Ultrastructural Evaluation of Mouse Oocytes Exposed In Vitro to Different Concentrations of the Fungicide Mancozeb
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. In Vitro Maturation of Oocyte-Cumulus Cell Complexes and Experimental Protocol
2.4. Light Microscopy (LM) and Transmission Electron Microscopy (TEM)
2.5. Morphometric Analysis
2.6. Statistical Analysis
3. Results
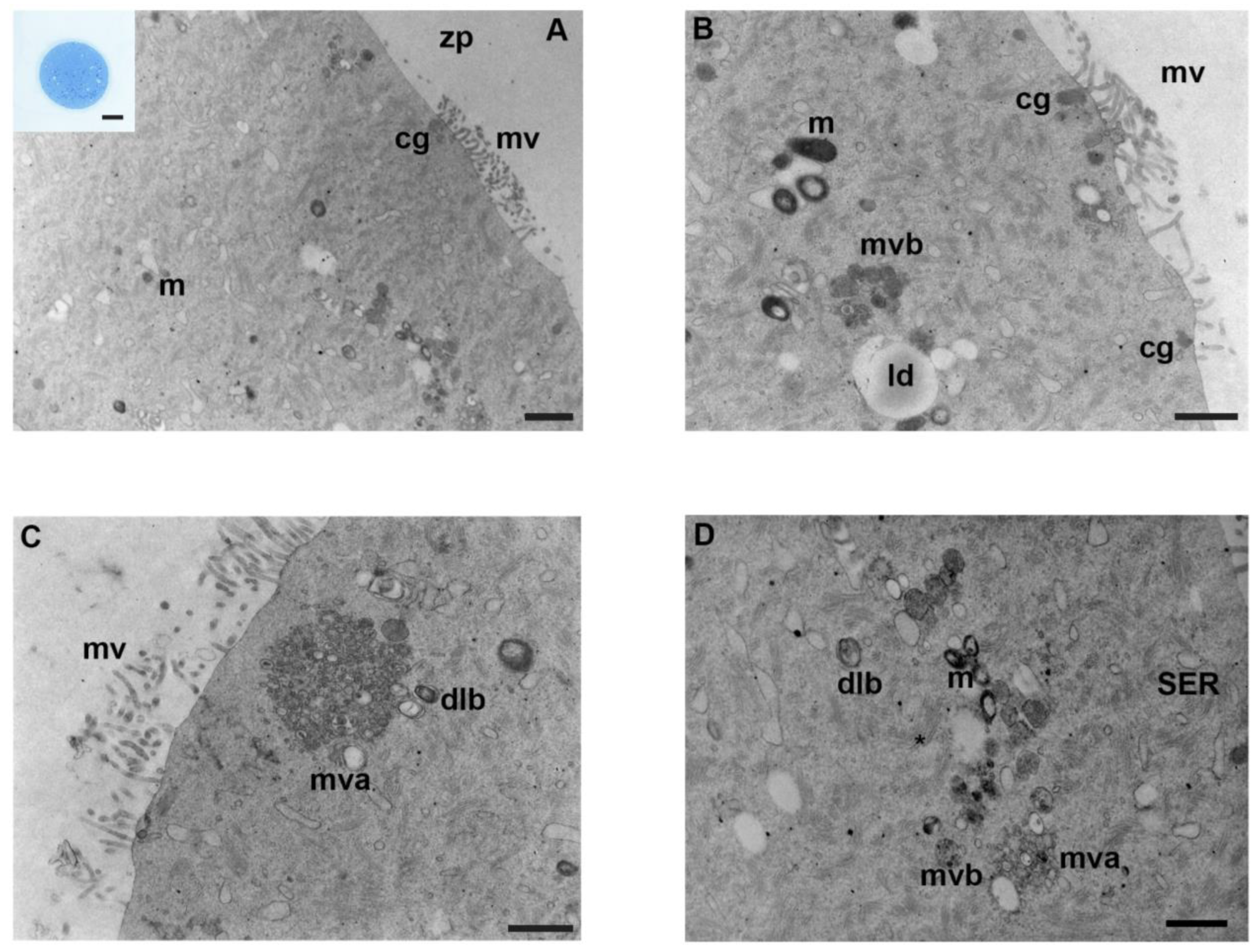
3.1. Controls
3.2. Mancozeb 0.001 µg/mL
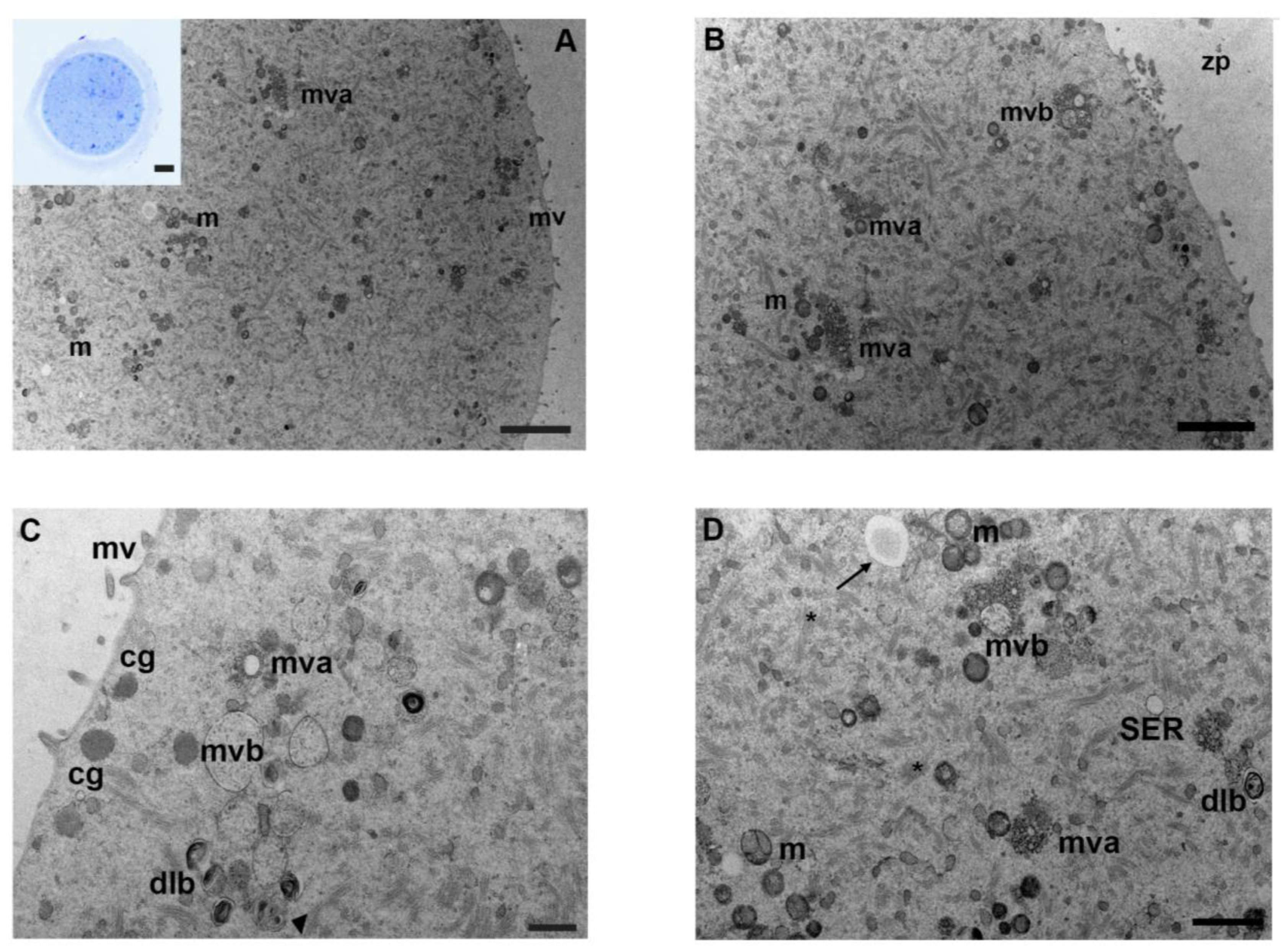
