Phytoremediation of Wastewater Containing Lead and Manganese Ions Using Algae
Abstract
Simple Summary
Abstract
1. Introduction
| Heavy Metals | Main Sources [18] | Main Organ and System Affected [18] | Permitted Quantities [mg/dm3] [17] |
|---|---|---|---|
| Lead (Pb2+) | Alloys, solder, lead-based batteries, ammunition, rust inhibitors, cable coating pigments, glazes, and plastic stabilizers. | Liver, bones, brain, kidneys, spleen, lungs, hematological system, immunological system, reproductive system, and cardiovascular system. | 0.20 |
| Manganese (Mn2+) | Batteries, iron and steel alloys, glass, various cleaning supplies, fireworks, fertilizers, fungicides, varnish, livestock feeding supplements, and cosmetics. | Brain and respiratory tract. | 1.00 |
| Copper (Cu2+) | Electronic and cables industry and corroded plumbing systems. | Brain, liver, cornea, kidneys, lungs, gastrointestinal system, hematological system, and immunological system. | 0.10 |
| Nickel (Ni2+) | Production of stainless steel and nickel alloys. | Kidneys, lungs, gastrointestinal system, and skin. | 0.50 |
| Arsenic (As+) | Glass and electronics production. | Lungs, skin, kidneys, brain, cardiovascular system, metabolic system, endocrine system, and immunological system. | 0.10 |
| Zinc (Zn2+) | Rubber products, brass coating, and some cosmetics. | Stomach and skin. | 0.50 |
| Cadmium (Cd2+) | Paints, batteries, corroded galvanized pipes, plastics industry, steel industry, and metal refineries. | Liver, bones, lungs, kidneys, brain, cardiovascular system, and immunological system. | 0.20 |
| Chromium (Cr) Cr3+/Cr3++Cr6+/Cr6+ | Steelworks, pulp mills, and tanneries. | Lungs, skin, liver, kidneys, pancreas, brain, taste, reproductive system, and gastrointestinal system. | -/1.00/0.10 |
| Mercury (Hg2+) | Electrolytic production of caustic soda and chlorine, electrical appliances, refineries, laboratory apparatus, industrial and control instruments. | Lungs, brain, liver, kidneys, cardiovascular system, immunological system, and reproductive system. | 0.05 |
2. Materials and Methods
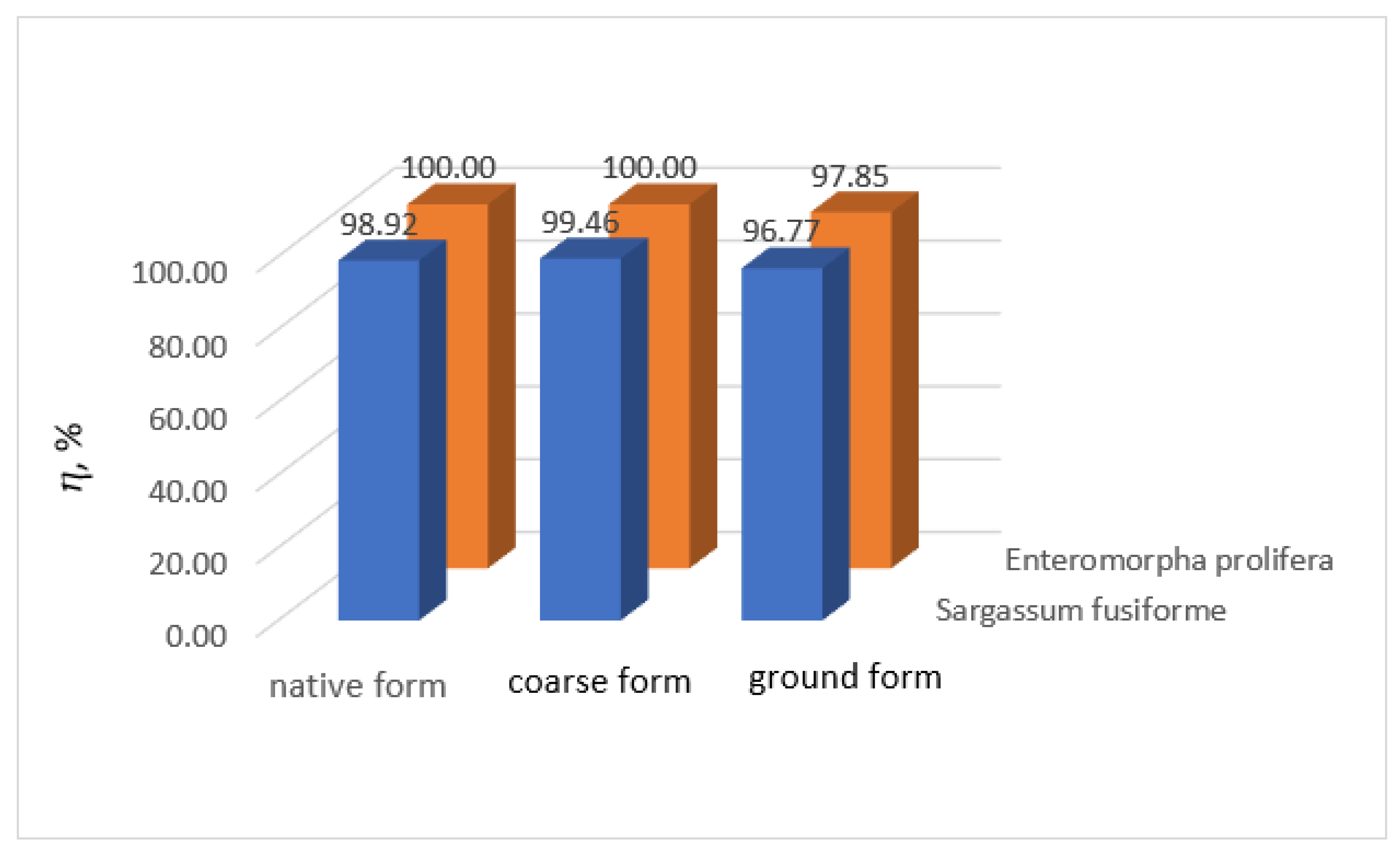
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhattacharjee, C.; Dutta, S.; Saxena, V.K. A review on biosorptive removal of dyes and heavy metals from wastewater using watermelon rind as biosorbent. Environ. Adv. 2020, 2, 100007. [Google Scholar] [CrossRef]
- Mishra, S.; Cheng, L.; Maiti, A. The utilization of agro-biomass/byproducts for effective bio-removal of dyes from dyeing wastewater: A comprehensive review. J. Environ. Chem. Eng. 2021, 9, 104901. [Google Scholar] [CrossRef]
- Martini, S.; Roni, K.A. The existing technology and the application of digital artificial intelligent in the wastewater treatment area: A review paper. J. Phys. Conf. Ser. 2021, 012013. [Google Scholar] [CrossRef]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of heavy metal ions by various low-cost adsorbents: A review. Int. J. Environ. Anal. Chem. 2020, 102, 342–379. [Google Scholar] [CrossRef]
