Simple Summary
Roundworms of the genus Oswaldocruzia are common parasites of the small intestine of amphibians and reptiles. Our recent study revealed that only one species, Oswaldocruzia filiformis, parasitizes anurans, lizards and snakes in European Russia. In this work we studied nematodes of the genus Oswaldocruzia in the European green toads from the Middle Volga region using traditional morphological and molecular genetic methods. Descriptions, original drawings, and data on molecular phylogenetic analysis for studied nematodes were produced. The results of our study showed that green toads are parasitized by two Oswaldocruzia species, Oswaldocruzia ukrainae and O. filiformis. We found broad variability in morphology of the studied nematodes, which can lead to inaccurate identification of closely related species of Oswaldocruzia. The data obtained in our study should contribute to the knowledge of Oswaldocruzia nematodes and host-parasite associations in general.
Abstract
Nematodes of the genus Oswaldocruzia are common parasites of the small intestine of amphibians and reptiles. Our recent molecular analysis of Oswaldocruzia nematodes revealed that only Oswaldocruzia filiformis, which possesses high morphological variability, parasitizes amphibians and reptiles in European Russia. Here we present the study of Oswaldocruzia nematodes from the European green toad Bufotes viridis (Anura, Bufonidae) collected at different localities of the Middle Volga region in 2018–2022. We analyzed the morphological characteristics of the Oswaldocruzia spp. taxonomy together with novel molecular phylogenetic data. The data on phylogenetic analysis (based on partial CoxI mtDNA gene sequences) showed that Bufotes viridis is parasitized by two Oswaldocruzia species, the host-specific parasite Oswaldocruzia ukrainae and species generalist Oswaldocruzia filiformis. Broad morphological variability was revealed in O. ukrainae nematodes both from the same host specimen and from various toad individuals from different localities. Our results highlight the need for further biodiversity research of morphologically similar Oswaldocruzia species from amphibians and reptiles in the Western Palearctic using molecular genetic methods.
1. Introduction
The distribution range of the European green toad, Bufotes viridis Laurenti, 1768 (Amphibia: Bufonidae), runs from eastern France to the Balkans and European Russia. Previously, this toad species also inhabited the Middle East and Central Asia, reaching western China and northwestern India, as well as in the south to North Africa. Nowadays, toad populations inhabiting North Africa, the Balearic Islands and Asia are considered separate species [1,2].
Nematodes from the genus Oswaldocruzia Travassos, 1917 are one of the most common parasites of B. viridis. These nematodes parasitize the small intestine of amphibians and reptiles, mainly in anurans. This genus includes about 90 species distributed worldwide [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37].
In Russia, three species, Oswaldocruzia filiformis (Goeze, 1782), Oswaldocruzia ukrainae Iwanitzky, 1928, and Oswaldocruzia iwanitzkyi Sudarikov, 1951, were found in B. viridis [3,38,39]. Nematodes of the genus Oswaldocruzia have broad morphological variability [40,41,42], which may lead to inaccurate species affiliation of Oswaldocruzia spp. from various host species. As a result, a number of previously described Oswaldocruzia species were proposed to be reduced as synonyms of the first described species, O. filiformis [3,33,43].
In most cases, only O. filiformis was recorded in the green toad B. viridis from various regions of European Russia. This nematode species was identified in B. viridis from the Republic of Tatarstan [44,45], Republic of Bashkortostan [46,47,48,49], Tambov [50], Samara [51,52,53,54,55], Nizhny Novgorod [56], Orenburg [57,58] and Astrakhan [38,39] regions. Outside of Russia, O. filiformis has been found in B. viridis from Ukraine [3], Belarus [59,60], Bulgaria [61], Turkey [14,25,26,62] and Uzbekistan [13,63].
Ivanitsky [64] described three species of Oswaldocruzia in B. viridis, namely O. fulleborni, O. ukrainae and O. skrjabini. Sudarikov [65] revised the latter species as Oswaldocruzia iwanitzkyi Sudarikov, 1951. According to Ryzhikov et al. [3], O. fulleborni is morphologically very similar to O. filiformis.
In Russia, the nematode O. ukrainae was previously identified in B. viridis only in the Stavropol, Volgograd and Astrakhan regions [3,38,39]. Oswaldocruzia ukrainae has also been recorded in toads from the Poltava region of Ukraine [33], Uzbekistan [13,63] and Tunisia [4]. In addition, O. iwanitzkyi, a new species for Russia, was revealed in B. viridis from the Astrakhan region [38,39]. This species has previously been recorded only in green toads of Ukraine [64] and Slovakia [66]. During the helminthological study of toads in the type area, Svitin [33] found only O. ukrainae in B. viridis. Therefore, the validity of O. iwanitzkyi requires clarification.
The species O. ukrainae is a parasite specific to B. viridis and has not been recorded in other amphibian species. On the contrary, the nematode O. filiformis is a species generalist parasitizing most species of amphibians in the Palaearctic. This species greatly exceeds all other nematodes of amphibians in terms of the number of hosts. Twelve amphibian species have been registered as hosts for O. filiformis in Russia and neighboring countries [3,52].
We have previously identified Oswaldocruzia nematodes from various amphibian species in the Middle Volga region as O. filiformis [35,55,67,68,69]. Our recent molecular and morphological analysis of Oswaldocruzia nematodes revealed that only one species, O. filiformis, parasitizes amphibians and reptiles in European Russia [40]. Unfortunately, that study did not include Oswaldocruzia specimens parasitizing the European green toads, B. viridis.
The purpose of this study was species identification of Oswaldocruzia nematodes parasitizing B. viridis in European Russia using morphological and molecular phylogenetic analysis. We also compared the newly obtained results with our previous data.
2. Materials and Methods
2.1. Nematode Collection and Examination
Nematodes of the genus Oswaldocruzia were collected from the small intestine of B. viridis at six localities in the Middle Volga region (European Russia) in 2018–2022: vicinity of Spassk city (Penza region, 53°54′49″ N, 43°12′36″ E), Nikolaevka village (Republic of Mordovia, 54°8′34″ N, 45°8′55″ E), Lykovshina village (Republic of Mordovia, 54°18′45″ N, 45°29′13″ E), Togliatti city (Samara region, 53°33′6″ N, 49°12′40″ E), Samara city (53°12′27″ N, 50°9′18″ E) and Oktyabrskiy township (Samara region, 53°25′33″ N, 52°3′48″ E). Data on the geographic origin of the examined Oswaldocruzia nematodes are presented in Figure 1 and Table 1.
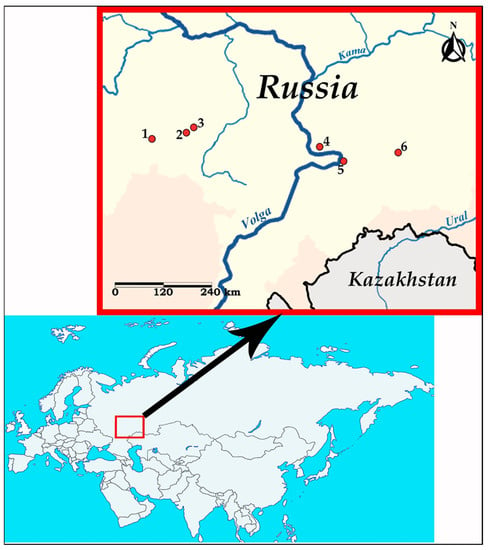
Figure 1.
