Long-Term Daytime Warming Rather Than Nighttime Warming Alters Soil Microbial Composition in a Semi-Arid Grassland
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Soil Microclimate, PLFA Analysis, and Plant Cover Measurement
2.4. Data Analysis
3. Results
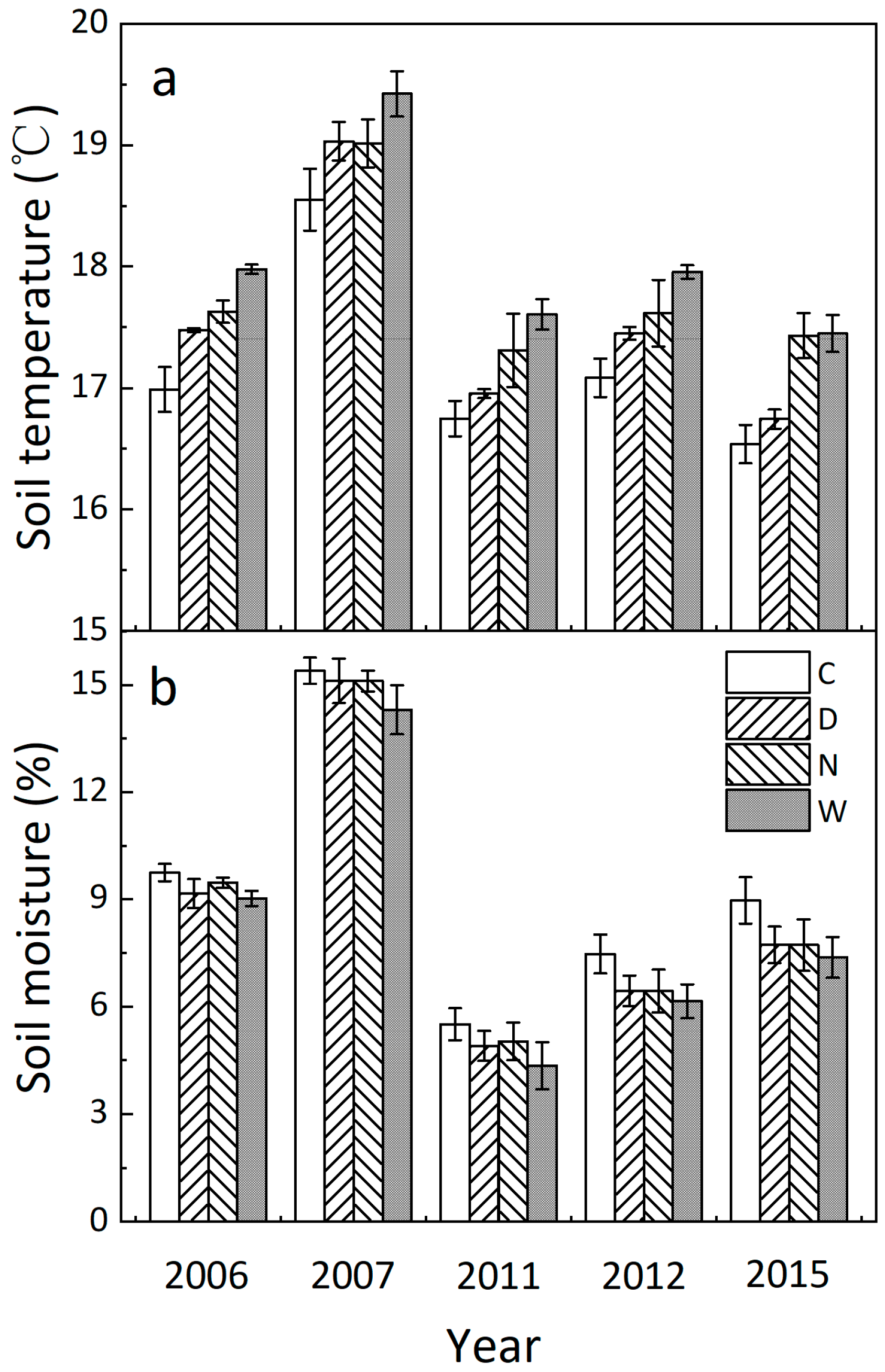
3.1. Soil Microclimate
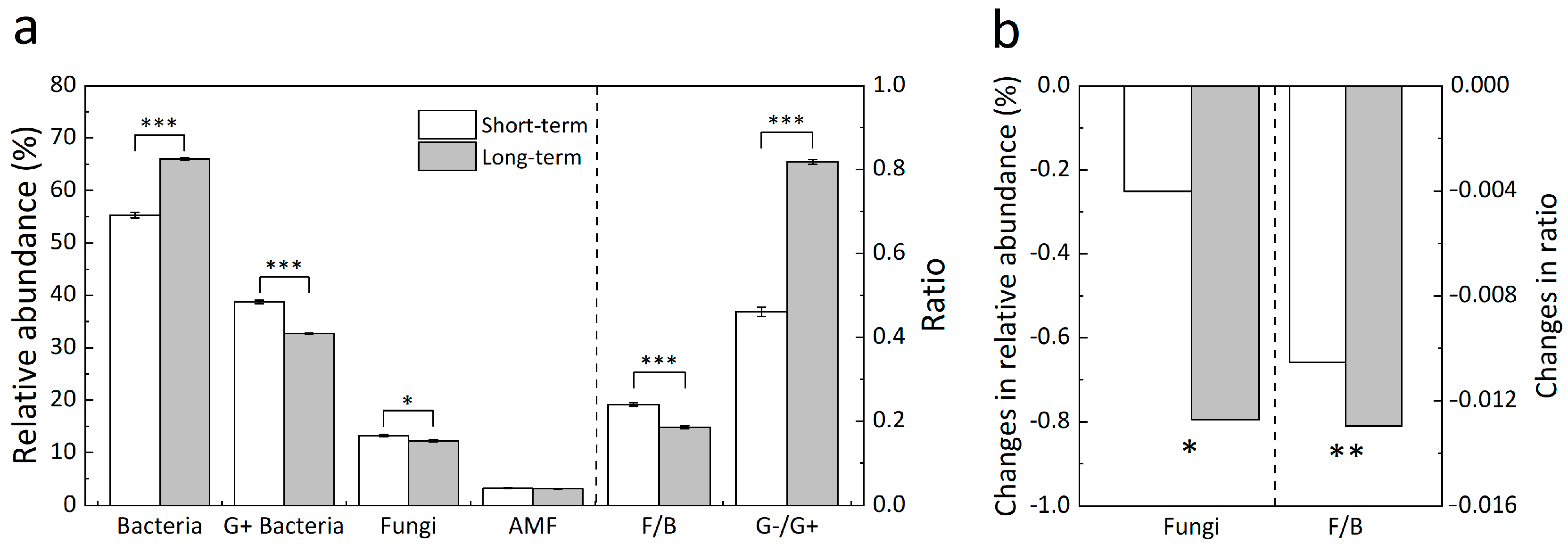
3.2. Soil Microbial Composition
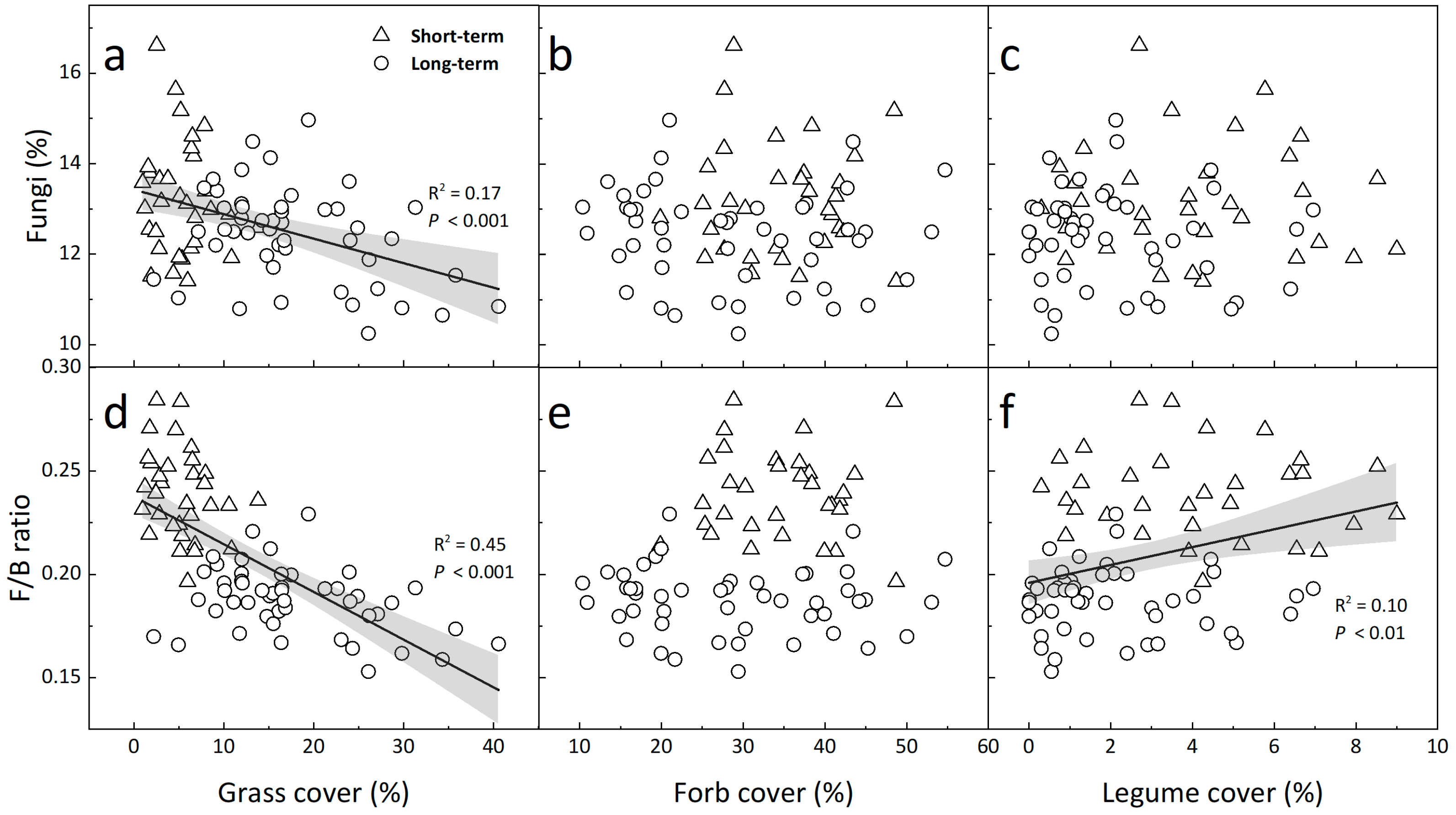
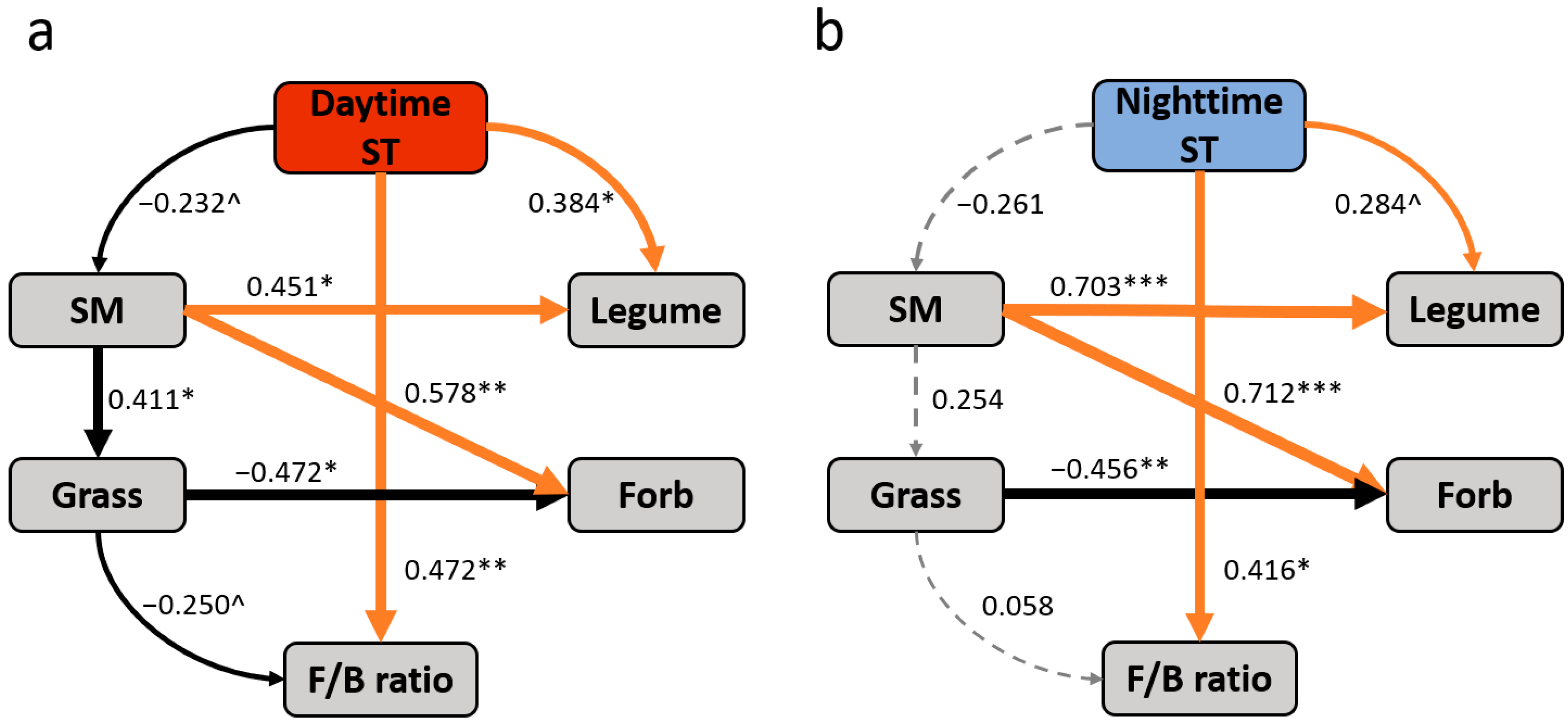
3.3. Relationships of Microbial Composition with Soil Microclimate and Plant Cover
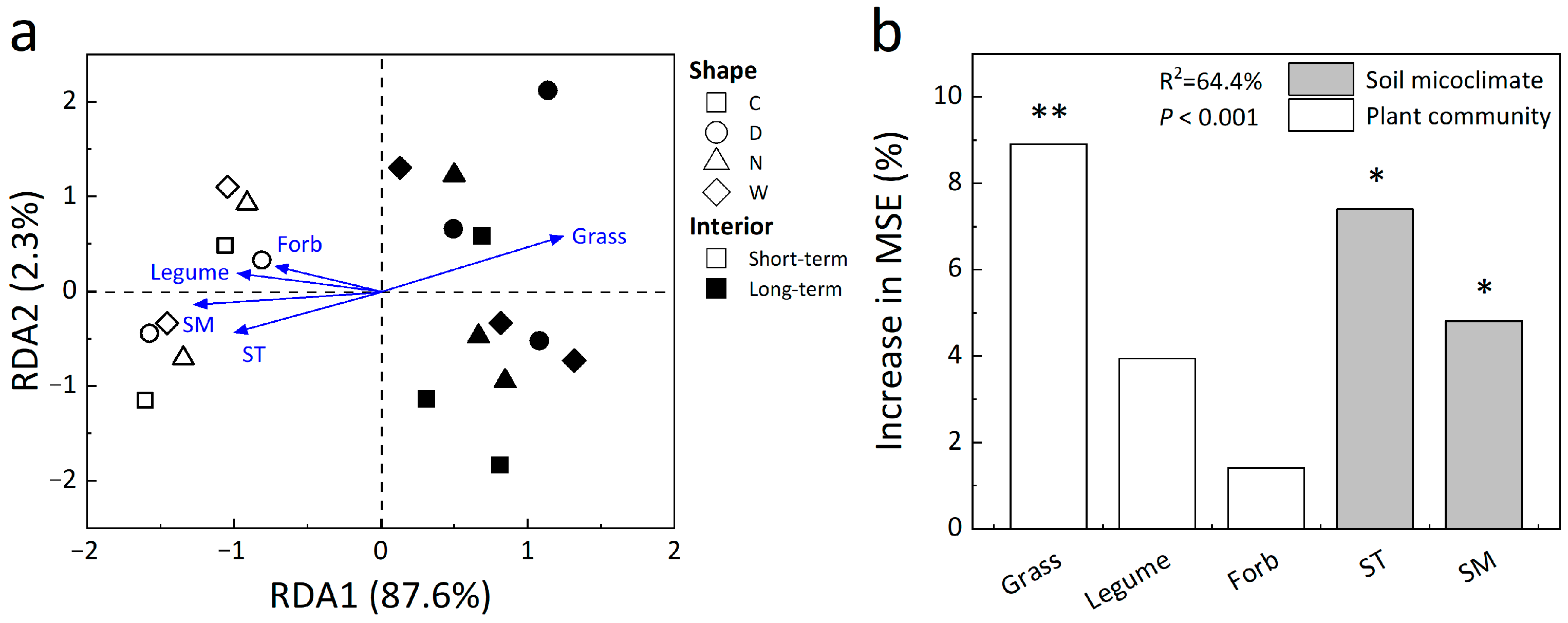
3.4. Controls of Abiotic and Biotic Factors on Soil Microbial Composition
4. Discussion
4.1. Short-and Long-Term Warming on Soil Microbial Composition
4.2. Asymmetrically Diurnal Warming on Soil Microbial Composition
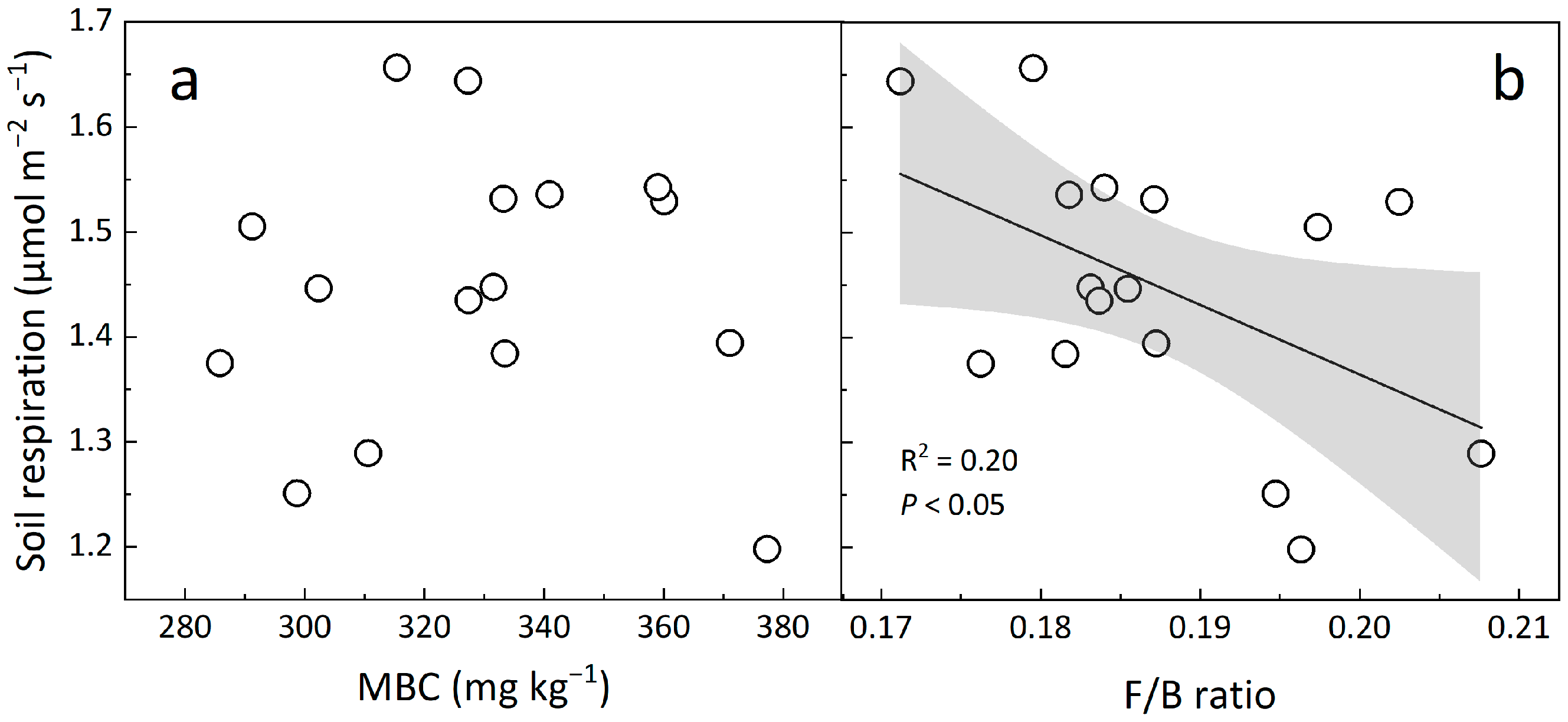
4.3. Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Melillo, J.; Steudler, P.; Aber, J.; Newkirk, K.; Lux, H.; Bowles, F.; Catricala, C.; Magill, A.; Ahrens, T.; Morrisseau, S. Soil warming and carbon-cycle feedbacks to the climate system. Science 2002, 298, 2173. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, J.; Tian, D.; Luo, Y.; Xue, X.; Peng, F.; He, J.; Liu, L.; Jiang, L.; Wang, X.; et al. Sustained increases in soil respiration accompany increased carbon input under long-term warming across global grasslands. Geoderma 2022, 428, 116157. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Luo, Y. Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Li, Z.; Hao, Y.; Wang, J.; Ru, J.; Song, J.; Wan, S. Litter removal exerts greater effects on soil microbial community than understory removal in a subtropical-warm temperate climate transitional forest. For. Ecol. Manag. 2022, 505, 119867. [Google Scholar] [CrossRef]
- Romero-Olivares, A.; Allison, S.; Treseder, K. Soil microbes and their response to experimental warming over time: A meta-analysis of field studies. Soil Biol. Biochem. 2017, 107, 32–40. [Google Scholar] [CrossRef]
- Che, R.; Wang, S.; Wang, Y.; Xu, Z.; Cui, X. Total and active soil fungal community profiles were significantly altered by six years of warming but not by grazing. Soil Biol. Biochem. 2019, 139, 107611. [Google Scholar] [CrossRef]
- Zhang, N.; Xia, J.; Yu, X.; Ma, K.; Wan, S. Soil microbial community changes and their linkages with ecosystem carbon exchange under asymmetrically diurnal warming. Soil Biol. Biochem. 2011, 43, 2053–2059. [Google Scholar] [CrossRef]
- Gao, Y.; Ding, J.; Yuan, M.; Chiariello, N.; Yang, Y. Long-term warming in a mediterranean-type grassland affects soil bacterial functional potential but not bacterial taxonomic composition. NPJ Biofilms Microbiomes 2021, 7, 17. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, L.; Wang, J.; Zhang, Y.; Xiao, C. Effects of warming on the bacterial community and its function in a temperate steppe. Sci. Total Environ. 2021, 792, 148409. [Google Scholar] [CrossRef]
