Pre-Learning Stress That Is Temporally Removed from Acquisition Impairs Fear Learning
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Procedures
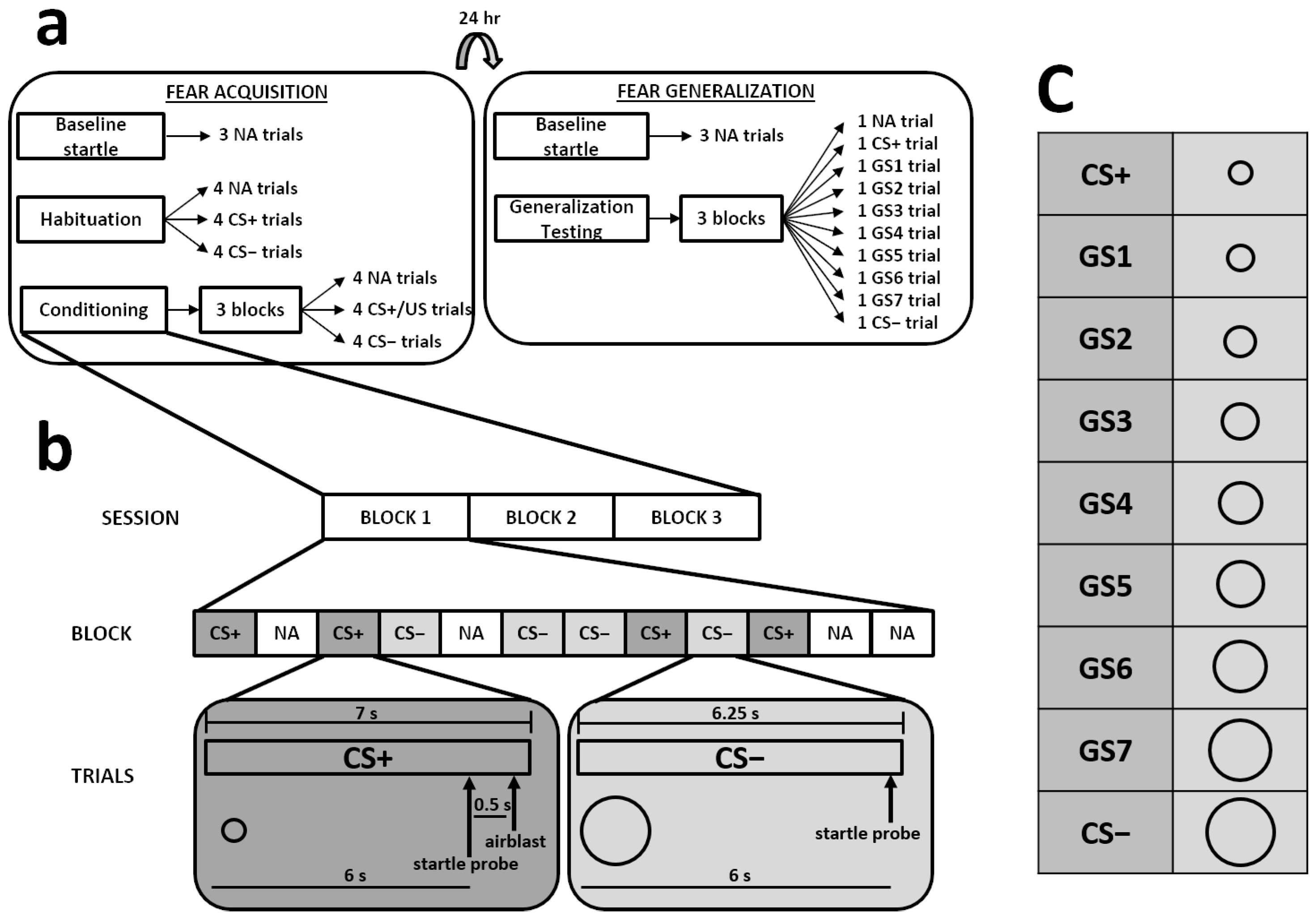
2.2.1. Socially Evaluated Cold Pressor Test (SECPT)
2.2.2. Subjective and Objective Stress Response Measures
2.2.3. Differential Fear Conditioning Paradigm
2.3. Statistical Analyses
| Title 1 | Minute 1 | Minute 2 | Minute 3 |
|---|---|---|---|
| Painfulness (scale of 0–10) | |||
| Responders | |||
| Males | 6.27 (0.41) | 5.62 (0.46) | 5.46 (0.50) |
| Females | 5.79 (0.40) | 6.21 (0.45) | 6.50 (0.48) |
| Non-Responders | |||
| Males | 6.67 (0.30) | 6.71 (0.34) | 6.83 (0.37) |
| Females | 6.48 (0.23) | 6.88 (0.26) | 7.10 (0.29) |
| No Stress | |||
| Males | 0.21 (0.22) | 0.11 (0.25) | 0.16 (0.27) |
| Females | 0.16 (0.16) | 0.11 (0.18) | 0.13 (0.20) |
| Stressfulness (scale of 0–10) | |||
| Responders | |||
| Males | 6.15 (0.52) | 5.15 (0.57) | 5.08 (0.60) |
| Females | 4.79 (0.50) | 5.43 (0.55) | 5.50 (0.58) |
| Non-Responders | |||
| Males | 6.25 (0.38) | 6.33 (0.42) | 6.04 (0.44) |
| Females | 5.98 (0.29) | 6.12 (0.31) | 6.19 (0.33) |
| No Stress | |||
| Males | 0.34 (0.28) | 0.32 (0.31) | 0.30 (0.33) |
| Females | 0.42 (0.20) | 0.41 (0.22) | 0.39 (0.24) |
3. Results
3.1. Subjective and Objective Stress Response Measures
3.1.1. Subjective Pain and Stress Ratings
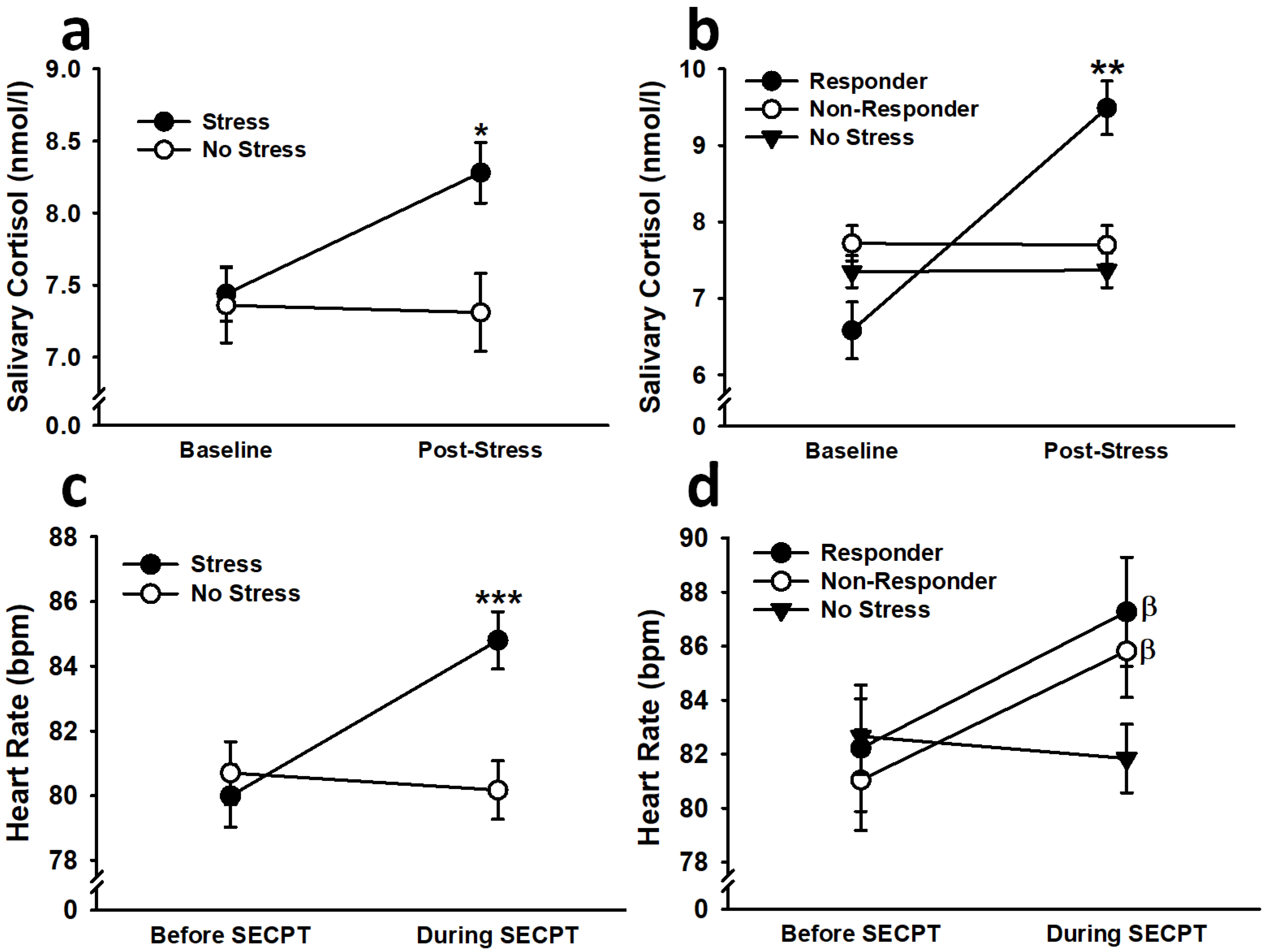
3.1.2. Cortisol
3.1.3. Heart Rate
3.2. Fear Acquisition
3.2.1. Fear-Potentiated Startle
3.2.2. Skin Conductance Response
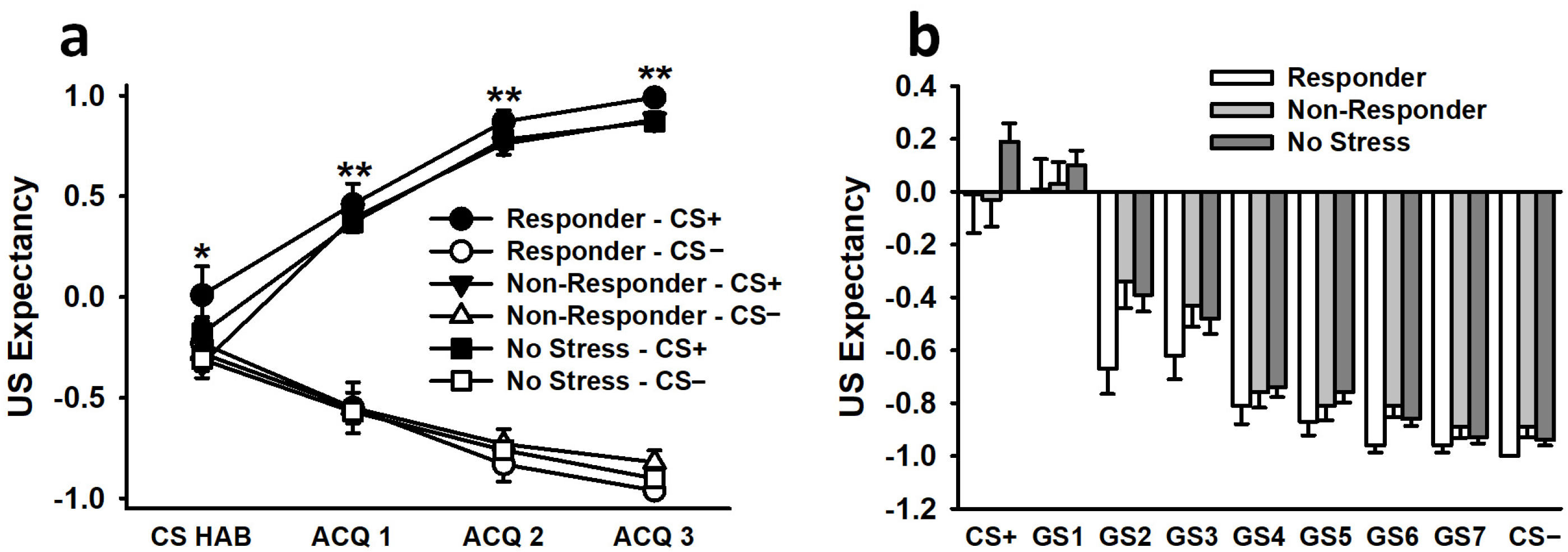
3.2.3. US Expectancy Ratings
3.3. Fear Generalization
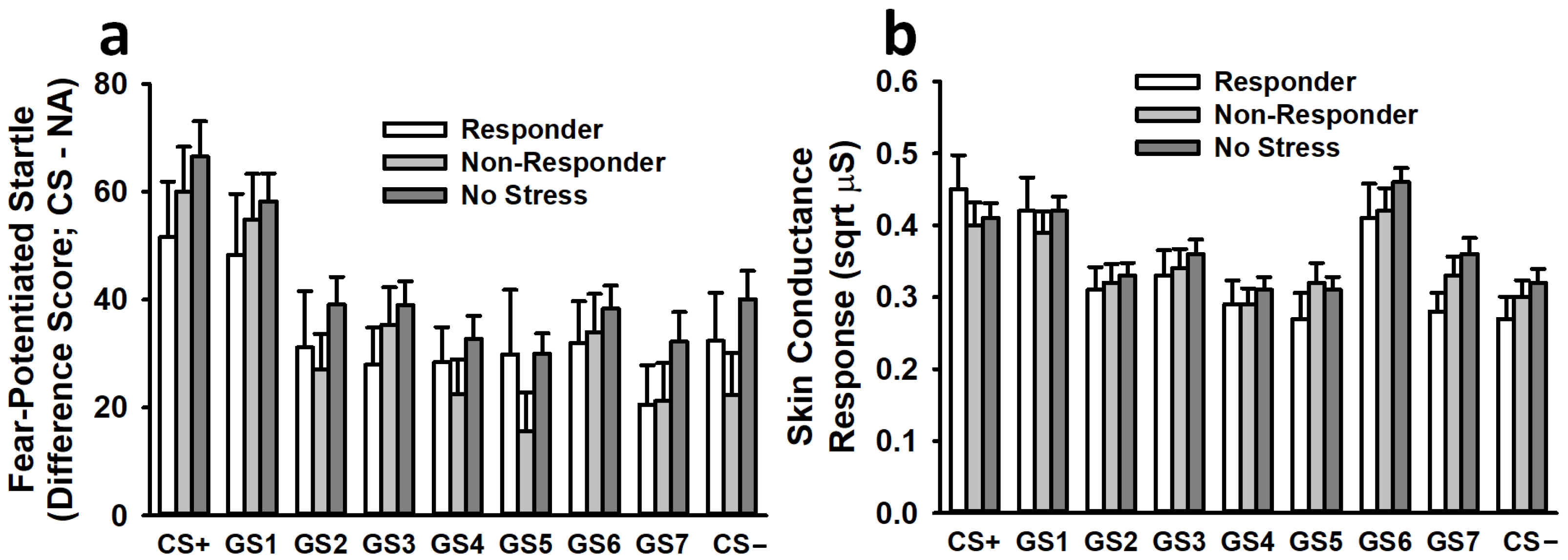
3.3.1. Fear-Potentiated Startle
3.3.2. Skin Conductance Response
3.3.3. US Expectancy Ratings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diamond, D.M.; Campbell, A.M.; Park, C.R.; Halonen, J.; Zoladz, P.R. The temporal dynamics model of emotional memory processing: A synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural Plast. 2007, 2007, 60803. [Google Scholar] [CrossRef]
- Schwabe, L.; Joels, M.; Roozendaal, B.; Wolf, O.T.; Oitzl, M.S. Stress effects on memory: An update and integration. Neurosci. Biobehav. Rev. 2012, 36, 1740–1749. [Google Scholar] [CrossRef]
- Schwabe, L.; Hermans, E.J.; Joels, M.; Roozendaal, B. Mechanisms of memory under stress. Neuron 2022, 110, 1450–1467. [Google Scholar] [CrossRef]
- Shields, G.S.; Sazma, M.A.; McCullough, A.M.; Yonelinas, A.P. The effects of acute stress on episodic memory: A meta-analysis and integrative review. Psychol. Bull. 2017, 143, 636–675. [Google Scholar] [CrossRef]
- Cadle, C.E.; Zoladz, P.R. Stress time-dependently influences the acquisition and retrieval of unrelated information by producing a memory of its own. Front. Psychol. 2015, 6, 910. [Google Scholar] [CrossRef]
- Quaedflieg, C.W.; Schwabe, L.; Meyer, T.; Smeets, T. Time dependent effects of stress prior to encoding on event-related potentials and 24h delayed retrieval. Psychoneuroendocrinology 2013, 38, 3057–3069. [Google Scholar] [CrossRef]
- Vogel, S.; Schwabe, L. Stress in the zoo: Tracking the impact of stress on memory formation over time. Psychoneuroendocrinology 2016, 71, 64–72. [Google Scholar] [CrossRef]
