Circulating Small RNA Profiling of Patients with Alveolar and Cystic Echinococcosis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. RNA Isolation, Library Construction, and Small RNA Sequencing
2.3. Small RNA Sequencing Data Pre-Processing and Analysis
2.3.1. Identification of miRNAs
2.3.2. Identification of Non-miRNA sRNAs
2.3.3. Expression and Correlation Analyses
3. Results
3.1. Characteristics of Patients
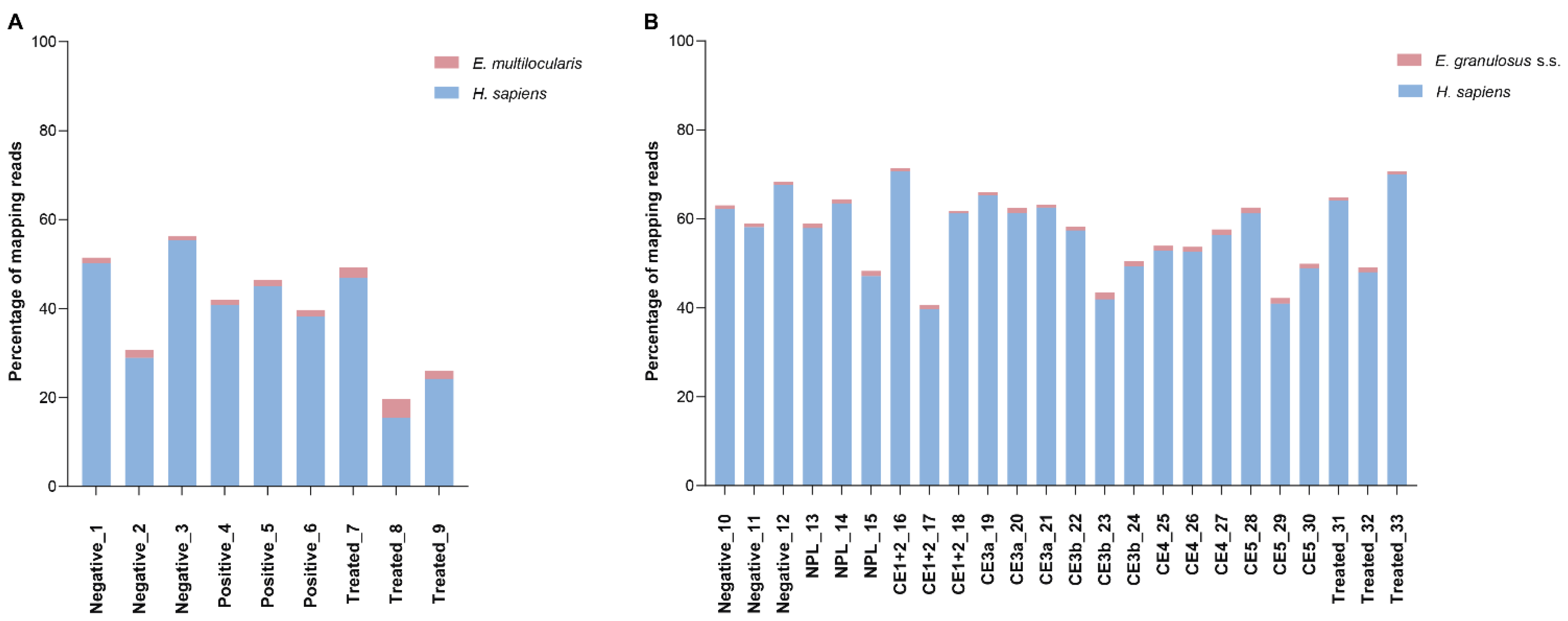
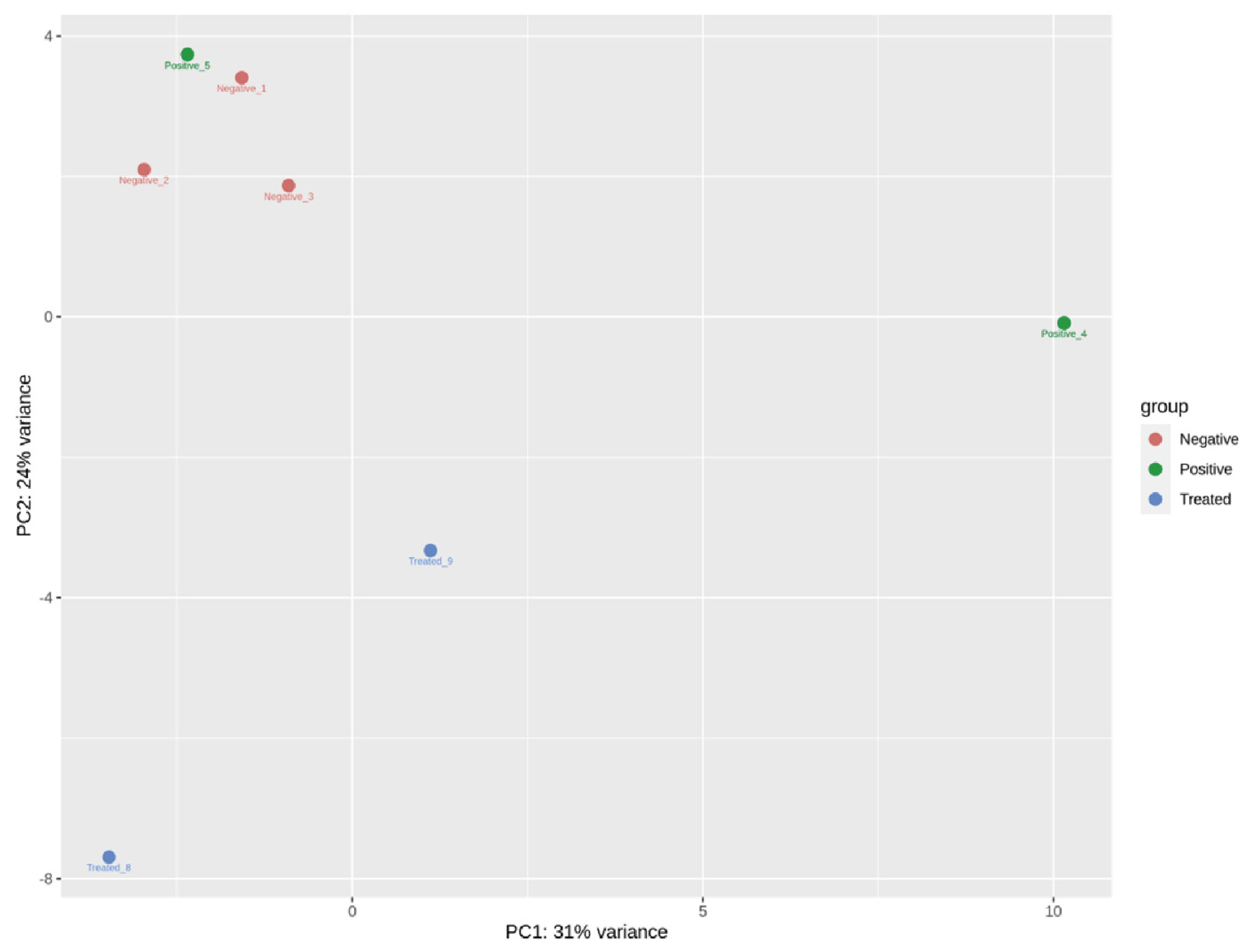
3.2. Overall Sequencing Results
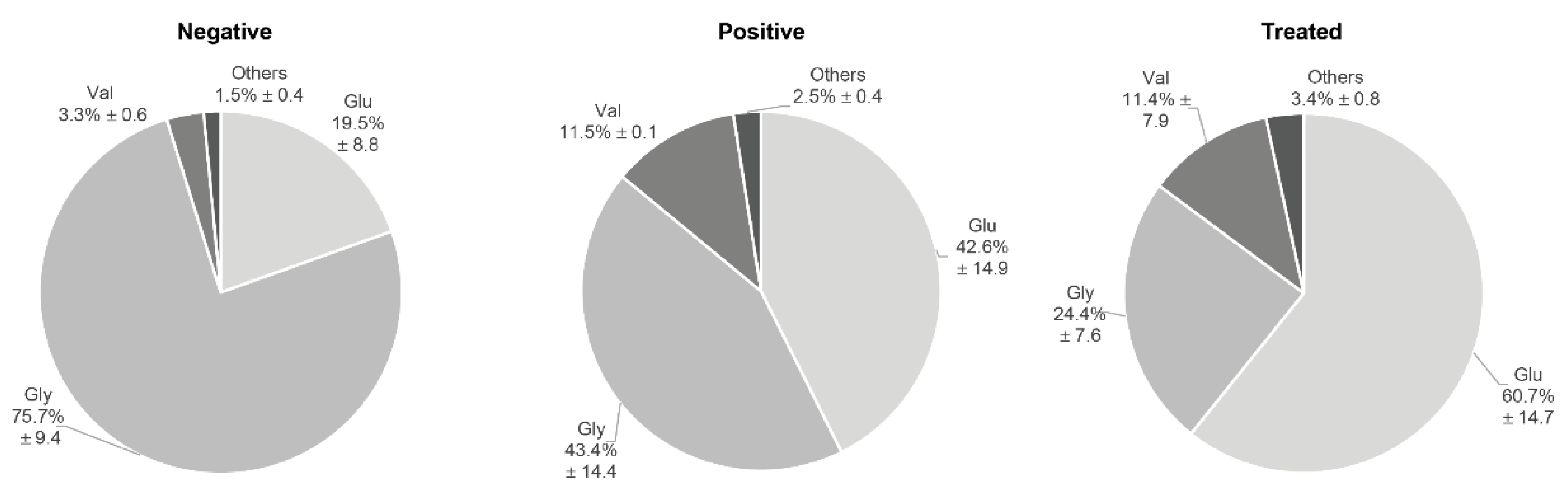
3.3. Circulating Endogenous sRNA Profile in AE Patients
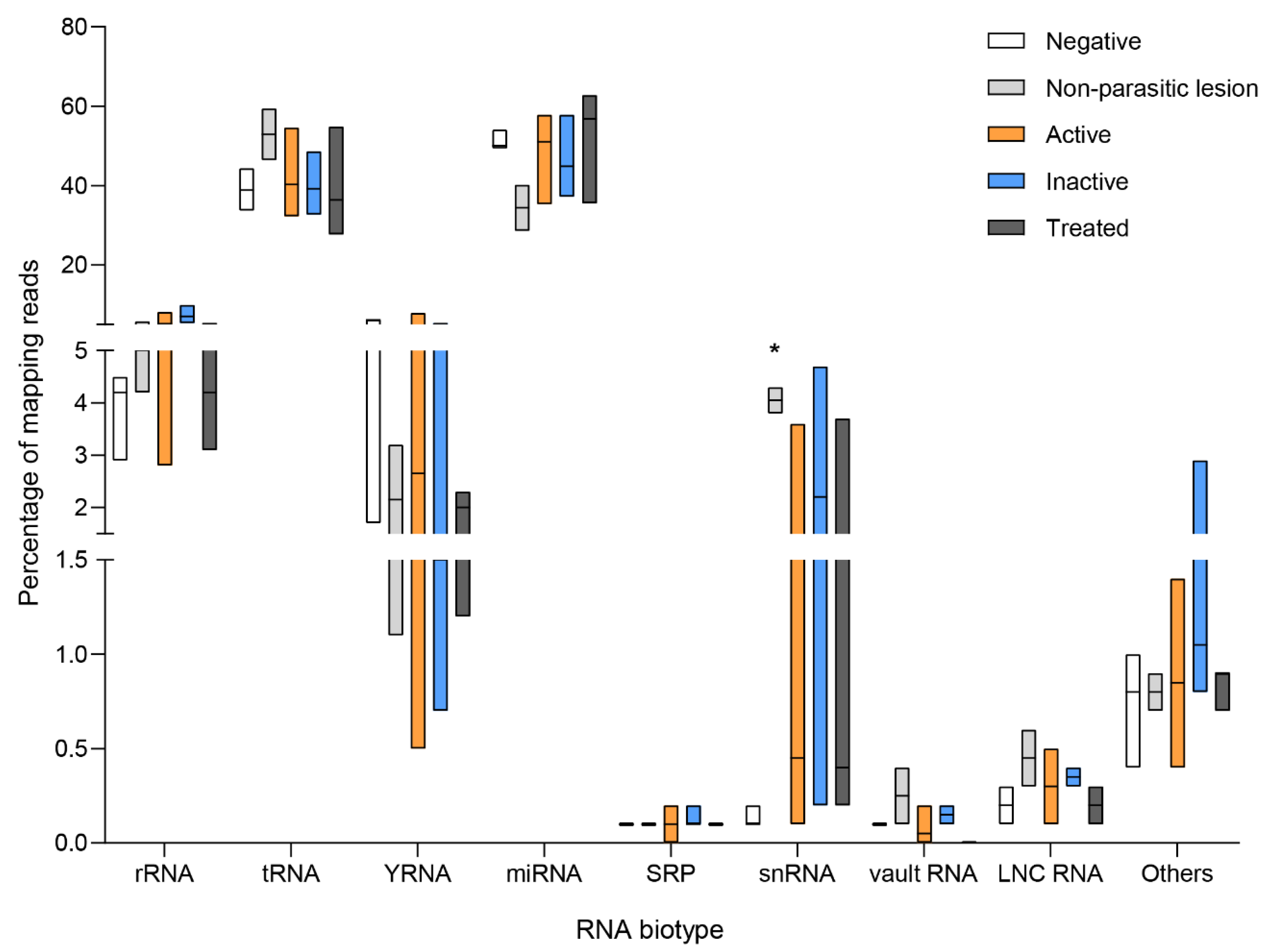
3.4. Circulating Endogenous sRNA Profile in CE Patients
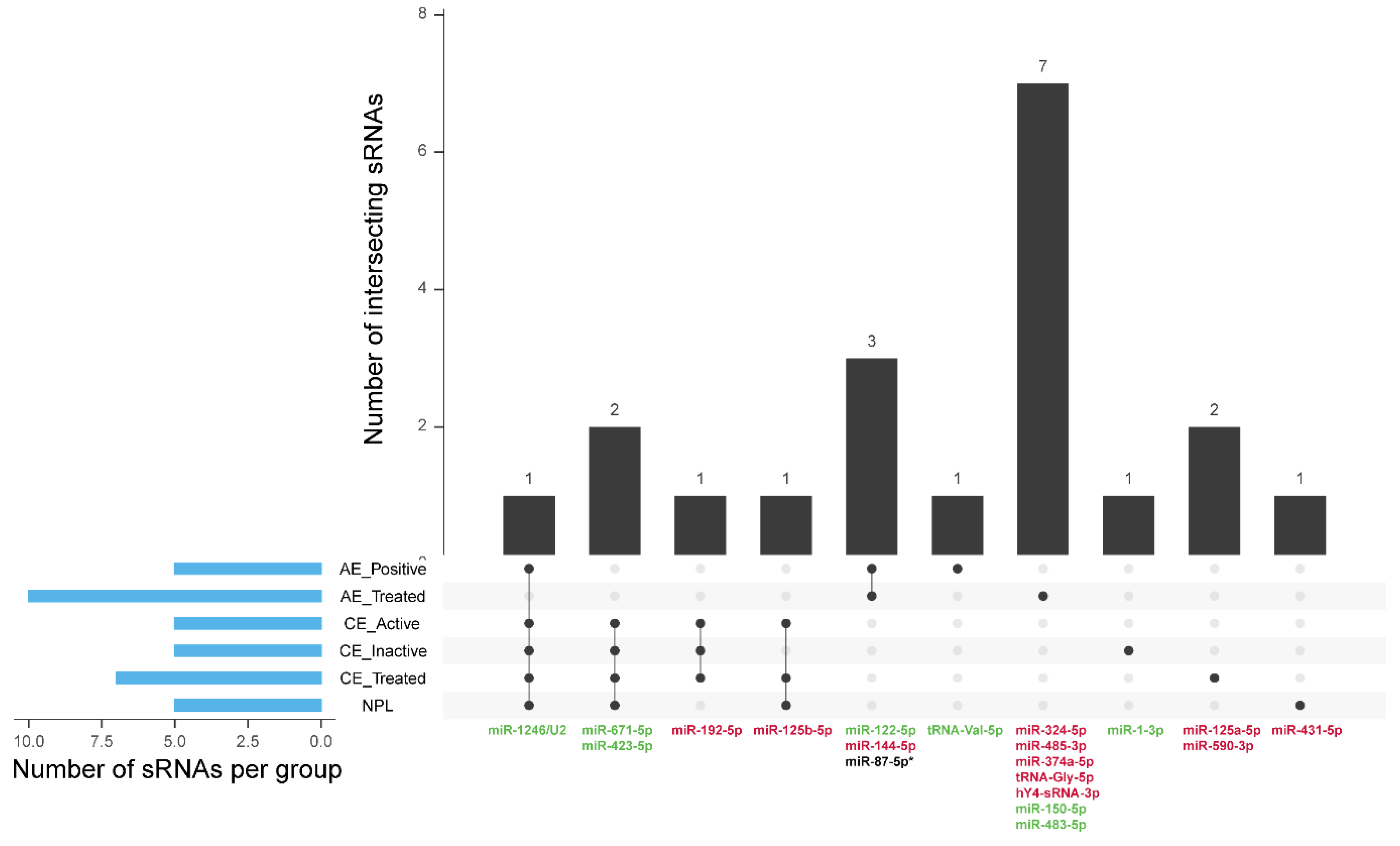
3.5. Parasite sRNAs
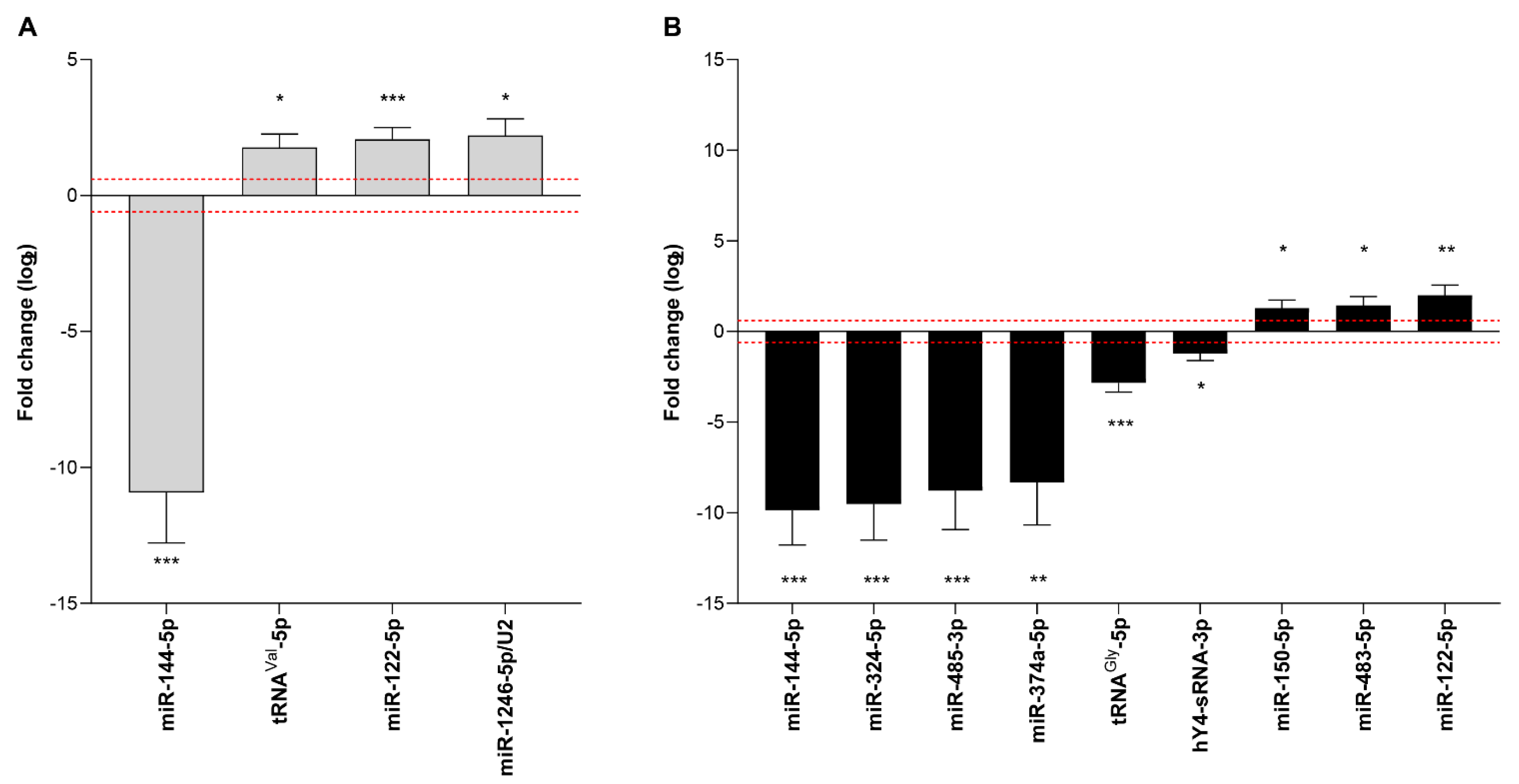
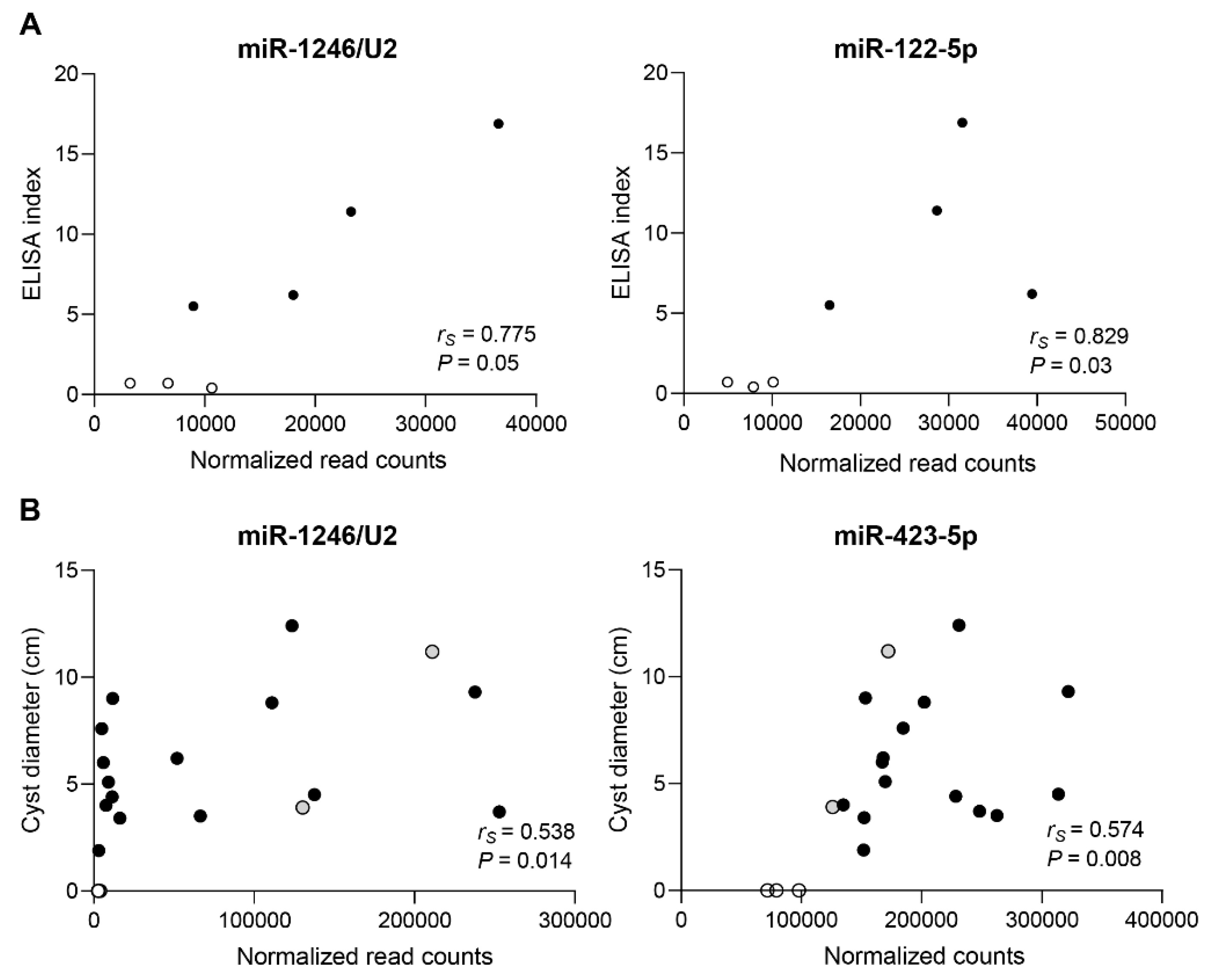
3.6. Diagnostic Potential of Endogenous Circulating sRNAs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maldonado, L.L.; Assis, J.; Araújo, F.M.G.; Salim, A.C.M.; Macchiaroli, N.; Cucher, M.; Camicia, F.; Fox, A.; Rosenzvit, M.; Oliveira, G.; et al. The Echinococcus Canadensis (G7) Genome: A Key Knowledge of Parasitic Platyhelminth Human Diseases. BMC Genom. 2017, 18, 204. [Google Scholar] [CrossRef] [PubMed]
- Tsai, I.J.; Zarowiecki, M.; Holroyd, N.; Garciarrubio, A.; Sánchez-Flores, A.; Brooks, K.L.; Tracey, A.; Bobes, R.J.; Fragoso, G.; Sciutto, E.; et al. The Genomes of Four Tapeworm Species Reveal Adaptations to Parasitism. Nature 2013, 496, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Gottstein, B.; Soboslay, P.; Ortona, E.; Wang, J.; Siracusano, A.; Vuitton, D. Immunology of Alveolar and Cystic Echinococcosis (AE and CE). Adv. Parasitol. 2017, 96, 1–54. [Google Scholar] [CrossRef] [PubMed]
- Mehlhorn, H.; Eckert, J.; Thompson, R.C. Proliferation and Metastases Formation of Larval Echinococcus Multilocularis. II. Ultrastructural Investigations. Z. Parasitenkd. 1983, 69, 749–763. [Google Scholar] [CrossRef] [PubMed]
- Koziol, U.; Rauschendorfer, T.; Zanon Rodríguez, L.; Krohne, G.; Brehm, K. The Unique Stem Cell Sysem of the Immortal Larva of the Human Parasite Echinococcus Multilocularis. Evodevo 2014, 5, 10. [Google Scholar] [CrossRef]
- Frider, B.; Larrieu, E.; Odriozola, M. Long-Term Outcome of Asymptomatic Liver Hydatidosis. J. Hepatol. 1999, 30, 228–231. [Google Scholar] [CrossRef]
- Larrieu, E.; Uchiumi, L.; Salvitti, J.C.; Sobrino, M.; Panomarenko, O.; Tissot, H.; Mercapide, C.H.; Sustercic, J.; Arezo, M.; Mujica, G.; et al. Epidemiology, Diagnosis, Treatment and Follow-up of Cystic Echinococcosis in Asymptomatic Carriers. Trans. R Soc. Trop. Med. Hyg. 2019, 113, 74–80. [Google Scholar] [CrossRef]
- Brunetti, E.; White, A.C. Cestode Infestations: Hydatid Disease and Cysticercosis. Infect. Dis. Clin. N. Am. 2012, 26, 421–435. [Google Scholar] [CrossRef]
- Eckert, J.; Thompson, R.C.A. Historical Aspects of Echinococcosis. Adv. Parasitol. 2017, 95, 1–64. [Google Scholar] [CrossRef]
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Gottstein, B.; Wang, J.; Blagosklonov, O.; Grenouillet, F.; Millon, L.; Vuitton, D.A.; Müller, N. Echinococcus Metacestode: In Search of Viability Markers. Parasite 2014, 21, 63. [Google Scholar] [CrossRef]
- Brunetti, E.; Kern, P.; Vuitton, D.A.; Writing Panel for the WHO-IWGE. Expert Consensus for the Diagnosis and Treatment of Cystic and Alveolar Echinococcosis in Humans. Acta Trop. 2010, 114, 1–16. [Google Scholar] [CrossRef]
- Vola, A.; Manciulli, T.; de Silvestri, A.; Lissandrin, R.; Mariconti, M.; Siles-Lucas, M.; Brunetti, E.; Tamarozzi, F. Diagnostic Performances of Commercial ELISA, Indirect Hemagglutination, and Western Blot in Differentiation of Hepatic Echinococcal and Non-Echinococcal Lesions: A Retrospective Analysis of Data from a Single Referral Centre. Am. J. Trop. Med. Hyg. 2019, 101, 1345–1349. [Google Scholar] [CrossRef]
- Tamarozzi, F.; Silva, R.; Fittipaldo, V.A.; Buonfrate, D.; Gottstein, B.; Siles-Lucas, M. Serology for the Diagnosis of Human Hepatic Cystic Echinococcosis and Its Relation with Cyst Staging: A Systematic Review of the Literature with Meta-Analysis. PLoS Negl. Trop. Dis. 2021, 15, e0009370. [Google Scholar] [CrossRef]
- Tamarozzi, F.; Longoni, S.S.; Vola, A.; Degani, M.; Tais, S.; Rizzi, E.; Prato, M.; Scarso, S.; Silva, R.; Brunetti, E.; et al. Evaluation of Nine Commercial Serological Tests for the Diagnosis of Human Hepatic Cyst Echinococcosis and the Differential Diagnosis with Other Focal Liver Lesions: A Diagnostic Accuracy Study. Diagnostics 2021, 11, 167. [Google Scholar] [CrossRef]
- Siles-Lucas, M.; Casulli, A.; Conraths, F.J.; Müller, N. Laboratory Diagnosis of Echinococcus Spp. in Human Patients and Infected Animals. Adv. Parasitol. 2017, 96, 159–257. [Google Scholar] [CrossRef]
- Stojkovic, M.; Adt, H.M.; Rosenberger, K.; Boubaker, G.; Hernandez-Gonzalez, A.; Junghanss, T.; Zwahlen, M.; Siles-Lucas, M. Follow-up of Surgically Treated Patients with Cystic Echinococcosis: Can Novel Recombinant Antigens Compete with Imaging? Analysis of a Patient Cohort. Trop. Med. Int. Health 2017, 22, 614–621. [Google Scholar] [CrossRef]
- Lissandrin, R.; Tamarozzi, F.; Piccoli, L.; Tinelli, C.; de Silvestri, A.; Mariconti, M.; Meroni, V.; Genco, F.; Brunetti, E. Factors Influencing the Serological Response in Hepatic Echinococcus Granulosus Infection. Am. J. Trop. Med. Hyg. 2016, 94, 166–171. [Google Scholar] [CrossRef]
- Schuhbaur, J.; Schweizer, M.; Philipp, J.; Schmidberger, J.; Schlingeloff, P.; Kratzer, W. Long-Term Follow-up of Liver Alveolar Echinococcosis Using Echinococcosis Multilocularis Ultrasound Classification. World J. Gastroenterol. 2021, 27, 6939–6950. [Google Scholar] [CrossRef]
- Grüner, B.; Schmidberger, J.; Drews, O.; Kratzer, W.; Gräter, T. Imaging in Alveolar Echinococcosis (AE): Comparison of Echinococcus Multilocularis Classification for Computed-Tomography (EMUC-CT) and Ultrasonography (EMUC-US). Radiol. Infect. Dis. 2017, 4, 70–77. [Google Scholar] [CrossRef]
- Brumpt, É.; Liu, W.; Graeter, T.; Calame, P.; Rong, S.; Jiang, Y.; Li, W.; Bao, H.; Delabrousse, É. Kodama-XUUB: An Informative Classification for Alveolar Echinococcosis Hepatic Lesions on Magnetic Resonance Imaging. Parasite 2021, 28, 66. [Google Scholar] [CrossRef]
- Kronenberg, P.A.; Deibel, A.; Gottstein, B.; Grimm, F.; Müllhaupt, B.; Meyer Zu Schwabedissen, C.; Aitbaev, S.; Omorov, R.A.; Abdykerimov, K.K.; Minbaeva, G.; et al. Serological Assays for Alveolar and Cystic Echinococcosis—A Comparative Multi-Test Study in Switzerland and Kyrgyzstan. Pathogens 2022, 11, 518. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [PubMed]
- Tosar, J.P.; Witwer, K.; Cayota, A. Revisiting Extracellular RNA Release, Processing, and Function. Trends Biochem. Sci. 2021, 46, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Godoy, P.M.; Bhakta, N.R.; Barczak, A.J.; Cakmak, H.; Fisher, S.; MacKenzie, T.C.; Patel, T.; Price, R.W.; Smith, J.F.; Woodruff, P.G.; et al. Large Differences in Small RNA Composition Between Human Biofluids. Cell Rep. 2018, 25, 1346–1358. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Khoshbakht, T.; Hussen, B.M.; Taheri, M.; Samadian, M. A Review on the Role of MiR-1246 in the Pathoetiology of Different Cancers. Front. Mol. Biosci. 2022, 8, 1233. [Google Scholar] [CrossRef]
- Klingenberg, M.; Matsuda, A.; Diederichs, S.; Patel, T. Non-Coding RNA in Hepatocellular Carcinoma: Mechanisms, Biomarkers and Therapeutic Targets. J. Hepatol. 2017, 67, 603–618. [Google Scholar] [CrossRef]
- Ren, F.J.; Yao, Y.; Cai, X.Y.; Fang, G.Y. Emerging Role of MiR-192-5p in Human Diseases. Front. Pharm. 2021, 12, 160. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Kim, D.K.; Lee, J.; Simpson, R.J.; Lötvall, J.; Gho, Y.S. EVpedia: A Community Web Resource for Prokaryotic and Eukaryotic Extracellular Vesicles Research. Semin. Cell Dev. Biol. 2015, 40, 4–7. [Google Scholar] [CrossRef]
- Sotillo, J.; Robinson, M.W.; Kimber, M.J.; Cucher, M.; Ancarola, M.E.; Nejsum, P.; Marcilla, A.; Eichenberger, R.M.; Tritten, L. The Protein and MicroRNA Cargo of Extracellular Vesicles from Parasitic Helminths—Current Status and Research Priorities. Int. J. Parasitol. 2020, 50, 635–645. [Google Scholar] [CrossRef]
- Dos Santos, G.B.; Monteiro, K.M.; da Silva, E.D.; Battistella, M.E.; Ferreira, H.B.; Zaha, A. Excretory/Secretory Products in the Echinococcus Granulosus Metacestode: Is the Intermediate Host Complacent with Infection Caused by the Larval Form of the Parasite? Int. J. Parasitol. 2016, 46, 843–856. [Google Scholar] [CrossRef]
- Ancarola, M.E.; Marcilla, A.; Herz, M.; Macchiaroli, N.; Pérez, M.; Asurmendi, S.; Brehm, K.; Poncini, C.; Rosenzvit, M.; Cucher, M. Cestode Parasites Release Extracellular Vesicles with MicroRNAs and Immunodiagnostic Proteins Cargo. Int. J. Parasitol. 2017, 47, 675–686. [Google Scholar] [CrossRef]
- Ancarola, M.E.; Lichtenstein, G.; Herbig, J.; Holroyd, N.; Mariconti, M.; Brunetti, E.; Berriman, M.; Albrecht, K.; Marcilla, A.; Rosenzvit, M.C.; et al. Extracellular Non-Coding RNA Signatures of the Metacestode Stage of Echinococcus Multilocularis. PLoS Negl. Trop. Dis. 2020, 14, e0008890. [Google Scholar] [CrossRef]
- Siles-Lucas, M.; Sánchez-Ovejero, C.; González-Sánchez, M.; González, E.; Falcón-Pérez, J.M.; Boufana, B.; Fratini, F.; Casulli, A.; Manzano-Román, R. Isolation and Characterization of Exosomes Derived from Fertile Sheep Hydatid Cysts. Vet. Parasitol. 2017, 236, 22–33. [Google Scholar] [CrossRef]
- Zheng, Y.; Guo, X.; Su, M.; Guo, A.; Ding, J.; Yang, J.; Xiang, H.; Cao, X.; Zhang, S.; Ayaz, M.; et al. Regulatory Effects of Echinococcus Multilocularis Extracellular Vesicles on RAW264.7 Macrophages. Vet. Parasitol. 2017, 235, 29–36. [Google Scholar] [CrossRef]
- Nicolao, M.C.; Rodriguez Rodrigues, C.; Cumino, A.C. Extracellular Vesicles from Echinococcus Granulosus Larval Stage: Isolation, Characterization and Uptake by Dendritic Cells. PLoS Negl. Trop. Dis. 2019, 13, e0007032. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, W.; Cui, F.; Shi, C.; Ma, Y.; Yu, Y.; Zhao, W.; Zhao, J. Extracellular Vesicles Derived from Echinococcus Granulosus Hydatid Cyst Fluid from Patients: Isolation, Characterization and Evaluation of Immunomodulatory Functions on T Cells. Int. J. Parasitol. 2019, 49, 1029–1037. [Google Scholar] [CrossRef]
- Zhang, X.; Gong, W.; Cao, S.; Yin, J.; Zhang, J.; Cao, J.; Shen, Y. Comprehensive Analysis of Non-Coding RNA Profiles of Exosome-Like Vesicles From the Protoscoleces and Hydatid Cyst Fluid of Echinococcus Granulosus. Front. Cell Infect. Microbiol. 2020, 10, 316. [Google Scholar] [CrossRef]
- Yang, J.; Wu, J.; Fu, Y.; Yan, L.; Li, Y.; Guo, X.; Zhang, Y.; Wang, X.; Shen, Y.; Cho, W.C.; et al. Identification of Different Extracellular Vesicles in the Hydatid Fluid of Echinococcus Granulosus and Immunomodulatory Effects of 110 K EVs on Sheep PBMCs. Front. Immunol. 2021, 12, 315. [Google Scholar] [CrossRef]
- Jeong, M.J.; Kang, S.A.; Choi, J.H.; Lee, D.I.; Yu, H.S. Extracellular Vesicles of Echinococcus Granulosus Have Therapeutic Effects in Allergic Airway Inflammation. Parasite Immunol. 2021, 43, e12872. [Google Scholar] [CrossRef]
- Ding, J.; He, G.; Wu, J.; Yang, J.; Guo, X.; Yang, X.; Wang, Y.; Kandil, O.M.; Kutyrev, I.; Ayaz, M.; et al. MiRNA-Seq of Echinococcus Multilocularis Extracellular Vesicles and Immunomodulatory Effects of MiR-4989. Front. Microbiol. 2019, 10, 2707. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zheng, Y. Expression Profiling of Circulating MiRNAs in Mouse Serum in Response to Echinococcus Multilocularis Infection. Parasitology 2017, 144, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhu, Y.; Wu, J.; Bai, M.; Xin, Y.; Wang, Q.; Zhao, J. Expression Profiling of Exosomal MiRNAs Derived from Different Stages of Infection in Mice Infected with Echinococcus Granulosus Protoscoleces Using High-Throughput Sequencing. Parasitol. Res. 2022, 121, 1993–2008. [Google Scholar] [CrossRef] [PubMed]
- Orsten, S.; Baysal, İ.; Yabanoglu-Ciftci, S.; Ciftci, T.; Azizova, A.; Akinci, D.; Akyon, Y.; Akhan, O. MicroRNA Expression Profile in Patients with Cystic Echinococcosis and Identification of Possible Cellular Pathways. J. Helminthol. 2021, 95, e1. [Google Scholar] [CrossRef]
- Mariconti, M.; Vola, A.; Manciulli, T.; Genco, F.; Lissandrin, R.; Meroni, V.; Rosenzvit, M.; Tamarozzi, F.; Brunetti, E. Role of MicroRNAs in Host Defense against Echinococcus Granulosus Infection: A Preliminary Assessment. Immunol. Res. 2019, 67, 93–97. [Google Scholar] [CrossRef]
- Helbig, M.; Frosch, P.; Kern, P.; Frosch, M. Serological Differentiation between Cystic and Alveolar Echinococcosis by Use of Recombinant Larval Antigens. J. Clin. Microbiol. 1993, 31, 3211–3215. [Google Scholar] [CrossRef]
- Tappe, D.; Grüner, B.; Kern, P.; Frosch, M. Evaluation of a Commercial Echinococcus Western Blot Assay for Serological Follow-up of Patients with Alveolar Echinococcosis. Clin. Vaccine Immunol. 2008, 15, 1633–1637. [Google Scholar] [CrossRef]
- Rossi, P.; Tamarozzi, F.; Galati, F.; Akhan, O.; Cretu, C.M.; Vutova, K.; Siles-Lucas, M.; Brunetti, E.; Casulli, A.; Angheben, A.; et al. The European Register of Cystic Echinococcosis, ERCE: State-of-the-Art Five Years after Its Launch. Parasite Vectors 2020, 13, 236. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J 2011, 17, 10. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Friedländer, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N.; Friedlander, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. MiRDeep2 Accurately Identifies Known and Hundreds of Novel MicroRNA Genes in Seven Animal Clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. MiRBase: From MicroRNA Sequences to Function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and Applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Schurch, N.J.; Schofield, P.; Gierliński, M.; Cole, C.; Sherstnev, A.; Singh, V.; Wrobel, N.; Gharbi, K.; Simpson, G.G.; Owen-Hughes, T.; et al. How Many Biological Replicates Are Needed in an RNA-Seq Experiment and Which Differential Expression Tool Should You Use? RNA 2016, 22, 839–851. [Google Scholar] [CrossRef]
- Bermúdez-Barrientos, J.R.; Ramírez-Sánchez, O.; Chow, F.W.-N.; Buck, A.H.; Abreu-Goodger, C. Disentangling SRNA-Seq Data to Study RNA Communication between Species. Nucleic Acids Res. 2020, 48, e21. [Google Scholar] [CrossRef]
- Macchiaroli, N.; Cucher, M.; Zarowiecki, M.; Maldonado, L.; Kamenetzky, L.; Rosenzvit, M.C. MicroRNA Profiling in the Zoonotic Parasite Echinococcus Canadensis Using a High-Throughput Approach. Parasite Vectors 2015, 8, 83. [Google Scholar] [CrossRef]
- Moshiri, F.; Salvi, A.; Gramantieri, L.; Sangiovanni, A.; Guerriero, P.; de Petro, G.; Bassi, C.; Lupini, L.; Sattari, A.; Cheung, D.; et al. Circulating MiR-106b-3p, MiR-101-3p and MiR-1246 as Diagnostic Biomarkers of Hepatocellular Carcinoma. Oncotarget 2018, 9, 15350. [Google Scholar] [CrossRef]
- Xu, Y.F.; Hannafon, B.N.; Khatri, U.; Gin, A.; Ding, W.Q. The Origin of Exosomal MiR-1246 in Human Cancer Cells. RNA Biol. 2019, 16, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jeppesen, D.K.; Higginbotham, J.N.; Graves-Deal, R.; Trinh, V.Q.; Ramirez, M.A.; Sohn, Y.; Neininger, A.C.; Taneja, N.; McKinley, E.T.; et al. Supermeres Are Functional Extracellular Nanoparticles Replete with Disease Biomarkers and Therapeutic Targets. Nat. Cell Biol. 2021, 23, 1240–1254. [Google Scholar] [CrossRef]
- Jopling, C.L. Liver-Specific MicroRNA-122: Biogenesis and Function. RNA Biol. 2012, 9, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Gröger, L.; Tschernig, T.; Solomon, J.; Laham, O.; Schaum, N.; Wagner, V.; Kern, F.; Schmartz, G.P.; Li, Y.; et al. MiRNATissueAtlas2: An Update to the Human MiRNA Tissue Atlas. Nucleic Acids Res. 2022, 50, D211. [Google Scholar] [CrossRef]
- Fründt, T.; Krause, L.; Hussey, E.; Steinbach, B.; Köhler, D.; von Felden, J.; Schulze, K.; Lohse, A.W.; Wege, H.; Schwarzenbach, H. Diagnostic and Prognostic Value of Mir-16, Mir-146a, Mir-192 and Mir-221 in Exosomes of Hepatocellular Carcinoma and Liver Cirrhosis Patients. Cancers 2021, 13, 2484. [Google Scholar] [CrossRef] [PubMed]
- Loukachov, V.v.; van Dort, K.A.; Maurer, I.; Takkenberg, R.B.; de Niet, A.; Reesink, H.W.; Willemse, S.B.; Kootstra, N.A. Identification of Liver and Plasma MicroRNAs in Chronic Hepatitis B Virus Infection. Front. Cell Infect. Microbiol. 2022, 12, 632. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, S.; Mayr, C.; Bartel, D.P.; Lodish, H.F. MiR-150, a MicroRNA Expressed in Mature B and T Cells, Blocks Early B Cell Development When Expressed Prematurely. Proc. Natl. Acad. Sci. USA 2007, 104, 7080–7085. [Google Scholar] [CrossRef]
- Lee, H.-M.; Kim, T.-S.; Jo, E.-K. MiR-146 and MiR-125 in the Regulation of Innate Immunity and Inflammation. BMB Rep. 2016, 49, 311–318. [Google Scholar] [CrossRef]
- Sun, C.M.; Wu, J.; Zhang, H.; Shi, G.; Chen, Z.T. Circulating MiR-125a but Not MiR-125b Is Decreased in Active Disease Status and Negatively Correlates with Disease Severity as Well as Inflammatory Cytokines in Patients with Crohn’s Disease. World J. Gastroenterol. 2017, 23, 7888–7898. [Google Scholar] [CrossRef]
- Castoldi, M.; Kordes, C.; Sawitza, I.; Häussinger, D. Isolation and Characterization of Vesicular and Non-Vesicular MicroRNAs Circulating in Sera of Partially Hepatectomized Rats. Sci. Rep. 2016, 6, 31869. [Google Scholar] [CrossRef]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 Complexes Carry a Population of Circulating MicroRNAs Independent of Vesicles in Human Plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef]
- Roberts, T.C.; Godfrey, C.; McClorey, G.; Vader, P.; Briggs, D.; Gardiner, C.; Aoki, Y.; Sargent, I.; Morgan, J.E.; Wood, M.J.A. Extracellular MicroRNAs Are Dynamic Non-Vesicular Biomarkers of Muscle Turnover. Nucleic Acids Res. 2013, 41, 9500–9513. [Google Scholar] [CrossRef] [PubMed]
- Driedonks, T.A.P.; Nolte-T’Hoen, E.N.M. Circulating Y-RNAs in Extracellular Vesicles and Ribonucleoprotein Complexes; Implications for the Immune System. Front. Immunol. 2019, 10, 3164. [Google Scholar] [CrossRef]
- Dhahbi, J.M. 5′ TRNA Halves: The next Generation of Immune Signaling Molecules. Front. Immunol. 2015, 6, 74. [Google Scholar] [CrossRef]
- Fu, H.; Feng, J.; Liu, Q.; Sun, F.; Tie, Y.; Zhu, J.; Xing, R.; Sun, Z.; Zheng, X. Stress Induces TRNA Cleavage by Angiogenin in Mammalian Cells. FEBS Lett. 2009, 583, 437–442. [Google Scholar] [CrossRef]
- Selitsky, S.R.; Baran-Gale, J.; Honda, M.; Yamane, D.; Masaki, T.; Fannin, E.E.; Guerra, B.; Shirasaki, T.; Shimakami, T.; Kaneko, S.; et al. Small TRNA-Derived RNAs Are Increased and More Abundant than MicroRNAs in Chronic Hepatitis B and C. Sci. Rep. 2015, 5, 7675. [Google Scholar] [CrossRef]
- Quintana, J.F.; Makepeace, B.L.; Babayan, S.A.; Ivens, A.; Pfarr, K.M.; Blaxter, M.; Debrah, A.; Wanji, S.; Ngangyung, H.F.; Bah, G.S.; et al. Extracellular Onchocerca-Derived Small RNAs in Host Nodules and Blood. Parasite Vectors 2015, 8, 58. [Google Scholar] [CrossRef]


| Group | Patient Number | Gender | Age | Total Larval Antigens ELISA Index | EG 55 Index | EM 10 Index | Observations |
|---|---|---|---|---|---|---|---|
| 1 | F | 46 | 0.4 | - | - | - | |
| Negative | 2 | F | 78 | 0.7 | - | - | - |
| 3 | F | 33 | 0.7 | - | - | - | |
| 4 | F | 49 | 16.9 | 0.6 | 8.1 | - | |
| Positive | 5 | F | 81 | 11.4 | 0.3 | 0.4 | - |
| 6 | F | 87 | 18.0 | 0.5 | 2.1 | - | |
| 7 | F | 69 | 1.2 | 0.4 | 0.4 | after ABZ treatment | |
| Treated | 8 | F | 57 | 5.5 | 0.7 | 2.7 | during ABZ treatment |
| 9 | F | 61 | 6.2 | 3.5 | 4.1 | during ABZ treatment |
| Group | Patient Number | Gender | Age | ELISA Index | Lesion Diameter (cm) | Stage | Observations |
|---|---|---|---|---|---|---|---|
| Negative | 10 | F | 33 | 0.47 | - | - | - |
| 11 | F | 49 | 0.35 | - | - | - | |
| 12 | M | 30 | 0.33 | - | - | - | |
| Non-parasitic lesion | 13 | F | 41 | 0.45 | 2.6 | Hepatic adenoma | - |
| 14 | M | 68 | 0.34 | 3.9 | Biliary cysts | - | |
| 15 | F | 68 | 0.38 | 11.2 | Not parasitic lesion | - | |
| CE1+2 | 16 | M | 33 | 6.93 | 6.2 | 2 | - |
| 17 | M | 34 | 0.35 | 8.8 | 1 | - | |
| 18 | F | 39 | 2.75 | 9.3 | 2 | - | |
| CE3a | 19 | F | 36 | 1 | 4 | 3a | - |
| 20 | F | 70 | 0.40 | 4.9 | 3a | - | |
| 21 | M | 29 | 2.00 | 5.1 | 3a | - | |
| CE3b | 22 | M | 63 | 5.18 | 7.6 | 3b | - |