3.3. Mancozeb 0.01 µg/mL
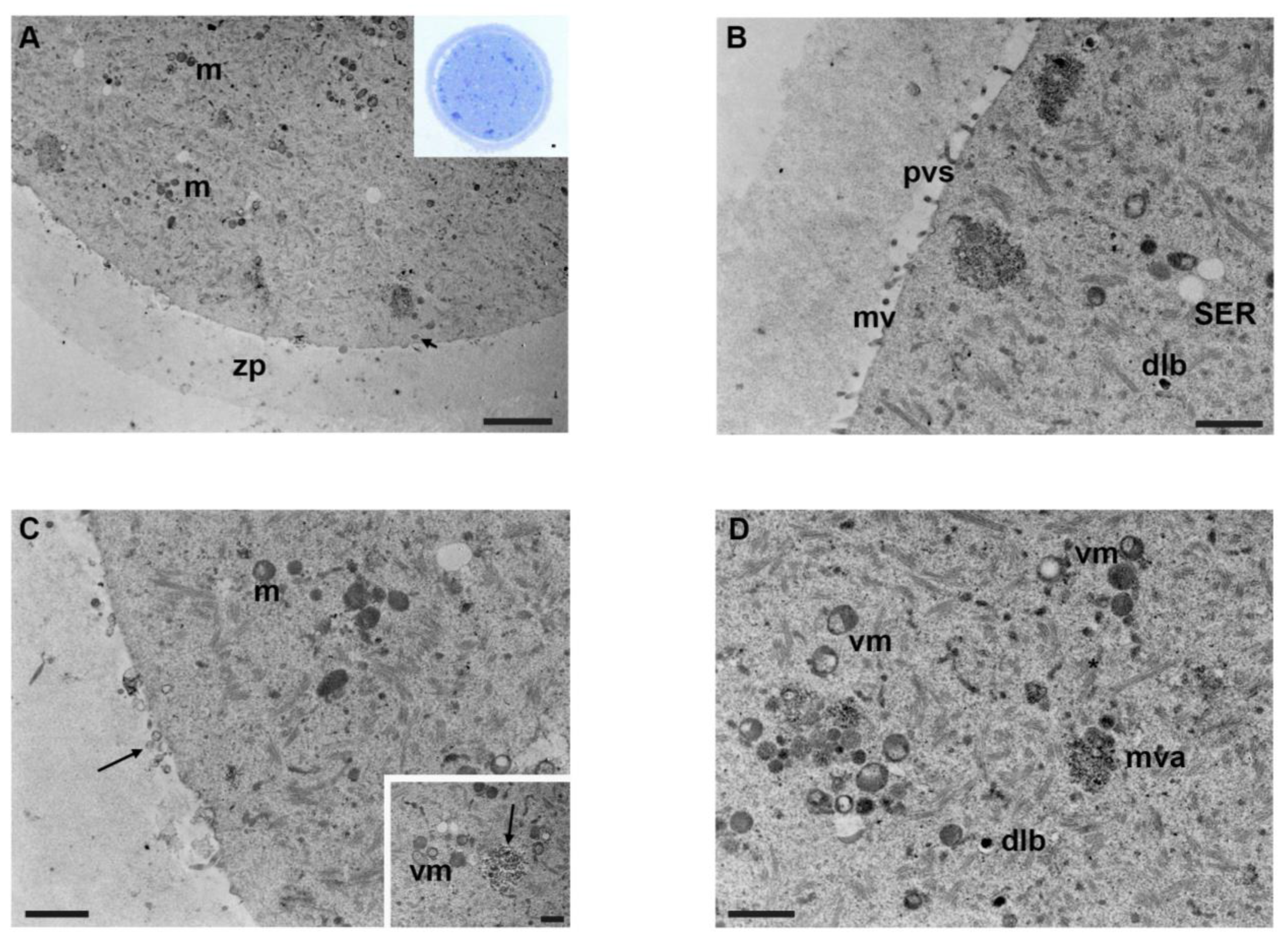
3.4. Mancozeb 0.1 µg/mL
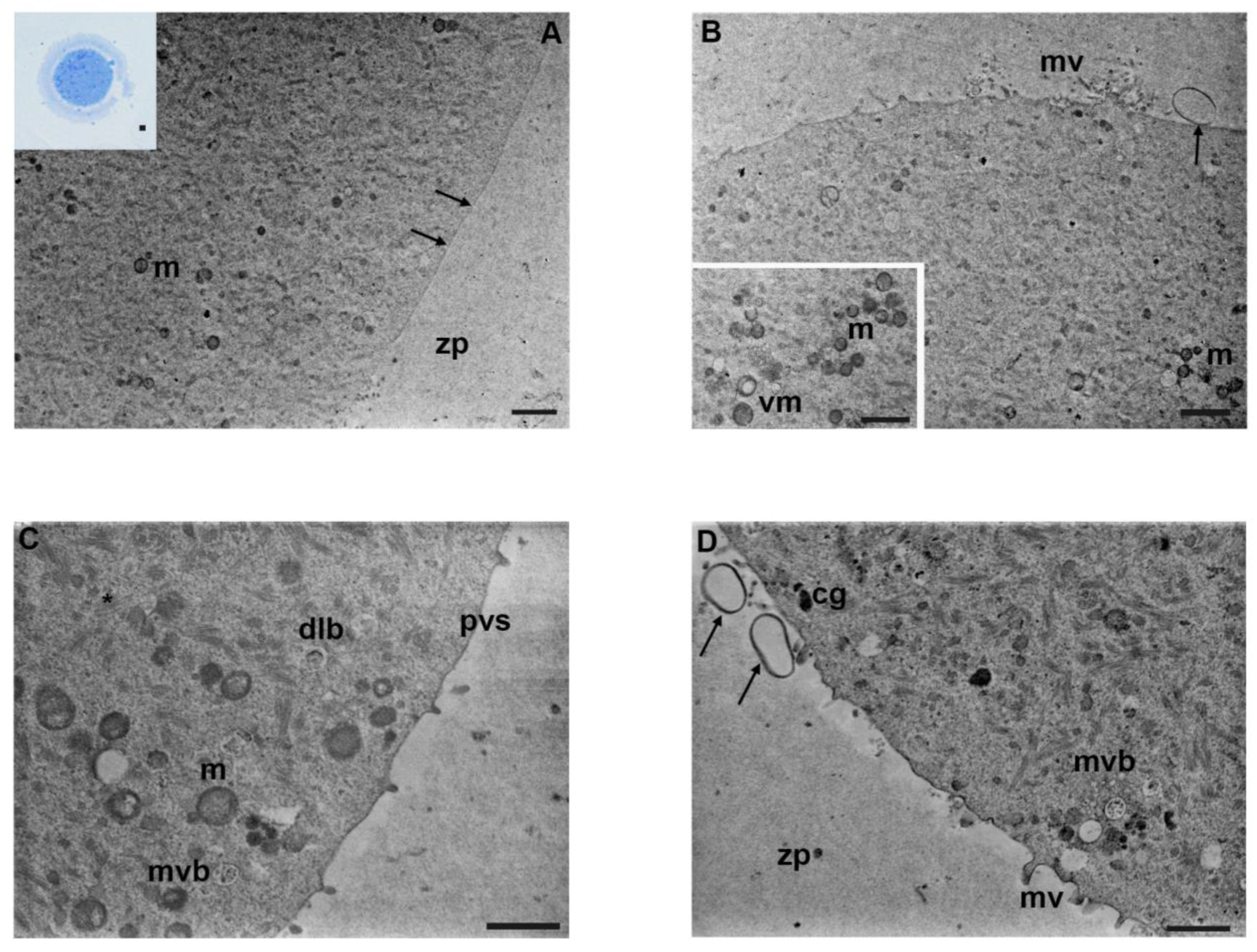
3.5. Mancozeb 1 µg/mL
3.6. Morphometric Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canossa, E.; Angiuli, G.; Garasto, G.; Buzzoni, A.; De Rosa, E. Dosage indicators in farm workers exposed to mancozeb. [Article in Italian]. La Med. Del. Lav. 1993, 84, 42–50. [Google Scholar]
- Miles, M.; Kemmitt, G. Field studies to determine mancozeb-based spray programmes with minimal impact on predatory mites in European vine cultivation. Commun. Agric. Appl. Biol. Sci. 2005, 70, 559–567. [Google Scholar] [PubMed]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef] [PubMed]
- EPA Environmental Protection Agency; Mancozeb Reregistration Eligibility Decision (RED). Available online: https://www.federalregister.gov/documents/2005/12/28/05-24465/mancozeb-reregistration-eligibility-decision (accessed on 28 December 2005).