- Zoladz, P.R.; Dailey, A.M.; Nagle, H.E.; Fiely, M.K.; Mosley, B.E.; Brown, C.M.; Duffy, T.J.; Scharf, A.R.; Earley, M.B.; Rorabaugh, B.R. FKBP5 polymorphisms influence pre-learning stress-induced alterations of learning and memory. Eur. J. Neurosci. 2017, 45, 648–659. [Google Scholar] [CrossRef]
- Zoladz, P.R.; Kalchik, A.E.; Hoffman, M.M.; Aufdenkampe, R.L.; Lyle, S.M.; Peters, D.M.; Brown, C.M.; Cadle, C.E.; Scharf, A.R.; Dailey, A.M.; et al. ADRA2B deletion variant selectively predicts stress-induced enhancement of long-term memory in females. Psychoneuroendocrinology 2014, 48, 111–122. [Google Scholar] [CrossRef]
- Zoladz, P.R.; Clark, B.; Warnecke, A.; Smith, L.; Tabar, J.; Talbot, J.N. Pre-learning stress differentially affects long-term memory for emotional words, depending on temporal proximity to the learning experience. Physiol. Behav. 2011, 103, 467–476. [Google Scholar] [CrossRef]
- Zoladz, P.R.; Duffy, T.J.; Mosley, B.E.; Fiely, M.K.; Nagle, H.E.; Scharf, A.R.; Brown, C.M.; Earley, M.B.; Rorabaugh, B.R.; Dailey, A.M. Interactive influence of sex, stressor timing, and the BclI glucocorticoid receptor polymorphism on stress-induced alterations of long-term memory. Brain Cogn. 2019, 133, 72–83. [Google Scholar] [CrossRef]
- Riggenbach, M.R.; Weiser, J.N.; Mosley, B.E.; Hipskind, J.J.; Wireman, L.E.; Hess, K.L.; Duffy, T.J.; Handel, J.K.; Kaschalk, M.G.; Reneau, K.E.; et al. Immediate pre-learning stress enhances baseline startle response and fear acquisition in a fear-potentiated startle paradigm. Behav. Brain Res. 2019, 371, 111980. [Google Scholar] [CrossRef]
- Zoladz, P.R.; Warnecke, A.J.; Woelke, S.A.; Burke, H.M.; Frigo, R.M.; Pisansky, J.M.; Lyle, S.M.; Talbot, J.N. Pre-learning stress that is temporally removed from acquisition exerts sex-specific effects on long-term memory. Neurobiol. Learn. Mem. 2013, 100, 77–87. [Google Scholar] [CrossRef]
- Dolfen, N.; King, B.R.; Schwabe, L.; Swinnen, S.; Albouy, G. Glucocorticoid response to stress induction prior to learning is negatively related to subsequent motor memory consolidation. Neurobiol. Learn. Mem. 2019, 158, 32–41. [Google Scholar] [CrossRef]
- Akirav, I.; Richter-Levin, G. Mechanisms of amygdala modulation of hippocampal plasticity. J. Neurosci. 2002, 22, 9912–9921. [Google Scholar] [CrossRef]
- Akirav, I.; Richter-Levin, G. Biphasic modulation of hippocampal plasticity by behavioral stress and basolateral amygdala stimulation in the rat. J. Neurosci. 1999, 19, 10530–10535. [Google Scholar] [CrossRef]
- Joels, M.; Fernandez, G.; Roozendaal, B. Stress and emotional memory: A matter of timing. Trends Cogn. Sci. 2011, 15, 280–288. [Google Scholar] [CrossRef]
- Zoladz, P.R.; Peters, D.M.; Kalchik, A.E.; Hoffman, M.M.; Aufdenkampe, R.L.; Woelke, S.A.; Wolters, N.E.; Talbot, J.N. Brief, pre-learning stress reduces false memory production and enhances true memory selectively in females. Physiol. Behav. 2014, 128, 270–276. [Google Scholar] [CrossRef]
- Zoladz, P.R.; Dailey, A.M.; Nagle, H.E.; Fiely, M.K.; Mosley, B.E.; Brown, C.M.; Duffy, T.J.; Scharf, A.R.; Earley, M.B.; Rorabaugh, B.R. ADRA2B deletion variant influences time-dependent effects of pre-learning stress on long-term memory. Neurobiol. Learn. Mem. 2017, 140, 71–81. [Google Scholar] [CrossRef]