Map of sampling sites in the Middle Volga region (European Russia). Red circles indicate sampling localities: 1—Vicinity of Spassk (Penza region); 2—Nikolaevka village (Republic of Mordovia); 3—Lykovshina village (Republic of Mordovia); 4—Togliatti (Samara region); 5—Samara city; and 6—Oktyabrskiy township (Samara region).

Table 1.
List of Oswaldocruzia specimens, with museum and laboratory specimen numbers and GenBank accession numbers according to the geographic origin.
No amphibians were intentionally killed for this research. In this work, we studied only road-killed toads. Several dead amphibians were kindly provided by the rural residents. The necropsy was performed on toads approximately 1–8 h after their death. Only alive motile adult nematodes were collected for further investigation. Some nematodes were collected from toads and preserved in 96% ethanol for further molecular phylogenetic analysis. For the morphological analysis, the nematodes were killed by heating in water, then parasites were cleared in lactic acid. In this work we studied and measured 59 Oswaldocruzia nematodes, including 26 males and 33 females.
Microscopic examination, morphological measurements and drawings of the nematodes were performed using MBI-9 light microscope, drawing tube RA-7 and the Levenhuk M500 BASE Digital Camera. We made apical and transverse nematode sections manually using a razor blade.
The synlophe and spicules were described in accordance with Durette-Desset [72]. The nomenclature of the male caudal bursa is specified following Durette-Desset and Chabaud [73]. The studied morphological characteristics were the shape of cephalic vesicle, the shape of lateral alae at mid-esophagus level, the number of crests in the level of mid-body, the shape and structure of male caudal bursa, the shape of male spicules, the shape and structure of the dorsal ray of bursa. We also examined such morphometric features as body length, width at mid-body level, length of esophagus, length and width of cephalic vesicle, tail length, spicule length, egg length and width, and distances from the anterior end of the body to the vulva, excretory pore and nerve ring.
2.2. DNA Extraction, Amplification, Sequencing, and Phylogenetic Analysis
In order to obtain partial CoxI mtDNA sequences, seven specimens of ethanol-fixed Oswaldocruzia were dried at 37 °C in a dry block heater for 1 h. The specimens were then transferred to clear 500 µL tubes with a mixture of 48 µL 0.1% Chelex-100 and 2 µL Proteinase K (concentration 10 mg/mL) and incubated for 2 h at 55 °C and 25 min at 95 °C to stop proteinase activity. After that, the water solution of the total DNA was transferred to a sterile 500 µL tube and frozen at −20 °C. The amplification primer pair was JB3 (5′-TTT TTT GGG CAT CCT GAG GTT TAT-3′) and JB4,5 (5′-TAA AGA AAG AAC ATA ATG AAA ATG-3′) [74]. PCR was performed in 25 µL of reaction mix per PCR-tube in a BioRad C1000 thermal cycler. The following parameters were used: initial denaturation (3 min at 95 °C) followed by 35 cycles of 20 s at 95 °C, 20 s at 53 °C and 40 s at 72 °C, with 5 min at 72 °C for final extension [40]. Amplicons were directly sequenced using ABI-PRISM 3500xl sequencer (Life Technologies, Carlsbad, Massachusetts, USA) with the same primers. The sequences were mounted in general alignment with other nematode species, which were downloaded from GenBank NCBI using a custom R script [75,76] (Table 1). The sequences were automatically aligned using Muscle algorithm [77] as implemented in SeaView 4.0 [78]; the alignment was then trimmed manually. The phylogenetic analysis was performed using the maximum likelihood method at Cipres portal [79] with GTR+G+I model and a non-parametric bootstrap with 1000 pseudoreplicates. Bayesian analysis was performed with MrBayes on XSEDE 3.2.7a (GTR+G+I model). The model for phylogenetic analysis was chosen with MrModeltest 2.3 [80]. Trees were run as two separate chains (default heating parameters) for 15,000,000 generations. The quality of the chains was estimated using built-in MrBayes tools and, additionally, using the Tracer 1.6 package [81]. Based on the estimates, the first 25,000 generations were discarded for burn-in.
The p-distances were calculated using MEGA11 software [82] with standard parameters. Results of p-distance estimation were used as input data for ‘ComplexHeatmap’ library in R programming language [76,83].
3. Results
We found the nematodes O. ukrainae during a helminthological study of B. viridis from various localities of the Middle Volga region (Figure 1). These specimens were identified in toads from the Republic Mordovia and Samara region. At the same time, during the study of one individual of B. viridis from the vicinity of the Spassk city (Penza region), one male of O. filiformis was revealed.
3.1. Morphological Description of Nematodes
3.1.1. Morphology of Oswaldocruzia ukrainae
The body of the nematode is thin and elongated, with maximum width at mid-length. The anterior body end has a cephalic vesicle that is variable in shape (Figure 2b and Figure 3h).
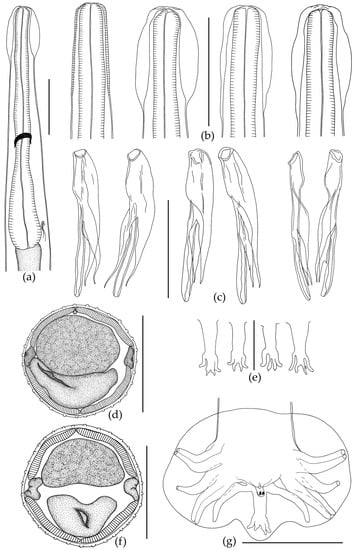
Figure 2.
Oswaldocruzia ukrainae male from Bufotes viridis. (a) Anterior body end, lateral view; (b) variation of cephalic vesicle; (c) spicules from different male specimens; (d) transverse section at mid-body, 32 crests; (e) variability of dorsal ray of bursa; (f) transverse section at mid-body, 35 crests; (g) caudal bursa, ventral view. (a–d,f,g) Scale bar, 0.1 mm; (e) scale bar, 0.05 mm.
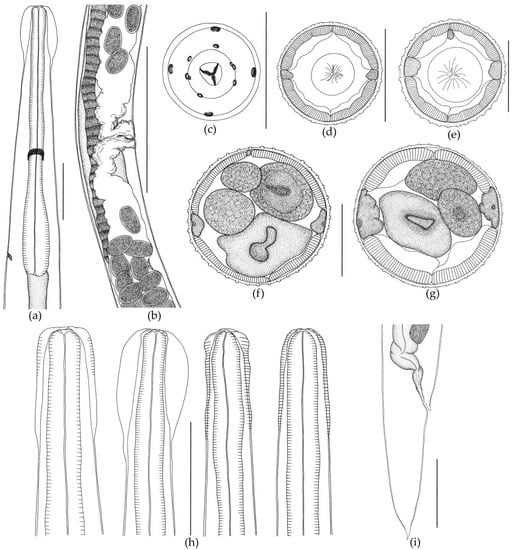
Figure 3.