- Shukla, Y.; Arora, A. Transplacental carcinogenic potential of the carbamate fungicide mancozeb. J. Environ. Pathol. Toxicol. Oncol. 2001, 20, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Domico, L.M.; Cooper, K.R.; Bernard, L.P.; Zeevalk, G.D. Reactive oxygen species generation by the ethylene-bis-dithiocarbamate (EBDC) fungicide mancozeb and its contribution to neuronal toxicity in mesencephalic cells. Neurotoxicology 2007, 28, 1079–1091. [Google Scholar] [CrossRef]
- Pavlovic, V.; Cekic, S.; Ciric, M.; Krtinic, D.; Jovanovic, J. Curcumin attenuates Mancozeb-induced toxicity in rat thymocytes through mitochondrial survival pathway. Food Chem. Toxicol. 2016, 88, 105–111. [Google Scholar] [CrossRef]
- Cecconi, S.; Paro, R.; Rossi, G.; Macchiarelli, G. The effects of the endocrine disruptors dithiocarbamates on the mammalian ovary with particular regard to mancozeb. Curr. Pharm. Des. 2007, 13, 2989–3004. [Google Scholar] [CrossRef]
- Merklinger-Gruchala, A.; Jasienska, G.; Kapiszewska, M. Effect of air pollution on menstrual cycle length-a prognostic factor of women’s reproductive health. Int. J. Environ. Res. Public Health 2017, 14, 816. [Google Scholar] [CrossRef]
- Green, M.P.; Harvey, A.J.; Finger, B.J.; Tarulli, G.A. Endocrine disrupting chemicals: Impacts on human fertility and fecundity during the peri-conception period. Environ. Res. 2021, 194, 110694. [Google Scholar] [CrossRef]
- You, H.H.; Song, G. Review of endocrine disruptors on male and female reproductive systems. Comp. Biochem. Physiol. C. Toxicol. Pharm. 2021, 244, 109002. [Google Scholar] [CrossRef]
- Spencer, S.W.; Karaca, H. Remediation of Fungicide Residues on Fresh Produce by Use of Gaseous Ozone. Environ. Sci. Technol. 2011, 45, 6961–6969. [Google Scholar]
- European Food Safety Authority (EFSA); Abdourahime, H.; Anastassiadou, M.; Arena, M.; Auteri, D.; Barmaz, S.; Brancato, A.; Bura, L.; Carrasco Cabrera, L.; Chaideftou, E.; et al. Peer review of the pesticide risk assessment of the active substance mancozeb. EFSA J. 2020, 18, e05755. [Google Scholar]
- Skalny, A.; Aschner, M.; Paoliello, M.; Santamaria, A.; Nikitina, N.; Rejniuk, V.; Jiang, Y.; Rocha, J.; Tinkov, A. Endocrine-disrupting activity of mancozeb. Arh. Farm. 2021, 71, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Aprea, C.; Betta, A.; Catenacci, G.; Lotti, A.; Minoia, C.; Passini, W.; Pavan, I.; Saverio, F.; Roggi, C.; Ruggeri, R.; et al. Reference values of urinary ethylenethiourea in four regions of Italy (multicentric study). Sci. Total. Environ. 1996, 192, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Colosio, C.; Fustinoni, S.; Birindelli, S.; Bonomi, I.; De Paschale, G.; Mammone, T.; Tiramani, M.; Vercelli, F.; Visentin, S.; Maroni, M. Ethylenethiourea in urine as an indicator of exposure to mancozeb in vineyard workers. Toxicol. Lett. 2002, 134, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Mandic-Rajcevic, S.; Rubino, F.M.; Ariano, E.; Cottica, D.; Neri, S.; Colosio, C. Environmental and biological monitoring for the identification of main exposure determinants in vineyard mancozeb applicators. J. Expo. Sci. Environ. Epidemiol. 2018, 28, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, Z.; Fan, Z.; Fang, Y.; He, L.; Peng, M.; Chen, Y.; Hu, Z.; Zhao, K.; Zhang, H.; et al. Cardiac developmental toxicity and transcriptome analyses of zebrafish (Danio rerio) embryos exposed to Mancozeb. Ecotoxicol. Environ. Saf. 2021, 226, 112798. [Google Scholar] [CrossRef]
- Archer, E.; van Wyk, J. The potential anti-androgenic effect of agricultural pesticides used in the Western Cape: In vitro investigation of mixture effects. Water SA 2014, 41, 129. [Google Scholar] [CrossRef]
- U.S. EPA/OPP (U.S. Environmental Protection Agency/Office of Pesticide Programs). Registration Review: Conventional Cases Schedule: 2012–2015. Available online: https://www.epa.gov/oppsrrd1/registration_review/schedule.htm (accessed on 9 July 2013).
- Kollman, W.S. Summary of Assembly Bill 1807/3219, Pesticide Air Monitoring Results; Department of Pesticide Regulation: Sacramento, CA, USA, 1995. [Google Scholar]
- World Health Organization. Dithiocarbamates Pesticides Ethylenethiourea, and Propylenenthiourea: A General Introduction. Environ Health Criteria 78 Geneva; World Health Organization: Geneva, Switzerland, 1988. [Google Scholar]
- International Agency for Research on Cancer Advisory Group Report of the Advisory Group to Recommend Priorities for the IARC Monographs during 2020–2024. Available online: https://www.iarc.who.int/news-events/report-of-the-advisory-group-to-recommend-priorities-for-the-iarc-monographs-during-2020-2024/ (accessed on 2 July 2022).
