Biophysical, Biochemical, and Photochemical Analyses Using Reflectance Hyperspectroscopy and Chlorophyll a Fluorescence Kinetics in Variegated Leaves
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material and Experimental Design
2.2. Spectral Data Collection
2.3. Fluorescence OJIP Data Collection
2.4. Biophysical Parameters of Leaves
2.5. Profile of the Pigments Extracted
2.5.1. Chlorophyll and Carotenoid Quantification
2.5.2. Flavonoid and Anthocyanin Quantification
2.5.3. Total Soluble Phenolic Compound Quantification
2.5.4. DPPH Free Radical Scavenging Activity
- AbsDPPH = absorbance of DPPH
- Abssample = absorbance DPPH after 60 min
2.6. Wavelength Selection Using Algorithms for Biophysical Parameters
2.7. Hyperspectral Vegetation Index for the Most Responsive Wavelength
2.8. Statistical Analyses
2.8.1. Univariate Statistical Analysis
2.8.2. Multivariate Statistical Analysis
3. Results
3.1. Chromaticity Indexes by Color and Pigment Concentration in Leaves
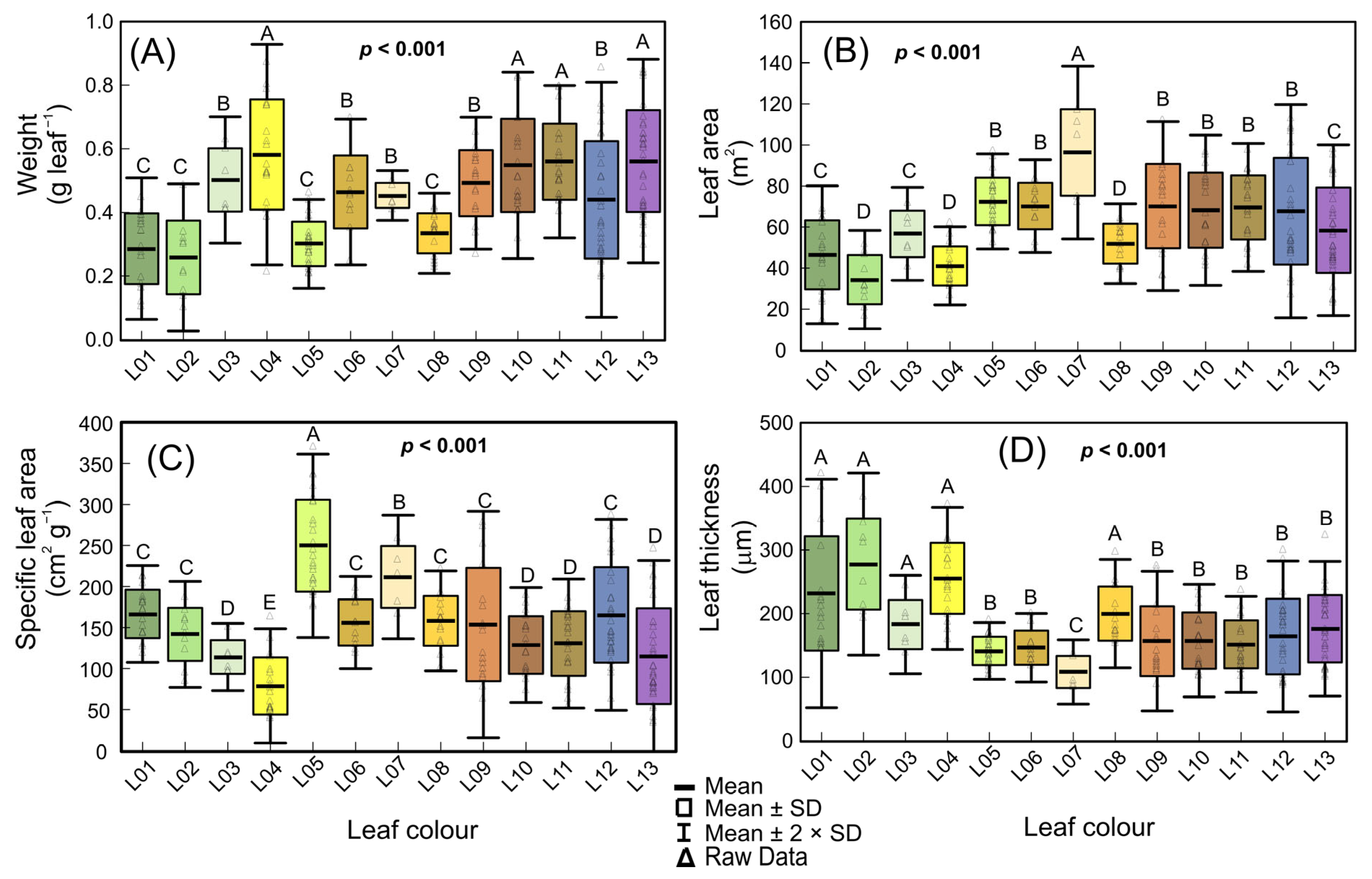
3.2. Biophysical Changes by Variegated Leaves
3.3. Hyperspectral Reflectance in Variegated Leaves
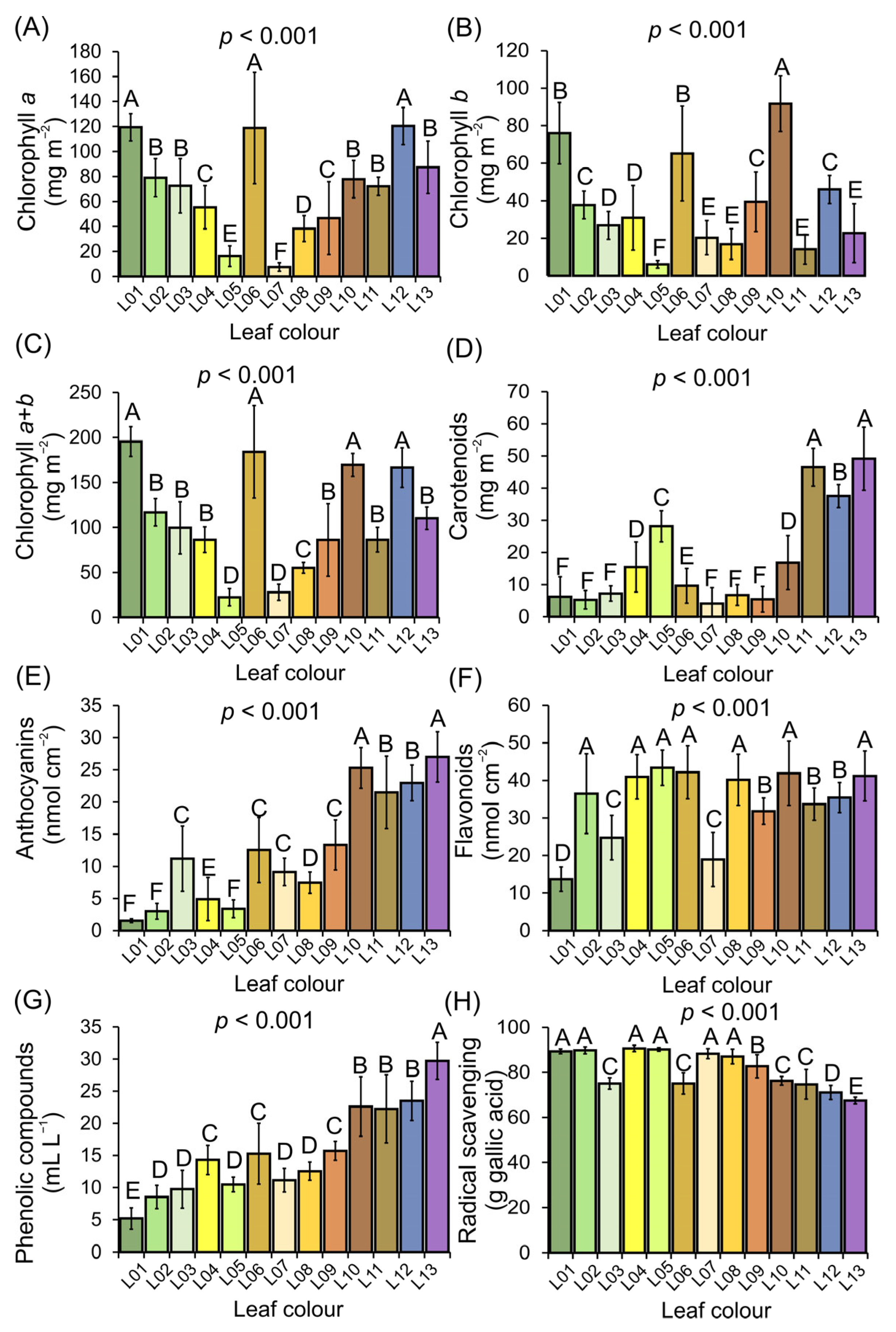
3.4. Profiling of Pigments and Free Radical Scavenging in Variegated Leaves
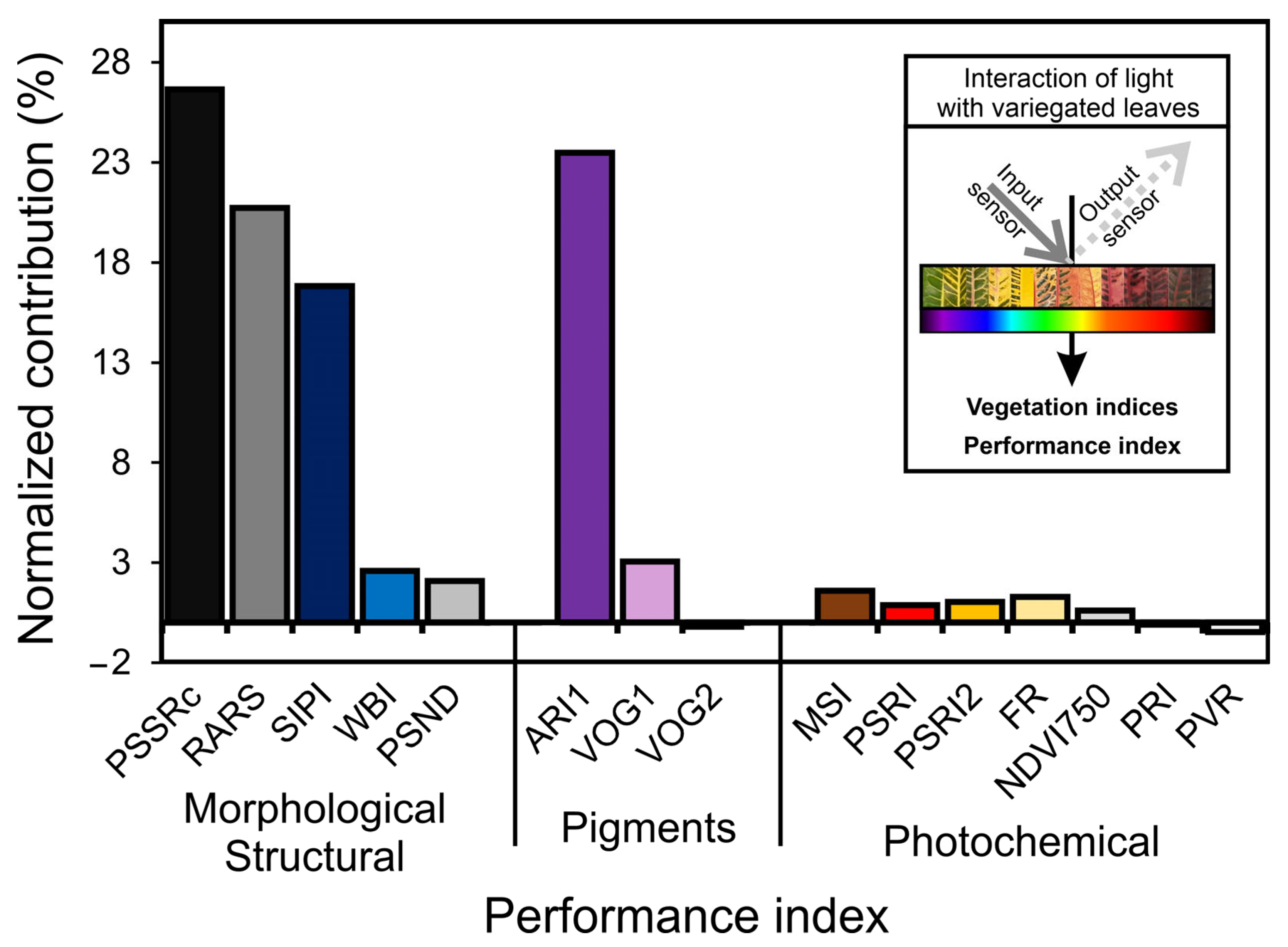
3.5. Vegetation Indexes and Putative Contribution for Biophysical, Biochemical, and Photochemical Parameters for Variegated Leaves
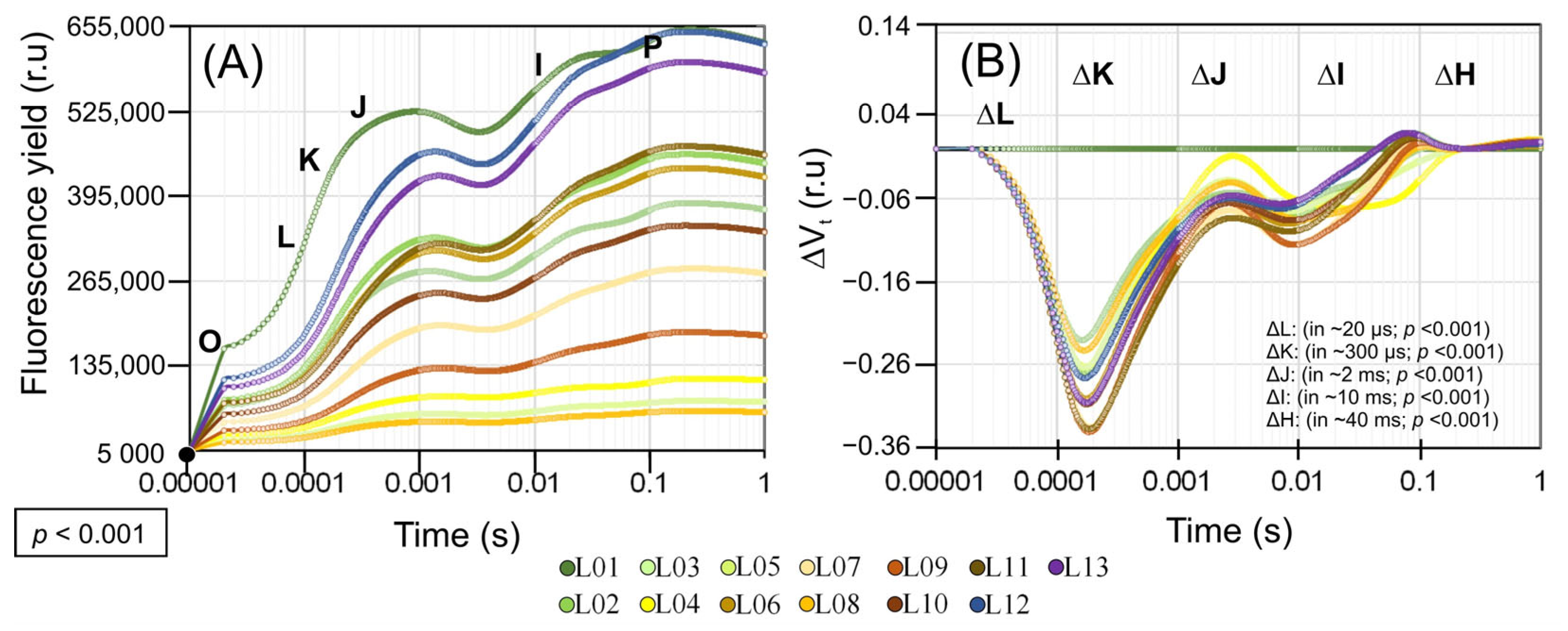
3.6. OJIP Chlorophyll a Fluorescence Kinetics
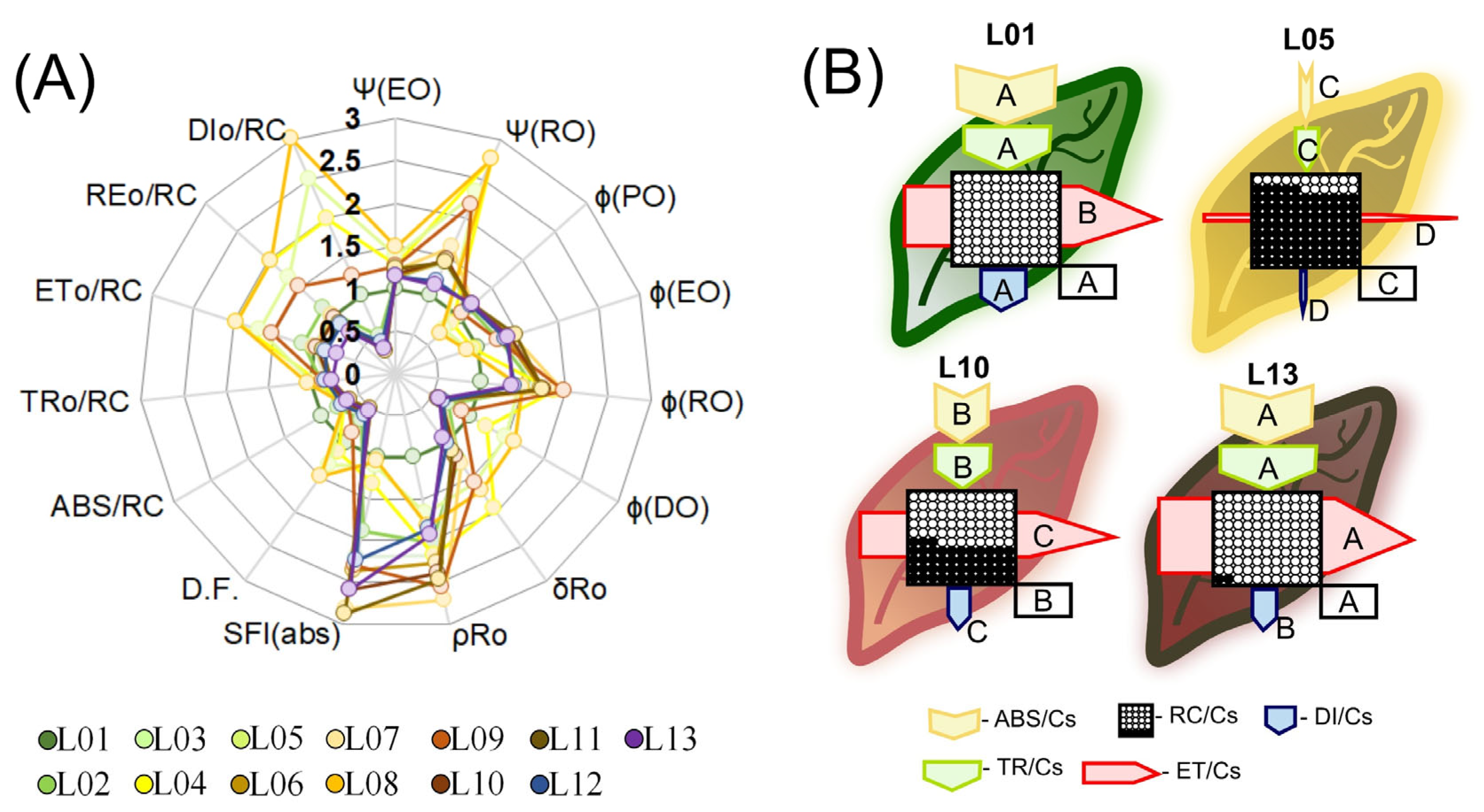
3.7. Target Modeling of the Fluorescence Kinetics of Phenomenological Energy Fluxes in Variegated Leaves
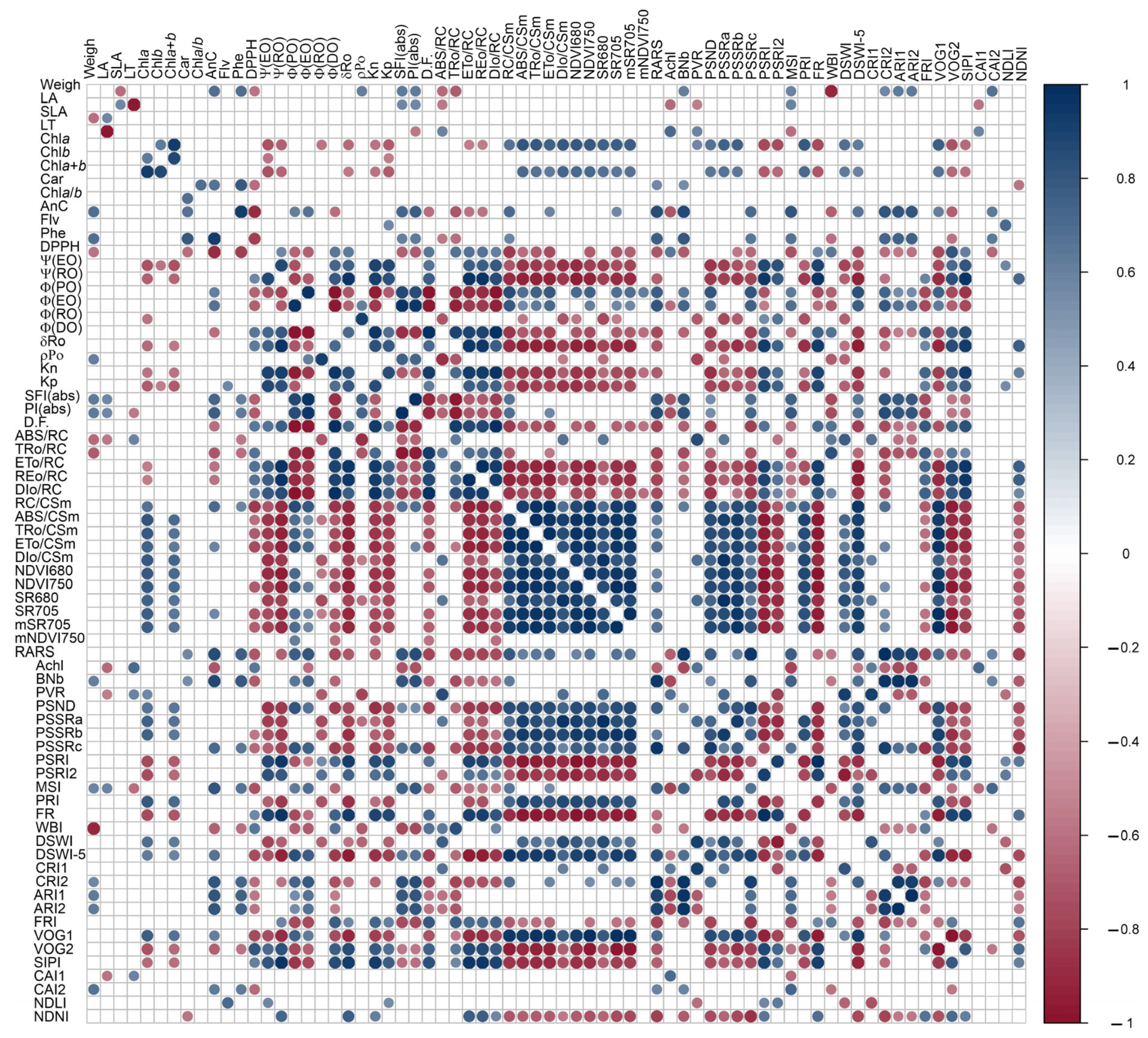
3.8. Hyperspectral Reflectance, Fluorescence Kinetics, Variables Total Performance and Pearson’s Correlation Coefficients Associated with Biophysical, Biochemical and Photochemical Parameters
4. Discussion
4.1. The Effect of Morphological, Physiological, and Anatomical Attributes on the Vegetation Indexes
4.2. The JIP Test Parameters Indicate Dissipation to the Thermally Based Vegetation Indexes
4.3. PRI and PSSRc Indexes Are Strongly Associated with VIS–NIR–SWIR Bands
4.4. PSSRc Photosynthetic Apparatus Could Be Measured Using Fluorescence Techniques Based on Vegetation Indexes in Variegated Leaves
4.5. Novel Perspective of Hyperspectral–Fluorescence Techniques
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Onoda, Y.; Wright, I.J.; Evans, J.R.; Hikosaka, K.; Kitajima, K.; Niinemets, Ü.; Poorter, H.; Tosens, T.; Westoby, M. Physiological and Structural Tradeoffs Underlying the Leaf Economics Spectrum. New Phytol. 2017, 214, 1447–1463. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, A.K.; Buonaiuto, D.M.; Chamberlain, C.J.; Morales-Castilla, I.; Wolkovich, E.M. Spatial and Temporal Shifts in Photoperiod with Climate Change. New Phytol. 2021, 230, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Falcioni, R.; Moriwaki, T.; Bonato, C.M.; de Souza, L.A.; Nanni, M.R.; Antunes, W.C. Distinct Growth Light and Gibberellin Regimes Alter Leaf Anatomy and Reveal Their Influence on Leaf Optical Properties. Environ. Exp. Bot. 2017, 140, 86–95. [Google Scholar] [CrossRef]