Oswaldocruzia ukrainae female from Bufotes viridis. (a) Anterior body end, lateral view; (b) region of vulva and ovejector, left lateral view; (c) head, apical view; (d) transverse section at the level of mid-esophagus, 33 crests; (e) transverse section at the level of mid-esophagus, 35 crests; (f) transverse section at the level of mid-body, 42 crests; (g) transverse section at the level of mid-body, 46 crests; (h) variability of cephalic vesicle; (i) tail, left lateral view. (a,f–i) Scale bar, 0.1 mm; (b) scale bar, 0.5 mm; (c,f,e) scale bar, 0.05 mm.
Shape of vesicle is variable even in specimens of O. ukrainae from one individual toad. The cuticle forms continuous longitudinal ridges beginning behind the cephalic vesicle and running along the whole nematode body, forming a synlophe, which is symmetrical. Lateral alae are absent (Figure 3). The number of crests at mid-length of the body varies depending on sex of nematodes (Figure 2 and Figure 3, Table 2 and Table 3). Ridges are not continuous from the anterior end of body to the tail. They can end in one area, and then appear in another body part. The anterior end of the body is rounded. The triangular oral opening is surrounded by four large cephalic papillae and six external-labial papillae, of which the lateral papillae are close to small, not always distinguishable amphids (Figure 3). The esophagus is club-shaped, thin, cylindrical in the anterior part and wide in the posterior part. The posterior end of the esophagus is rounded with a posterior bulb (Figure 2 and Figure 3). Excretory pore position varies within the posterior third of the esophagus. The nerve ring surrounding the esophagus in the middle part is slightly closer to its posterior third (Figure 2 and Figure 3).

Table 2.
Morphometry of Oswaldocruzia ukrainae males.

Table 3.
Morphometry of Oswaldocruzia ukrainae females.
Male. The body ends with a wide caudal bursa that is symmetrical and three-lobed (two lateral and one dorsal). According to the classification of Durette-Desset and Chabaud [73], it belongs to type II. Ray 9 in male bursa always have a more or less S-shaped bend. Ray 10 always has extra branches of variable size (Figure 2). The genital cone is well developed, with two papillae (Figure 2). Gubernaculum is absent. Spicules are approximately equal and surrounded by a thin membrane. According to Durette-Desset [72], spicules are non-idiomorphic, with three branches: the internal branch is wide in the anterior part and narrows towards the posterior sharp end (Figure 2). The central branch is the longest, is rounded at the end, and has no extra offshoots. The external branch is narrow, sharp at the end; at the level of the last third it has a small offshoot (Figure 2). Measurements are presented in Table 2.
Female. The body is always larger than in males, tapering to the posterior end. The tail has a thin tip (Figure 3). The female reproductive system, like in all Oswaldocruzia species, is amphidelphic. Vulva is wide, opening postequatorially, behind the body at mid-length. The muscular vagina passes into paired ovejectors, which have powerful sphincters (Figure 3). The anterior ovary forms numerous loops in the anterior body part. The ovary reaches the level of esophagus and returns. The posterior ovary extends to the body end, strongly bends and turns forward slightly twisting, going beyond the level of the vulva. The uterus of adult females is filled with eggs. All eggs in vulva, ovejector and uterus were found at morula stage. Measurements of the main characteristics are presented in Table 3.
3.1.2. Morphology of Oswaldocruzia filiformis Male
The body is thin and elongated. The cephalic vesicle presents on the anterior body end (Figure 4). The cuticle forms longitudinal ridges starting behind the cephalic vesicle and running along the whole body. The anterior end of the body is rounded. The triangular oral opening has four large cephalic papillae and six externo-labial papillae. The esophagus is thin, cylindrical in the anterior part and wide in the posterior part. The posterior end of the esophagus forms a bulb (Figure 4).
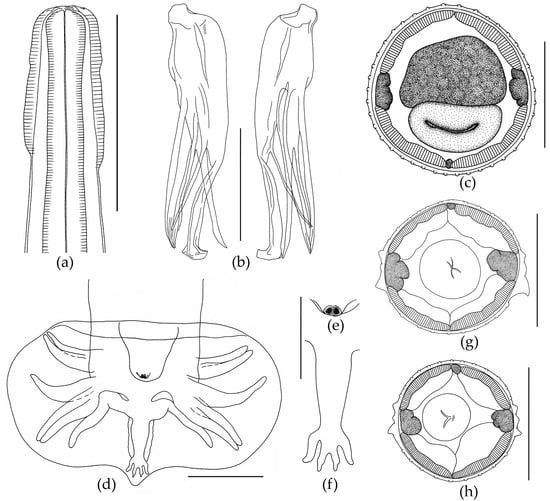
Figure 4.
Oswaldocruzia filiformis male from Bufotes viridis. (a) Cephalic vesicle; (b) spicules, lateral view; (c) transverse section at the level of mid-body, 40 crests; (d) caudal bursa, ventral view; (e) genital cone, ventral view; (f) dorsal ray of bursa; (g,h) transverse section at the level of mid-esophagus. (a–f) Scale bar, 0.1 mm; (g,h) scale bar, 0.05 mm.
The excretory pore is located at the level of the posterior third of the esophagus. The esophagus is surrounded by a nerve ring at the level of its middle part. Synlophe is symmetrical. On transverse sections, at the level of the second third of the esophagus, small cervical alae are clearly visible, formed by three slightly enlarged crests (Figure 4). Lateral alae consist of three ridges, with a more developed ventral crest. Two much smaller ridges (dorsal and central) are directly above the large ventral crest (Figure 4). At the level of the anterior part of the intestine, the lateral alae transform into simple ridges. The number of crests at mid-length of the body is 40 (Figure 4). A wide caudal bursa is at the end of the body. The caudal bursa is three-lobed, symmetrical and belongs to type II [73]. Ray 9 is in the form of an S-shaped bend. Extra branches on ray 10 are not expressed (Figure 4). The genital cone is well developed and has two papillae (Figure 4). According to Durette-Desset [72], spicules are idiomorphic, approximately equal and surrounded by a thin membrane. Each spicule has three branches, namely “blade”, “fork” and “shoe”. The end of the blade is visually divided into two branches, as well as distal parts of branches that visually split into two narrow offshoots. In fact, the branches do not split, but are tightly pressed against each other. The fork is divided into two branches approximately at mid-length. The shoe has a thin branch at mid-length and is slightly bent at its distal end (Figure 4). Measurements are presented in Table 4.

Table 4.
Morphometry of Oswaldocruzia filiformis from Bufotes viridis.
3.2. Molecular Phylogenetic Analysis
According to the results of molecular phylogenetic analysis, two clusters of specimens with almost full support are clearly distinguishable (Figure 5).
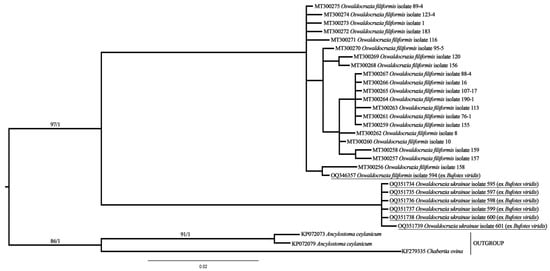
Figure 5.