- Lovallo, W.R.; Robinson, J.L.; Glahn, D.C.; Fox, P.T. Acute effects of hydrocortisone on the human brain: An fMRI study. Psychoneuroendocrinology 2010, 35, 15–20. [Google Scholar] [CrossRef]
- Merz, C.J.; Wolf, O.T.; Schweckendiek, J.; Klucken, T.; Vaitl, D.; Stark, R. Stress differentially affects fear conditioning in men and women. Psychoneuroendocrinology 2013, 38, 2529–2541. [Google Scholar] [CrossRef] [PubMed]
- Antov, M.I.; Wolk, C.; Stockhorst, U. Differential impact of the first and second wave of a stress response on subsequent fear conditioning in healthy men. Biol. Psychol. 2013, 94, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Bentz, D.; Michael, T.; Wilhelm, F.H.; Hartmann, F.R.; Kunz, S.; von Rohr, I.R.; de Quervain, D.J. Influence of stress on fear memory processes in an aversive differential conditioning paradigm in humans. Psychoneuroendocrinology 2013, 38, 1186–1197. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.D.; Payne, J.D.; Nadel, L.; Jacobs, W.J. Stress differentially modulates fear conditioning in healthy men and women. Biol. Psychiatry 2006, 59, 516–522. [Google Scholar] [CrossRef]
- Zorawski, M.; Blanding, N.Q.; Kuhn, C.M.; LaBar, K.S. Effects of stress and sex on acquisition and consolidation of human fear conditioning. Learn. Mem. 2006, 13, 441–450. [Google Scholar] [CrossRef]
- Antov, M.I.; Stockhorst, U. Stress exposure prior to fear acquisition interacts with estradiol status to alter recall of fear extinction in humans. Psychoneuroendocrinology 2014, 49, 106–118. [Google Scholar] [CrossRef]
- Antov, M.I.; Melicherova, U.; Stockhorst, U. Cold pressor test improves fear extinction in healthy men. Psychoneuroendocrinology 2015, 54, 54–59. [Google Scholar] [CrossRef]
- Sep, M.S.C.; Gorter, R.; van Ast, V.A.; Joels, M.; Geuze, E. No time-dependent effects of psychosocial stress on fear contextualization and generalization: A randomized-controlled study with healthy participants. Chronic Stress 2019, 3, 2470547019896547. [Google Scholar] [CrossRef]
- Duits, P.; Cath, D.C.; Lissek, S.; Hox, J.J.; Hamm, A.O.; Engelhard, I.M.; van den Hout, M.A.; Baas, J.M. Updated meta-analysis of classical fear conditioning in the anxiety disorders. Depress. Anxiety 2015, 32, 239–253. [Google Scholar] [CrossRef]
- Lissek, S.; Powers, A.S.; McClure, E.B.; Phelps, E.A.; Woldehawariat, G.; Grillon, C.; Pine, D.S. Classical fear conditioning in the anxiety disorders: A meta-analysis. Behav. Res. Ther. 2005, 43, 1391–1424. [Google Scholar] [CrossRef]
- Lissek, S.; van Meurs, B. Learning models of PTSD: Theoretical accounts and psychobiological evidence. Int. J. Psychophysiol. 2015, 98, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Jasnow, A.M.; Lynch, J.F., 3rd; Gilman, T.L.; Riccio, D.C. Perspectives on fear generalization and its implications for emotional disorders. J. Neurosci. Res. 2017, 95, 821–835. [Google Scholar] [CrossRef] [PubMed]
- Kheirbek, M.A.; Klemenhagen, K.C.; Sahay, A.; Hen, R. Neurogenesis and generalization: A new approach to stratify and treat anxiety disorders. Nat. Neurosci. 2012, 15, 1613–1620. [Google Scholar] [CrossRef]
- Lissek, S. Toward an account of clinical anxiety predicated on basic, neurally mapped mechanisms of Pavlovian fear-learning: The case for conditioned overgeneralization. Depress. Anxiety 2012, 29, 257–263. [Google Scholar] [CrossRef]