- Saleska, S.; Shaw, M.; Fischer, M.; Dunne, J.; Still, C.; Holman, M.; Harte, J. Plant community composition mediates both large transient decline and predicted long-term recovery of soil carbon under climate warming. Global Biogeochem. Cycles 2002, 16, 1055. [Google Scholar] [CrossRef]
- Guo, X.; Feng, J.; Shi, Z.; Zhou, X.; Yuan, M.; Tao, X.; Hale, L.; Yuan, T.; Wang, J.; Qin, Y.; et al. Climate warming leads to divergent succession of grassland microbial communities. Nat. Clim. Chang. 2018, 8, 813–818. [Google Scholar] [CrossRef]
- Yuan, M.; Guo, X.; Wu, L.; Zhang, Y.; Zhou, J. Climate warming enhances microbial network complexity and stability. Nat. Clim. Chang. 2021, 11, 343–348. [Google Scholar] [CrossRef]
- Wang, F.; Li, Z.; Su, F.; Guo, H.; Wang, P.; Guo, J.; Zhu, W.; Wang, Y.; Hu, S. Sensitive groups of bacteria dictate microbial functional responses to short-term warming and N input in a semiarid grassland. Ecosystems 2021, 25, 1346–1357. [Google Scholar] [CrossRef]
- Xia, J.; Han, Y.; Zhang, Z.; Wan, S. Effects of diurnal warming on soil respiration are not equal to the summed effects of daytime and nighttime warming in a temperate steppe. Biogeosciences 2009, 6, 1361–1370. [Google Scholar] [CrossRef]
- Peng, S.; Piao, S.; Ciais, P.; Myneni, R.; Chen, A.; Chevallier, F.; Dolman, A.; Janssens, I.; Peñuelas, J.; Zhang, G.; et al. Asymmetric effects of daytime and night-time warming on Northern Hemisphere vegetation. Nature 2013, 501, 88–92. [Google Scholar] [CrossRef]