Data on the molecular phylogenetic analysis of Oswaldocruzia spp. based on CoxI mtDNA gene sequences. ML/BI values are shown. Supports of deeply branched clades are not given due to its polyphyly and several mismatches between ML and BI analysis.
One cluster includes specimens of O. filiformis, and the other is a clade of O. ukrainae. One of the newly obtained sequences, belonging to O. filiformis, clustered together with all previously generated ones, although this specimen was collected from B. viridis, a typical host for O. ukrainae.
The presence of two clusters is also confirmed by the results of p-distance estimation. On average, the p-distance among specimens of the same species is about 0.01, but approximately 0.11 between O. filiformis and O. ukrainae (Figure 6). As in phylogenetic reconstruction, a single specimen of O. filiformis collected from B. viridis is clustered together with other representatives of this species from different hosts.
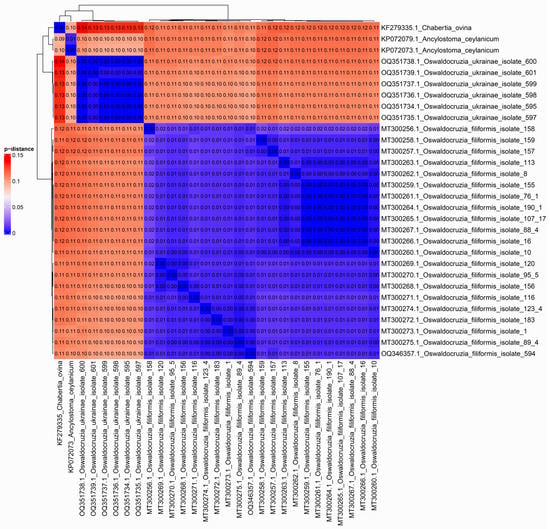
Figure 6.
Heatmap based on pairwise distances between CoxI mtDNA sequences of specimens under consideration. Numeric values of p-distances were translated into color: the greater the differences, the warmer the color. The specific values in the comparison pairs are indicated in the cells.
4. Discussion
Here we present a morphological description of two species of Oswaldocruzia nematodes of the European green toad Bufotes viridis inhabiting the Middle Volga region (European Russia) and new molecular phylogenetic data for these species. The combined use of both molecular and morphological methods allowed the reliable identification of Oswaldocruzia spp.
Morphological and molecular data obtained in our work showed that Bufotes viridis in European Russia is parasitized by two species of the Oswaldocruzia genus, O. ukrainae and O. filiformis. Oswaldocruzia ukrainae is the only species of the genus in the Western Palearctic with non-idiomorphic spicules formed by three branches [33,72]. The nematode specimens examined in our work correspond to earlier morphological descriptions of O. ukrainae [3,33,64].
Some differences in the morphometric characteristics of the nematodes O. ukrainae were revealed, as in previous studies of O. filiformis [40,41,42]. Oswaldocruzia ukrainae specimens from different B. viridis individuals collected at various localities of the Middle Volga region and even from one host individual also exhibit broad morphometric variability. We found variability of such morphometric characteristics as the body size, cephalic vesicles and spicules, the distances from the anterior end of the body to the excretory pore, nerve ring and vulva (Table 2 and Table 3). Variability in the number of ridges in the nematode mid-body was also revealed. In this study, the number of ridges in O. ukrainae males varies from 32 to 37, as well as in females from 40 to 48 (Table 2 and Table 3). We determined that not all crests extend continuously from the anterior body end to the tail. Some ridges may disappear and not reach the posterior body end. At the same time, new crests may appear elsewhere in the body. This may be one of the reasons for previously noticed variations in the number of crests. Moreover, the number of crests in Oswaldocruzia nematodes varies in young and mature individuals as well as in males and females, as we have shown earlier [40,41,42]. We believe that this is directly correlated with the size of Oswaldocruzia nematodes. Therefore, the largest Oswaldocruzia females will always have the maximum number of ridges in the mid-body. This is also revealed in the case of the nematode age. Thus, young small-sized nematodes will always have fewer crests than large adults. Egg sizes remained relatively stable (Table 2 and Table 3). Variability in the morphology of different O. ukrainae individuals was also revealed, as in the case of O. filiformis [40,41,42]. Thus, the shape of the head vesicle in individuals of both sexes varies greatly in nematodes from different toad individuals and from the same host specimen (Figure 2).
The spicules in O. ukrainae males were similar in structure and shape, but we noted differences in their sizes from different nematode individuals (Table 2). The shape and structure of the caudal bursa in O. ukrainae males were constant. However, the shape and structure of the dorsal ray of the bursa, which includes the 9 and 10 rays, are varied. All O. ukrainae males had an extra offshoot on the 10th ray of different size and shape (Figure 2). In females, the distance from the anterior end of the body to the vulva varied broadly and depended on the total length of the nematodes. Expectedly, this index was the highest in the largest nematode females (Table 3). In addition to O. ukrainae specimens, we also revealed one O. filiformis male in the examined individuals of B. viridis (Table 2 and Figure 3). Previously, we recorded O. filiformis in B. viridis from European Russia [42]. The description of specimen of O. filiformis studied in our work did not differ from the previous morphological descriptions of this species [3,33,40,41,42]. Thus, we confirmed the possibility of parasitism of both Oswaldocruzia species in the green toad B. viridis. Previously, the parasitism of these nematode species in B. viridis was reported by Ivanitsky [64], Ryzhikov et al. [3], Vashetko and Siddikov [13], Andreev [38] and Ikromov [63].
Thus, findings of O. filiformis in B. viridis are not uncommon and are probably related to the results of syntopic habitation with other amphibian species, especially during the breeding season in semiaquatic habitats. Infection of B. viridis with the nematode O. filiformis can also occur in habitats where it lives together with the Common toad, Bufo bufo (Linnaeus, 1758).
Bufotes viridis and B. bufo are sympatric species with overlapping ranges. In European Russia, the territory of the joint habitat of both toad species coincides with the boundaries of the forest-steppe zone. Both toad species lead the same ground-burrow, twilight lifestyle. These synanthropic species inhabit the same anthropogenic environments such as parks, gardens, cellars and agricultural landscapes, and often prefer them to natural habitats [52,84,85,86,87,88]. The similarity in lifestyles contributes to the infection of toads with common helminth species, including the nematode O. filiformis. Nematodes of this genus have a direct life cycle. Therefore, simple oral contact of toads with infective nematode larvae on a moist substrate, for example, feeding on terrestrial invertebrates, is sufficient to infect the host.