- Kerur, S.S.; Bandekar, S.; Hanagadakar, M.S.; Nandi, S.S.; Ratnamala, G.M.; Hegde, P.G. Removal of hexavalent Chromium Industry treated water and Wastewater: A review. Mater. Today Proc. 2021, 42, 1112–1121. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, H.; Li, W.; Ding, X.; Chu, H. Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. J. Anal. Appl. Pyrolysis 2021, 155, 105081. [Google Scholar] [CrossRef]
- Bilal, M.; Ihsanullah, I.; Younas, M.; Ul Hassan Shah, M. Recent advances in applications of low-cost adsorbents for the removal of heavy metals from water: A critical review. Sep. Purif. Technol. 2021, 278, 119510. [Google Scholar] [CrossRef]
- Tamjidi, S.; Esmaeili, H. Chemically modified CaO/Fe3O4 nanocomposite by sodium dodecyl sulfate for Cr(III) removal from water. Chem. Eng. Technol. 2019, 42, 607–616. [Google Scholar] [CrossRef]
- El Gheriany, I.A.; El Saqa, F.A.; Amer, A.A.E.R.; Hussein, M. Oil spill sorption capacity of raw and thermally modified orange peel waste. Alex. Eng. J. 2020, 59, 925–932. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K. A review on heavy metal ions and dye adsorption from water by agricultural solid waste adsorbents. Water Air Soil. Pollut. 2018, 229, 225. [Google Scholar] [CrossRef]
- Foday, E.H., Jr.; Bo, B.; Xu, X. Removal of Toxic Heavy Metals from Contaminated Aqueous Solutions Using Seaweeds: A Review. Sustainability 2021, 13, 12311. [Google Scholar] [CrossRef]
- Gümüş, N.E.; Aşıkkutlu, B.; Keskinkaya, H.B.; Akköz, C. Comparison of heavy metal absorption of some algae isolated from Altınapa Dam Lake (Konya). J. Anatol. Environ. Anim. Sci. 2021, 1, 50–56. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Parisa, T.A.; Islam, N.; Kusumo, F.; Inayat, A.; Le, V.G.; Badruddin, I.A.; Khan, T.M.Y.; Ong, H.C. Progress and challenges of contaminate removal from wastewater using microalgae biomass. Chemosphere 2022, 286, 131656. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Chen, C. Biosorption of heavy metals by Saccharomyces cerevisiae: A review. Biotechnol. Adv. 2006, 24, 427–451. [Google Scholar] [CrossRef]
- Low, K.; Lee, C.; Liew, S. Sorption of cadmium and lead from aqueous solutions by spent grain. Process. Biochem. 2000, 36, 59–64. [Google Scholar] [CrossRef]
- Assi, M.A.; Hezmee, M.N.M.; Abd Wahid Haron, M.Y.M.; Sabri, M.A.R. The detrimental effects of lead on human and animal health. Vet. World 2016, 9, 660. [Google Scholar] [CrossRef]
- NTPA 001. VALORI LIMITA DE INCARCARE CU POLUANTI A APELOR UZATE INDUSTRIALE SI ORASENESTI EVACUATE IN RECEPTORI NATURALI. Available online: https://moleculah2o.com/2013/08/14/ntpa-001-valori-limita-de-incarcare-cu-poluanti-a-apelor-uzate-industriale-si-orasenesti-evacuate-in-receptori-naturali/ (accessed on 4 April 2023).