- Xia, J.; Chen, J.; Piao, S.; Ciais, P.; Luo, Y.; Wan, S. Terrestrial carbon cycle affected by non-uniform climate warming. Nat. Geosci. 2014, 7, 173–180. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, S.; Gao, Q.; Ganjurjav, H.; Wang, X.; Geng, W. “Rare biosphere” plays important roles in regulating soil available nitrogen and plant biomass in alpine grassland ecosystems under climate changes. Agric. Ecosyst. Environ. 2019, 279, 187–193. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Song, J.; Ru, J.; Zhou, Z.; Xia, J.; Dukes, J.; Wan, S. Nighttime warming enhances ecosystem carbon-use efficiency in a temperate steppe. Funct. Ecol. 2020, 34, 1721–1730. [Google Scholar] [CrossRef]
- Wan, S.; Xia, J.; Liu, W.; Niu, S. Photosynthetic overcompensation under nocturnal warming enhances grassland carbon sequestration. Ecology 2009, 90, 2700–2710. [Google Scholar] [CrossRef]
- Liu, J.; Long, Z.; Zhang, J.; Chen, C. Effects of nighttimely warming and nitrogen application on the diversity of soil fungi in winter wheat in the lower reach of the Yangtze River. Arch. Agron. Soil Sci. 2022, 68, 838–851. [Google Scholar] [CrossRef]
- Halász, J.; Kotroczó, Z.; Szabó, P.; Kocsis, T. Biomonitoring and assessment of dumpsites soil using phospholipid fatty acid analysis (PLFA) method—Evaluation of possibilities and limitations. Chemosensors 2022, 10, 409. [Google Scholar] [CrossRef]