- Lissek, S.; Rabin, S.; Heller, R.E.; Lukenbaugh, D.; Geraci, M.; Pine, D.S.; Grillon, C. Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. Am. J. Psychiatry 2010, 167, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Lissek, S.; Grillon, C. Learning models of PTSD. In The Oxford Handbook of Traumatic Stress Disorders; Beck, J.G., Sloan, D.M., Eds.; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Lissek, S.; Levenson, J.; Biggs, A.L.; Johnson, L.L.; Ameli, R.; Pine, D.S.; Grillon, C. Elevated fear conditioning to socially relevant unconditioned stimuli in social anxiety disorder. Am. J. Psychiatry 2008, 165, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Kaouane, N.; Porte, Y.; Vallee, M.; Brayda-Bruno, L.; Mons, N.; Calandreau, L.; Marighetto, A.; Piazza, P.V.; Desmedt, A. Glucocorticoids can induce PTSD-like memory impairments in mice. Science 2012, 335, 1510–1513. [Google Scholar] [CrossRef]
- Lemmens, A.; Beckers, T.; Dibbets, P.; Kang, S.; Smeets, T. Overgeneralization of fear, but not avoidance, following acute stress. Biol. Psychol. 2021, 164, 108151. [Google Scholar] [CrossRef]
- Dunsmoor, J.E.; Otto, A.R.; Phelps, E.A. Stress promotes generalization of older but not recent threat memories. Proc. Natl. Acad. Sci. USA 2017, 114, 9218–9223. [Google Scholar] [CrossRef]
- Kausche, F.M.; Zerbes, G.; Kampermann, L.; Muller, J.C.; Wiedemann, K.; Buchel, C.; Schwabe, L. Acute stress leaves fear generalization in healthy individuals intact. Cogn. Affect. Behav. Neurosci. 2021, 21, 372–389. [Google Scholar] [CrossRef]
- Besnard, A.; Sahay, A. Adult hippocampal neurogenesis, fear generalization, and stress. Neuropsychopharmacology 2016, 41, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Lange, I.; Goossens, L.; Michielse, S.; Bakker, J.; Lissek, S.; Papalini, S.; Verhagen, S.; Leibold, N.; Marcelis, M.; Wichers, M.; et al. Behavioral pattern separation and its link to the neural mechanisms of fear generalization. Soc. Cogn. Affect. Neurosci. 2017, 12, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Zoladz, P.; Reneau, K.; Weiser, J.; Cordes, C.; Virden, E.; Helwig, S.; Thebeault, C.; Pfister, C.; Getnet, B.; Boaz, K.; et al. Childhood maltreatment in females is associated with enhanced fear acquisition and an overgeneralization of fear. Brain Sci. 2022, 12, 1536. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, L.; Haddad, L.; Schachinger, H. HPA axis activation by a socially evaluated cold-pressor test. Psychoneuroendocrinology 2008, 33, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Zoladz, P.R.; Cadle, C.E.; Dailey, A.M.; Fiely, M.K.; Peters, D.M.; Nagle, H.E.; Mosley, B.E.; Scharf, A.R.; Brown, C.M.; Duffy, T.J.; et al. Blunted cortisol response to acute pre-learning stress prevents misinformation effect in a forced confabulation paradigm. Horm. Behav. 2017, 93, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gamwell, K.; Nylocks, M.; Cross, D.; Bradley, B.; Norrholm, S.D.; Jovanovic, T. Fear conditioned responses and PTSD symptoms in children: Sex differences in fear-related symptoms. Dev. Psychobiol. 2015, 57, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Glover, E.M.; Jovanovic, T.; Mercer, K.B.; Kerley, K.; Bradley, B.; Ressler, K.J.; Norrholm, S.D. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biol. Psychiatry 2012, 72, 19–24. [Google Scholar] [CrossRef]