Ivanitsky [64] described another species of Oswaldocruzia that parasitizes B. viridis on the territory of Ukraine, O. fulleborni, which has not been recorded since the initial description. The main differences of the species were the following: the absence of lateral alae; synlophe consists of 30–50 crests; the “blade” branch is rounded and does not split at the end. We have previously noted varying degrees of the lateral alae development in O. filiformis from various hosts and even from the same host specimen [40,41,42]. It should be noted that the lateral alae are clearly visible only on transverse sections of nematodes, which are not presented in Ivanitsky’s work [64]. We also showed that the number of ridges in O. filiformis individuals can vary depending on the sex and age of the nematodes themselves. Thus, the number of ridges in mid-body of males of O. filiformis from different amphibian species can vary from 33 to 58; and in that of females—from 39 to 78 [40,41,42]. Therefore, these two morphological characteristics cannot be used in the diagnosis of Oswaldocruzia species. According to the description and drawing of the spicules of Oswaldocruzia males by Ivanitsky [64], we did not find any differences compared to O. filiformis. The visible shape of the “blade” end of the branch varies depending on the position of the spicule. We already noted that the distal parts of the “blade” branch are divided into two thin offshoots, but it is not always clearly visible [40]. In fact, these branches are always tightly adjacent to each other. We did not observed separation or splitting of these branches on any of the spicules of O. filiformis males [40,41,42].
Judging by the description of O. fulleborni by Ivanitsky [64], Ryzhikov et al. [3] believed that there are no significant differences between O. filiformis and O. fulleborni, and considered these species to be synonymous. Ben Sliman with coauthors [7] considers this species valid due to the difference in the crest number at the level of the mid-body and the structure of the “blade” branch of the spicule. Nevertheless, we agree with the opinion of Ryzhikov et al. [3] and consider O. fulleborni to be a synonym of O. filiformis, until the opposite is proven. It should also be noted that Svitin [33] found only O. ukrainae in the green toads during the study of Oswaldocruzia from the type area of O. fulleborni and other parts of Bufotes viridis range. Due to morphological similarity with O. filiformis, the species O. ukrainae may be misidentified. Therefore, diagnosis of both species should take into account the morphological and morphometric variability. Apparently, the species O. ukrainae could be misdiagnosed as O. filiformis in most of the early studies of Oswaldocruzia nematodes from B. viridis.
When identifying Oswaldocruzia nematodes, special care should be paid to the presence/absence of lateral alae. This cuticular structure can be deformed during collection, fixation, or production of whole mounts. Therefore, it is necessary to make transverse sections of nematodes. As our studies have shown, the number of crests also cannot be a diagnostic feature, since the ranges of the number of crests in the two nematode species overlap (Table 2, Table 3 and Table 4) [40,42].
There are also relative differences in body size between the two nematode species. Adult males and females of O. ukrainae are somewhat smaller than mature O. filiformis individuals. Thus, adult O. ukrainae males were not found larger than 7.83, and the body length of the smallest males was 5.00 (Table 2). The size of adult O. filiformis males from different species of amphibians was 6.25–13.25 [40,42]. The females of O. ukrainae in our study were 9.25–11.55 in length (Table 3), while the body sizes of adult O. filiformis females were 9.96–24.50 [40,42]. Thus, the body sizes of the nematodes should be taken into account, but not as a main feature, since they overlap. The only reliable morphological features in the diagnosis of O. ukrainae and O. filiformis are the size and structure of the male spicules (Figure 2 and Figure 3, Table 3 and Table 4) [40,42].
5. Conclusions
Our morphological and molecular phylogenetic data showed that the European green toad, Bufotes viridis, in the Middle Volga region is parasitized by two species of the genus Oswaldocruzia, the host-specific parasite O. ukrainae and species generalist O. filiformis. We assessed morphological characteristics for a reliable identification of two closely related Oswaldocruzia species under consideration and showed that only size and structure of male spicules can serve as the species diagnosis. The analysis of the morphological features of O. ukrainae from B. viridis both from various toad individuals and from the same host specimen reveals broad morphological and morphometric variability. In addition, high variability in O. ukrainae nematodes was revealed in the green toads from different geographical localities. The obtained data expand our knowledge of amphibian nematodes. We presented the first record of O. ukrainae parasitizing amphibians of the Middle Volga region. The results of our studies emphasize the need to confirm the validity of Oswaldocruzia species by molecular methods.
Author Contributions
Conceptualization, N.Y.K., A.A.K. and S.V.S.; methodology, S.V.S. and N.Y.K.; software, S.V.S. and A.A.K.; validation, N.Y.K., S.V.S. and A.A.K.; formal analysis, N.Y.K., A.A.K., S.V.S. and I.V.C.; investigation, N.Y.K., S.V.S., I.V.C. and A.A.K.; resources, A.A.K. and S.V.S.; writing—original draft preparation, A.A.K., N.Y.K., S.V.S. and I.V.C.; writing—review and editing, N.Y.K., A.A.K., S.V.S. and I.V.C.; supervision, A.A.K.; project administration, N.Y.K., A.A.K. and I.V.C.; funding acquisition, N.Y.K., A.A.K. and S.V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All applicable international, national, and institutional guidelines for the use and care of wild animals were followed. No animal was killed with the purpose of helminth sampling for our study. Our research was conducted in compliance with the ethical standards of humane treatment of animals according to the recommended standards of the Directive of the European Parliament and of the Council of the European Union of 22 September 2010 “On the protection of animals used for scientific purposes” (EU Directive 2010/63/EU).
Informed Consent Statement
Not applicable.
Data Availability Statement
GenBank numbers are given in the relevant section of the manuscript. Any other data is available after a reasonable request.
Acknowledgments
The authors are deeply grateful to Alexander Ruchin (Joint Directorate of the Mordovia State Nature Reserve and National Park “Smolny”) and Alexey Knyazev (Samara State University) for his help in field work; as well as to Sofia Denisova (Saint Petersburg State University) for help in preparing the manuscript. The work was carried out on research theme No. 1021060107212-5-1.6.20;1.6.19 “Change, sustainability and biological diversity conservation under the global climate change impact and intense anthropogenic pressure on the ecosystems of the Volga River basin” of the Institute of Ecology of the Volga River Basin of RAS, a branch of the Samara Federal Research Center of the Russian Academy of Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dufresnes, C.; Mazepa, G.; Jablonski, D.; Oliveira, R.C.; Wenseleers, T.; Shabanov, D.A.; Auer, M.; Ernst, R.; Koch, C.; Ramírez-Chaves, H.E.; et al. Fifteen shades of green: The evolution of Bufotes toads revisited. Mol. Phylogenet. Evol. 2019, 141, 106615. [Google Scholar] [CrossRef] [PubMed]
- Bufotes viridis. Amphibian Species of the World 6.1, an Online Reference. American Museum of Natural History. Available online: https://amphibiansoftheworld.amnh.org/ (accessed on 22 January 2023).