- Yergeau, E.; Bokhorst, S.; Kang, S.; Zhou, J.; Greer, C.; Aerts, R.; Kowalchuk, G. Shifts in soil microorganisms in response to warming are consistent across a range of Antarctic environments. ISME J. 2012, 6, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xia, J.; Hui, D.; Zheng, M.; Wang, J.; Ru, J.; Wang, H.; Zhang, Q.; Yang, C.; Wan, S. Plant functional types regulate non-additive responses of soil respiration to 5-year warming and nitrogen addition in a semi-arid grassland. Funct. Ecol. 2021, 35, 2593–2603. [Google Scholar] [CrossRef]
- Xia, J.; Chen, S.; Wan, S. Impacts of day versus night warming on soil microclimate: Results from a semiarid temperate steppe. Sci. Total Environ. 2010, 408, 2807–2816. [Google Scholar] [CrossRef] [PubMed]
- Melillo, J.; Frey, S.; DeAngelis, K.; Werner, W.; Bernard, M.; Bowles, F.; Pold, G.; Knorr, M.; Grandy, A. Long-term pattern and magnitude of soil carbon feedback to the climate system in a warming world. Science 2017, 358, 101. [Google Scholar] [CrossRef]
- DeAngelis, K.; Pold, G.; Topçuoglu, B.; van Diepen, L.; Varney, R.; Blanchard, J.; Melillo, J.; Frey, S. Long-term forest soil warming alters microbial communities in temperate forest soils. Front. Microbiol. 2015, 6, 104. [Google Scholar] [CrossRef]
- Cabrol, L.; Poly, F.; Malhautier, L.; Pommier, T.; Lerondelle, C.; Verstraete, W.; Lepeuple, A.; Fanlo, J.; Le Roux, X. Management of microbial communities through transient disturbances enhances the functional resilience of nitrifying gas-biofilters to future disturbances. Environ. Sci. Technol. 2016, 50, 338–348. [Google Scholar] [CrossRef]