- Naef, A.A.Q.; Ramy, H.M.; Dahiru, U.L. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Nat. Partn. J. 2021, 36, 1–15. [Google Scholar]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar]
- Vijayaraghavan, K.; Yun, Y.S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar]
- Vasavi, A.; Usha, R.; Swamy, P.M. Phytoremediation—An overview review. J. Ind. Pollut. Control. 2010, 26, 83–88. [Google Scholar]
- Sharma, S.; Rana, S.; Thakkar, A.; Baldi, A.; Murthy, R.S.R.; Sharma, R.K. Physical, chemical and phytoremediation technique for removal of heavy metals. J. Heavy Met. Toxic. Dis. 2016, 1, 1–15. [Google Scholar]
- Wang, Q.; Cui, Y.; Dong, Y. Phytoremediation of polluted waters potentials and prospects of wetland plants. Acta Biotechnol. 2002, 22, 199–208. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Wani, P.A.; Oves, M. Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils. Environ. Chem. Lett. 2009, 7, 1–19. [Google Scholar] [CrossRef]
- Ma, Y.; Oliveira, R.S.; Freitas, H.; Zhang, C. Biochemical and molecular mechanisms of plant-microbe-metal interactions: Relevance for phytoremediation. Front. Plant. Sci. 2016, 7, 918. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Singh, S.P. A review on phytoremediation of heavy metals and utilization of it’s by products. Asian J. Energy Environ. 2005, 6, 1–18. [Google Scholar]
- Lasat, M.M. Phytoextraction of toxic metals. J. Environ. Qual. 2002, 31, 109–120. [Google Scholar]
- Maxted, A.P.; Black, C.R.; West, H.M.; Crout, N.M.J.; McGrath, S.P.; Young, S.D. Phytoextraction of cadmium and zinc from arable soils amended with sewage sludge using Thlaspi caerulescens: Development of a predictive model. Environ. Pollut. 2007, 150, 363–372. [Google Scholar] [CrossRef]
- Kabeyaa, F.I.; Pongrac, P.; Lange, B.; Faucon, M.-P.; van Elteren, J.T.; Šala, M.; Šelih, V.S.; Eeckhoudt, E.V.; Verbruggen, N. Tolerance and accumulation of cobalt in three species of Haumaniastrum and the influence of copper. Environ. Exp. Bot. 2018, 149, 27–33. [Google Scholar] [CrossRef]
- Sagner, S.; Kneer, R.; Wanner, G.; Cosson, J.-P.; Deus-Neumann, B.; Zenk, M.H. Hyperaccumulation, complexation and distribution of nickel in Sebertia Acuminata. Phytochem. 1998, 47, 339–347. [Google Scholar] [CrossRef]
- Awa, S.H.; Hadibarata, T. Removal of Heavy Metals in Contaminated Soil by Phytoremediation Mechanism: A Review. Water Air Soil. Pollut. 2020, 231, 47. [Google Scholar] [CrossRef]
