M.S.; Jo, P.G.; Choi, Y.K.; An, K.W.; Choi, C.Y. Characterization and mRNA expression of Mn-SOD and physiological responses to stresses in the Pacific oyster Crassostrea gigas. Mar. Biol. Res. 2009, 5, 451–461. [Google Scholar] [CrossRef]
- Imahori, Y.; Bai, J.; Baldwin, E. Antioxidative responses of ripe tomato fruit to postharvest chilling and heating treatments. Sci. Hortic. 2016, 198, 398–406. [Google Scholar] [CrossRef]
- Niu, D.D.; Liu, H.X.; Jiang, C.H.; Wang, Y.P.; Wang, Q.Y.; Jin, H.L.; Guo, J.H. The plant growth-promoting rhizobacterium Bacillus cereus AR156 induces systemic resistance in Arabidopsis thalianaby simultaneously activating Salicylate-and Jasmonate/Ethylene-dependent signaling pathways. Mol. Plant. Microbe Interact. 2011, 24, 533–542. [Google Scholar] [CrossRef]
- Lin, Z.F.; Zhong, S.L.; Grierson, D. Recent advances in ethylene research. J. Exp. Bot. 2009, 60, 3311–3336. [Google Scholar] [CrossRef]
- Ayaz, M.; Ali, Q.; Farzand, A.; Khan, A.R.; Ling, H.; Gao, X. Nematicidal volatiles from Bacillus atrophaeus GBSC56 promote growth and stimulate induced systemic resistance in tomato against Meloidogyne incognita. Int. J. Mol. Sci. 2021, 22, 5049. [Google Scholar] [CrossRef]
- Wang, C.J.; Wang, Y.Z.; Chu, Z.H.; Wang, P.S.; Liu, B.Y.; Li, B.Y.; Luan, B.H. Endophytic Bacillus amyloliquefaciens YTB1407 elicits resistance against two fungal pathogens in sweet potato (Ipomoea batatas (L.) Lam.). J. Plant. Physiol. 2020, 253, 153260. [Google Scholar] [CrossRef]
- Park, Y.G.; Mun, B.G.; Kang, S.M.; Hussain, A.; Shahzad, R.; Seo, C.W.; Yun, B.W. Bacillus aryabhattai SRB02 tolerates oxidative and nitrosative stress and promotes the growth of soybean by modulating the production of phytohormones. PLoS ONE 2017, 12, e0173203. [Google Scholar] [CrossRef]
- Ali, A.M.; Awad, M.Y.; Hegab, S.A.; Gawad, A.M.A.E.; Eissa, M.A. Effect of potassium solubilizing bacteria (Bacillus cereus) on growth and yield of potato. J. Plant. Nutr. 2021, 44, 411–420. [Google Scholar] [CrossRef]
- Ellis, C.M.; Nagpal, P.; Young, J.C.; Hagen, G.; Guilfoyle, T.J.; Reed, J.W. AUXIN RESPONSE FACTOR1 and AUXIN RESPONSE FACTOR2 regulate senescence and floral organ abscission in Arabidopsis thaliana. Trends Plant. Sci. 2005, 132, 4563–4574. [Google Scholar]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant. Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef]
- Krochko, J.E.; Abrams, G.D.; Loewen, M.K.; Abrams, S.R.; Cutler, A.J. (+)-Abscisic acid 8’-hydroxylase is a cytochrome P450 monooxygenase. Plant Physiol. 1998, 118, 849–860. [Google Scholar] [CrossRef]
- Qin, L.; Tian, P.; Cui, Q.; Hu, S.; Jian, W.; Xie, C.; Shen, H. Bacillus circulans GN03 alters the microbiota, promotes cotton seedling growth and disease resistance, and increases the expression of phytohormone synthesis and disease resistance-related genes. Front. Plant. Sci. 2021, 12, 644597. [Google Scholar] [CrossRef]
- Marion, L.; Paillisson, J.M. A mass balance assessment of the contribution of floating-leaved macrophytes in nutrient stocks in an eutrophic macrophyte-dominated lake. Aquat. Bot. 2003, 75, 249–260. [Google Scholar] [CrossRef]
- Benjamin, J.G.; Nielsen, D.C. Water deficit effects on root distribution of soybean, field pea and chickpea. Field Crop. Res. 2006, 97, 248–253. [Google Scholar] [CrossRef]
- Ansari, F.A.; Ahmad, I. Fluorescent Pseudomonas-FAP2 and Bacillus licheniformis interact positively in biofilm mode enhancing plant growth and photosynthetic attributes. Sci. Rep. 2019, 9, 4547. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.J.; Hossain, M.T.; Khan, A.; Kim, K.H.; Jeon, C.O.; Chung, Y.R. Bacillus oryzicola sp. nov., an endophytic bacterium isolated from the roots of rice with antimicrobial, plant growth promoting, and systemic resistance inducing activities in rice. J. Plant. Pathol. 2015, 31, 152–164. [Google Scholar] [CrossRef] [PubMed]
| Serial Number | Name | Parent Ion | Daughter Ion | Declustering Voltage | Collision Energy |
|---|---|---|---|---|---|
| 1 | IAA | 173.9 | 130; 128 | −49; −49 | −12; −24 |
| 2 | GA3 | 345 | 142.9; 238.9 | −78; −90 | −32; −20 |
| 3 | ABA | 264 | 153.1; 219.9 | −60; −67 | −16; −17 |
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| StNPR1 | TATTGGCTGCACGAAGTCAGT | CGCACCAAATCCTTCAGCAAA |
| StLOX2.1 | GCAGCTGTTAACTTTGGCCAA | CCACTCCCATTCTTCAGCTGT |
| StACO1 | TGACAAAGTGAGTGGCCTTCA | CCTCAAGTTGGTCACCAAGGT |
| StARF2 | GGGGATCTTCGTGTTGGAGTT | CCAAGCTGTTGCAAGTACACC |
| StGA20ox1 | TGATGGTGTCACTGGCTATGG | TCGAGCATGTTCAATTGGGGA |
| StNCED1 | GTACCGGAAAACCCAGTTTGC | CGAAAAGAGGGTTAGCTCCGT |
| StActin7 | TTTGCTGGTGATGATGCTCCT | AGCTTCATCACCCACATAGGC |
| StNPR1 | TATTGGCTGCACGAAGTCAGT | CGCACCAAATCCTTCAGCAAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Li, Q.; Zhang, D.; Zhao, Y.; Zhao, D.; Pan, Y.; Wang, J.; Yang, Z.; Zhu, J. Bacillus velezensis Strain HN-Q-8 Induced Resistance to Alternaria solani and Stimulated Growth of Potato Plant. Biology 2023, 12, 856. https://doi.org/10.3390/biology12060856
Bai X, Li Q, Zhang D, Zhao Y, Zhao D, Pan Y, Wang J, Yang Z, Zhu J. Bacillus velezensis Strain HN-Q-8 Induced Resistance to Alternaria solani and Stimulated Growth of Potato Plant. Biology. 2023; 12(6):856. https://doi.org/10.3390/biology12060856
Chicago/Turabian StyleBai, Xuefei, Qian Li, Dai Zhang, Yi Zhao, Dongmei Zhao, Yang Pan, Jinhui Wang, Zhihui Yang, and Jiehua Zhu. 2023. "Bacillus velezensis Strain HN-Q-8 Induced Resistance to Alternaria solani and Stimulated Growth of Potato Plant" Biology 12, no. 6: 856. https://doi.org/10.3390/biology12060856
APA StyleBai, X., Li, Q., Zhang, D., Zhao, Y., Zhao, D., Pan, Y., Wang, J., Yang, Z., & Zhu, J. (2023). Bacillus velezensis Strain HN-Q-8 Induced Resistance to Alternaria solani and Stimulated Growth of Potato Plant. Biology, 12(6), 856. https://doi.org/10.3390/biology12060856