- Ryzhikov, K.M.; Sharpilo, V.P.; Shevchenko, N.N. Helminths of Amphibians in the USSR Fauna; Nauka: Moscow, Russia, 1980; pp. 1–279. [Google Scholar]
- Baker, M.R. On three Oswaldocruzia spp. (Trichostrongyloidea: Molineidea) in amphibians from Africa. Can. J. Zool. 1981, 59, 246–251. [Google Scholar] [CrossRef]
- Durette-Desset, M.C.; Batcharov, A.; Ben Slimane, B.; Chabaud, A. Some Oswaldocruzia (Nematoda: Trichostrongyloidea) parasites of Amphibia in Bulgaria. Redescription of Oswaldocruzia bialata (Molin, 1860). Helminthologia 1993, 30, 99–104. [Google Scholar]
- Ben Slimane, B.; Durette-Desset, M.C. Quatre nouvelles espéces du genre Oswaldocruzia Travassos, 1917 (Nematoda: Trichostrongyloidea) parasites d’Amphibiens d’ Equateur. Rev. Suiss. Zool. 1993, 100, 113–136. [Google Scholar] [CrossRef]
- Ben Slimane, B.; Lluch, J.; Durette-Desset, M.C. Two new species of the genus Oswaldocruzia Travassos, 1917 (Nematode: Trichostrongylina: Molineidae) parasitizing Spanish amphibians. Res. Rev. Parasitol. 1995, 55, 209–215. [Google Scholar]
- Moravec, F.; Kaiser, H. Helminth parasites from West Indian frogs, with descriptions of two new species. Carib. J. Sci. 1995, 31, 252–268. [Google Scholar]
- Ben Slimane, B.; Durette-Desset, M.-C. Four new species of Oswaldocruzia (Nematoda: Trichostrongylina, Molineoidea) Parasitizing amphibians and lizards from Ecuador. Mem. Inst. Osw. Cruz 1996, 91, 317–328. [Google Scholar] [CrossRef]
- Ben Slimane, B.; Durette-Desset, M.-C. New Oswaldocruzia (Nematoda, Trichostrongylina, Molineoidea) parasites of amphibians from French Guyana and Ecuador. Misc. Zool. 1996, 19, 55–66. [Google Scholar]
- Ben Slimane, B.; Chabaud, A.G.; Durette-Desset, M.-C. Les Nématodes Trichostrongylina parasites d’Amphibiens et de Reptiles: Problèmes taxonomiques, phylétiques et biogéographiques. Syst. Parasitol. 1996, 35, 179–206. [Google Scholar] [CrossRef]
- Ben Slimane, B.; Guerrero, R.; Durette-Desset, M.C. Oswaldocruzia venezuelensis sp. n. (Nematoda: Trichostrongylina, Molineoidea), a parasite of Bufo marinus from Venezuela. Folia Parasitol. 1996, 43, 297–300. [Google Scholar]
- Vashetko, E.V.; Siddikov, H.B. The effect of the ecology of toads on the distributions of helmints. Turk. J. Zool. 1999, 23, 107–110. [Google Scholar]
- Yildirimhan, H.S. Researches on parasitic helminths of Bufo viridis Laurenti, 1768 (Anura: Amphibia). Turk. J. Zool. 1999, 23, 177–195. [Google Scholar]
- Kirillov, A.A. Helminth fauna of reptiles from Samara region. Proc. Sam. Sci. Cent. RAS 2000, 2, 324–329. [Google Scholar]
- Galli, P.; Crosa, G.; Gentilli, A.; Santagostino, M. New geographical records of parasitic nematodes from Bufo bufo in Italy. Parassitologia 2001, 43, 147–149. [Google Scholar] [PubMed]
- Bursey, C.R.; Goldberg, S.R. Cosmocerca vrcibradici n. sp. (Ascaridida: Cosmocercidae), Oswaldocruzia vitti n. sp. (Strongylida: Molineoidae), and other helminths from Prionodactylus eigenmanni and Prionodactylus oshaughnessyi (Sauria: Gymnophthalmidae) from Brazil and Ecuador. J. Parasitol. 2004, 90, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Bursey, C.R.; Goldberg, S.R. New species of Oswaldocruzia (Nematoda: Molineoidae), new species of Rhabdias (Nematoda: Rhabdiasidae), and other helminths in Rana cf. forreri (Anura: Ranidae) from Costa Rica. J. Parasitol. 2005, 91, 600–605. [Google Scholar] [CrossRef]
- Bursey, C.R.; Goldberg, S.R.; Vitt, L.J. New species of Oswaldocruzia (Nematoda: Molineidae) in Ameiva festiva (Squamata: Teiidae) from Nicaragua. J. Parasitol. 2006, 92, 350–352. [Google Scholar] [CrossRef]
- Durette-Desset, M.C.; Alves dos Anjos, L.; Vrcibradic, D. Three new species of the genus Oswaldocruzia Travassos, 1917 (Nematoda, Trichostrongylina, Molineoidea) parasites of Enyalius spp. (Iguanidae) from Brazil. Parasite 2006, 13, 115–125. [Google Scholar] [CrossRef]
- Bursey, C.R.; Goldberg, S.R.; Telford, S.R. Gastrointestinal helminths of 14 species of lizards from Panama with descriptions of five new species. Comp. Parasitol. 2007, 74, 108–140. [Google Scholar] [CrossRef]
- Murvanidze, L.; Nikolaishvili, K.; Lomidze, T. The annotated list of amphibians of Georgia. Proc. Inst. Zool. 2008, 23, 43–49. [Google Scholar]
- Santos, J.N.; Giese, E.G.; Maldonado, A.J.; Lanfredi, R.M. A new species of Oswaldocruzia (Molineidae: Nematoda) in Chaunus marinus (Amphibian: Bufonidae) (Linneaus, 1758) from Brazil. J. Parasitol. 2008, 94, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Schotthoefer, A.M.; Bolek, M.G.; Cole, R.; Beasley, V.R. Parasites of the Mink Frog (Rana septentrionalis) from Minnesota, U.S.A. Comp. Parasitol. 2009, 76, 240–246. [Google Scholar] [CrossRef]
- Düşen, S.; Oğuz, M.C. Metazoan endoparasites of three species of anurans collected from the Middle Black Sea Region of Turkey. Helminthologia 2010, 47, 226–232. [Google Scholar] [CrossRef]
- Dusen, S.; Oguz, M.C.; Barton, D.P.; Aral, A.; Sulekoglu, S.; Tepe, Y. Metazoan parasitological research on three species of anurans collected from Çanakkale Province, Northwestern Turkey. North-West. J. Zool. 2010, 6, 25–35. [Google Scholar]
- Mohammad, M.K.; Al-Moussawi, A.A.; Jasim, S.Y. Helminth parasites of the Green Toad Bufo viridis Laurenti, 1768 in Baghdad Area, Central Iraq. Egypt. Acad. J. Biol. Sci. 2010, 2, 17–25. [Google Scholar] [CrossRef]
- Bursey, C.R.; Goldberg, S.R. New species of Oswaldocruzia (Nematoda: Molineidae) and other helminths in Bolitoglossa subpalmata (Caudata: Plethodontidae) from Costa Rica. J. Parasitol. 2011, 97, 286–292. [Google Scholar] [CrossRef]
- Svitin, R.; Kuzmin, Y. Oswaldocruzia duboisi (Nematoda, Molineidae): Morphology, Hosts and Distribution in Ukraine. Vest. Zool. 2012, 46, 1–9. [Google Scholar] [CrossRef]