| 23 | F | 67 | 0.59 | 3.4 | 3b | - | |
| 24 | F | 67 | 0.28 | 4.4 | 3b | - | |
| CE4 | 25 | M | 61 | 0.34 | 4.5 | 4 | - |
| 26 | F | 59 | 0.46 | 3.7 | 4 | - | |
| 27 | F | 50 | 0.34 | 7.5 | 4 | - | |
| CE5 | 28 | F | 68 | 0.22 | 5.1 | 5 | - |
| 29 | F | 53 | 0.21 | 1.9 | 5 | - | |
| 30 | F | 70 | 0.23 | 3.5 | 5 | - | |
| Treated | 31 | F | 58 | 4.08 | 12.4 | 3b | during ABZ treatment |
| 32 | F | 40 | 8.07 | 9.0 | 3b | during ABZ treatment | |
| 33 | M | 50 | 0.45 | 6.0 | 3b | during ABZ treatment |
| Group | Patient Number | rRNA | tRNA | microRNA | SRP | Spliceosomal RNA |
|---|---|---|---|---|---|---|
| 1 | 28,518 | 180 | 0 | 0 | 0 | |
| Negative | 2 | 39,689 | 0 | 0 | 35 | 0 |
| 3 | 18,863 | 115 | 0 | 0 | 0 | |
| 4 | 19,534 | 58 | 34 | 0 | 0 | |
| Positive | 5 | 23,012 | 83 | 0 | 0 | 0 |
| 6 | 15,449 | 52 | 0 | 0 | 0 | |
| 7 | 56,494 | 2935 | 61 | 0 | 0 | |
| Treated | 8 | 138,623 | 28540 | 2265 | 0 | 0 |
| 9 | 36,387 | 133 | 0 | 0 | 57 |
| Group | Patient Number | rRNA | tRNA | microRNA |
|---|---|---|---|---|
| Negative | 10 | 14,779 | 27 | 0 |
| 11 | 15,372 | 238 | 70 | |
| 12 | 15,173 | 104 | 0 | |
| Non-parasitic lesion | 13 | 19,946 | 116 | 0 |
| 14 | 16,773 | 284 | 321 | |
| 15 | 19228 | 108 | 33 | |
| CE1+2 | 16 | 10,109 | 0 | 0 |
| 17 | 37,247 | 76 | 0 | |
| 18 | 14,461 | 89 | 0 | |
| CE3a | 19 | 11,498 | 60 | 0 |
| 20 | 19,668 | 60 | 0 | |
| 21 | 14,258 | 80 | 0 | |
| CE3b | 22 | 16,061 | 154 | 0 |
| 23 | 30,992 | 89 | 0 | |
| 24 | 23,763 | 231 | 0 | |
| CE4 | 25 | 19,168 | 63 | 0 |
| 26 | 18,725 | 58 | 0 | |
| 27 | 23,919 | 160 | 0 | |
| CE5 | 28 | 44,205 | 76 | 0 |
| 29 | 22,987 | 197 | 0 | |
| 30 | 18,785 | 153 | 0 | |
| Treated | 31 | 13,929 | 124 | 0 |
| 32 | 17,794 | 219 | 0 | |
| 33 | 9215 | 74 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cucher, M.A.; Mariconti, M.; Manciulli, T.; Vola, A.; Rosenzvit, M.C.; Brehm, K.; Kamenetzky, L.; Brunetti, E. Circulating Small RNA Profiling of Patients with Alveolar and Cystic Echinococcosis. Biology 2023, 12, 715. https://doi.org/10.3390/biology12050715
Cucher MA, Mariconti M, Manciulli T, Vola A, Rosenzvit MC, Brehm K, Kamenetzky L, Brunetti E. Circulating Small RNA Profiling of Patients with Alveolar and Cystic Echinococcosis. Biology. 2023; 12(5):715. https://doi.org/10.3390/biology12050715
Chicago/Turabian StyleCucher, Marcela A., Mara Mariconti, Tommaso Manciulli, Ambra Vola, Mara C. Rosenzvit, Klaus Brehm, Laura Kamenetzky, and Enrico Brunetti. 2023. "Circulating Small RNA Profiling of Patients with Alveolar and Cystic Echinococcosis" Biology 12, no. 5: 715. https://doi.org/10.3390/biology12050715
APA StyleCucher, M. A., Mariconti, M., Manciulli, T., Vola, A., Rosenzvit, M. C., Brehm, K., Kamenetzky, L., & Brunetti, E. (2023). Circulating Small RNA Profiling of Patients with Alveolar and Cystic Echinococcosis. Biology, 12(5), 715. https://doi.org/10.3390/biology12050715