- Maddox, S.A.; Kilaru, V.; Shin, J.; Jovanovic, T.; Almli, L.M.; Dias, B.G.; Norrholm, S.D.; Fani, N.; Michopoulos, V.; Ding, Z.; et al. Estrogen-dependent association of HDAC4 with fear in female mice and women with PTSD. Mol. Psychiatry 2018, 23, 658–665. [Google Scholar] [CrossRef]
- Michopoulos, V.; Norrholm, S.D.; Stevens, J.S.; Glover, E.M.; Rothbaum, B.O.; Gillespie, C.F.; Schwartz, A.C.; Ressler, K.J.; Jovanovic, T. Dexamethasone facilitates fear extinction and safety discrimination in PTSD: A placebo-controlled, double-blind study. Psychoneuroendocrinology 2017, 83, 65–71. [Google Scholar] [CrossRef]
- Norrholm, S.D.; Jovanovic, T.; Olin, I.W.; Sands, L.A.; Karapanou, I.; Bradley, B.; Ressler, K.J. Fear extinction in traumatized civilians with posttraumatic stress disorder: Relation to symptom severity. Biol. Psychiatry 2011, 69, 556–563. [Google Scholar] [CrossRef]
- Norrholm, S.D.; Glover, E.M.; Stevens, J.S.; Fani, N.; Galatzer-Levy, I.R.; Bradley, B.; Ressler, K.J.; Jovanovic, T. Fear load: The psychophysiological over-expression of fear as an intermediate phenotype associated with trauma reactions. Int. J. Psychophysiol. 2015, 98, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Fani, N.; King, T.Z.; Brewster, R.; Srivastava, A.; Stevens, J.S.; Glover, E.M.; Norrholm, S.D.; Bradley, B.; Ressler, K.J.; Jovanovic, T. Fear-potentiated startle during extinction is associated with white matter microstructure and functional connectivity. Cortex 2015, 64, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Norrholm, S.D.; Jovanovic, T.; Vervliet, B.; Myers, K.M.; Davis, M.; Rothbaum, B.O.; Duncan, E.J. Conditioned fear extinction and reinstatement in a human fear-potentiated startle paradigm. Learn. Mem. 2006, 13, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Struyf, D.; Zaman, J.; Hermans, D.; Vervliet, B. Gradients of fear: How perception influences fear generalization. Behav. Res. Ther. 2017, 93, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Fraunfelter, L.; Gerdes, A.B.M.; Alpers, G.W. Fear one, fear them all: A systematic review and meta-analysis of fear generalization in pathological anxiety. Neurosci. Biobehav. Rev. 2022, 139, 104707. [Google Scholar] [CrossRef]
- Grasser, L.R.; Saad, B.; Bazzi, C.; Suhaiban, H.A.; Mammo, D.F.; Izar, R.; Rass, N.A.; Winters, S.J.; Nashef, R.; Ali, A.A.; et al. The fear that remains: Associations between trauma, related psychopathology, and fear-potentiated startle in youth resettled as refugees. Dev. Psychobiol. 2023, 65, e22385. [Google Scholar] [CrossRef] [PubMed]
- Reist, C.; Jovanovic, T.; Kantarovich, D.; Weingast, L.; Hollifield, M.; Novin, M.; Khalaghizadeh, S.; Jafari, B.; George, R.; Riser, M.; et al. An analysis of fear inhibition and fear extinction in a sample of veterans with obstructive sleep apnea (OSA): Implications for co-morbidity with post-traumatic stress disorder (PTSD). Behav. Brain Res. 2021, 404, 113172. [Google Scholar] [CrossRef]
- Jovanovic, T.; Stenson, A.F.; Thompson, N.; Clifford, A.; Compton, A.; Minton, S.; van Rooij, S.J.F.; Stevens, J.S.; Lori, A.; Nugent, N.; et al. Impact of ADCYAP1R1 genotype on longitudinal fear conditioning in children: Interaction with trauma and sex. Neuropsychopharmacology 2020, 45, 1603–1608. [Google Scholar] [CrossRef]