- Guerrero, R. Two new species of Oswaldocruzia (Nematoda: Trichostrongylina: Molineoidea) parasites of the cane toad Rhinella marina (Amphibia: Anura) from Peru. Acta Parasitol. 2013, 58, 30–36. [Google Scholar] [CrossRef]
- Svitin, R.S. New Data on the morphology and distribution of Oswaldocruzia skrjabini (Nematoda, Molineidae). Vest. Zool. 2015, 46, 195–203. [Google Scholar] [CrossRef]
- Herczeg, D.; Vörös, J.; Végvári, Z.; Kuzmin, Y.; Brooks, D.R. Helminth Parasites of the Pelophylax esculentus Complex (Anura: Ranidae) in Hortobágy National Park (Hungary). Comp. Parasitol. 2016, 83, 36–48. [Google Scholar] [CrossRef]
- Svitin, R. Nematodes of the genus Oswaldocruzia Travassos, 1917 of Western Palaearctic. Ph.D. Thesis, Institute of Zoology, Kyiv, Ukraine, 2016. [Google Scholar]
- Willkens, Y.; Maldonado, A.; dos Santos, J.N.; Maschio, G.F.; Melo, F.T.D.V. Redescription of Oswaldocruzia chambrieri (Strongylida: Molineidae) from Rhinella margaritifera (Anura: Bufonidae) in Caxiuanã National Forest, Brazil. Acta Parasitol. 2016, 61, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Kirillov, A.A.; Kirillova, N.Y. Overview of helminths in reptiles of the National Park «Samarskaya Luka» (Russia). Nat. Conserv. Res. 2018, 3 (Suppl. 1), 73–82. [Google Scholar] [CrossRef]
- Larrat, Y.M.; Melo, F.T.D.V.; Gomes, T.F.F.; Wilkens, Y.; dos Santos, J.N. Oswaldocruzia lanfrediae n. sp. (Strongylida: Molineidae), a parasite of Leptodactylus paraensis Heyer (Anura: Leptodactylidae) in Brazil. Syst. Parasitol. 2018, 95, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Sinsch, U.; Heneberg, P.; Těšínský, M.; Balczun, C.; Scheid, P. Helminth endoparasites of the smooth newt Lissotriton vulgaris: Linking morphological identification and molecular data. J. Helminthol. 2019, 93, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Andreev, V.Y. To the helminth fauna of the green toad (Bufo viridis Laur.). In Ecological and Biological Problems of the Caspian Sea Basin: Materials of VIII International Conference (11–12 October 2005); Pilipenko, V.N., Ed.; Astrakhan University: Astrakhan, Russia, 2005; pp. 3–5. [Google Scholar]
- Kalmykov, A.P.; Semenova, N.N.; Ivanov, V.M. Helminths in the Volga Delta Ecosystem. Volume 2. Nematodes; Print: Izhevsk, Russia, 2017; pp. 1–350. [Google Scholar]
- Kirillova, N.Y.; Kirillov, A.A.; Shchenkov, S.V.; Chikhlyaev, I.V. Oswaldocruzia filiformis sensu lato (Nematoda: Molineidae) from amphibians and reptiles of European Russia: Morphological and molecular data. Nat. Cons. Res. 2020, 5, 41–56. [Google Scholar] [CrossRef]
- Kirillova, N.Y.; Kirillov, A.A. Morphological variability of Oswaldocruzia filiformis (Nematoda: Molineidae) in reptiles inhabiting the protected areas of the Republic of Mordovia (Russia). IOP Conf. Ser. Earth Environ. Sci. 2020, 607, 012007. [Google Scholar] [CrossRef]
- Kirillova, N.Y.; Kirillov, A.A.; Chikhlyaev, I.V. Morphological variability of Oswaldocruzia filiformis (Nematoda: Molineidae) in amphibians from European Russia. IOP Conf. Ser. Earth Environ. Sci. 2021, 818, 012018. [Google Scholar] [CrossRef]
- Moravec, F.; Vojtkova, L. Variabilität von zwei Nematodenarten Oswaldocruzia filiformis (Goeze, 1782) und Oxysomatium brevicaudatum (Zeder, 1800). In Der Gemeinsamen Parasiten der Europäischen Amphibien und Reptilien. Scripta Fac. Sci. Natur. Univ. Purk. Brun. Biol. 1975, 2, 61–76. [Google Scholar]
- Shaldybin, S.L. To the parasite fauna of anurans in the Volga-Kama reserve. In Issues of Herpetology; Darevsky, I.S., Ed.; Nauka: Leningrad, Russia, 1977; pp. 228–230. [Google Scholar]
- Smirnova, M.I.; Gorshkov, P.K.; Sizova, V.G. Helminth Fauna of Tailless Amphibians in Tatarstan Republic; Institute of Biology, Kazan Branch of Academy of Sciences of the USSR: Kazan, Russia, 1987; pp. 3–19. [Google Scholar]
- Ayupov, K.V.; Valiullin, S.M.; Khaziev, G.Z.; Bayanov, M.G.; Kazadaev, V.I.; Antonov, N.P. Helminths of animals, humans and plants in the Bashkirian ASSR. In Helminths of Animals, Humans and Plants in the Southern Urals; Bayanov, M.G., Ed.; Bashkiria Branch of Academy of Sciences of the USSR: Ufa, Russia, 1974; pp. 8–29. [Google Scholar]
- Bayanov, M.G.; Yumagulova, G.R. Helminths in anurans from various habitats. Res. Biol. Res. 2000, 6, 153–155. [Google Scholar]
- Petrova, S.V.; Bayanov, M.G. Helminths in toads (Amphibia, Bufonidae) in Bashkiria. Res. Biol. Res. 2000, 6, 155–157. [Google Scholar]
- Yumagulova, G.R. Helminths of amphibians in the Southern Urals. Ph.D. Thesis, Bashkirian State University, Ufa, Russia, 2000. [Google Scholar]
- Ravkovskaya, E.A.; Polyakova, N.A.; Terekhina, M.S.; Pyatova, M.V.; Lada, G.A. The first data about the helminths in the green toad Bufotes viridis (Laurenti, 1768) in the Tambov region. In Recent Problems of Parasitology and Ecology: Readings Dedicated to the Memory of S.S. Shulman; Kirillov, A.A., Ed.; Anna: Togliatti, Russia, 2018; pp. 223–228. [Google Scholar]
- Vekhnik, V.P.; Golovatyuk, L.V.; Goreslavets, I.N.; Dyuzhaeva, I.V.; Zharikov, V.V.; Zinchenko, T.D.; Kirillov, A.A.; Kirillova, N.Y.; Krasnobaev, Y.P.; Krasnobaeva, T.P.; et al. Cadastre of Invertebtates of the Samarskaya Luka; Ofort: Samara, Russia, 2007; pp. 1–471. [Google Scholar]
- Fayzulin, A.I.; Chikhlyaev, I.V.; Kuzovenko, A.E. Amphibians of the Samara Region; Kassandra: Togliatti, Russia, 2013; pp. 1–140. [Google Scholar]
- Chikhlyaev, I.V.; Fayzulin, A.I.; Kuzovenko, A.E. The analysis of helminthes fauna of a green toad Bufotes viridis (Laurenti, 1768) on the urbanized territories of the Samara region. Proc. Sam. Sci Cent. RAS 2017, 19, 178–184. [Google Scholar]
- Chikhlyaev, I.V.; Kirillova, N.Y.; Kirillov, A.A. Overview of helminths of amphibians (Amphibia) from Samara region. Proc. Sam. Sci. Cent. RAS 2018, 20, 385–400. [Google Scholar]