- Falcioni, R.; Moriwaki, T.; Benedito, E.; Bonato, C.M.; de Souza, L.A.; Antunes, W.C. Increased Gibberellin Levels Enhance Light Capture Efficiency in Tobacco Plants and Promote Dry Matter Accumulation. Theor. Exp. Plant Physiol. 2018, 30, 235–250. [Google Scholar] [CrossRef]
- Falcioni, R.; Moriwaki, T.; Pattaro, M.; Herrig Furlanetto, R.; Nanni, M.R.; Camargos Antunes, W. High Resolution Leaf Spectral Signature as a Tool for Foliar Pigment Estimation Displaying Potential for Species Differentiation. J. Plant Physiol. 2020, 249, 153161. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Fortini, E.A.; Müller, L.A.d.C.; Batista, D.S.; Vieira, L.M.; Silva, P.O.; Amaral, C.H.d.; Poethig, R.S.; Otoni, W.C. Leaf Development Stages and Ontogenetic Changes in Passionfruit (Passiflora edulis Sims.) Are Detected by Narrowband Spectral Signal. J. Photochem. Photobiol. B Biol. 2020, 209, 111931. [Google Scholar] [CrossRef]
- Kume, A. Importance of the Green Color, Absorption Gradient, and Spectral Absorption of Chloroplasts for the Radiative Energy Balance of Leaves. J. Plant Res. 2018, 131, 501–514. [Google Scholar] [CrossRef]
- Samborska, I.A.; Kalaji, H.M.; Sieczko, L.; Borucki, W.; Mazur, R.; Kouzmanova, M.; Goltsev, V. Can Just One-Second Measurement of Chlorophyll a Fluorescence Be Used to Predict Sulphur Deficiency in Radish (Raphanus sativus L. Sativus) Plants? Curr. Plant Biol. 2019, 19, 100096. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Račková, L.; Paganová, V.; Swoczyna, T.; Rusinowski, S.; Sitko, K. Can Chlorophyll-a Fluorescence Parameters Be Used as Bio-Indicators to Distinguish between Drought and Salinity Stress in Tilia cordata Mill? Environ. Exp. Bot. 2018, 152, 149–157. [Google Scholar] [CrossRef]
- Mollick, A.S.; Shimoji, H.; Denda, T.; Yokota, M.; Yamasaki, H. Croton Codiaeum variegatum (L.) Blume Cultivars Characterized by Leaf Phenotypic Parameters. Sci. Hortic. 2011, 132, 71–79. [Google Scholar] [CrossRef]
- Hatier, J.H.B.; Clearwater, M.J.; Gould, K.S. The Functional Significance of Black-Pigmented Leaves: Photosynthesis, Photoprotection and Productivity in Ophiopogon planiscapus ‘Nigrescens’. PLoS ONE 2013, 8, e67850. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, G.; Yang, X.; Li, Z.; Feng, H.; Xu, B.; Zhao, X. Monitoring Ratio of Carbon to Nitrogen (C/N) in Wheat and Barley Leaves by Using Spectral Slope Features with Branch-and-Bound Algorithm. Sci. Rep. 2018, 8, 10034. [Google Scholar] [CrossRef] [PubMed]