- Domico, L.M.; Zeevalk, G.D.; Bernard, L.P.; Cooper, K.R. Acute neurotoxic effects of mancozeb and maneb in mesencephalic neuronal cultures are associated with mitochondrial dysfunction. Neurotoxicology 2006, 27, 816–825. [Google Scholar] [CrossRef]
- Calviello, G.; Piccioni, E.; Boninsegna, A.; Tedesco, B.; Maggiano, N.; Serini, S.; Wolf, F.I.; Palozza, P. DNA damage and apoptosis induction by the pesticide mancozeb in rat cells: Involvement of the oxidative mechanism. Toxicol. Appl. Pharm. 2006, 211, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [PubMed]
- Cabry, R.; Merviel, P.; Madkour, A.; Lefranc, E.; Scheffler, F.; Desailloud, R.; Bach, V.; Benkhalifa, M. The impact of endocrine disruptor chemicals on oocyte/embryo and clinical outcomes in IVF. Endocr. Connect. 2020, 9, 134–142. [Google Scholar] [CrossRef]
- Jacobsen, P.R.; Axelstad, M.; Boberg, J.; Isling, L.K.; Christiansen, S.; Mandrup, K.R.; Berthelsen, L.O.; Vinggaard, A.M.; Hass, U. Persistent developmental toxicity in rat offspring after low dose exposure to a mixture of endocrine disrupting pesticides. Reprod. Toxicol. 2012, 34, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Hass, U.; Boberg, J.; Christiansen, S.; Jacobsen, P.R.; Vinggaard, A.M.; Taxvig, C.; Poulsen, M.E.; Herrmann, S.S.; Jensen, B.H.; Petersen, A.; et al. Adverse effects on sexual development in rat offspring after low dose exposure to a mixture of endocrine disrupting pesticides. Reprod. Toxicol. 2012, 34, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Runkle, J.; Flocks, J.; Economos, J.; Dunlop, A.L. A systematic review of Mancozeb as a reproductive and developmental hazard. Environ. Int. 2017, 99, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Paro, R.; Tiboni, G.M.; Buccione, R.; Rossi, G.; Cellini, V.; Canipari, R.; Cecconi, S. The fungicide mancozeb induces toxic effects on mammalian granulosa cells. Toxicol. Appl. Pharm. 2012, 260, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Ali, W.; Singh, R.; Bhui, K.; Tyagi, S.; Al-Khedhairy, A.A.; Srivastava, P.K.; Musarrat, J.; Shukla, Y. Mancozeb-induced genotoxicity and apoptosis in cultured human lymphocytes. Life Sci. 2012, 90, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Akthar, I.; Wang, Z.; Wijayagunawardane, M.P.B.; Ratnayake, C.J.; Siriweera, E.H.; Lee, K.F.; Kodithuwakku, S.P. In vitro and in vivo impairment of embryo implantation by commonly used fungicide Mancozeb. Biochem. Biophys. Res. Commun. 2020, 527, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kottawatta, K.S.A.; Kodithuwakku, S.P.; Fernando, T.S.; Lee, Y.L.; Ng, E.H.Y.; Yeung, W.S.B.; Lee, K.F. The fungicide Mancozeb reduces spheroid attachment onto endometrial epithelial cells through downregulation of estrogen receptor β and integrin β3 in Ishikawa cells. Ecotoxicol. Environ. Saf. 2021, 208, 111606. [Google Scholar] [CrossRef]
- Greenlee, A.R.; Ellis, T.M.; Berg, R.L. Low-dose agrochemicals and lawn-care pesticides induce developmental toxicity in murine preimplantation embryos. Environ. Health Perspect. 2004, 112, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Dinisri, I.; Kodikara, S.; Prasadani, M.; Pathirana, I.; Rathnayake, C.; Alexander, B.; Lee, K.F.; Kodithuwakku, S.P. Impairment of caprine oocyte maturation in vitro and alteration of granulosa cells functions by widely used fungicide mancozeb. Trop. Anim. Health Prod. 2021, 53, 406. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Zhang, Y.; Wen, R.; Zhang, L.; Wang, X. Low level of mancozeb exposure affects ovary in mice. Ecotoxicol. Environ. Saf. 2022, 239, 113670. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Palmerini, M.G.; Macchiarelli, G.; Buccione, R.; Cecconi, S. Mancozeb adversely affects meiotic spindle organization and fertilization in mouse oocytes. Reprod. Toxicol. 2006, 22, 51–55. [Google Scholar] [CrossRef]
- Rossi, G.; Buccione, R.; Baldassarre, M.; Macchiarelli, G.; Palmerini, M.G.; Cecconi, S. Mancozeb exposure in vivo impairs mouse oocyte fertilizability. Reprod. Toxicol. 2006, 21, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Iorio, R.; Castellucci, A.; Rossi, G.; Cinque, B.; Cifone, M.G.; Macchiarelli, G.; Cecconi, S. Mancozeb affects mitochondrial activity, redox status and ATP production in mouse granulosa cells. Toxicol. In Vitro 2015, 30, 438–445. [Google Scholar] [CrossRef]
- Palmerini, M.G.; Belli, M.; Nottola, S.A.; Miglietta, S.; Bianchi, S.; Bernardi, S.; Antonouli, S.; Cecconi, S.; Familiari, G.; Macchiarelli, G. Mancozeb impairs the ultrastructure of mouse granulosa cells in a dose-dependent manner. J. Reprod. Dev. 2018, 64, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Nottola, S.A.; Macchiarelli, G.; Coticchio, G.; Bianchi, S.; Cecconi, S.; De Santis, L.; Scaravelli, G.; Flamigni, C.; Borini, A. Ultrastructure of human mature oocytes after slow cooling cryopreservation using different sucrose concentrations. Hum. Reprod. 2007, 22, 1123–1133. [Google Scholar] [CrossRef]