- Orcutt, H.K.; Hannan, S.M.; Seligowski, A.V.; Jovanovic, T.; Norrholm, S.D.; Ressler, K.J.; McCanne, T. Fear-potentiated startle and fear extinction in a sample of undergraduate women exposed to a campus mass shooting. Front. Psychol. 2017, 7, 2031. [Google Scholar] [CrossRef]
- Glover, E.M.; Phifer, J.E.; Crain, D.F.; Norrholm, S.D.; Davis, M.; Bradley, B.; Ressler, K.J.; Jovanovic, T. Tools for translational neuroscience: PTSD is associated with heightened fear responses using acoustic startle but not skin conductance measures. Depress. Anxiety 2011, 28, 1058–1066. [Google Scholar] [CrossRef]
- Jovanovic, T.; Sakoman, A.J.; Kozaric-Kovacic, D.; Mestrovic, A.H.; Duncan, E.J.; Davis, M.; Norrholm, S.D. Acute stress disorder versus chronic posttraumatic stress disorder: Inhibition of fear as a function of time since trauma. Depress. Anxiety 2013, 30, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Morrison, K.E.; Stenson, A.F.; Marx-Rattner, R.; Carter, S.; Michopoulos, V.; Gillespie, C.F.; Powers, A.; Huang, W.; Kane, M.A.; Jovanovic, T.; et al. Developmental timing of trauma in women predicts unique extracellular vesicle proteome signatures. Biol. Psychiatry 2022, 91, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, L.; Bohringer, A.; Chatterjee, M.; Schachinger, H. Effects of pre-learning stress on memory for neutral, positive and negative words: Different roles of cortisol and autonomic arousal. Neurobiol. Learn. Mem. 2008, 90, 44–53. [Google Scholar] [CrossRef]
- Norrholm, S.D.; Jovanovic, T.; Briscione, M.A.; Anderson, K.M.; Kwon, C.K.; Warren, V.T.; Bosshardt, L.; Bradley, B. Generalization of fear-potentiated startle in the presence of auditory cues: A parametric analysis. Front. Behav. Neurosci. 2014, 8, 361. [Google Scholar] [CrossRef] [PubMed]
- Bradford, D.E.; Starr, M.J.; Shackman, A.J.; Curtin, J.J. Empirically based comparisons of the reliability and validity of common quantification approaches for eyeblink startle potentiation in humans. Psychophysiology 2015, 52, 1669–1681. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, T.; Keyes, M.; Fiallos, A.; Myers, K.M.; Davis, M.; Duncan, E.J. Fear potentiation and fear inhibition in a human fear-potentiated startle paradigm. Biol. Psychiatry 2005, 57, 1559–1564. [Google Scholar] [CrossRef]
- Jovanovic, T.; Norrholm, S.D.; Keyes, M.; Fiallos, A.; Jovanovic, S.; Myers, K.M.; Davis, M.; Duncan, E.J. Contingency awareness and fear inhibition in a human fear-potentiated startle paradigm. Behav. Neurosci. 2006, 120, 995–1004. [Google Scholar] [CrossRef]
- Jovanovic, T.; Norrholm, S.D.; Fennell, J.E.; Keyes, M.; Fiallos, A.M.; Myers, K.M.; Davis, M.; Duncan, E.J. Posttraumatic stress disorder may be associated with impaired fear inhibition: Relation to symptom severity. Psychiatry Res. 2009, 167, 151–160. [Google Scholar] [CrossRef]
- Merz, C.J.; Tabbert, K.; Schweckendiek, J.; Klucken, T.; Vaitl, D.; Stark, R.; Wolf, O.T. Investigating the impact of sex and cortisol on implicit fear conditioning with fMRI. Psychoneuroendocrinology 2010, 35, 33–46. [Google Scholar] [CrossRef]