- Braga, P.; Crusiol, L.G.T.; Nanni, M.R.; Caranhato, A.L.H.; Fuhrmann, M.B.; Nepomuceno, A.L.; Neumaier, N.; Farias, J.R.B.; Koltun, A.; Gonçalves, L.S.A.; et al. Vegetation Indices and NIR-SWIR Spectral Bands as a Phenotyping Tool for Water Status Determination in Soybean. Precis. Agric. 2021, 22, 249–266. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Ustin, S. Applications of Leaf Optics, 1st ed.; Cambridge University Press: Davis, CA, USA, 2019. [Google Scholar]
- Chicati, M.S.; Nanni, M.R.; Chicati, M.L.; Furlanetto, R.H.; Cezar, E.; De Oliveira, R.B. Hyperspectral Remote Detection as an Alternative to Correlate Data of Soil Constituents. Remote Sens. Appl. Soc. Environ. 2019, 16, 100270. [Google Scholar] [CrossRef]
- de Sá, N.C.; Castro, P.; Carvalho, S.; Marchante, E.; López-Núñez, F.A.; Marchante, H. Mapping the Flowering of an Invasive Plant Using Unmanned Aerial Vehicles: Is There Potential for Biocontrol Monitoring? Front. Plant Sci. 2018, 9, 293. [Google Scholar] [CrossRef]
- Nanni, M.R.; Cezar, E.; da Silva Junior, C.A.; Silva, G.F.C.; da Silva Gualberto, A.A. Partial Least Squares Regression (PLSR) Associated with Spectral Response to Predict Soil Attributes in Transitional Lithologies. Arch. Agron. Soil Sci. 2018, 64, 682–695. [Google Scholar] [CrossRef]
- Thornley, R.; Gerard, F.F.; White, K.; Verhoef, A. Intra-Annual Taxonomic and Phenological Drivers of Spectral Variance in Grasslands. Remote Sens. Environ. 2022, 271, 112908. [Google Scholar] [CrossRef]
- Kováč, D.; Veselovská, P.; Klem, K.; Večeřová, K.; Ač, A.; Peñuelas, J.; Urban, O. Potential of Photochemical Reflectance Index for Indicating Photochemistry and Light Use Efficiency in Leaves of European Beech and Norway Spruce Trees. Remote Sens. 2018, 10, 1202. [Google Scholar] [CrossRef]
- Sukhova, E.; Sukhov, V. Relation of Photochemical Reflectance Indices Based on Different Wavelengths to the Parameters of Light Reactions in Photosystems I and II in Pea Plants. Remote Sens. 2020, 12, 1312. [Google Scholar] [CrossRef]
- Sobejano-Paz, V.; Mikkelsen, T.N.; Baum, A.; Mo, X.; Liu, S.; Köppl, C.J.; Johnson, M.S.; Gulyas, L.; García, M. Hyperspectral and Thermal Sensing of Stomatal Conductance, Transpiration, and Photosynthesis for Soybean and Maize under Drought. Remote Sens. 2020, 12, 3182. [Google Scholar] [CrossRef]
- Mittenzwey, K.-H.; GitelSon, A.A.; Lopatchenko, A.A.; Sukhorukov, B.L.; Voigt, T. In-Situ Monitoring of Water Quality on the Basis of Spectral Reflectance. Ship-Borne Experiments for the Development of Remote Sensing Algorithms Especially for the Estimation of Algae Content in Natural Waters. Int. Rev. Gesamten Hydrobiol. Hydrogr. 1988, 73, 61–72. [Google Scholar] [CrossRef]
- Gitelson, A. Nondestructive Estimation of Foliar Pigments (Chlorophylls, Carotenoids, and Anthocyanins) Contents: Evaluating a Semianalytical Three-Band Model. In Hyperspectral Remote Sensing of Vegetation; Thenkabail, P.S., Lyon, J.G., Huete, A., Eds.; CRC Press: New York, NY, USA, 2011; p. 782. [Google Scholar]