- Khalili, M.A.; Maione, M.; Palmerini, M.G.; Bianchi, S.; Macchiarelli, G.; Nottola, S.A. Ultrastructure of human mature oocytes after vitrification. Eur. J. Histochem. 2012, 56, e38. [Google Scholar] [CrossRef] [PubMed]
- Coticchio, G.; Dal Canto, M.; Fadini, R.; Mignini Renzini, M.; Guglielmo, M.C.; Miglietta, S.; Palmerini, M.G.; Macchiarelli, G.; Nottola, S.A. Ultrastructure of human oocytes after in vitro maturation. Mol. Hum. Reprod. 2016, 22, 110–118. [Google Scholar] [CrossRef]
- Palmerini, M.G.; Zhurabekova, G.; Balmagambetova, A.; Nottola, S.A.; Miglietta, S.; Belli, M.; Bianchi, S.; Cecconi, S.; Di Nisio, V.; Familiari, G.; et al. The pesticide Lindane induces dose-dependent damage to granulosa cells in an in vitro culture. Reprod. Biol. 2017, 17, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Leoni, G.G.; Palmerini, M.G.; Satta, V.; Succu, S.; Pasciu, V.; Zinellu, A.; Carru, C.; Macchiarelli, G.; Nottola, S.A.; Naitana, S.; et al. Differences in the kinetic of the first meiotic division and in active mitochondrial distribution between prepubertal and adult oocytes mirror differences in their developmental competence in a sheep model. PLoS ONE 2015, 10, e0124911. [Google Scholar] [CrossRef] [PubMed]
- Belli, M.; Zhang, L.; Liu, X.; Donjacour, A.; Ruggeri, E.; Palmerini, M.G.; Nottola, S.A.; Macchiarelli, G.; Rinaudo, P. Oxygen concentration alters mitochondrial structure and function in in vitro fertilized preimplantation mouse embryos. Hum. Reprod. 2019, 34, 601–611. [Google Scholar] [CrossRef]
- Bianchi, V.; Macchiarelli, G.; Borini, A.; Lappi, M.; Cecconi, S.; Miglietta, S.; Familiari, G.; Nottola, S.A. Fine morphological assessment of quality of human mature oocytes after slow freezing or vitrification with a closed device: A comparative analysis. Reprod. Biol. Endocrinol. 2014, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Nordby, K.C.; Andersen, A.; Irgens, L.M.; Kristensen, P. Indicators of mancozeb exposure in relation to thyroid cancer and neural tube defects in farmers’ families. Scand. J. Work. Environ. Health 2005, 31, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Axelstad, M.; Boberg, J.; Nellemann, C.; Kiergaard, M.; Jacobsen, P.R.; Christiansen, S.; Hougaard, K.S.; Hass, U. Exposure to the widely used fungicide mancozeb causes thyroid hormone disruption in rat dams but no behavioral effects in the offspring. Toxicol. Sci. 2011, 120, 439–446. [Google Scholar] [CrossRef]
- Mahadevaswami, M.P.; Jadaramkunti, U.C.; Hiremath, M.B.; Kaliwal, B.B. Effect of mancozeb on ovarian compensatory hypertrophy and biochemical constituents in hemicastrated albino rat. Reprod. Toxicol. 2000, 14, 127–134. [Google Scholar] [CrossRef]
- Baligar, P.N.; Kaliwal, B.B. Morphometric analysis of follicular growth and biochemical constituents in albino rats exposed to mancozeb. J. Basic. Clin. Physiol. Pharmacol. 2004, 15, 241–262. [Google Scholar] [CrossRef]
- Baligar, P.N.; Kaliwal, B.B. Induction of gonadal toxicity to female rats after chronic exposure to mancozeb. Ind. Health 2001, 39, 235–243. [Google Scholar] [CrossRef]
- Esposito, G.; Vitale, A.M.; Leijten, F.P.; Strik, A.M.; Koonen-Reemst, A.M.; Yurttas, P.; Robben, T.J.; Coonrod, S.; Gossen, J.A. Peptidylarginine deiminase (PAD) 6 is essential for oocyte cytoskeletal sheet formation and female fertility. Mol. Cell. Endocrinol. 2007, 273, 25–31. [Google Scholar] [CrossRef]
- Yurttas, P.; Vitale, A.M.; Fitzhenry, R.J.; Cohen-Gould, L.; Wu, W.; Gossen, J.A.; Coonrod, S.A. Role for PADI6 and the cytoplasmic lattices in ribosomal storage in oocytes and translational control in the early mouse embryo. Development 2008, 135, 2627–2636. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Boiani, M.; Redi, C.; Monti, M. Cytoplasmic lattices are not linked to mouse 2-cell embryos developmental arrest. Eur. J. Histochem. 2018, 62, 2972. [Google Scholar] [CrossRef] [PubMed]
- Bebbere, D.; Albertini, D.F.; Coticchio, G.; Borini, A.; Ledda, S. The subcortical maternal complex: Emerging roles and novel perspectives. Mol. Hum. Reprod. 2021, 27, gaab043. [Google Scholar] [CrossRef]
- Motta, P.M.; Nottola, S.A.; Familiari, G.; Makabe, S.; Stallone, T.; Macchiarelli, G. Morphodynamics of the follicular-luteal complex during early ovarian development and reproductive life. Int. Rev. Cytol. 2003, 223, 177–288. [Google Scholar]
- Pepling, M.E.; Wilhelm, J.E.; O’Hara, A.L.; Gephardt, G.W.; Spradling, A.C. Mouse oocytes within germ cell cysts and primordial follicles contain a Balbiani body. Proc. Natl. Acad. Sci. USA 2007, 104, 187–192. [Google Scholar] [CrossRef]