- Wani, K.A.; Sofi, Z.M.; Malik, J.A.; Wani, J.A. Phytoremediation of Heavy Metals Using Salix (Willows), Chapter 9. Bioremediation Biotechnol. 2020, 2, 161–174. [Google Scholar]
- Mahdi, B.A.; Jaafar, R.S. The ability of Corn waste products to adsorb lead ions from the industrial wastewater. Marsh Bull. 2020, 15, 19–28. [Google Scholar]
- Bortoloti, G.A.; Baron, D. Phytoremediation of toxic heavy metals by Brassica plants: A biochemical and physiological approach. Environ. Adv. 2022, 8, 100204. [Google Scholar] [CrossRef]
- Zhao, X.; Joo, J.C.; Kim, J.Y. Evaluation of heavy metal phytotoxicity to Helianthus annuus L. using seedling vigor index-soil model. Chemosphere 2021, 275, 130026. [Google Scholar] [CrossRef]
- Lone, M.I.; He, Z.L.; Stoffella, P.J.; Yang, X.E. Phytoremediation of heavy metal polluted soils and water: Progresses and perspectives. J. Zhejiang Univ. Sci. B. 2008, 9, 210–220. [Google Scholar] [CrossRef]
- Tang, S.; Xi, L.; Zheng, J.; Li, H. Response to elevated CO2 of Indian mustard and sunflower growing on copper contaminated soil. Bull. Environ. Contam. Toxicol. 2003, 71, 988–997. [Google Scholar] [CrossRef]
- Mahajan, P.; Kaushal, J. Role of phytoremediation in reducing cadmium toxicity in soil and water. J. Toxicol. 2018, 2018, 4864365. [Google Scholar] [CrossRef]
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of heavy metals: A promising tool for clean-up of polluted environment? Front. Plant. Sci. 2018, 9, 1476. [Google Scholar] [CrossRef]
- Rezania, S.; Taib, S.M.; Din, M.F.M.; Dahalan, F.A.; Kamyab, H. Comprehensive review on phytotechnology: Heavy metals removal by diverse aquatic plants species from wastewater. J. Hazard. Mater. 2016, 318, 587–599. [Google Scholar] [CrossRef]
- DalCorso, G.; Fasani, E.; Manara, A.; Visioli, G.; Furini, A. Heavy metal pollutions: State of the art and innovation in phytoremediation. Int. J. Molec. Sci. 2019, 20, 3412. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Cai, G. An application of phytoremediation to river pollution remediation. Procedia Environ. Sci. 2011, 10, 1904–1907. [Google Scholar] [CrossRef]
- Rajkumar, M.; Sandhya, S.; Prasad, M.N.V.; Freitas, H. Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotech. Adv. 2012, 30, 1562–1574. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Singh, G.; Jadeja, R.N. Phytoremediation for Heavy Metal Removal. Poll. Water Manag. 2021, 6, 128–150. [Google Scholar]
- Davis, T.A.; Volesky, B.; Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef]