- Luo, C.; Xu, G.; Wang, Y.; Wang, S.; Lin, X.; Hu, Y.; Zhang, Z.; Chang, X.; Duan, J.; Su, A.; et al. Effects of grazing and experimental warming on DOC concentrations in the soil solution on the Qinghai-Tibet plateau. Soil Biol. Biochem. 2009, 41, 2493–2500. [Google Scholar] [CrossRef]
- Yuste, J.; Penuelas, J.; Estiarte, M.; Garcia-Mas, J.; Mattana, S.; Ogaya, R.; Pujol, M.; Sardans, J. Drought-resistant fungi control soil organic matter decomposition and its response to temperature. Glob. Chang. Biol. 2011, 17, 1475–1486. [Google Scholar] [CrossRef]
- Ball, B.; Convey, P.; Feeser, K.; Nielsen, U.; Van Horn, D. Environmental harshness mediates the relationship between aboveground and belowground communities in Antarctica. Soil Biol. Biochem. 2022, 164, 108493. [Google Scholar] [CrossRef]
- García-Palacios, P.; Vandegehuchte, M.; Shaw, E.; Dam, M.; Post, K.; Ramirez, K.; Sylvain, Z.; Milanode Tomasel, C.; Wall, D. Are there links between responses of soil microbes and ecosystem functioning to elevated CO2, N deposition and warming? A global perspective. Glob. Chang. Biol. 2015, 21, 1590–1600. [Google Scholar] [CrossRef] [PubMed]
- Seaton, F.; Reinsch, S.; Goodall, T.; White, N.; Jones, D.; Griffiths, R.; Creer, S.; Smith, A.; Emmett, B.; Robinson, D. Long-term drought and warming alter soil bacterial and fungal communities in an upland heathland. Ecosystems 2021, 25, 1279–1294. [Google Scholar] [CrossRef]