- Stark, R.; Wolf, O.T.; Tabbert, K.; Kagerer, S.; Zimmermann, M.; Kirsch, P.; Schienle, A.; Vaitl, D. Influence of the stress hormone cortisol on fear conditioning in humans: Evidence for sex differences in the response of the prefrontal cortex. Neuroimage 2006, 32, 1290–1298. [Google Scholar] [CrossRef]
- Merz, C.J.; Tabbert, K.; Schweckendiek, J.; Klucken, T.; Vaitl, D.; Stark, R.; Wolf, O.T. Oral contraceptive usage alters the effects of cortisol on implicit fear learning. Horm. Behav. 2012, 62, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.; Falls, W.A.; Campeau, S.; Kim, M. Fear-potentiated startle: A neural and pharmacological analysis. Behav. Brain Res. 1993, 58, 175–198. [Google Scholar] [CrossRef] [PubMed]
- Grillon, C.; Baas, J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin. Neurophysiol. 2003, 114, 1557–1579. [Google Scholar] [CrossRef]
- van Ast, V.A.; Vervliet, B.; Kindt, M. Contextual control over expression of fear is affected by cortisol. Front. Behav. Neurosci. 2012, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Morey, R.A.; Haswell, C.C.; Stjepanovic, D.; Mid-Atlantic, M.W.; Dunsmoor, J.E.; LaBar, K.S. Neural correlates of conceptual-level fear generalization in posttraumatic stress disorder. Neuropsychopharmacology 2020, 45, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Lissek, S.; Bradford, D.E.; Alvarez, R.P.; Burton, P.; Espensen-Sturges, T.; Reynolds, R.C.; Grillon, C. Neural substrates of classically conditioned fear-generalization in humans: A parametric fMRI study. Soc. Cogn. Affect. Neurosci. 2014, 9, 1134–1142. [Google Scholar] [CrossRef]
- Cha, J.; Greenberg, T.; Carlson, J.M.; Dedora, D.J.; Hajcak, G.; Mujica-Parodi, L.R. Circuit-wide structural and functional measures predict ventromedial prefrontal cortex fear generalization: Implications for generalized anxiety disorder. J. Neurosci. 2014, 34, 4043–4053. [Google Scholar] [CrossRef]
- Onat, S.; Buchel, C. The neuronal basis of fear generalization in humans. Nat. Neurosci. 2015, 18, 1811–1818. [Google Scholar] [CrossRef]
- de Voogd, L.D.; Murray, Y.P.J.; Barte, R.M.; van der Heide, A.; Fernandez, G.; Doeller, C.F.; Hermans, E.J. The role of hippocampal spatial representations in contextualization and generalization of fear. Neuroimage 2020, 206, 116308. [Google Scholar] [CrossRef]
- Dunsmoor, J.E.; Prince, S.E.; Murty, V.P.; Kragel, P.A.; LaBar, K.S. Neurobehavioral mechanisms of human fear generalization. Neuroimage 2011, 55, 1878–1888. [Google Scholar] [CrossRef]
- Morey, R.A.; Dunsmoor, J.E.; Haswell, C.C.; Brown, V.M.; Vora, A.; Weiner, J.; Stjepanovic, D.; Wagner, H.R., 3rd; LaBar, K.S. Fear learning circuitry is biased toward generalization of fear associations in posttraumatic stress disorder. Transl. Psychiatry 2015, 5, e700. [Google Scholar] [CrossRef] [PubMed]
- Myers, C.E.; Hopkins, R.O.; DeLuca, J.; Moore, N.B.; Wolansky, L.J.; Sumner, J.M.; Gluck, M.A. Learning and generalization deficits in patients with memory impairments due to anterior communicating artery aneurysm rupture or hypoxic brain injury. Neuropsychology 2008, 22, 681–686. [Google Scholar] [CrossRef]