- Mestechkina, N.M.; Shcherbukhin, V.D. Sulfated polysaccharides and their anticoagulant activity: A review. Appl. Biochem. Microbiol. 2010, 46, 267–273. [Google Scholar] [CrossRef]
- Yende, S.R.; Harle, U.N.; Chaugule, B.B. Therapeutic potential and health benefits of Sargassum species. Pharmacogn. Rev. 2014, 8, 1–7. [Google Scholar] [CrossRef]
- Kokubu, S.; Nishihara, G.N.; Watanabe, Y.; Tsuchiya, Y.; Amamo, Y.; Terada, R. The effect of irradiance and temperature on the photosynthesis of a native alga Sargassum fusiforme (Fucales) from Kagoshima, Japan. Phycologia 2015, 54, 235–247. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Fu, Z.; Ye, C.; Zhang, R.; Song, Y.; Zhang, Y.; Li, H.; Ying, H.; Liu, H. 24 (S)-Saringosterol from edible marine seaweed Sargassum fusiforme is a novel selective LXRβ agonist. J. Agric. Food Chem. 2014, 62, 6130–6137. [Google Scholar] [CrossRef]
- Li, J.; Ren, J.; Cui, R.; Yu, K.; Zhao, Y. Optical imaging spectroscopy coupled with machine learning for detecting heavy metal of plants: A review. Front. Plant. Sci. 2022, 13, 1–13. [Google Scholar] [CrossRef]
- Gong, L.; Qiu, D.; Yao, X.; Yang, G. Determination of Heavy Metals in the Plant Sample Pretreatment Methods. J. Phys. Conf. Ser. 2020, 1549, 022008. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.; Du, C.; Mou, H.; Wang, P. Compositional and structural characteristics of sulfated polysaccharide from Enteromorpha prolifera. Carbohydr. Polym. 2017, 165, 221–228. [Google Scholar] [CrossRef]
- Liu, D.; Keesing, J.K.; He, P.; Wang, Z.; Shi, Y.; Wang, Y. The world’s largest macroalgal bloom in the Yellow Sea, China: Formation and implications. Estuar. Coast. Shelf Sci. 2013, 129, 2–10. [Google Scholar] [CrossRef]
- Wei, J.; Wang, S.; Liu, G.; Pei, D.; Liu, Y.; Liu, Y.; Di, D. Polysaccharides from Enteromorpha prolifera enhance the immunity of normal mice. Int. J. Biol. Macromol. 2014, 64, 1–5. [Google Scholar] [CrossRef]
- Qiao, L.; Li, Y.; Chi, Y.; Ji, Y.; Gao, Y.; Hwang, H.; Aker, W.G.; Wang, P. Rheological properties, gelling behavior and texture characteristics of polysaccharide from Enteromorpha prolifera. Carbohydr. Polym. 2016, 136, 1307–1314. [Google Scholar] [CrossRef]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs. 2011, 9, 1056–1100. [Google Scholar] [CrossRef]
- Collins, K.G.; Fitzgerald, G.F.; Stanton, C.; Ross, R.P. Looking beyond the terrestrial: The potential of seaweed derived bioactives to treat non-communicable diseases. Mar. Drugs. 2016, 14, 60. [Google Scholar] [CrossRef]