- Morrison, E.; Pringle, A.; Linda, T.; Grandy, A.; Melillo, J.; Frey, S. Warming alters fungal communities and litter chemistry with implications for soil carbon stocks. Soil Biol. Biochem. 2019, 132, 120–130. [Google Scholar] [CrossRef]
- Verbrigghe, N.; Meeran, K.; Bahn, M.; Canarini, A.; Fransen, E.; Fuchslueger, L.; Ingrisch, J.; Janssens, I.; Richter, A.; Sigurdsson, B.; et al. Long-term warming reduced microbial biomass but increased recent plant-derived C in microbes of a subarctic grassland. Soil Biol. Biochem. 2022, 167, 108590. [Google Scholar] [CrossRef]
- Tatsuhiro, E.; Katsuharu, S. How do arbuscular mycorrhizal fungi handle phosphate? New insight into fine-tuning of phosphate metabolism. New Phytol. 2018, 220, 1116–1121. [Google Scholar]
- Barrett, G.; Campbell, C.; Hodge, A. The direct response of the external mycelium of arbuscular mycorrhizal fungi to temperature and the implications for nutrient transfer. Soil Biol. Biochem. 2014, 78, 109–117. [Google Scholar] [CrossRef]
- Crowther, T.; van den Hoogen, J.; Wan, J.; Mayes, M.; Keiser, A.; Mo, L.; Averill, C.; Maynard, D. The global soil community and its influence on biogeochemistry. Science 2019, 365, 6455. [Google Scholar] [CrossRef]

| Source of Variations | ST | SM | Bacteria | G+ Bacteria | Fungi | AMF | F/B Ratio | G−/G+ Ratio | |
|---|---|---|---|---|---|---|---|---|---|
| All 5 years | D | 18.6 *** | 7.85 ** | 1.28 | 1.54 | 3.76 * | 0.06 | 7.53 ** | 0.08 |
| N | 64.1 *** | 5.94 * | 0.08 | 0.20 | 0.29 | 1.57 | 0.53 | 0.15 | |