- Gitelson, A.; Chivkunova, O.; Zhigalova, T.; Solovchenko, A. In Situ Optical Properties of Foliar Flavonoids: Implication for Non-Destructive Estimation of Flavonoid Content. J. Plant Physiol. 2017, 218, 258–264. [Google Scholar] [CrossRef]
- Gitelson, A.; Solovchenko, A.; Viña, A. Foliar Absorption Coefficient Derived from Reflectance Spectra: A Gauge of the Efficiency of in Situ Light-Capture by Different Pigment Groups. J. Plant Physiol. 2020, 254, 153277. [Google Scholar] [CrossRef]
- Falqueto, A.R.; da Silva Júnior, R.A.; Gomes, M.T.G.; Martins, J.P.R.; Silva, D.M.; Partelli, F.L. Effects of Drought Stress on Chlorophyll a Fluorescence in Two Rubber Tree Clones. Sci. Hortic. 2017, 224, 238–243. [Google Scholar] [CrossRef]
- Stirbet, A.; Lazár, D.; Kromdijk, J. Govindjee Chlorophyll a Fluorescence Induction: Can Just a One-Second Measurement Be Used to Quantify Abiotic Stress Responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Falcioni, R.; Antunes, W.C.; Demattê, J.A.M.; Nanni, M.R. A Novel Method for Estimating Chlorophyll and Carotenoid Concentrations in Leaves: A Two Hyperspectral Sensor Approach. Sensors 2023, 23, 3843. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The Fluorescence Transient as a Tool to Characterize and Screen Photosynthetic Samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaptation, 1st ed.; CRC Press: London, UK, 2000; pp. 443–480. [Google Scholar]
- Sitko, K.; Rusinowski, S.; Kalaji, H.M.; Szopiński, M.; Małkowski, E. Photosynthetic Efficiency as Bioindicator of Environmental Pressure in A. halleri. Plant Physiol. 2017, 175, 290–302. [Google Scholar] [CrossRef]
- Gitelson, A.; Solovchenko, A. Non-Invasive Quantification of Foliar Pigments: Possibilities and Limitations of Reflectance- and Absorbance-Based Approaches. J. Photochem. Photobiol. B Biol. 2018, 178, 537–544. [Google Scholar] [CrossRef]
- Falcioni, R.; Moriwaki, T.; Gibin, M.S.; Vollmann, A.; Pattaro, M.C.; Giacomelli, M.E.; Sato, F.; Nanni, M.R.; Antunes, W.C. Classification and Prediction by Pigment Content in Lettuce (Lactuca sativa L.) Varieties Using Machine Learning and ATR-FTIR Spectroscopy. Plants 2022, 11, 3413. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Ragaee, S. Antioxidant Activity and Nutrient Composition of Selected Cereals for Food Use. Food Chem. 2006, 98, 32–38. [Google Scholar] [CrossRef]
- Crusiol, L.G.T.; Nanni, M.R.; Furlanetto, R.H.; Sibaldelli, R.N.R.; Sun, L.; Gonçalves, S.L.; Foloni, J.S.S.; Mertz-Henning, L.M.; Nepomuceno, A.L.; Neumaier, N.; et al. Assessing the Sensitive Spectral Bands for Soybean Water Status Monitoring and Soil Moisture Prediction Using Leaf-Based Hyperspectral Reflectance. Agric. Water Manag. 2023, 277, 108089. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Pearson Education: Upper Saddle River, NJ, USA, 2010; ISBN 0-13-100846-3. [Google Scholar]