- Xie, C.; Zhang, Y.; Niu, K.; Liang, X.; Wang, H.; Shan, J.; Wu, X. Enteromorpha polysaccharide-zinc replacing prophylactic antibiotics contributes to improving gut health of weaned piglets. Anim. Nutr. 2021, 7, 641–649. [Google Scholar] [CrossRef]
- Putri, L.S.E.; Syafiqa, E. The adsorption of heavy metals from industrial wastewater using Sargassum Crassifolium. Int. J. Geomate 2019, 17, 21–27. [Google Scholar]
- Jeba, D.; Sangeetha, K.; Suganthi, B. Biosorption of Heavy Metal Lead from Aqueous Solution by Non-living Biomass of Sargassum myriocystum. Int. J. Appl. Innov. Eng. Manag. 2014, 3, 39–45. [Google Scholar]
- Sheng, P.X.; Ting, Y.-P.; Chen, J.P.; Hong, L. Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: Characterization of biosorptive capacity and investigation of mechanisms. J. Colloid. Interface Sci. 2004, 275, 131–141. [Google Scholar] [CrossRef]
- Montazer-Rahmati, M.M.; Rabbani, P.; Abdolali, A.; Keshtkar, A.R. Kinetics and equilibrium studies on biosorption of cadmium, lead, and nickel ions from aqueous solutions by intact and chemically modified brown algae. J. Hazard. Mater. 2011, 185, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Vafajooa, L.; Cheraghia, R.; Dabbaghb, R.; McKay, G. Removal of cobalt (II) ions from aqueous solutions utilizing the pre-treated 2-Hypnea Valentiae algae: Equilibrium, thermodynamic, and dynamic studies. Chem. Eng. J. 2018, 331, 39–47. [Google Scholar] [CrossRef]
- Jayakumar, V.; Govindaradjane, S.; Rajasimman, M. Efficient adsorptive removal of Zinc by green marine macro alga Caulerpa scalpelliformis –Characterization, Optimization, Modeling, Isotherm, Kinetic, Thermodynamic, Desorption and Regeneration Studies. Surf. Interfaces 2021, 22, 100798. [Google Scholar] [CrossRef]
- Yang, J.; Cao, J.; Xing, G.; Yuan, H. Lipid production combined with biosorption and bioaccumulation of cadmium, copper, manganese and zinc by oleaginous microalgae Chlorella minutissima UTEX2341. Bioresour. Technol. 2015, 175, 537–544. [Google Scholar] [CrossRef]
- El Nemr, A.; El-Sikaily, A.; Khaled, A.; Abdelwahab, O. Removal of toxic chromium from aqueous solution, wastewater and saline water by marine red alga Pterocladia capillacea and its activated carbon. Arab. J. Chem. 2015, 8, 105–117. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Kyzas, G.Z. Progress in batch biosorption of heavy metals onto algae. J. Mol. Liq. 2015, 209, 77–86. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, J.; Kandasamy, S.; He, Z. Hydrothermal liquefaction of fresh lemon-peel and Spirulina platensis blending-operation parameter and biocrude chemistry investigation. Energy 2020, 193, 116645. [Google Scholar] [CrossRef]