- Nottola, S.A.; Coticchio, G.; De Santis, L.; Macchiarelli, G.; Maione, M.; Bianchi, S.; Iaccarino, M.; Flamigni, C.; Borini, A. Ultrastructure of human mature oocytes after slow cooling cryopreservation with ethylene glycol. Reprod. Biomed. Online 2008, 17, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Dvorák, M. Ultrastructure and quantitative analysis of mouse and human oocytes. Prog. Clin. Biol. Res. 1989, 296, 273–280. [Google Scholar]
- Makabe, S.; Naguro, T.; Nottola, S.A.; Van Blerkom, J. Atlas of Human Female Reproductive Function: Ovarian Development to Early Embryogenesis After In Vitro Fertilization; Taylor & Francis: London, UK, 2006. [Google Scholar]
- Crocco, M.; Alberio, R.H.; Lauria, L.; Mariano, M.I. Effect of serum on the mitochondrial active area on developmental days 1 to 4 in in vitro-producedbovine embryos. Zygote 2011, 19, 297–306. [Google Scholar] [CrossRef]
- Dadarwal, D.; Adams, G.P.; Hyttel, P.; Brogliatti, G.M.; Caldwell, S.; Singh, J. Organelle reorganization in bovine oocytes during dominant follicle-growth and regression. Reprod. Biol. Endocrinol. 2015, 13, 124. [Google Scholar] [CrossRef]
- Belli, M.; Rinaudo, P.; Palmerini, M.G.; Ruggeri, E.; Antonouli, S.; Nottola, S.A.; Macchiarelli, G. Pre-Implantation Mouse Embryos Cultured In Vitro under Different Oxygen Concentrations Show Altered Ultrastructures. Int. J. Environ. Res. Public Health 2020, 17, 3384. [Google Scholar] [CrossRef]
- Belli, M.; Palmerini, M.G.; Bianchi, S.; Bernardi, S.; Khalili, M.A.; Nottola, S.A.; Macchiarelli, G. Ultrastructure of mitochondria of human oocytes in different clinical conditions during assisted reproduction. Arch. Biochem. Biophys. 2021, 703, 108854. [Google Scholar] [CrossRef]
- Babayev, E.; Seli, E. Oocyte mitochondrial function and reproduction. Curr. Opin. Obs. Gynecol. 2015, 27, 175–181. [Google Scholar] [CrossRef]
- Nicosia, S.V.; Wolf, D.P.; Inoue, M. Cortical granule distribution and cell surface characteristics in mouse eggs. Dev. Biol. 1977, 57, 56–74. [Google Scholar] [CrossRef] [PubMed]
- Sathanathan, A.H.; Trounson, A.O. Ultrastructrure of cortical granule release and zona interaction in monospermic and polyspermic human ova fertilized in vitro. Gamete Res. 1982, 6, 225–234. [Google Scholar] [CrossRef]
- Sathanathan, A.H.; Trounson, A.O. Ultrastructural observations on cortical granules in human follicular oocytes cultured in vitro. Gamete Res. 1982, 5, 191–198. [Google Scholar] [CrossRef]
- Motta, P.M.; Nottola, S.A.; Micara, G.; Familiari, G. Ultrastructure of human unfertilized oocytes and polyspermic embryos in an IVF-ET program. Ann. N. Y. Acad. Sci. 1988, 541, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Familiari, G.; Heyn, R.; Relucenti, M.; Nottola, S.A.; Sathanathan, A.H. Ultrastructural dynamics of human reproduction, from ovulation to fertilization and early embryo development. Int. Rev. Cytol. 2006, 249, 53–141. [Google Scholar] [PubMed]
- Sathananthan, A.H.; Selvaraj, K.; Girijashankar, M.L.; Ganesh, V.; Selvaraj, P.; Trounson, A.O. From oogonia to mature oocytes: Inactivation of the maternal centrosome in humans. Microsc. Res. Tech. 2006, 69, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Cappa, A.I.; De Paola, M.; Wetten, P.; De Blas, G.A.; Michaut, M.A. Live imaging of cortical granule exocytosis reveals that in vitro matured mouse oocytes are not fully competent to secrete their content. Biol. Open. 2018, 7, bio031872. [Google Scholar] [CrossRef]
- De Paola, M.; Miró, M.P.; Ratto, M.; Bátiz, L.F.; Michaut, M.A. Pleiotropic effects of alpha-SNAP M105I mutation on oocyte biology: Ultrastructural and cellular changes that adversely affect female fertility in mice. Sci. Rep. 2019, 9, 17374. [Google Scholar] [CrossRef]
- Yanagimachi, R. Sperm-egg association in animals. Curr. Top. Dev. Biol. 1978, 12, 83–105. [Google Scholar] [PubMed]
- Wilson, N.F.; Snell, W.J. Microvilli and cell-cell fusion during fertilization. Trends Cell Biol. 1998, 8, 93–96. [Google Scholar] [CrossRef]
- Runge, K.E.; Evans, J.E.; He, Z.Y.; Gupta, S.; McDonald, K.L.; Stahlberg, H.; Primakoff, P.; Myles, D.G. Oocyte CD9 is enriched on the microvillar membrane and required for normal microvillar shape and distribution. Dev. Biol. 2007, 304, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Benammar, A.; Ziyyat, A.; Lefèvre, B.; Wolf, J.P. Tetraspanins and Mouse Oocyte Microvilli Related to Fertilizing Ability. Reprod. Sci. 2017, 24, 1062–1069. [Google Scholar] [CrossRef]
- Ghadially, F.N. Ultrastructural Pathology of the Cell and Matrix, 3rd ed.; Butterworths: Oxford, UK, 1997; Volume 2, Chapter 7: Lysosomes; pp. 602–608. [Google Scholar]
- Uchiyama, Y.; Shibata, M.; Koike, M.; Yoshimura, K.; Sasaki, M. Autophagy-physiology and pathophysiology. Histochem. Cell. Biol. 2008, 129, 407–420. [Google Scholar] [CrossRef]