- Myers, C.E.; Kluger, A.; Golomb, J.; Ferris, S.; de Leon, M.J.; Schnirman, G.; Gluck, M.A. Hippocampal atrophy disrupts transfer generalization in nondemented elderly. J. Geriatr. Psychiatry Neurol. 2002, 15, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Wild, J.M.; Blampied, N.M. Hippocampal lesions and stimulus generalization in rats. Physiol. Behav. 1972, 9, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Bucci, D.J.; Saddoris, M.P.; Burwell, R.D. Contextual fear discrimination is impaired by damage to the postrhinal or perirhinal cortex. Behav. Neurosci. 2002, 116, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Wolf, O.T. Memories of and influenced by the Trier Social Stress Test. Psychoneuroendocrinology 2019, 105, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, D.M.; Berke, E.T. Gender-and sex-based contributors to sex differences in PTSD. Curr. Psychiatry Rep. 2020, 22, 19. [Google Scholar] [CrossRef]
- Kornfield, S.L.; Hantsoo, L.; Epperson, C.N. What does sex have to do with it? The role of sex as a biological variable in the development of posttraumatic stress disorder. Curr. Psychiatry Rep. 2018, 20, 39. [Google Scholar] [CrossRef]
- Hsu, C.K.; Kleim, B.; Nicholson, E.L.; Zuj, D.V.; Cushing, P.J.; Gray, K.E.; Clark, L.; Felmingham, K.L. Sex differences in intrusive memories following trauma. PLoS ONE 2018, 13, e0208575. [Google Scholar] [CrossRef]
- Kamboj, S.K.; Oldfield, L.; Loewenberger, A.; Das, R.K.; Bisby, J.; Brewin, C.R. Voluntary and involuntary emotional memory following an analogue traumatic stressor: The differential effects of communality in men and women. J. Behav. Ther. Exp. Psychiatry 2014, 45, 421–426. [Google Scholar] [CrossRef]
- Rattel, J.A.; Wegerer, M.; Miedl, S.F.; Blechert, J.; Grunberger, L.M.; Craske, M.G.; Wilhelm, F.H. Peritraumatic unconditioned and conditioned responding explains sex differences in intrusions after analogue trauma. Behav. Res. Ther. 2019, 116, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Wessel, I.; Overwijk, S.; Verwoerd, J.; de Vrieze, N. Pre-stressor cognitive control is related to intrusive cognition of a stressful film. Behav. Res. Ther. 2008, 46, 496–513. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoladz, P.R.; Cordes, C.N.; Weiser, J.N.; Reneau, K.E.; Boaz, K.M.; Helwig, S.J.; Virden, E.M.; Thebeault, C.K.; Pfister, C.L.; Getnet, B.A.; et al. Pre-Learning Stress That Is Temporally Removed from Acquisition Impairs Fear Learning. Biology 2023, 12, 775. https://doi.org/10.3390/biology12060775
Zoladz PR, Cordes CN, Weiser JN, Reneau KE, Boaz KM, Helwig SJ, Virden EM, Thebeault CK, Pfister CL, Getnet BA, et al. Pre-Learning Stress That Is Temporally Removed from Acquisition Impairs Fear Learning. Biology. 2023; 12(6):775. https://doi.org/10.3390/biology12060775
Chicago/Turabian StyleZoladz, Phillip R., Chloe N. Cordes, Jordan N. Weiser, Kassidy E. Reneau, Kayla M. Boaz, Sara J. Helwig, Emma M. Virden, Caitlin K. Thebeault, Cassidy L. Pfister, Bruktawit A. Getnet, and et al. 2023. "Pre-Learning Stress That Is Temporally Removed from Acquisition Impairs Fear Learning" Biology 12, no. 6: 775. https://doi.org/10.3390/biology12060775
APA StyleZoladz, P. R., Cordes, C. N., Weiser, J. N., Reneau, K. E., Boaz, K. M., Helwig, S. J., Virden, E. M., Thebeault, C. K., Pfister, C. L., Getnet, B. A., Niese, T. D., Parker, S. L., Stanek, M. L., Long, K. E., Norrholm, S. D., & Rorabaugh, B. R. (2023). Pre-Learning Stress That Is Temporally Removed from Acquisition Impairs Fear Learning. Biology, 12(6), 775. https://doi.org/10.3390/biology12060775