- Kandasamy, S.; Narayanan, M.; He, Z.; Liu, G.; Ramakrishnan, M.; Thangavel, P.; Pugazhendhi, A.; Raja, R.; Carvalho, I.S. Current strategies and prospects in algae for remediation and biofuels: An overview. Biocatal. Agric. Biotechnol. 2021, 35, 102045. [Google Scholar] [CrossRef]
- Romera, E.; González, F.; Ballester, A.; Blázquez, M.L.; Muñoz, J.A. Comparative Study of Biosorption of Heavy Metals Using Different Types of Algae. Bioresour. Technol. 2007, 98, 3344–3353. [Google Scholar] [CrossRef]
- He, J.; Chen, J.P. A Comprehensive Review on Biosorption of Heavy Metals by Algal Biomass: Materials, Performances, Chemistry, and Modeling Simulation Tools. Bioresour. Technol. 2014, 160, 67–78. [Google Scholar] [CrossRef]
- Raize, O.; Argaman, Y.; Yannai, S. Mechanisms of Biosorption of Different Heavy Metals by Brown Marine Macroalgae. Biotechnol. Bioeng. 2004, 87, 451–458. [Google Scholar] [CrossRef]
- Flouty, R.; Estephane, G. Bioaccumulation and biosorption of copper and lead by a unicellular algae Chlamydomonas reinhardtii in single and binary metal systems: A comparative study. J. Environ. Manag. 2012, 111, 106–114. [Google Scholar] [CrossRef]

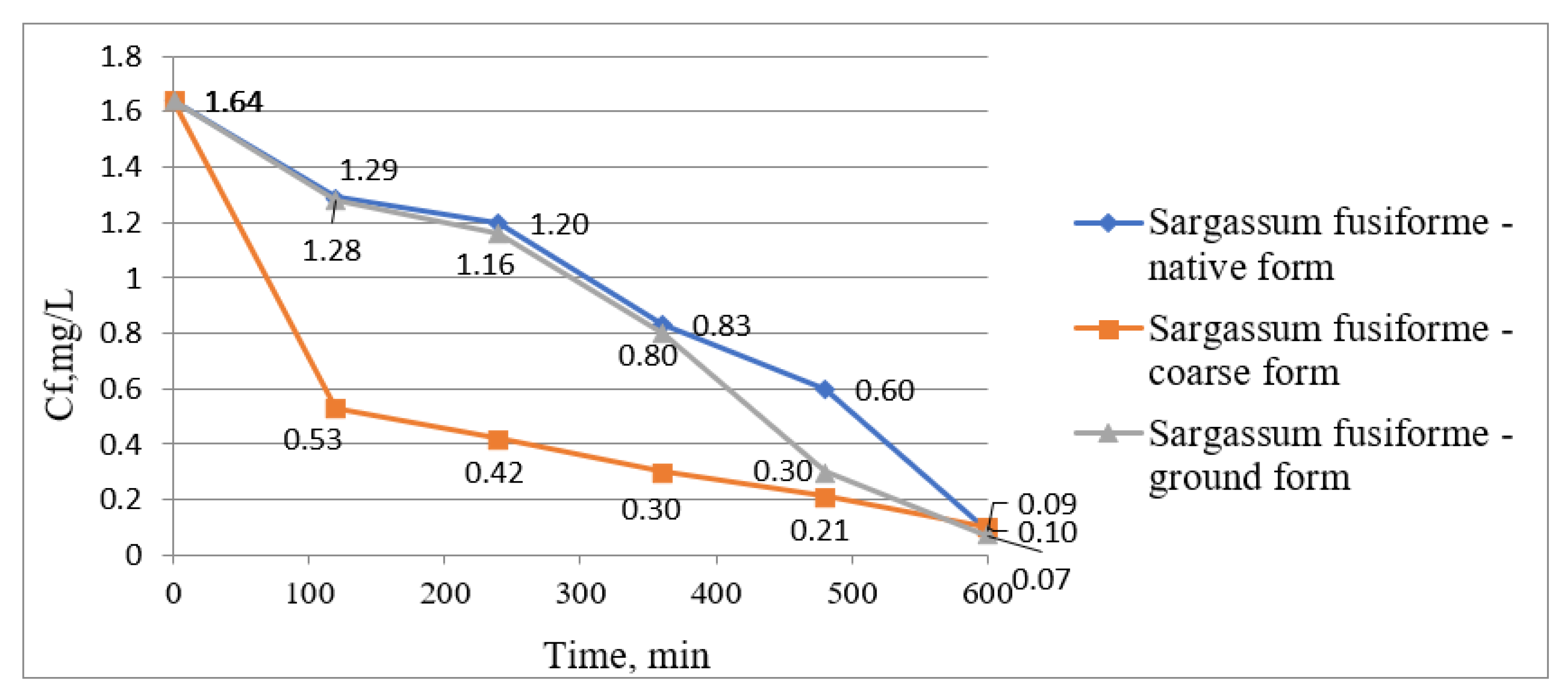
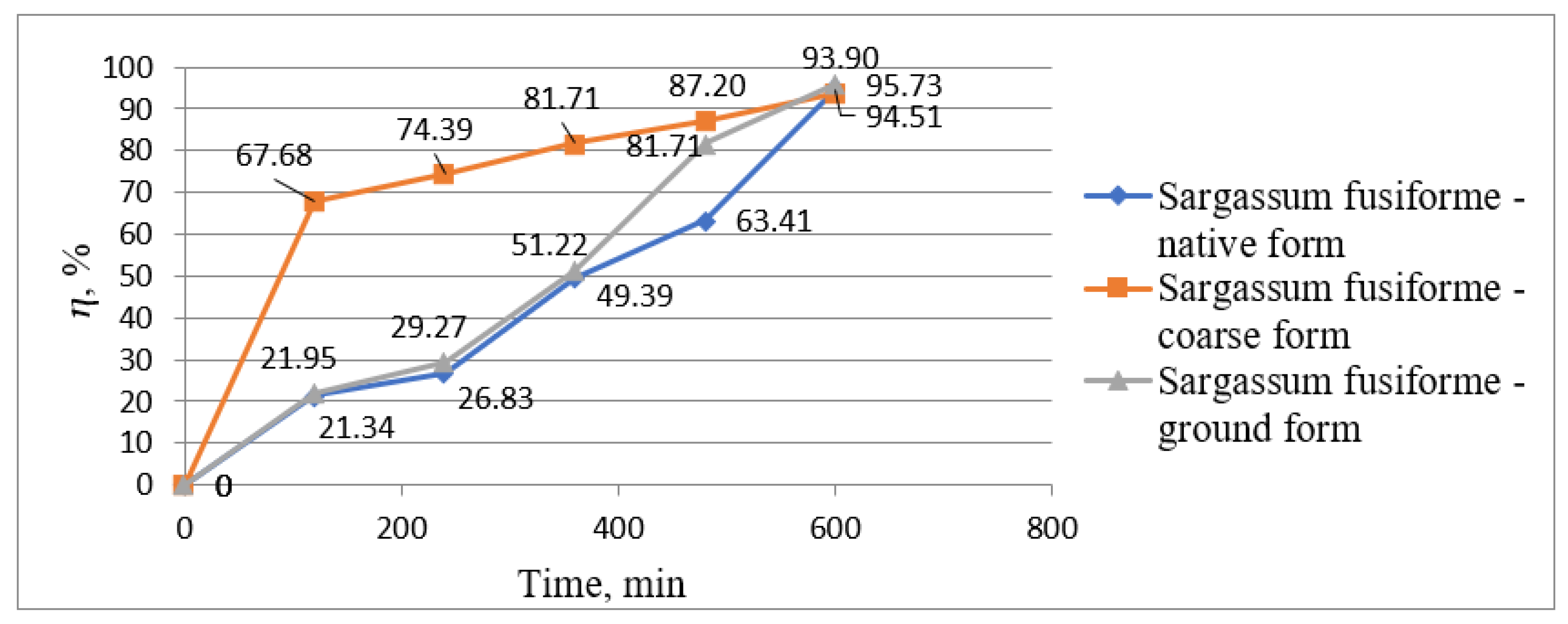



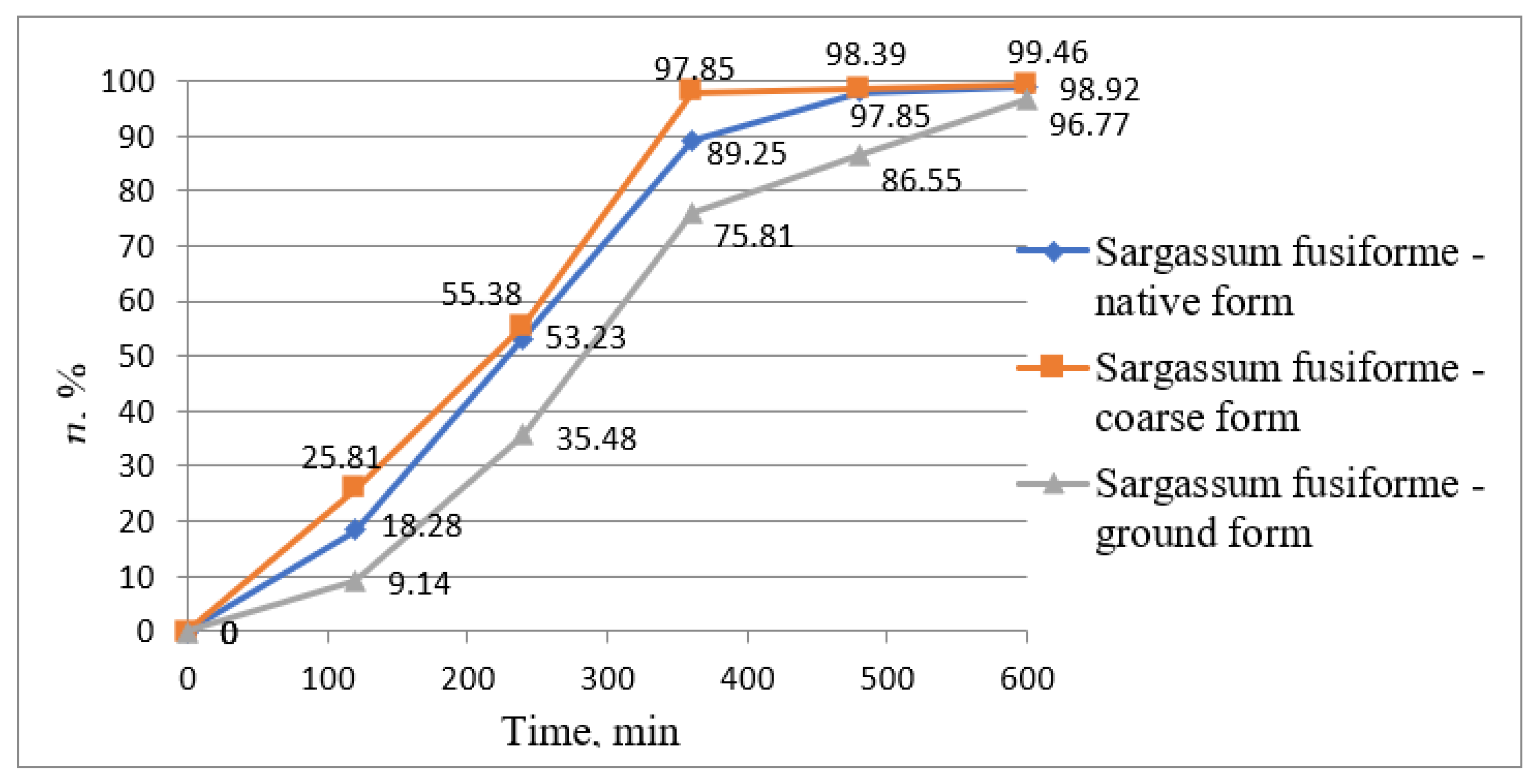
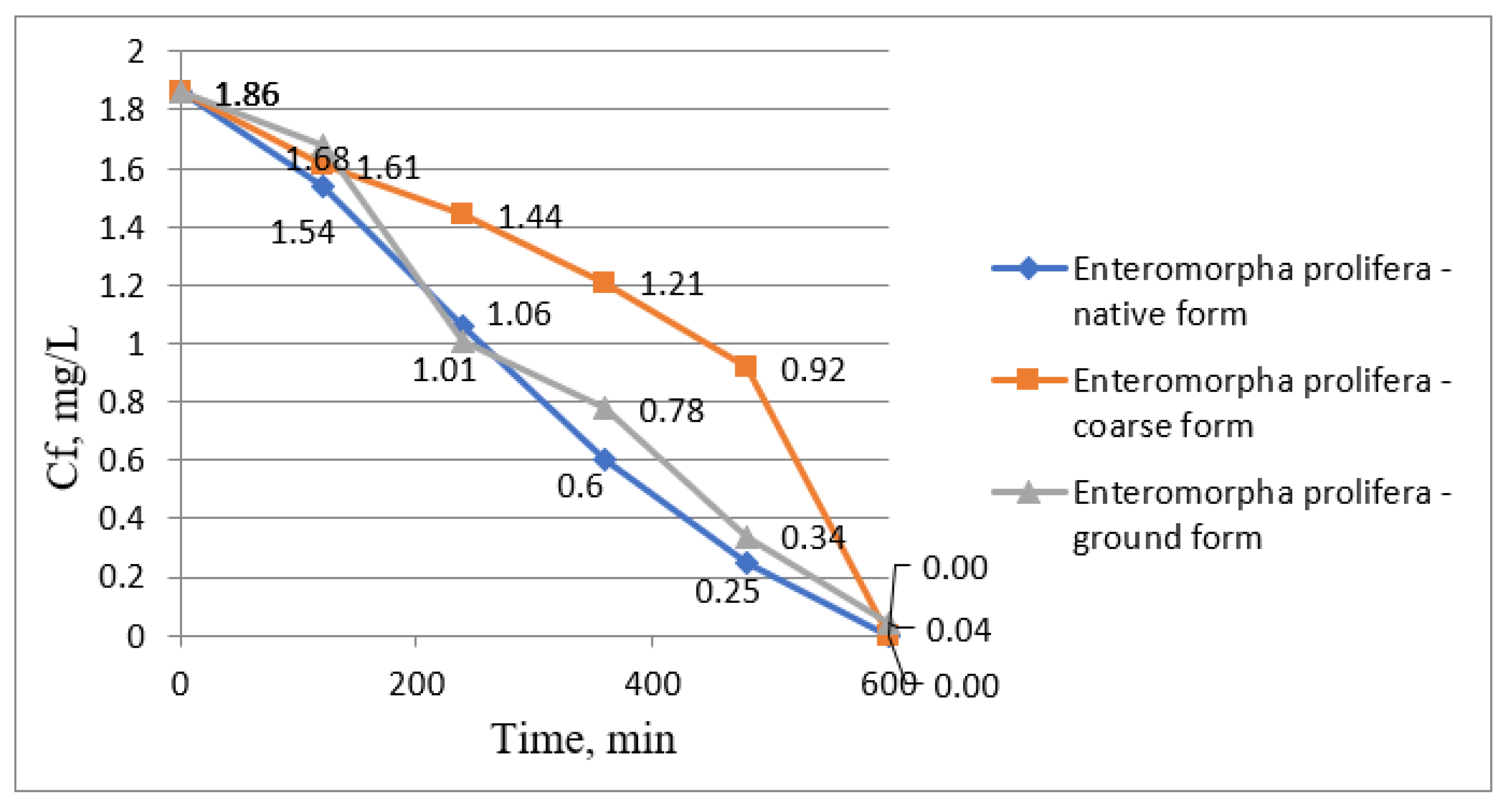


| Water Treatment Methods | Advantages | Disadvantages |
|---|---|---|
| Coagulation/ flocculation | Sludge settling, process simplicity | Generation of large volume of sludge, high operational costs |
| Ion exchange | Simple equipment, easy control and maintenance | High operational costs |
| Photocatalysis | No sludge production, less harmful byproduct, instant removal of metals and organic pollutants | Limited application |
| Flotation | High efficiency for removing pollutants, rapid operation, inexpensive | High cost of operation and maintenance |
| Electrochemical treatment | Efficient technology for the recycling/recovery of valuable metals | High capital and operating costs |
| Algae | Heavy Metals | Time [min] | pH | Temperature [°C] | Yield [%] | Ref. |
|---|---|---|---|---|---|---|
| Sargassum crassifolium | Cd (II), Hg (II), Pb (II) | 60 | 2, 3, 4, 5, 9 | - | 75.00–99.05 | [59] |
| Sargassum myriocystum | Pb (II) | 60 | 5 | 25 | 89.75 | [60] |