- Kirillov, A.A.; Kirillova, N.Y.; Chikhlyaev, I.V. Parasites of Vertebrates of the Samara Region; Polyar: Togliatti, Russia, 2018; pp. 1–304. [Google Scholar]
- Kostyunin, V.M. Helminth Fauna of Land Vertebrates in the Middle Volga Region; Nizhny Novgorod State Pedagogical University: Nizhny Novgorod, Russia, 2010; pp. 1–225. [Google Scholar]
- Davletbakova, G.M.; Yumagulova, G.R. Helminths of tailless amphibians in the Orenburg region. In Ecological Collection Papers: Proceedings of Young Scientists of the Volga Region; Saxonov, S.V., Ed.; Cassandra: Togliatti, Russia, 2013; pp. 31–34. [Google Scholar]
- Burakova, A.V.; Vershinin, V.L. Analysis of the parasite fauna of syntopically inhabiting representatives of anurans. Vest. St. Petersb. Univ. Ser. 3. Biol. 2016, 3, 31–36. [Google Scholar] [CrossRef]
- Shimalov, V.V.; Shimalov, V.T. Helminth fauna of toads in Belorussian Polesie. Parasitol. Res. 2001, 87, 84. [Google Scholar] [CrossRef] [PubMed]
- Shimalov, V.V. Helminth fauna of amphibians (Vertebrata: Amphibia) in the Republic of Belarus. Parazitologiia 2009, 43, 118–129. [Google Scholar]
- Buchvarov, G.K. Catalog of Helminths of Amphibians in Bulgaria; Plovdiv University, P. Hilendarski: Plovdiv, Bulgaria, 1977; pp. 1–53. [Google Scholar]
- Schad, G.A.; Kuntz, R.E.; Wells, W.H. Nematode Parasites from Turkish Vertebrates: An Annotated List. Can. J. Zool. 1960, 38, 949–963. [Google Scholar] [CrossRef]
- Ikromov, E.F. Some patterns of infection of amphibians with helminths depending on age. World Sci. Discov. 2010, 9, 33–36. [Google Scholar]
- Ivanitsky, S.V. Materials for the helminth fauna of Ukrainian vertebrates (fauna of cestodes, nematodes and acanthocephalans). Proc. Kharkov Vet. Inst. 1940, 19, 129–155. [Google Scholar]
- Sudarikov, V.E. Helminth fauna of vertebrates in the Middle Volga region. Proc. Helminthol. Lab. Acad. Sci. USSR 1951, 5, 326–330. [Google Scholar]
- Kozak, A. Helminth fauna of frogs from the surroundings of Kosice. Biologia 1966, 21, 606–611. [Google Scholar]
- Chikhlyaev, I.V.; Ruchin, A.B.; Fayzulin, A.I. The helminth fauna study of European common toad in the Volga Basin. Nat. Environ. Poll. Tech. 2016, 15, 1103–1109. [Google Scholar]
- Chikhlyaev, I.V.; Ruchin, A.B. An Overview of the Helminths of Moor Frog Rana arvalis Nilsson, 1842 (Amphibia: Anura) in the Volga Basin. Diversity 2021, 13, 61. [Google Scholar] [CrossRef]
- Chikhlyaev, I.V.; Ruchin, A.B. Ecological Analysis and Biodiversity of the Helminth Community of the Pool Frog Pelophylax lessonae (Amphibia: Anura) from Floodplain and Forest Water Bodies. Diversity 2022, 14, 247. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, G.-H.; Zhao, G.-H.; Cai, J.-Z.; Zhu, X.-Q.; Qian, A.-D. Genetic differences between Chabertia ovina and C. erschowirevealed by sequence analysis of four mitochondrial genes. Mitochondrial DNA 2013, 26, 167–170. [Google Scholar] [CrossRef]
- Hu, W.; Yu, X.; Wu, S.; Tan, L.; Song, M.; Abdulahi, A.; Wang, Z.; Jiang, B.; Li, G. Levels of Ancylostoma infections and phylogenetic analysis of cox 1 gene of A. ceylanicum in stray cat faecal samples from Guangzhou, China. J. Helminthol. 2016, 90, 392–397. [Google Scholar] [CrossRef]
- Durette-Desset, M.-C. Trichostrongyloid Nematodes and their Vertebrate Hosts: Reconstruction of the Phylogeny of a Parasitic Group. Adv. Parasitol. 1985, 24, 239–306. [Google Scholar] [CrossRef]
- Durette-Desset, M.-C.; Chabaud, A.G. Nouvel essai de classification des Nématodes Trichostrongyloidea. Ann. Parasitol. Hum. Comp. 1981, 56, 297–312. [Google Scholar] [CrossRef]
- Bowles, J.; Blair, D.; McManus, D. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol. Biochem. Parasitol. 1992, 54, 165–173. [Google Scholar] [CrossRef]
- Paradis, E.; Schliep, K. ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available online: http://www.r-project.org/index.html (accessed on 22 January 2023).
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView Version 4: A Multiplatform Graphical User Interface for Sequence Alignment and Phylogenetic Tree Building. Mol. Biol. Evol. 2010, 27, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 45–52. [Google Scholar] [CrossRef]
- Nylander, J.A.A. MrModeltest v2. Program Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Gu, Z. Complex heatmap visualization. Imeta 2022, 1, 1–15. [Google Scholar] [CrossRef]
- Stöck, M.; Roth, P.; Podloucky, R.; Grossenbacher, K. Wechselkröten unter Berücksichtigung von Bufo viridis viridis Laurenti, 1768; Bufo variabilis (Pallas, 1769); Bufo boulengeri Lataste, 1879; Bufo balearicus Böttger, 1880 und Bufo siculus Stöck, Sicilia, Belfiore, Lo Brutto, Lo Valvo und Arculeo, 2008. In Handbuch der Reptilien und Amphibien Europas, Band 5/II, Froschlurche (Anura) II (Hylidae, Bufonidae); Grossenbacher, K., Ed.; AULA-Verlag: Wiebelsheim, Germany, 2009; pp. 413–498. [Google Scholar]
- Kovacs, E.-H.; Sas, I. Aspects of breeding activity of Bufo viridis in an urban habitat: A case study in Oradea, Romania. Biharean Biol. 2010, 4, 73–77. [Google Scholar]
- Kuzmin, S.L. Amphibians of the Former USSR, 2nd ed.; KMK: Moscow, Russia, 2012; pp. 1–370. [Google Scholar]
- Kaczmarski, M.; Szala, K.; Kloskowski, J. Early onset of breeding season in the green toad Bufotes viridis in Western Poland. Herpetozoa 2019, 32, 109–112. [Google Scholar] [CrossRef]
- Konowalik, A.; Najbar, A.; Konowalik, K.; Dylewski, Ł.; Frydlewicz, M.; Kisiel, P.; Starzecka, A.; Zaleśna, A.; Kolenda, K. Amphibians in an urban environment: A case study from a central European city (Wrocław, Poland). Urban Ecosyst. 2019, 23, 235–243. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).