- Fine, P.V.A.; Salazar, D.; Martin, R.E.; Metz, M.R.; Misiewicz, T.M.; Asner, G.P. Exploring the Links between Secondary Metabolites and Leaf Spectral Reflectance in a Diverse Genus of Amazonian Trees. Ecosphere 2021, 12, e03362. [Google Scholar] [CrossRef]
- Croce, R.; van Amerongen, H. Light Harvesting in Oxygenic Photosynthesis: Structural Biology Meets Spectroscopy. Science 2020, 369, eaay2058. [Google Scholar] [CrossRef]
- Ertani, A.; Francioso, O.; Ferrari, E.; Schiavon, M.; Nardi, S. Spectroscopic-Chemical Fingerprint and Biostimulant Activity of a Protein-Based Product in Solid Form. Molecules 2018, 23, 1031. [Google Scholar] [CrossRef]
- Sinanoglou, V.J.; Zoumpoulakis, P.; Fotakis, C.; Kalogeropoulos, N.; Sakellari, A.; Karavoltsos, S.; Strati, I.F. On the Characterization and Correlation of Compositional, Antioxidant and Colour Profile of Common and Balsamic Vinegars. Antioxidants 2018, 7, 139. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Xu, W.; Liu, A.; Chen, S. COMT1 Silencing Aggravates Heat Stress-Induced Reduction in Photosynthesis by Decreasing Chlorophyll Content, Photosystem II Activity, and Electron Transport Efficiency in Tomato. Front. Plant Sci. 2018, 9, 998. [Google Scholar] [CrossRef]
- Moriwaki, T.; Falcioni, R.; Giacomelli, M.E.; Gibin, M.S.; Sato, F.; Nanni, M.R.; Lima, S.M.; da Cunha Andrade, L.H.; Baesso, M.L.; Antunes, W.C. Chloroplast and Outside-Chloroplast Interference of Light inside Leaves. Environ. Exp. Bot. 2023, 208, 105258. [Google Scholar] [CrossRef]
- Coast, O.; Shah, S.; Ivakov, A.; Gaju, O.; Wilson, P.B.; Posch, B.C.; Bryant, C.J.; Negrini, A.C.A.; Evans, J.R.; Condon, A.G.; et al. Predicting Dark Respiration Rates of Wheat Leaves from Hyperspectral Reflectance. Plant Cell Environ. 2019, 42, 2133–2150. [Google Scholar] [CrossRef]
- Kitazaki, K.; Fukushima, A.; Nakabayashi, R.; Okazaki, Y.; Kobayashi, M.; Mori, T.; Nishizawa, T.; Reyes-Chin-Wo, S.; Michelmore, R.W.; Saito, K.; et al. Metabolic Reprogramming in Leaf Lettuce Grown Under Different Light Quality and Intensity Conditions Using Narrow-Band LEDs. Sci. Rep. 2018, 8, 7914. [Google Scholar] [CrossRef]
- Miao, Y.X.; Wang, X.Z.; Gao, L.H.; Chen, Q.Y.; Qu, M. Blue Light Is More Essential than Red Light for Maintaining the Activities of Photosystem II and I and Photosynthetic Electron Transport Capacity in Cucumber Leaves. J. Integr. Agric. 2016, 15, 87–100. [Google Scholar] [CrossRef]
- Matsuda, R.; Ohashi-Kaneko, K.; Fujiwara, K.; Kurata, K. Effects of Blue Light Deficiency on Acclimation of Light Energy Partitioning in PSII and CO2 Assimilation Capacity to High Irradiance in Spinach Leaves. Plant Cell Physiol. 2008, 49, 664–670. [Google Scholar] [CrossRef]
- Jia, M.; Li, D.; Colombo, R.; Wang, Y.; Wang, X.; Cheng, T.; Zhu, Y.; Yao, X.; Xu, C.; Ouer, G.; et al. Quantifying Chlorophyll Fluorescence Parameters from Hyperspectral Reflectance at the Leaf Scale under Various Nitrogen Treatment Regimes in Winter Wheat. Remote Sens. 2019, 11, 2838. [Google Scholar] [CrossRef]
- Bloem, E.; Gerighausen, H.; Chen, X.; Schnug, E. The Potential of Spectral Measurements for Identifying Glyphosate Application to Agricultural Fields. Agronomy 2020, 10, 1409. [Google Scholar] [CrossRef]
- Wang, D.; Cao, W.; Zhang, F.; Li, Z.; Xu, S.; Wu, X. A Review of Deep Learning in Multiscale Agricultural Sensing. Remote Sens. 2022, 14, 559. [Google Scholar] [CrossRef]
- Bag, P.; Chukhutsina, V.; Zhang, Z.; Paul, S.; Ivanov, A.G.; Shutova, T.; Croce, R.; Holzwarth, A.R.; Jansson, S. Direct Energy Transfer from Photosystem II to Photosystem I Confers Winter Sustainability in Scots Pine. Nat. Commun. 2020, 11, 6388. [Google Scholar] [CrossRef]
- Jin, J.; Arief Pratama, B.; Wang, Q. Tracing Leaf Photosynthetic Parameters Using Hyperspectral Indices in an Alpine Deciduous Forest. Remote Sens. 2020, 12, 1124. [Google Scholar] [CrossRef]
- Kokaly, R.F.; Skidmore, A.K. Plant Phenolics and Absorption Features in Vegetation Reflectance Spectra near 1.66 Μm. Int. J. Appl. Earth Obs. Geoinf. 2015, 43, 55–83. [Google Scholar] [CrossRef]
- Häme, T.; Sirro, L.; Kilpi, J.; Seitsonen, L.; Andersson, K.; Melkas, T. A Hierarchical Clustering Method for Land Cover Change Detection and Identification. Remote Sens. 2020, 12, 1751. [Google Scholar] [CrossRef]
- Ripoll, J.; Bertin, N.; Bidel, L.P.R.; Urban, L. A User’s View of the Parameters Derived from the Induction Curves of Maximal Chlorophyll a Fluorescence: Perspectives for Analyzing Stress. Front. Plant Sci. 2016, 7, 1679. [Google Scholar] [CrossRef]
- Burton-Smith, R.N.; Watanabe, A.; Tokutsu, R.; Song, C.; Murata, K.; Minagawa, J. Structural Determination of the Large Photosystem II–Light Harvesting Complex II Supercomplex of Chlamydomonas reinhardtii Using Non-Ionic Amphipol. J. Biol. Chem. 2019, 294, 15003–15013. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Shen, J.S.; Gu, M.; Wang, J.; Cheng, T.R.; Pan, H.T.; Zhang, Q.X. Leaf Coloration and Photosynthetic Characteristics of Hybrids between Forsythia ‘Courtaneur’ and Forsythia Koreana ‘Suwon Gold’. HortScience 2017, 52, 1661–1667. [Google Scholar] [CrossRef]
- Falcioni, R.; Moriwaki, T.; Antunes, W.C.; Nanni, M.R. Rapid Quantification Method for Yield, Calorimetric Energy and Chlorophyll a Fluorescence Parameters in Nicotiana tabacum L. Using Vis-NIR-SWIR Hyperspectroscopy. Plants 2022, 11, 2406. [Google Scholar] [CrossRef]
- Bussotti, F.; Desotgiu, R.; Pollastrini, M.; Cascio, C. The JIP Test: A Tool to Screen the Capacity of Plant Adaptation to Climate Change. Scand. J. For. Res. 2010, 25, 43–50. [Google Scholar] [CrossRef]
- Castro, F.A.; Campostrini, E.; Netto, A.T.; Viana, L.H. Relationship between Photochemical Efficiency (JIP-Test Parameters) and Portable Chlorophyll Meter Readings in Papaya Plants. Braz. J. Plant Physiol. 2011, 23, 295–304. [Google Scholar] [CrossRef]
- Thornley, R.H.; Verhoef, A.; Gerard, F.F.; White, K. The Feasibility of Leaf Reflectance-Based Taxonomic Inventories and Diversity Assessments of Species-Rich Grasslands: A Cross-Seasonal Evaluation Using Waveband Selection. Remote Sens. 2022, 14, 2310. [Google Scholar] [CrossRef]
- Sexton, T.; Sankaran, S.; Cousins, A.B. Predicting Photosynthetic Capacity in Tobacco Using Shortwave Infrared Spectral Reflectance. J. Exp. Bot. 2021, 72, 4373–4383. [Google Scholar] [CrossRef]
- Feng, W.; Yao, X.; Tian, Y.; Cao, W.; Zhu, Y. Monitoring Leaf Pigment Status with Hyperspectral Remote Sensing in Wheat. Aust. J. Agric. Res. 2008, 59, 748–760. [Google Scholar] [CrossRef]
- Shurygin, B.; Chivkunova, O.; Solovchenko, O.; Solovchenko, A.; Dorokhov, A.; Smirnov, I.; Astashev, M.E.; Khort, D. Comparison of the Non-Invasive Monitoring of Fresh-Cut Lettuce Condition with Imaging Reflectance Hyperspectrometer and Imaging PAM-Fluorimeter. Photonics 2021, 8, 425. [Google Scholar] [CrossRef]
- Sasidharan, R.; Chinnappa, C.C.; Staal, M.; Elzenga, J.T.M.; Yokoyama, R.; Nishitani, K.; Voesenek, L.A.C.J.; Pierik, R. Light Quality-Mediated Petiole Elongation in Arabidopsis during Shade Avoidance Involves Cell Wall Modification by Xyloglucan Endotransglucosylase/Hydrolases. Plant Physiol. 2010, 154, 978–990. [Google Scholar] [CrossRef]
- Srivastava, L. Plant Growth and Development: Hormones and Environment, 1st ed.; Srivastava, L., Ed.; Academic Press: New York, NY, USA, 2002. [Google Scholar]
- Gitelson, A.; Merzlyak, M.N. Spectral Reflectance Changes Associated with Autumn Senescence of Aesculus hippocastanum L. and Acer platanoides L. Leaves. Spectral Features and Relation to Chlorophyll Estimation. J. Plant Physiol. 1994, 143, 286–292. [Google Scholar] [CrossRef]
- Chappelle, E.W.; Kim, M.S.; McMurtrey, J.E. Ratio Analysis of Reflectance Spectra (RARS): An Algorithm for the Remote Estimation of the Concentrations of Chlorophyll A, Chlorophyll B, and Carotenoids in Soybean Leaves. Remote Sens. Environ. 1992, 39, 239–247. [Google Scholar] [CrossRef]
- Pontius, J.; Martin, M.; Plourde, L.; Hallett, R. Ash Decline Assessment in Emerald Ash Borer-Infested Regions: A Test of Tree-Level, Hyperspectral Technologies. Remote Sens. Environ. 2008, 112, 2665–2676. [Google Scholar] [CrossRef]
- Metternicht, G. Vegetation Indices Derived from High-Resolution Airborne Videography for Precision Crop Management. Int. J. Remote Sens. 2003, 24, 2855–2877. [Google Scholar] [CrossRef]
- Blackburn, G.A. Spectral Indices for Estimating Photosynthetic Pigment Concentrations: A Test Using Senescent Tree Leaves. Int. J. Remote Sens. 1998, 19, 657–675. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Chivkunova, O.B.; Solovchenko, A.E.; Naqvi, K.R. Light Absorption by Anthocyanins in Juvenile, Stressed, and Senescing Leaves. J. Exp. Bot. 2008, 59, 3903–3911. [Google Scholar] [CrossRef]
- Garbulsky, M.F.; Peñuelas, J.; Gamon, J.; Inoue, Y.; Filella, I. The Photochemical Reflectance Index (PRI) and the Remote Sensing of Leaf, Canopy and Ecosystem Radiation Use Efficiencies. A Review and Meta-Analysis. Remote Sens. Environ. 2011, 115, 281–297. [Google Scholar] [CrossRef]
- Lang, M.; Stober, F.; Lichtenthaler, H.K. Fluorescence Emission Spectra of Plant Leaves and Plant Constituents. Radiat. Environ. Biophys. 1991, 30, 333–347. [Google Scholar] [CrossRef]
- Stimson, H.C.; Breshears, D.D.; Ustin, S.L.; Kefauver, S.C. Spectral Sensing of Foliar Water Conditions in Two Co-Occurring Conifer Species: Pinus edulis and Juniperus monosperma. Remote Sens. Environ. 2005, 96, 108–118. [Google Scholar] [CrossRef]
- Apan, A.; Held, A.; Phinn, S.; Markley, J. Formulation and Assessment of Narrow-Band Vegetation Indices from EO-1 Hype-rion Imagery for Discriminating Sugarcane Disease. In Proceedings of the Spatial Sciences Institute Biennial Conference (SSC 2003): Spatial Knowledge Without Boundaries, Canberra, Australia, 22–27 September 2003; pp. 1–13. [Google Scholar]
- Nagler, P.L.; Inoue, Y.; Glenn, E.P.; Russ, A.L.; Daughtry, C.S.T. Cellulose Absorption Index (CAI) to Quantify Mixed Soil–Plant Litter Scenes. Remote Sens. Environ. 2003, 87, 310–325. [Google Scholar] [CrossRef]
- Serrano, L.; Peñuelas, J.; Ustin, S.L. Remote Sensing of Nitrogen and Lignin in Mediterranean Vegetation from AVIRIS Data: Decomposing Biochemical from Structural Signals. Remote Sens. Environ. 2002, 81, 355–364. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falcioni, R.; Antunes, W.C.; Demattê, J.A.M.; Nanni, M.R. Biophysical, Biochemical, and Photochemical Analyses Using Reflectance Hyperspectroscopy and Chlorophyll a Fluorescence Kinetics in Variegated Leaves. Biology 2023, 12, 704. https://doi.org/10.3390/biology12050704
Falcioni R, Antunes WC, Demattê JAM, Nanni MR. Biophysical, Biochemical, and Photochemical Analyses Using Reflectance Hyperspectroscopy and Chlorophyll a Fluorescence Kinetics in Variegated Leaves. Biology. 2023; 12(5):704. https://doi.org/10.3390/biology12050704
Chicago/Turabian StyleFalcioni, Renan, Werner Camargos Antunes, José A. M. Demattê, and Marcos Rafael Nanni. 2023. "Biophysical, Biochemical, and Photochemical Analyses Using Reflectance Hyperspectroscopy and Chlorophyll a Fluorescence Kinetics in Variegated Leaves" Biology 12, no. 5: 704. https://doi.org/10.3390/biology12050704
APA StyleFalcioni, R., Antunes, W. C., Demattê, J. A. M., & Nanni, M. R. (2023). Biophysical, Biochemical, and Photochemical Analyses Using Reflectance Hyperspectroscopy and Chlorophyll a Fluorescence Kinetics in Variegated Leaves. Biology, 12(5), 704. https://doi.org/10.3390/biology12050704