| Sargassum sp./Padina sp. | Pb (II), Cu (II) | ~60 | 5 | - | 90.00 | [61] |
| Cd (II), Zn (II), Ni (II) | 5.5 | |||||
| Formaldehyde-treated Cystoseira indica biomass | Cd (II), Ni (II) | ~180 | 6 | 25 | 90.00 | [62] |
| Formaldehyde-treated Nizimuddinia zanardini biomass | Pb (II) | 5.5 | ||||
| Formaldehyde-treated 2-Hypnea valintiae | Co (II) | 120 | 6 | 25 | ~90.00 | [63] |
| Caulerpa scalpelliformis | Zn (II) | - | 5.7 | 30 | 89.60 | [64] |
| Chlorella minutissima | Zn (II) | 20 | 6 | 28 | 62.00 | [65] |
| Pterocladia capillacea | Cr (III) | 45 | 1 | 25 | 80.00–85.00 | [66] |
| Spirulina platensis | Cu (II) | 90 | 7 | 37 | 90.60 | [67] |
| Scenedesmus quadricauda | Cr (VI) | - | 1 | - | 60.00 | |
| Scenedesmus quadricauda | Cr (III) | - | 6 | - | 85.00 | |
| Dunaliela | Cd (II), Pb (II), Ni (II), Cr (II), Zn (II), Cu (II) | - | - | - | 74.00–95.00 | |
| Jania rubens | Hg (II) | 60 | 6 | - | 54.00–71.00 | |
| Ulva lactuca | Cr (VI) | 15–180 | 5 | - | 96.00 | |
| Ulva lactuca | Hg (II) | 60 | 6 | - | 60.00–86.00 | |
| Sphaerococcus coronopifolius | Hg (II) | 60 | 6 | - | 70.00–90.00 | |
| Azolla fliculoides | Cr (VI) | 100 | 2 | - | 83.00 | |
| Caulerpa fastigiata | Pb (II) | 90 | 5 | - | 70.00–82.00 | |
| Sargassum myriocystum | Pb (II) | 60 | 5 | 25 | 87.00 | |
| Osmundea pinnatifda | Cu (II) | 60 | 5 | - | 70.00 | |
| Osmundea pinnatifda | Cd (II) | 60 | 5 | - | 75.00 | |
| Cystoseira indica | Co (II), Cu (II) | 70 | - | 45 | 90.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaconu, L.I.; Covaliu-Mierlă, C.I.; Păunescu, O.; Covaliu, L.D.; Iovu, H.; Paraschiv, G. Phytoremediation of Wastewater Containing Lead and Manganese Ions Using Algae. Biology 2023, 12, 773. https://doi.org/10.3390/biology12060773
Diaconu LI, Covaliu-Mierlă CI, Păunescu O, Covaliu LD, Iovu H, Paraschiv G. Phytoremediation of Wastewater Containing Lead and Manganese Ions Using Algae. Biology. 2023; 12(6):773. https://doi.org/10.3390/biology12060773
Chicago/Turabian StyleDiaconu, Loredana Ioana, Cristina Ileana Covaliu-Mierlă, Oana Păunescu, Leon Dumitru Covaliu, Horia Iovu, and Gigel Paraschiv. 2023. "Phytoremediation of Wastewater Containing Lead and Manganese Ions Using Algae" Biology 12, no. 6: 773. https://doi.org/10.3390/biology12060773
APA StyleDiaconu, L. I., Covaliu-Mierlă, C. I., Păunescu, O., Covaliu, L. D., Iovu, H., & Paraschiv, G. (2023). Phytoremediation of Wastewater Containing Lead and Manganese Ions Using Algae. Biology, 12(6), 773. https://doi.org/10.3390/biology12060773