- Yi, J.; Tang, X.M. Functional implication of autophagy in steroid-secreting cells of the rat. Anat. Rec. 1995, 242, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Morelli, E.; Ginefra, P.; Mastrodonato, V.; Beznoussenko, G.V.; Rusten, T.E.; Bilder, D.; Stenmark, H.; Mironov, A.A.; Vaccari, T. Multiple functions of the SNARE protein Snap29 in autophagy, endocytic, and exocytic trafficking during epithelial formation in Drosophila. Autophagy 2014, 10, 2251–2268. [Google Scholar] [CrossRef]
- Bianchi, S.; Nottola, S.A.; Torge, D.; Palmerini, M.G.; Necozione, S.; Macchiarelli, G. Association between Female Reproductive Health and Mancozeb: Systematic Review of Experimental Models. Int. J. Environ. Res. Public Health 2020, 17, 2580. [Google Scholar] [CrossRef] [PubMed]
- Kidder, G.M.; Vanderhayen, B.C. Bidirectional communication between oocytes and follicles cells: Ensuring oocyte developmental competence. Can. J. Physiol. Pharm. 2010, 88, 399–413. [Google Scholar] [CrossRef]
- Canipari, R.; Cellini, V.; Cecconi, S. The ovary feels fine when paracrine and autocrine networks cooperate with gonadotropins in the regulation of folliculogenesis. Curr. Pharm. Des. 2012, 18, 245–255. [Google Scholar] [PubMed]
- Dumesic, D.A.; Meldrum, D.R.; Katz-Jaffe, M.G.; Krisher, R.L.; Schoolcraft, W.B. Oocytes environment: Follicular fluid and cumuls cells are critical for oocyte health. Fertil. Steril. 2015, 103, 3030–3316. [Google Scholar] [CrossRef] [PubMed]
- Campen, K.A.; McNatty, K.P.; Pitman, J.L. A protective role of cumulus cells after short-term exposure of rat cumulus cell-oocyte complexes to lifestyle or environmental contaminants. Reprod. Toxicol. 2017, 69, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Pocar, P.; Nestler, D.; Risch, M.; Fischer, B. Apoptosis in bovine cumulus-oocyte complexes after exposure to polychlorinated biphenyl mixtures during in vitro maturation. Reproduction 2005, 130, 857–868. [Google Scholar] [CrossRef] [PubMed]

| Mancozeb | |||||
|---|---|---|---|---|---|
| Control | 0.001 µg/mL | 0.01 µg/mL | 0.1 µg/mL | 1 µg/mL | |
| Cytoplasmic lattice | Uniformly distributed | Uniformly distributed | Uniformly distributed | Uniformly distributed | Uniformly distributed |
| Mitochondria | Round to ovoid shaped, with dense matrix | Round to ovoid shaped | Increased vacuolated forms | Increased vacuolated forms | Prevalent vacuolated forms |
| Cortical Granules | Round, dark electron density | Round, dark electron density | Round, dark electron density | Round, dark electron density | Round, dark electron density |
| Microvilli | Long, and thin | Thicker | Short and thick | Short and thick | Flattened |
| Zona pellucida | Dense | Dense | Dense | Thin and dense | Thin and dense |
| Mancozeb | |||||
|---|---|---|---|---|---|
| Control | 0.001 µg/mL | 0.01 µg/mL | 0.1 µg/mL | 1 µg/mL | |
| Mitochondria (50 µm2) | 28 ± 7.131 a | 24.13 ± 5.436 a,b | 23.38 ± 8.684 a,b | 21.13 ± 4.794 a,b | 18.5 ± 5.175 b |
| Multivesicular bodies and dense lamellar bodies (50 µm2) | 7 ± 1.581 | 8.6 ± 1.517 | 7.4 ± 1.517 | 7.2 ± 0.836 | 7.4 ± 1.14 |
| Autophagic vesicles (50 µm2) | 0.6 ± 0.547 | 0.8 ± 0.836 | 1.4 ± 0.547 | 1.6 ± 0.547 | 1.8 ± 0.836 |
| SER (50 µm2) | 3 ± 1 | 2.8 ± 1.789 | 2.4 ± 1.14 | 1.8 ± 0.836 | 0.8 ± 0.836 |
| Cortical granules/10 µm | 3 ±0.707 a | 3.2 ± 0.837 a,b | 1.6 ± 0.894 a,c | 1.6 ± 1.14 a,c | 0.8 ± 0.837 c |
| Microvilli/10 µm | 15.6 ± 2.408 a | 14.4 ± 2.702 a,b | 11 ± 2.345 b | 11.4 ± 1.517 a,b | 6 ± 2.449 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatti, M.; Belli, M.; De Rubeis, M.; Khalili, M.A.; Familiari, G.; Nottola, S.A.; Macchiarelli, G.; Hajderi, E.; Palmerini, M.G. Ultrastructural Evaluation of Mouse Oocytes Exposed In Vitro to Different Concentrations of the Fungicide Mancozeb. Biology 2023, 12, 698. https://doi.org/10.3390/biology12050698
Gatti M, Belli M, De Rubeis M, Khalili MA, Familiari G, Nottola SA, Macchiarelli G, Hajderi E, Palmerini MG. Ultrastructural Evaluation of Mouse Oocytes Exposed In Vitro to Different Concentrations of the Fungicide Mancozeb. Biology. 2023; 12(5):698. https://doi.org/10.3390/biology12050698
Chicago/Turabian StyleGatti, Marta, Manuel Belli, Mariacarla De Rubeis, Mohammad Ali Khalili, Giuseppe Familiari, Stefania Annarita Nottola, Guido Macchiarelli, Edmond Hajderi, and Maria Grazia Palmerini. 2023. "Ultrastructural Evaluation of Mouse Oocytes Exposed In Vitro to Different Concentrations of the Fungicide Mancozeb" Biology 12, no. 5: 698. https://doi.org/10.3390/biology12050698
APA StyleGatti, M., Belli, M., De Rubeis, M., Khalili, M. A., Familiari, G., Nottola, S. A., Macchiarelli, G., Hajderi, E., & Palmerini, M. G. (2023). Ultrastructural Evaluation of Mouse Oocytes Exposed In Vitro to Different Concentrations of the Fungicide Mancozeb. Biology, 12(5), 698. https://doi.org/10.3390/biology12050698