| Y | 93.3 *** | 233 *** | 104 *** | 53.1 *** | 8.30 *** | 17.3 *** | 38.8 *** | 166 *** | |
| D × N | 0.20 | 0.29 | 1.82 | 0.76 | 0.53 | 0.04 | 0.03 | 0.00 | |
| D × Y | 0.70 | 0.05 | 0.52 | 1.01 | 0.60 | 1.01 | 0.47 | 1.38 | |
| N × Y | 0.71 | 0.19 | 1.91 | 1.16 | 0.38 | 0.95 | 0.74 | 1.10 | |
| D × N × Y | 0.11 | 0.34 | 0.41 | 0.22 | 0.39 | 1.21 | 0.22 | 0.45 | |
| Short term | D | 14.3 *** | 3.27 ^ | 1.37 | 0.64 | 0.28 | 0.60 | 2.52 | 0.06 |
| N | 19.2 *** | 1.67 | 0.25 | 0.48 | 0.93 | 1.84 | 2.02 | 2.55 | |
| Y | 170 *** | 372 *** | 4.26 * | 2.17 | 0.05 | 3.37 ^ | 2.02 | 0.09 | |
| D × N | 0.20 | 0.10 | 0.80 | 0.43 | 0.19 | 0.17 | 0.01 | 0.12 | |
| D × Y | 0.01 | 0.00 | 0.10 | 1.08 | 0.68 | 0.77 | 0.57 | 1.48 | |
| N × Y | 0.39 | 0.33 | 1.08 | 1.48 | 0.10 | 0.04 | 0.17 | 0.39 | |
| D × N × Y | 0.02 | 0.32 | 0.02 | 0.24 | 0.11 | 1.96 | 0.06 | 0.80 | |
| Long term | D | 6.24 * | 4.75 * | 0.05 | 1.93 | 4.39 * | 1.12 | 5.11 * | 0.36 |
| N | 45.5 *** | 4.27 * | 2.36 | 0.31 | 0.01 | 0.00 | 0.09 | 0.90 | |
| Y | 9.57 *** | 29.5 *** | 6.35 ** | 3.04 ^ | 11.8 *** | 100 *** | 9.65 *** | 1.14 | |
| D × N | 0.05 | 0.68 | 1.42 | 0.51 | 0.35 | 0.18 | 0.09 | 0.12 | |
| D × Y | 0.54 | 0.02 | 0.67 | 3.35* | 0.47 | 2.13 | 0.66 | 1.94 | |
| N × Y | 0.77 | 0.06 | 5.72 ** | 1.15 | 0.37 | 2.48 | 0.52 | 0.20 | |
| D × N × Y | 0.19 | 0.22 | 1.80 | 0.09 | 0.75 | 0.79 | 0.41 | 0.36 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Ru, J.; Song, J.; Qiu, X.; