Simple Summary
The shrimp Penaeus aztecus, native to the western Atlantic, was first reported in the Mediterranean Sea (Bay of Antalya, Southern Turkey) in 2010. In the following years, it proved its invasiveness with multiple records from all over the Mediterranean except the westernmost sector and the North Adriatic Sea. Several pieces of evidence suggest that the unintentional transport of larvae in the ballast waters of transoceanic vessels departing from the U.S. West Coast, instead of the escape of adults from unreported experimental shrimp farming, is the more likely pathway of the introduction of P. aztecus in the Mediterranean Sea. The accurate scrutiny of scientific literature on non-indigenous species brought to light an earlier (2005) arrival in the Black Sea, which passed unnoticed as the shrimps were misidentified as Penaeus semisulcatus, also a non-indigenous species, which is established and exploited in the Levant Sea since 90 years. But it is native to the Indo-Pacific region, other misidentifications were also found, therefore morphological characters allowing correct identification of the two species and of the autochthonous Penaeus kerathurus are illustrated. Non-indigenous species are among the descriptors adopted in the Marine Strategy Framework Directive for determining the good environmental status of marine waters in the European States, hence the importance of their correct identification.
Abstract
The shrimp Penaeus aztecus, native to the western Atlantic, was first reported in the eastern Mediterranean Sea in 2010. New records, from different Mediterranean localities, multiplied in the following years. The accurate search of the literature on non-indigenous species discovered it was misidentified more than once as another alien shrimp, P. semisulcatus, native to the Indo-Pacific region, with the result that its earlier presence in the Black Sea went unnoticed. Morphological characteristics allowing the identification of these two species, the autochthonous P. kerathurus and two other alien Penaeus species present in the Mediterranean, are reprised. The present distribution of P. aztecus based on literature records and surveys carried out in the northern and central Adriatic between 2016 and 2021 is mapped. The unintentional transport of larvae carried in ballast water by transoceanic vessels departing from the U.S. East Coast is suggested as the most probable introduction pathway. The significance of the correct identification of non-indigenous species, a “Descriptor” adopted in the Marine Strategy Framework Directive for determining the good environmental status of marine waters in the European States, is emphasized.
1. Introduction
The unintentional introduction of non-indigenous species (NIS) in marine habitats as a consequence of maritime activities is a worldwide phenomenon. The evolution of naval architecture, with the adoption of tanks for the temporary storage of large volumes of seawater to ballast unloaded cargo vessels together with the progressive increase of vessel speed, gave new opportunities to plankton and larval stages of benthic species to be displaced outside their native range and to settle in new habitats [1]. Ship canals, opened to facilitate maritime traffics, also facilitate the introduction of NIS. The Suez Canal, progressively enlarged since its opening in 1869 [2], has reconnected the Atlanto-Mediterranean and the Indian-Red Sea biota, which have been separated for several million years. A large number of Red Sea immigrants, “Lessepsian immigrants” [3], have entered the Mediterranean through this waterway and have progressively changed the biota of the eastern Mediterranean coastal waters. Substantial differences between the eastern and western Mediterranean are observed in the total number of NIS, their native regions, and pathways of introduction [4,5].
The “brown shrimp” Penaeus aztecus Ives, 1891 is native to the NW Atlantic and Gulf of Mexico, where it is a very important fishery resource, with annual landings of over 40,000 tons [6]. Its presence in the Mediterranean Sea was reported for the first time in 2010 [7] from several specimens collected in the Gulf of Antalya (South Turkey) since December 2009. The unintentional transport of larvae via ballast waters was suggested as the most probable vector of introduction [7]. Only 3 years later, P. aztecus became common, not only in the coastal waters of southern Turkey [8], but also in the northern Aegean Sea (Thermaikos Gulf) [9]. A single specimen was also caught in the South Adriatic (Boka Kotorska) [10]. In the following years, the capture of one, or a few specimens, was reported from several localities all over the Mediterranean Sea and the hypothesis of escapes from aquaculture plants was also suggested [11,12,13].
To follow the chronology of the spreading of P. aztecus in the Mediterranean and its present distribution, an in-depth scrutiny of the literature was carried out. It also discovered the species was misidentified as Penaeus semisulcatus de Haan, 1844, in the Black Sea [14,15] and Central Mediterranean [16].
In this age of globalization, species native from all over the world can easily reach our shores. Therefore, it is fundamental that the new records of alien species include detailed illustrations of the specimens examined. It allows taxonomists to detect possible misidentifications, otherwise perpetuated by their inclusion in regional lists of alien species, as in the herein-discussed cases of P. aztecus.
2. Materials and Methods
We suspected that the report of Penaeus semisulcatus from the Gulf of Taranto (West Ionian Sea) published in 2015 [16] was the result of a misidentification of P. aztecus; therefore, in the summer of 2016, a leaflet with photos of distinctive characters of the shrimps was produced and sent to a friend, a skilled artisanal fisher in Roccella Ionica (Ionian Sea). Quite soon, we received photos and the first specimen caught with a trammel net. At the same time, skippers of bottom trawlers in Ancona (Central Adriatic Sea) told us that occasionally they noted single “Mazzancolle”—the Italian commercial name for the autochthonous P. kerathurus (Forskål, 1775)—with uniform color, without the typical dark transversal bands. Therefore, in October 2016, the same leaflet was circulated in the wholesale fish markets of Ancona and San Benedetto del Tronto, the largest in the Central Adriatic, where large quantities of “Mazzancolle” are auctioned daily. Shortly after, we received shrimp specimens from both markets, with the indication of the fishing area where they were caught.
All shrimps received were preserved in 80% ethanol and stored in the CF Decapoda collection, which is to be transferred to the Museo Civico di Storia Naturale in Verona, Italy.
We also received various reports from fishers, but not supported by physical specimens; these are not included here.
The number of records of an NIS may indicate its dispersal capability, but they are a poor index of its actual abundance. To obtain insight into the relative abundance of P. aztecus versus the autochthonous P. kerathurus, here we examine the data collected between 2016 and 2021 during the fishery surveys “SoleMon”. It is carried out in the Northern and Central Adriatic (GSA 17), to assess the abundance of some flatfish stock as well as commercial invertebrates [17]. This survey is carried out yearly in late autumn, when juveniles of common sole, common cuttlefish, and shrimp have left their nursery areas (lagoons and coastal waters) and are recruited to the fishery. In each survey, about 70 stations—located between the Italian and Slovenian coast and the limit of Croatian territorial waters, depth range 10–80 m—have been sampled with two “rapido”, professional beam trawls (width 3.6 m) rigged with iron teeth along the lower leading edge [17] (Annex 1), towed for 30 min at an average speed of 5.5 knots (about 10 km/h). In each sampling station, penaeid shrimps have been identified to species, sexed, counted, and measured.
The review of the literature and databases on NIS, present on the Web, brought to light other erroneous reports. Therefore, to facilitate the identification of P. aztecus from P. semisulcatus, the autochthonous P. kerathurus, and two other alien Penaeus species present in the Mediterranean Sea (namely P. pulchricaudatus Stebbing, 1914 and P. hator Burkenroad, 1959, both native to the Indian Ocean), more evident distinctive characters are summarized and illustrated.
3. Results
3.1. Species Identification
In the earlier records from the Mediterranean Sea, the shrimp species herein considered has been reported under the name Farfantepenaeus aztecus (Ives, 1891), following the nomenclature adopted by Pérez-Farfante and Kensley [18]. They split the genus Penaeus Fabricius, 1798 into six genera, based on morphological differences. In a phylogenetic molecular study, published in 2011 [19], these genera were again lumped into the genus Penaeus s.l.; thereafter, the species was reported under the name Penaeus aztecus Ives, 1891. In a very recent comprehensive phylogenetic molecular investigation, Yang et al. [20], while keeping all the species in the genus Penaeus s.l., showed that up to 11 clades can be recognized within the genus. Chan [21] morphologically characterized these clades, regarded as subgenera of Penaeus s.l., and reinstated Farfantepenaeus Burukovsky, 1972 at the subgenus level. In this note, we use the genus name Penaeus for all the species considered.
The 13 shrimps examined have been identified as adult Penaeus aztecus based on a set of morphological characters reported in the literature [22] and the comparison with the material of P. aztecus, P. semisulcatus, P. hator, P. pulchricaudatus, and P. kerathurus present in the Decapoda collection of the senior author (CF).
Material examined, Penaeus aztecus:
Western Ionian Sea: 1♂ c.l. 30 mm, off Roccella Ionica, depth 10 m, 30 July 2016, trammel net, D1962; 2♀ c.l. 36–41 mm, same locality, July 2017, D1961.
Central Adriatic Sea: 4♀ c.l. 41.3–44.5 mm, Ancona, about 6 miles (11 km) offshore, depth 30–35 m, October 2016, coastal trawlers, D1959; 1♀ c.l. 43 mm, S. Benedetto del Tronto, about 6 miles (11 km) offshore, depth 30 m, 11 November 2016, coastal trawler, D1960; 1♂ c.l. 29.3 mm, S. Benedetto del Tronto, about 3 miles (5.5 km) offshore, depth 15 m, 30 July 2017, beam trawler, D2136; 2♀ c.l. 48–53 mm, off Porto Civitanova, 13 September 2017, coastal trawler, D2137; 1♀ c.l. 40 mm, 43°20′ N 13°59′ E (SoleMon 2020 St. 67), depth 52–53 m, 15 December 2020, D2138; 1♀ c.l. 40 mm, 43°42.4′ N 13°41.9′ E (SoleMon 2021 St. 36), depth 52–53 m, 28 November 2021, D2139.
Comparative material:
Penaeus aztecus: 1♂ c.l. 10.5 mm, 2♀ c.l. 10.0–11.8 mm, 29°30′ N 91°52′ W, Missisipi Delta, USA, depth 2 m, 30 May 1999, D1775; 2♂, 1♀, 36°49′ N 30°58′ E, Gulf of Antalya East Mediterranean, depth 30 m, 26 June 2010, D2100.
Penaeus semisulcatus: 1♂ c.l. 35.6 mm, 1♀ c.l. 43.2 mm, 31°15′ N 32°41′ E, Israel, East Mediterranean, depth 16 m, 31 October 1975, D255; 3♂ c.l. 30.8–42.1 mm, 1♀ c.l. 48.5 mm, 36°47′ N 31°15′ E, Gulf of Antalya, East Mediterranean, depth 40 m, 22 June 2010, D2153.
Penaeus kerathurus: 2♂ c.l. 24.6–30.2 mm, 2♀ c.l. 21.3–31.8 mm, 43°45′ N 13°18′ E, Central Adriatic, depth 14 m, 17 December 1984, D1313; 1♂ c.l. 39.3 mm, 1♀ c.l. 48 mm, 43°06.1′ N 13°54.3′ E, Central Adriatic, depth 12 m, 29 August 2001, D2031.
Penaeus hator: 1♂ c.l. 37.9 mm, 1♀ c.l. 39.0 mm, 36°49′ N 31°02′ E, Gulf of Antalya, East Mediterranean, depth 30 m, 21 April 2011, D2155.
Penaeus pulchricaudatus: 1♂ c.l. 43 mm, 1♀ c.l. 56.5 mm, off Bardawil lagoon, East Mediterranean, depth 18 m, 27 January 1979, D1931.
In freshly caught P. aztecus, the body color is light brown to rose with minute reddish chromatophores, and uropods have reddish distal margins, whereas P. semisulcatus is olive-brown, with slightly darker transverse bands and reddish setal fringe of uropods, and P. kerathurus is light brown with dark brown interrupted transverse bands (may fade after long storage in ice, but always remain visible), the uropods are distally bluish (Figure 1). The color pattern in P. pulchricaudatus is similar to that of P. kerathurus, except for the uninterrupted dark brown bands on abdominal somites. P. hator presents a cream color body with short narrow vertical brown stripes on abdominal pleurae.
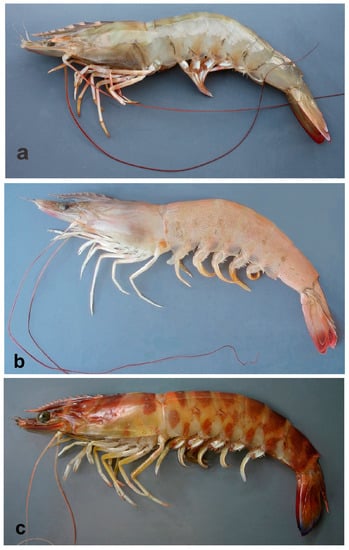
Figure 1.
Colors in life: (a) Penaeus semisulcatus, eastern Mediterranean Gulf of Antalya; (b) Penaeus aztecus, Adriatic Sea off Ancona; (c) Penaeus kerathurus, Adriatic Sea off Ancona.
Preserved specimens, with colors faded, can be easily identified on a set of morphological characters (Figure 2):
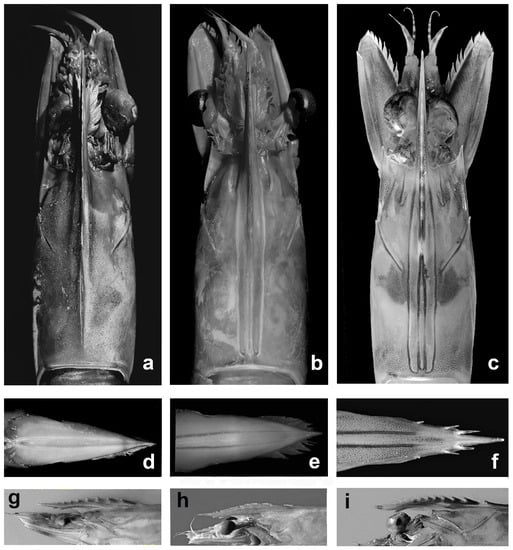
Figure 2.
Penaeus semisulcatus (a,d,g); Penaeus aztecus (b,e,h); Penaeus kerathurus (c,f,i); carapace dorsal view (a–c); telson (d–f); rostrum side view (g–i).
- The adrostral groove and crest end about at 2/3 of the carapace length in P. semisulcatus, whereas extend almost to the posterior margin of the carapace in P. aztecus and P. kerathurus, as well as in P. pulchricaudatus and P. hator;
- The ventral margin of the rostrum bears two, occasionally three, teeth in P. aztecus, versus three to four in P. semisulcatus, and only one in P. kerathurus, P. pulchricaudatus, and P. hator;
- The lateral margins of telson are devoid of teeth or spines in P. aztecus and P. semisulcatus, whereas are distally armed with three pairs of movable spines in P. kerathurus, P. pulchricaudatus, and P. hator;
- The mesial margin of both coxa and basis of the first and second pereopods are armed with acute teeth in P. kerathurus, whereas the other four species lack coxal teeth on the first and second pereopods; only P. aztecus has an ischial tooth on the first pereopods;
- The last abdominal somite in P. aztecus presents a well-defined dorsolateral sulcus, lacking in the other four species.
3.2. Previous Misidentifications
The accurate scrutiny of the existing literature on Mediterranean NIS, primarily carried out to investigate the spreading of P. aztecus, discovered that it has been repeatedly misidentified as P. semisulcatus de Haan, 1844, an NIS native to the Red Sea and the Indian Ocean.
It was reported twice under the latter name from the eastern Black Sea: first in 2006 [14], four years before the first Mediterranean record [7], and again in 2017 [15], well in advance of the “first” record of P. aztecus in 2019 off the Turkish Black Sea coast [23]. Khvorov et al. [14] reported the capture in October 2005 of eight specimens of a penaeid shrimp near Bolshoi Sochi, about 150 km from Novorossiysk (the largest commercial Russian port in the Black Sea), and identified them as P. semisulcatus. In addition to the description (in Russian), they published photos of the morphological details of the specimens examined that allowed their identification as P. aztecus: the carapace in dorsal view shows the adrostral groove and crest extending almost to the posterior margin of the carapace [14] (Figure 3A); the telson has unarmed lateral margins [14] (Figure 3E); pereopod I has two teeth (ischial and basial) and pereopod II only one tooth [14] (Figure 3K).
The second Black Sea report, based on one female caught in September 2014 near the port of Batumi (Georgia) at the extreme East of the Black Sea, about 320 km from the previous locality, was again misidentified as P. semisulcatus, but its identity with P. aztecus is evident from the photos of the specimen [15] (Figures 2–4, in the latter the images probably became distorted in page composition).
The above misidentifications led to the inclusion of P. semisulcatus in the list of alien species in Russian seas [24] (Table 1) and in other reviews of Black Sea decapods fauna [25] and Black Sea NIS [26].
In a short note, without shrimp figure or description, Arnesano et al. [16] reported 147 specimens of P. semisulcatus, examined in the autumn of 2014 during the monitoring of commercial catches of fishing vessels working in the north-western Ionian Sea (Gulf of Taranto). In a subsequent report of the same monitoring program for the years 2014–2018, Donnaloia et al. [27] cited only P. aztecus for the localities referred to in the previous note and added new ones. The finding in 2016 of P. aztecus in the Gulf of Corigliano by Renda and Crocetta [28] and the specimens collected in the summer of 2016 in the nearby Roccella Ionica grounds, reported by this study, are pieces of evidence that the report of P. semisulcatus in the north-western Ionian Sea [16] was based on a misidentification of P. aztecus. The above misidentification [16] led to the inclusion of P. semisulcatus in the “New Sightings” of the Italy national report in the ICES WGITMO Report 2016 [29] (p. 105). This error was recognized and corrected in the subsequent ICES WGITMO Report 2017 [30] (p. 74).
3.3. Spreading of Penaeus aztecus in the Mediterranean Sea
The literature review suggests that the records from the eastern Black Sea [14,15], reported nine years apart, represent two independent introductions of P. aztecus—probably via ballast waters—that did not give origin to any established population.
The first records of P. aztecus in the different sectors of the Mediterranean Sea, summarized in Table 1 and mapped in Figure 3, evidence very rapid colonization. Only three years after its first record in 2010 in the Gulf of Antalya [7], a significant population was already established all along the Turkish Mediterranean shelf, from Finike to the Gulf of Iskenderun [8]. By 2013, P. aztecus was also recorded in the Thermaikos Gulf (northern Aegean Sea) [9]. The proximity to the port of Thessaloniki, the second commercial port of Greece, again let us guess an introduction of larvae via ballast waters or an unaided expansion of the Turkish population.
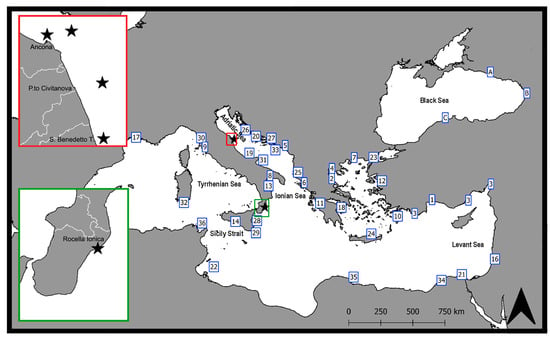
Figure 3.
First records of Penaeus aztecus, illustrating its spreading in the Mediterranean region. Insets with stars: new records (present study) for west-central Adriatic Sea and Ionian Sea. (See Table 1 for the references of the coded records).
The species also very rapidly spread westward, with multiple records since 2015, in the northern part of the Strait of Sicily [31] and in the Tyrrhenian Sea [32]. One specimen was also collected in 2015 in the Gulf of Lion [11], about 60 miles from Marseille, the main French port in the Mediterranean, not followed by additional records. Up to now, this is the westernmost record of the species in the Mediterranean Sea.
The invasion of P. aztecus in the southern rim of the Mediterranean Sea shows a similar pattern, with the first capture in 2015 off Israel [11], and the following year off Nile Delta (Egypt) [33] and in the Gulf of Gabes (South Tunisia) [34].
In 2013, captures of single adult specimens of P. aztecus were recorded for the first time from the south-eastern side of the Adriatic Sea (Boka Kotorska) [10] and the north-eastern side of the Ionian Sea (off Korfu Island) [35], suggesting an arrival of larvae either carried via ballast waters or drifted by the Levantine current entering in the Adriatic through the Otranto Strait, and flowing northward along the western coast of the Balkan peninsula [36].
The various published reports—all based on one or a few specimens—from different localities in Central Adriatic [12,13] as well as our unpublished records suggest that by 2016, the species was already established in the basin on the eastern and western sides, up to the latitude of 44° N.

Table 1.
First records of Penaeus aztecus in the Black Sea (letters) and the Mediterranean Sea (numbers), ordered by date. Record codes assigned to the localities as shown in Figure 1. Subsequent records from the same or nearby localities are not listed.
Table 1.
First records of Penaeus aztecus in the Black Sea (letters) and the Mediterranean Sea (numbers), ordered by date. Record codes assigned to the localities as shown in Figure 1. Subsequent records from the same or nearby localities are not listed.
| Record Code | Date Collection | Locality | Reference |
|---|---|---|---|
| A | 2005 | Black Sea: off Lazarevskoe (Russia) | Khvorov et al., 2006 [14] * |
| B | 2014 | Black Sea: Batumi (Georgia) | Guchmanidze et al., 2017 [15] * |
| C | 2017 | Black Sea: Bozkurt (Turkey) | Gönülal & Türetken, 2019 [23] |
| 1 | 2009 | Levant Sea, Gulf of Antalya | Deval et al., 2010 [7] |
| 2 | 2012 | Aegean Sea: lagunes of Thermaikos Gulf | Nikolopoulou et al., 2013 [9] |
| 3 | 2012 | Levant Sea: Mersin, Finike, Iskenderun | Gökoglu & Özvarol, 2013 [8] |
| 4 | 2013 | Aegean Sea: Thermaikos Gulf | Kevrekidis, 2014 [37] |
| 5 | 2013 | Adriatic Sea East: Boka Kotorska | Marković et al., 2014 [10] |
| 6 | 2013 | Ionian Sea East: Korfu | Kapiris & Apostolidis, 2014 [35] |
| 7 | 2013 | Aegean Sea: Nestos River estuary | Minos et al., 2015 [38] |
| 8 | 2014 | Ionian Sea West: Gulf of Taranto | Arnesano et al., 2015 [16] * |
| 9 | 2014 | Tyrrhenian Sea: Castiglione della Pescaia | Cruscanti et al., 2015 [32] |
| 10 | 2014 | Aegean Sea: off Chalki Island | Kondylatos & Corsini-Foka, 2015 [39] |
| 11 | 2015 | Ionian Sea East: off Kyllini | Zenetos & Giavasi, 2015 [40] |
| 12 | 2015 | Aegean Sea: Çandarlı Bay | Bakir & Aydin, 2016 [41] |
| 13 | 2015 | Ionian Sea West: Gulf of Corigliano | Renda & Crocetta, 2016 [28] |
| 14 | 2015 | Sicily: P.to Empedocle, Mazara del Vallo | Scannella et al., 2017 [31] |
| 15 | 2015 | Ionian Sea West: off Augusta | Donnaloia et al., 2019 [27] |
| 16 | 2015 | Levant Sea: Palmahim | Galil et al., 2017 [11] |
| 17 | 2015 | Gulf of Lion: Le Grau du Roi, | Galil et al., 2017 [11] |
| 18 | 2016 | Aegean Sea: Argolicos Gulf, Vivari lagoon | Kapiris & Minos, 2017 [42] |
| 19 | 2016 | Adriatic Sea West: off Termoli | Zava et al., 2018 [12] |
| 20 | 2016 | Adriatic Sea East: Hvarski kanal | Ugarković & Crocetta, 2021 [13] |
| 21 | 2016 | Egypt: Nile Delta, Damietta | Sadek et al., 2018 [33] |
| 22 | 2016 | Tunisia South: Gulf of Gabes | Ben Jarray et al., 2019 [34] |
| 23 | 2016 | Aegean Sea: Ibrice | Gönülal & Türetken, 2019 [23] |
| 24 | 2017 | Aegean Sea: off Heraklion (Crete Is.) | Kampouris et al., 2018a [43] |
| 25 | 2018 | Adriatic Sea East: Vlora Bay | Kampouris et al., 2018b [44] |
| 26 | 2018 | Adriatic Sea East: Murtersko more | Ugarković & Crocetta, 2021 [13] |
| 27 | 2018 | Adriatic Sea East: Neretvanski kanal | Ugarković & Crocetta, 2021 [13] |
| 28 | 2018 | Ionian Sea West: off Augusta | Pipitone & Lombardo, 2019 [45] |
| 29 | 2018 | Ionian Sea West: off Marzamemi | Kampouris et al., 2018b [44] |
| 30 | 2018 | Ligurian Sea: off Livorno | Ligas et al., 2019 [46] |
| 31 | 2018 | Adriatic Sea West: Gulf of Manfredonia | Donnaloia et al., 2019 [27] |
| 32 | 2019 | Sardinia: off Cape Teulada | Mulas et al., 2019 [47] |
| 33 | 2019 | Adriatic Sea East: off Cavtat | Ugarković & Crocetta, 2021 [13] |
| 34 | 2019 | Egypt: Nile Delta, Abu-Qir | El Deeb et al., 2020 [48] |
| 35 | 2020 | Libya: Gulf of Bomba, Umm-Hufayn lagoon | Abdulrraziq et al., 2021 [49] |
| 36 | 2020 | Tunisia North | Ben Abdallah Ben Hady Hamida et al., 2020 [50] |
* Reported as Penaeus semisulcatus.
However, the data of the SoleMon survey (carried out yearly in the North and Central Adriatic Sea from 2016 to 2021 (Table 2)) evidence that it is still very rare compared with the autochthonous species (one specimen of P. aztecus caught in the years 2020 and 2021 versus 1864 and 2124 specimens of P. kerathurus, respectively).

Table 2.
Abundance of P. kerathurus and P. aztecus in the stations sampled in the North and Central Adriatic (GSA 17) by the fishery survey “SoleMon” in the years 2016–2021.
It is worth noting that in GSA17, the stock of the autochthonous P. kerathurus markedly increased in this century [51]. For example, the quantities auctioned in the Ancona gross market rose from 17 tons in 2000 to 82 tons in 2022, with a peak of 95 tons in 2018.
4. Discussion
At the collection of a new NIS, it is possible that the species—particularly if native to distant regions—is misidentified, or even described as a new taxon, as in the case of Lysmata arvoredensis Giraldes, Macedo, Brandão, Baeza & Freire, 2018. It was described as a new species from the West Atlantic Brasilian coast and later placed in the synonymy of Lysmata uncicornis Holthuis & Maurin, 1952, native to the East Atlantic African coast [52]. A species misidentification introduces a “false positive” error in species distribution modelling [53].
Before the appearance of P. aztecus, among the penaeid species recorded in the Mediterranean Sea (autochthonous and alien), only P. semisulcatus was characterized by the presence of more than one tooth on the lower margin of the rostrum and an unarmed telson. These “distinguishing characters” mentioned in the “CIESM Atlas of exotic species in the Mediterranean—Crustaceans” [54] may have led to the misidentifications, passed unnoticed till now, with the consequent listing of P. semisulcatus in the regional lists of NIS [24,25,26] and also in the AquaNIS database [55].
Penaeus semisulcatus is an earlier Lessepsian immigrant in the Levant Sea, where it is targeted by local fishers since the 1930′s [56,57]. Despite its long-dating presence in the Levant Sea, it did not spread northward into the Aegean Sea [58] (Table 1), [59] (Appendix 1). It is present in the trawling grounds off the Nile Delta [60], but we found no records of its presence westward, except in the species list of an ecological study of a benthic community—which was invaded by the alien alga Caulerpa cylindracea—in the Gulf of Salerno (Tyrrhenian Sea) [61] (Appendix). As is often the case with non-taxonomic papers, no vouchered specimens were available to verify whether this report is another misidentification.
In the eastern Mediterranean, the brown shrimp—P. aztecus—has proven remarkable invasiveness, quickly becoming of economic value as a fishery resource or as a source of wild fry [33] for the developing shrimp farming industry in Egypt [62]. In other Mediterranean basins, such as the Adriatic Sea, it is still rare. Although it was collected already in 2016 (present records) off Ancona (about at latitude 44° N), it has never been collected further North. The shallow depths and the climatic conditions—winter bottom sea temperatures as low as 10 °C at 30 m depth [63]—are likely to prevent its settlement. The species has not yet been reported from the westernmost part of the Mediterranean (Spain, Algeria, Morocco), where climatic conditions seem favorable, and we may expect its record in the near future.
Significant longshore movements of P. aztecus were reported in the Gulf of Mexico (native area) in a study carried out between 1978 and 1980 [64], with over 71,000 tagged brown shrimps released in different sites of the offshore fishing grounds, and a percentage of recapture of over 12%. Traveled distances of 596 and 528 km from the release point were recorded for two specimens recaptured after 430 and 400 days at sea, respectively. Even considering this capacity of natural dispersion, the multiple records of P. aztecus—only 5 years from the first record in the Gulf of Antalya [7]—from sites far away, such as the North Tyrrhenian Sea and the Gulf of Lion, suggest that multiple introduction events have been at the origin of its spreading in the Mediterranean Sea. Unfortunately, the records of P. aztecus, with the morphological identification corroborated by molecular data (COI or 16S rRNA sequence) [11], are too scanty to verify any hypothesis. An extensive molecular study of the P. aztecus populations through the Mediterranean Sea and the comparison with the genetic sequences available for its American native range may provide insights into the number and origin of introduction events and genetic connectivity among populations, as recently done for another West Atlantic invasive immigrant, the crab Callinectes sapidus Rathbun, 1896 [65].
A high number of the NIS species established in the Mediterranean Sea are native to the Indo-West-Pacific region and entered via the Suez Canal, such as P. semisulcatus. Others arrived through different introduction pathways from the world oceans. Various studies addressed the role of the different introduction pathways and/or of the native region on the observed distribution of alien species in the Mediterranean region [5,66,67]. Except in the case of intentional introductions of alien species, for which official records may be available, the introduction pathway in the other cases remains an educated/speculative guess. Penaeus aztecus is no exception.
In the first record of P. aztecus in the Mediterranean Sea, the introduction of larvae via ballast water was suggested as the most likely introduction pathway [7]. Subsequently, other Authors [11,12,13,32] suggested escapes from unreported “clandestine” shrimp farming activities.
The evidence from the history of some shrimp farming attempts in the Mediterranean scarcely support the hypothesis that escape from confinement at aquaculture facilities were the origin of the introduction and spreading of P. aztecus in the Mediterranean.
In response to the high demand for penaeid shrimps by the European market, projects to develop shrimp farming in the Mediterranean were launched in the 1970s in various countries such as France [68] and Italy [69]. In the beginning, P. japonicus Spence Bate, 1888 was imported from Japan to raise “in loco” the breeding stock necessary to produce shrimp fry. Between 1982 and 1985, about 1.5 million postlarvae (P17–P31) of P. japonicus were produced under controlled laboratory conditions by the research center established by the Italian National Research Council in Lesina. These postlarvae were released in spring and harvested in late summer in a “restocking” experiment in the coastal lagoons of Lesina and Varano (Adriatic Sea); small numbers were also intentionally released at sea near lagoon entrances [70]. After these releases, one single adult of P. japonicus was caught in the open sea (depth 25 m) in front of the lagoons in December 1985 [71]. A large shrimp hatchery—with a production potential of 6 million postlarvae per year—was established in Sardinia in the 1990s but ceased activity in 2006 [72] before the record of P. aztecus in the Mediterranean. A few smaller hatcheries, often inside large fish aquaculture plants, are still present in Italy and other Mediterranean countries, and some cases of unreported “clandestine” import of different species—potential candidates for shrimp aquaculture—have been evidenced [73,74]. The possibility that they also imported P. aztecus to experiment with its farming cannot be ruled out.
However, we consider it improbable that aquaculture entrepreneurs in different Mediterranean countries (Turkey, Greece, Italy, and France) almost simultaneously imported P. aztecus, with subsequent events of escape from confinement. In addition, the choice of P. aztecus seems unlikely as, to our knowledge, it is currently not used in industrial shrimp aquaculture. It was introduced in New Caledonia and French Polynesia (with other alien penaeid shrimps) during the early experiments to develop local shrimp farming [75], but due to its low performance, it was quickly set aside in favor of P. stylirostris Stimpson, 1871, which currently accounts for the bulk of production of New Caledonian shrimp farms [76].
The earlier record of P. aztecus (misidentified as P. semisulcatus) in the Black Sea [14], climatically not suited for penaeid shrimp farming, further supports the hypothesis that its presence originated from introductions of larvae/postlarvae via the ballast water of transoceanic vessels departed from the US East coast.
Also worthy of note is the capture in 2018 of a juvenile female (TL 115 mm), tentatively identified with P. aztecus, at the mouth of Schelde River (North Sea) near Antwerpen, the main harbor in Belgium [77]. Most likely, it was introduced at the larval stage via ballast water either by a transoceanic vessel departed from the U.S. East Coast, or by a ship of the many lines connecting Mediterranean and North Sea harbors.
Concerns that the presence of P. aztecus may “negatively” affect the autochthonous P. kerathurus have been expressed [37]; however, at present, no experimental evidence is available. The ecological niche of P. aztecus is similar to that of the Penaeus species (native or alien) already present in the Mediterranean. Therefore, in areas where a self-sustained population of P. aztecus is present, competition for space or food resources seems likely. Local climatology and edaphic conditions, in synergy with the impact of the fishing effort exerted on these highly prized resources, will ultimately determine the abundance of one or another species, probably without substantial changes in ecosystem functioning.
5. Conclusions
According to the Marine Strategy Framework Directive, “Non-indigenous species introduced by human activities” is one of the descriptors for determining the good environmental status of marine waters in the EU States [78] and has to be periodically evaluated [79]. Therefore, the correct species identification, hence precise knowledge of its ecological niche in the native region and probable introduction pathways, is fundamental to adopt the more appropriate actions to limit species spreading, keeping in mind that marine spaces are not a backyard and eradication is impracticable once an alien species is established.
Author Contributions
Conceptualization, C.F.; methodology, C.F.; data curation, C.F. and M.S.; writing—original draft preparation, C.F.; writing—review and editing, C.F. and M.S.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used are available in the text.
Acknowledgments
We are most grateful to the fisherman Antonio Martino for sending us, on our request, the specimens from Roccella Ionica and to the fishers and staff of the gross fish markets of Ancona and San Benedetto del Tronto for the Adriatic specimens. We also thank Giuseppe Scarcella and all the participants in the SoleMon surveys for the specimens collected, and the Italian “Ministero dell’agricoltura, della sovranità alimentare e delle foreste” for the access to the data of the SoleMon surveys collected in the frame of the Italian Fishery Data Collection Programme (Programma Nazionale Raccolta Dati Alieutici). M.S. partecipated in the research leading to these results while enrolled in the Ph.D. Program “Innovative Technologies and Sustainable Use of Mediterranean Sea Fishery and Biological Resources—FishMed”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carlton, J.T. Transoceanic and interoceanic dispersal of coastal marine organisms: The biology of ballast water. Oceanogr. Mar. Biol. Ann. Rev. 1985, 23, 313–374. [Google Scholar]
- Galil, B.S.; Marchini, A.; Occhipinti Ambrogi, A.; Ojaveer, H. The enlargement of the Suez Canal—Erythraean introductions and management challenges. Manag. Biol. Invasions 2017, 8, 141–152. [Google Scholar] [CrossRef]
- Por, F.D. Tethys returns to the Mediterranean: Success and limits of tropical re-colonization. BioRisk 2009, 3, 5–19. [Google Scholar] [CrossRef]
- Galil, B.S. Taking stock: Inventory of alien species in the Mediterranean Sea. Biol. Invasions 2009, 11, 359–372. [Google Scholar] [CrossRef]
- Tsiamis, K.; Zenetos, A.; Deriu, I.; Gervasini, E.; Cardoso, A.C. The native distribution range of the European marine non-indigenous species. Aquat. Invasions 2018, 13, 187–198. [Google Scholar] [CrossRef]
- FAO. Global Capture Production. Fisheries and Aquaculture. Available online: https://www.fao.org/fishery/en/collection/capture?lang=en (accessed on 27 February 2023).
- Deval, M.C.; Kaya, Y.; Guven, O.; Gökoglu, M.; Froglia, C. An unexpected find of the western Atlantic shrimp, Farfantepenaeus aztecus (Ives, 1891) (Decapoda, Penaeidae) in Antalya Bay, eastern Mediterranean Sea. Crustaceana 2010, 83, 1531–1537. [Google Scholar] [CrossRef]
- Gökoglu, M.; Ozvarol, Y. Biogeographic expansion of Farfantepenaeus aztecus (Ives, 1891) (Decapoda: Penaeidae) in the Eastern Mediterranean Sea. Bilecenoglu, M.; Alfaya, J.; Azzurro, E.; Baldacconi, R.; Boyaci, Y.; Circosta, V.; Compagno, L.; Coppola, F.; Deidun, A.; Durgham, H.; et al. New Mediterranean Marine biodiversity records (December, 2013). Mediterr. Mar. Sci. 2013, 14, 475–476. [Google Scholar] [CrossRef]
- Nikolopoulou, I.; Baxevanis, A.D.; Kampouris, T.E.; Abatzopoulos, T.J. Farfantepenaeus aztecus (Ives, 1891) (Crustacea: Decapoda: Penaeidae) in N Aegean: First record in Greece by morphological and genetic features. J. Biol. Res. Thessaloniki 2013, 20, 367–375. [Google Scholar]
- Marković, O.; Gökoglu, M.; Petović, S.; Mandić, M. First record of the Northern brown shrimp, Farfantepenaeus aztecus (Ives, 1891) (Crustacea: Decapoda: Penaeidae) in the South Adriatic Sea, Montenegro. Mediterr. Mar. Sci. 2014, 15, 165–167. [Google Scholar] [CrossRef]
- Galil, B.S.; Innocenti, G.; Douek, J.; Paz, G.; Rinkevich, B. Foul play? On the rapid spread of the brown shrimp Penaeus aztecus Ives, 1891 (Crustacea, Decapoda, Penaeidae) in the Mediterranean, with new records from the Gulf of Lion and the southern Levant. Mar. Biodivers. 2017, 47, 979–985. [Google Scholar] [CrossRef]
- Zava, B.; Insacco, G.; Galil, B.S. The first record of the brown shrimp Penaeus aztecus Ives, 1891 in the central Adriatic coast of Italy. BioInvasions Rec. 2018, 7, 293–296. [Google Scholar] [CrossRef]
- Ugarković, P.; Crocetta, F. The brown shrimp Penaeus aztecus Ives, 1891 (Crustacea: Decapoda: Penaeidae) spreading northern in the Adriatic Sea: A first record from Croatia. BioInvasions Rec. 2021, 10, 636–643. [Google Scholar] [CrossRef]
- Khvorov, S.A.; Boltachev, A.R.; Reshetnikov, S.I.; Pashkov, A.N. First record of the green tiger prawn Penaeus semisulcatus (Penaeidae, Decapoda) in the Black Sea. Ekologija Morija 2006, 72, 65–69, (In Russian, with English Abstract and Figures Legend). [Google Scholar]
- Guchmanidze, A.; Slatkevich, S.V.; Boltachev, A.R. The first record of prawn Penaeus semisulcatus De Haan, 1844 (Decapoda, Penaeidae) near the coast of Georgia. Russ. J. Biol. Invasions 2017, 8, 14–17. [Google Scholar] [CrossRef]
- Arnesano, M.; Gaudio, P.; Zupa, W.; Casciaro, L.; Carbonara, P. Presence of Penaeus semisulcatus (Decapoda Peneidae) in the North-Western Ionian Sea (Central Mediterranean). Biol. Mar. Mediterr. 2015, 22, 157–159. [Google Scholar]
- SoleMon. Rapido Trawl Surveys in the Northern Adriatic Sea. In SoleMon Handbook Version 4; 2019; 49p, Available online: https://dcf-italia.cnr.it/rest/uploads/SOLEMON-Handbook_2019_Ver_4 (accessed on 10 January 2023).
- Pérez Farfante, I.; Kensley, B. Penaeoid and Sergestoid Shrimps and Prawns of the World. Key and Diagnoses for the Families and Genera. Mém. Mus. Natl. Hist. Nat. (A) Zool 1997, 175, 1–233. [Google Scholar]
- Ma, K.Y.; Chan, T.Y.; Chu, K.H. Refuting the six-genus classification of Penaeus s.l. (Dendrobranchiata, Penaeidae): A combined analysis of mitochondrial and nuclear genes. Zool. Scr. 2011, 40, 498–508. [Google Scholar] [CrossRef]
- Yang, C.H.; Ma, K.Y.; Chu, K.H.; Chan, T.Y. Making sense of the taxonomy of the most commercially important shrimps Penaeus Fabricius, 1798 s.l. (Crustacea: Decapoda: Penaeidae), a way forward. Aquaculture 2023, 563, 738955. [Google Scholar] [CrossRef]
- Chan, T.Y. New subgeneric names for the most commercially important shrimp genus Penaeus Fabricius, 1798 (Crustacea, Decapoda, Penaeidae). ZooKeys 2023, 1141, 29–40. [Google Scholar] [CrossRef]
- Pérez Farfante, I. Western Atlantic Shrimps of the Genus Penaeus. Fish. Bull. 1969, 67, i–x + 461–591. [Google Scholar]
- Gönülal, O.; Türetken, P.S.C. One of the most invasive alien species, Penaeus aztecus Ives, 1891 reached the Black Sea coasts. BioInvasions Rec. 2019, 8, 871–875. [Google Scholar] [CrossRef]
- Spiridonov, V.A.; Zalota, A.K. Understanding and forecasting dispersal of non-indigenous marine decapods (Crustacea: Decapoda) in East European and North Asian waters. J. Mar. Biol. Assoc. UK 2017, 97, 591–611. [Google Scholar] [CrossRef]
- Anosov, S.E.; Ignatyev, S.M. History of the studying of fauna of Ponticus decapods. Morskoi Biol. Zhurnal 2016, 1, 93–101, (In Russian, with English Abstract). [Google Scholar] [CrossRef]
- Shalovenkov, N. Distribution of Alien Zoobenthic Species on the Black Sea Shelf. Russ. J. Biol. Invasions 2022, 13, 123–139. [Google Scholar] [CrossRef]
- Donnaloia, M.; Casciaro, L.; Zupa, W.; Arnesano, M.; Gaudio, P.; Carbonara, P. New records of the alien species Penaeus aztecus (Decapoda: Penaeidae) in Ionian and South Adriatic seas. Biol. Mar. Mediterr. 2019, 26, 344–345. [Google Scholar]
- Renda, W.; Crocetta, F. The Northern brown shrimp Penaeus aztecus Ives, 1891 invades the Italian Ionian Sea. Mytilineou, C.; Akel, E.; Babali, N.; Balistreri, P.; Bariche, M.; Boyaci, Y.; Cilenti, L.; Constantinou, C.; Crocetta, F.; Çelik, M.; et al. New Mediterranean Biodiversity Records (November, 2016). Mediterr. Mar. Sci. 2016, 17, 800–801. [Google Scholar]
- ICES. Report of the Working Group on Introductions and Transfers of Marine Organisms (WGITMO), 16–18 March 2016, Olbia, Italy. ICES CM 2016/SSGEPI:10. 201pp. [CrossRef]
- ICES. Interim Report of the Working Group on Introductions and Transfers of Marine Organisms (WGITMO), 13–15 March 2017, Woods Hole, MA, USA. ICES CM 2017/SSGEPI:09. 139pp. [CrossRef]
- Scannella, D.; Falsone, F.; Geraci, M.L.; Froglia, C.; Fiorentino, F.; Giusto, G.B.; Zava, B.; Insacco, G.; Colloca, F. First report of Northern brown shrimp Penaeus aztecus Ives, 1891 in Strait of Sicily. BioInvasions Rec. 2017, 6, 67–77. [Google Scholar] [CrossRef]
- Cruscanti, M.; Innocenti, G.; Alvarado Bremer, J.R.; Galil, B.S. First report of the brown shrimp Penaeus aztecus Ives, 1891 (Crustacea, Decapoda, Penaeidae) in the Tyrrhenian Sea. Mar. Biodivers. Rec. 2015, 8, e81. [Google Scholar] [CrossRef]
- Sadek, S.; Abou El-Soud, W.; Galil, B.S. The brown shrimp Penaeus aztecus Ives, 1891 (Crustacea, Decapoda, Penaeidae) in the Nile Delta, Egypt: An exploitable resource for fishery and mariculture? BioInvasions Rec. 2018, 7, 51–54. [Google Scholar] [CrossRef]
- Ben Jarray, F.; Marouani, S.; Karaa, S.; Hentati, S.; Jarboui, O. First record of the brown shrimp Penaeus aztecus (Decapoda: Penaeidae) in the Gulf of Gabes (Central Mediterranean Sea, Tunisia). Cah. Biol. Mar. 2019, 60, 425–430. [Google Scholar] [CrossRef]
- Kapiris, K.; Apostolidis, C. Farfantepenaeus aztecus: A new alien decapod in the Ionian Sea. Kapiris, K.; Apostolidis, C.; Baldacconi, R.; Başusta, N.; Bilecenoglu, M.; Bitar, G.; Bobori, D.; Boyaci, Y.; Dimitriadis, C.; Djurović, M.; et al. New Mediterranean marine biodiversity records (April, 2014). Mediterr. Mar. Sci. 2014, 15, 209. [Google Scholar] [CrossRef]
- Civitarese, G.; Gačić, M.; Lipizer, M.; Eusebi Borzelli, G.L. On the impact of the Bimodal Oscillating System (BiOS) on the biogeochemistry and biology of the Adriatic and Ionian Seas (Eastern Mediterranean). Biogeosciences 2010, 7, 3987–3997. [Google Scholar] [CrossRef]
- Kevrekidis, K. The occurrence of the Atlantic penaeid prawn Farfantepenaeus aztecus (Ives, 1891) in the Thermaikos Gulf (Aegean Sea, eastern Mediterranean): Considerations on the potential establishment and impact on the autochthonous Melicertus kerathurus (Forskal, 1775). Crustaceana 2014, 87, 1606–1619. [Google Scholar] [CrossRef]
- Minos, G.; Kokokiris, L.; Imsiridou, A.; Karachle, P.K.; Kapiris, K. Notes on the distribution and biology of northern brown shrimp Farfantepenaeus aztecus (Ives, 1891) in the eastern Mediterranean. Turk. J. Zool. 2015, 39, 467–473. [Google Scholar] [CrossRef]
- Kondylatos, G.; Corsini-Foka, M. First record of Penaeus aztecus Ives, 1891 (Crustacea, Decapoda) and Melibe viridis (Kelaart, 1858) (Gastropoda, Nudibranchia) in the South-Eastern Aegean Sea (Greece). Zenetos, A.; Akel, E.; Apostolidis, C.; Bilecenoglu, M.; Bitar, G.; Buchet, V.; Chalari, N.; Corsini-Foka, M.; Crocetta, F.; Dogrammatzi, A.; et al. New Mediterranean Biodiversity Records (April 2015). Mediterr. Mar. Sci. 2015, 16, 278–279. [Google Scholar] [CrossRef]
- Zenetos, A.; Giavasi, M. Penaeus aztecus establishing in the Greek Ionian Sea. Crocetta, F.; Agius, D.; Balistreri, P.; Bariche, M.; Bayhan, Y.; Çakir, M.; Ciriaco, S.; Corsini-Foka, M.; Deidun, A.; EL Zrelli, R.; et al. New Mediterranean Biodiversity Records (October 2015). Mediterr. Mar. Sci. 2015, 16, 691. [Google Scholar] [CrossRef]
- Bakir, K.; Aydin, I. New localities in the Aegean Sea for alien shrimps Penaeus aztecus (Ives, 1891) and Metapenaeus affinis (H. Milne Edwards, 1837). Acta Adriat. 2016, 57, 273–280. [Google Scholar]
- Kapiris, K.; Minos, G. Weight-length relationship of the northern brown shrimp Penaeus aztecus Ives, 1891 (Decapoda: Penaeidae) from the Central Aegean Sea, Greece. Kapiris, K., Anastasopoulou, A., Kousteni, V., Lampri, P.-N., Minos, G., Mytilineou, C.; Oikonomidis, G. New Fisheries-related data from the Mediterranean Sea (December 2017). Mediterr. Mar. Sci. 2017, 18, 557–563. [Google Scholar]
- Kampouris, T.E.; Giovos, I.; Doumpas, N.; Sterioti, A.; Batjakas, I.E. First record of Penaeus pulchricaudatus (Stebbing, 1914) and the establishment of P. aztecus (Ives, 1891) and P. hathor (Burkenroad, 1959) in Cretan waters, Greece. J. Black Sea/Mediterr. Environ. 2018, 24, 199–211. [Google Scholar]
- Kampouris, T.E.; Tiralongo, F.; Golemaj, A.; Giovos, I.; Doumpas, N.; Batjakas, I.E. Penaeus aztecus Ives, 1891 (Decapoda, Dendrobranchiata, Penaeidae): On the range expansion in Sicilian waters and on the first record from Albanian coast. Int. J. Fish. Aquat. Stud. 2018, 6, 468–472. [Google Scholar]
- Pipitone, C.; Lombardo, A. The brown shrimp, Penaeus aztecus (Decapoda, Penaeidae) in southeastern Sicily: Further expansion of a non-indigenous species with a potential as a fishery resource. Stern, N., Badreddine, A., Bitar, G., Crocetta, F., Deidun, A., Dragičević, B., Dulčić, J., Durgham, H., Galil, B.S., Galiya, M.Y.; et al. New Mediterranean Biodiversity Records (July 2019). Mediterr. Mar. Sci. 2019, 20, 416. [Google Scholar] [CrossRef]
- Ligas, A.; De Carlo, F.; Massaro, A.; Musumeci, C.; Rossetti, I.; Sartini, M.; Sbrana, M.; Viva, C.; Sartor, P. L’espansione della mazzancolla americana, Penaeus aztecus Ives, 1891, nel mar Tirreno settentrionale e prima segnalazione della specie in mar Ligure. Biol. Mar. Mediterr. 2019, 26, 352–353. [Google Scholar]
- Mulas, A.; Bellodi, A.; Cau, A.; Cannas, R.; Marongiu, M.F.; Pesci, P.; Porcu, C.; Follesa, M.C. First records of Penaeus aztecus Ives, 1891 (Decapoda Penaeidae) in Sardinian waters (Central-Western Mediterranean). Biol. Mar. Mediterr. 2019, 26, 360–361. [Google Scholar]
- El-Deeb, R.S.; Sarhan, M.; Khafage, A.R.; Abdel Razek, F.A.; Abdel-Wahab, M.; Omar, H.A. Occurrence of Penaeus aztecus, Ives, 1891 (Crustacea: Decapoda: Penaeidae) in the coastal water of Alexandria, Egypt. Egypt. J. Aquat. Res. 2020, 46, 303–309. [Google Scholar] [CrossRef]
- Abdulrraziq, A.A.; Abdulghani, A.; Ibrahim, S.M.; Zava, B.; Deidun, A. First record of the northern brown shrimp Penaeus aztecus Ives, 1891 (Crustacea, Decapoda, Penaeidae) from Libyan waters. BioInvasions Rec. 2021, 10, 287–294. [Google Scholar] [CrossRef]
- Ben Abdallah Ben Hady Hamida, O.; Ben Hadj Hamida, N.; Chouikh, A.S.; Abidi, D.; Missaoui, H. Sur l’expansion de la crevette royale grise Penaeus aztecus (Ives, 1904) en Mediterranee et sa premiere observation au nord de la Tunisie. Bull. Inst. Natn. Scien. Technol. Mer de Salammbô. 2020, 47, 29–36. [Google Scholar]
- Froglia, C.; Scarcella, G.; Lucchetti, A. On the recent increase of Penaeus (Melicertus) kerathurus stock in northern and central Adriatic Sea: Possible explanations. Rapp. Com. Int. Mer Medit. 2013, 40, 780. [Google Scholar]
- Gonzalez-Ortegon, E.; Garcia-Raso, J.E.; Calado, R.; Lopez de la Rosa, I.; Guerrero, M.; Cuesta, J.A. Atlantic expansion of the African caridean shrimp Lysmata uncicornis Holthuis & Maurin, 1952 (Caridea: Lysmatidae). Mar. Biodivers. 2020, 50, 26. [Google Scholar] [CrossRef]
- Costa, H.; Foody, G.M.; Jimenez, S.; Silva, L. Impacts of Species Misidentification on Species Distribution Modeling with Presence-Only data. ISPRS Int. J. Geo-Inf. 2015, 4, 2496–2518. [Google Scholar] [CrossRef]
- Galil, B.; Froglia, C.; Noël, P. CIESM Atlas of Exotic Species in the Mediterranean. Vol. 2 Crustaceans: Decapods and Stomatopods; CIESM Publishers: Monaco, Italy, 2002; 192p. [Google Scholar]
- AquaNIS. Informatics System on Aquatic Non-Indigenous and Cryptogenic Species. Available online: http://www.corpi.ku.lt/databases/aquanis/ (accessed on 15 February 2023).
- Gruvel, A. Les États de Syrie. Richesses Marines et Fluviales, Exploitation Actuelle—Avenir; Société d’Editions Géographiques, Maritimes et Coloniales: Paris, France, 1931; 453p, 28 pls, 1 map. [Google Scholar]
- Can, M.F.; Mazlum, Y.; Demirci, A. The catch composition and catch per unit of swept area (CPUE) of penaeid shrimps in the bottom trawls from Iskenderun Bay, Turkey. Turk. J. Fish. Aquat. Sci. 2004, 4, 87–91. [Google Scholar]
- Çinar, M.E.; Bilecenoğlu, M.; Yokes, B.; Öztürk, B.; Taşkin, E.; Bakir, K.; Doğan, A.; Açik, S. Current status (as of end of 2020) of marine alien species in Turkey. PLoS ONE 2021, 16, e0251086. [Google Scholar] [CrossRef] [PubMed]
- Zenetos, A.; Karachle, P.K.; Corsini-Foka, M.; Gerovasileiou, V.; Simboura, N.; Xentidis, N.; Tsiamis, K. Is the trend in new introductions of marine non-indigenous species a reliable criterion for assessing good environmental status? The case study of Greece. Mediterr. Mar. Sci. 2020, 21, 775–793. [Google Scholar] [CrossRef]
- Wadie, W.F.; Abdel Razek, F.A. The effect of damming on the shrimp population in the South-eastern part of the Mediterranean Sea. Fish. Res. 1985, 3, 323–335. [Google Scholar] [CrossRef]
- Lorenti, M.; Gambi, M.C.; Guglielmo, R.; Patti, F.P.; Scipione, M.B.; Zupo, V.; Buia, M.C. Soft-bottom macrofaunal assemblages in the Gulf of Salerno, Tyrrhenian Sea, Italy, an area affected by the invasion of the seaweed Caulerpa racemosa var. cylindracea. Mar. Ecol. 2011, 32, 392–409. [Google Scholar] [CrossRef]
- Feidi, I. Will the new large-scale aquaculture projects make Egypt self sufficient in fish supplies? Mediterr. Fish. Aquac. Res. 2018, 1, 31–41. [Google Scholar]
- Russo, A.; Carniel, S.; Sclavo, M.; Krzelj, M. Climatology of the Northern-Central Adriatic Sea. In Modern Climatology; Wang, S.Y., Gillies, R.R., Eds.; InTech: London, UK, 2012; pp. 177–212. [Google Scholar]
- Sheridan, P.F.; Patella, F.J., Jr.; Baxter, N.; Emiliani, D.A. Movements of brown shrimp, Penaeus aztecus, and pink shrimp, P. duorarum, relative to the U.S.-Mexico border in the western Gulf of Mexico. Mar. Fish. Rev. 1987, 49, 14–19. [Google Scholar]
- Schubart, C.D.; Deli, T.; Mancinelli, G.; Cilenti, L.; Fernandez, A.G.; Falco, S.; Berger, S. Phylogeography of the Atlantic Blue Crab Callinectes sapidus (Brachyura: Portunidae) in the Americas versus the Mediterranean Sea: Determining Origins and Genetic Connectivity of a Large-Scale Invasion. Biology 2023, 12, 35. [Google Scholar] [CrossRef]
- Servello, G.; Andaloro, F.; Azzurro, E.; Castriota, L.; Catra, M.; Chiarore, A.; Crocetta, F.; D’Alessandro, M.; Denitto, F.; Froglia, C.; et al. Marine alien species in Italy: A contribution to the implementation of descriptor D2 of the Marine Strategy Framework Directive. Mediterr. Mar. Sci. 2019, 20, 1–48. [Google Scholar] [CrossRef]
- Galil, B.; Mienis, H.; Hoffman, R.; Goren, M. Non-indigenous species along the Israeli Mediterranean coast: Tally, policy, outlook. Hydrobiologia 2020, 848, 2011–2029. [Google Scholar] [CrossRef]
- Tournier, H. Conditions d’acclimatation des crevettes Penaeus kerathurus et P. japonicus dans les eaux du littoral Languedocien. Sci. Et Pêche 1972, 213, 1–13. [Google Scholar]
- Lumare, F. Diretrices seguidas en Italia para la cria de langostinos peneidos. Inf. Tecn. Inst. Inv. Pesq. 1983, 109, 1–16. [Google Scholar]
- Lumare, F. Restocking by Penaeus japonicus: A trend to the economic management of the Italian lagoons. Oebalia 1989, 14, 69–85. [Google Scholar]
- Lumare, F.; Casolino, G. First record of Penaeus japonicus Bate 1888 (Decapoda Natantia) along Italian coast. Oebalia 1986, 13, 179–183. [Google Scholar]
- Sanna, A. 2011 L’allevamento dei gamberi in Italia e nel Mediterraneo settentrionale. In Atti del Convegno “La Risorsa Crostacei nel Mediterraneo: Ricerca, Produzione e Mercato”; Veneto Agricoltura: Legnaro, Italy, 2011; pp. 49–59. Available online: https://www.venetoagricoltura.org/upload/pubblicazioni/Atti%20Convegno%20Crostacei/8%20Sanna.pdf (accessed on 10 January 2023).
- Özcan, T.; Galil, B.S.; Bakir, K.; Katağan, T. The first record of the banana prawn Fenneropenaeus merguiensis (De Man, 1888) (Crustacea: Decapoda: Penaeidae) from the Mediterranean Sea. Aquat. Invasions 2006, 1, 286–288. [Google Scholar] [CrossRef]
- Stentiford, G.D.; Lightner, D.V. Cases of white spot disease (WSD) in European shrimp farms. Aquaculture 2011, 319, 302–306. [Google Scholar] [CrossRef]
- AQUACOP. Maturation and Spawning in Captivity of Penaeid Shrimp: Penaeus merguiensis de Man Penaeus japonicus Bate Penaeus aztecus Ives Metapenaeus ensis de Hann Penaeus semisulcatus de Haan. J. World Aquac. Soc. 1975, 6, 123–132. [Google Scholar] [CrossRef]
- Della Patrona, L.; Brun, P. Elevage de la Crevette Bleue en Nouvelle-Calédonie. Litopenaeus Stylirostris. Bases Biologiques et Zootechnie; Ifremer: Paris, France, 2009; pp. iv + 1–312. Available online: https://archimer.ifremer.fr/doc/00251/36229/34789.pdf (accessed on 10 January 2023).
- Soors, J.; Breine, A.; D’Udekem d’Acoz, C.; Van der Bergh, E.; Van der Meutter, F.; Terrie, T. Penaeus aztecus Ives, 1891 (Crustacea, Decapoda), in the Scheldt estuary (Belgium): Isolated record or forerunner of a penaeid invasion? J. Exp. Mar. Biol. Ecol. 2020, 530–531, 151437. [Google Scholar] [CrossRef]
- EC. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 establishing a framework for community action in the field of marine environmental policy (Marine Strategy Framework Directive). OJEU 2008, L164, 19–40. [Google Scholar]
- EC. European Commission Decision (EU) 2017/848 of 17 May 2017 laying down criteria and methodological standards on good environmental status of marine waters and specifications and standardized methods for monitoring and assessment, and repealing Decision 2010/477/EU. OJEU 2017, L125, 43–74. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).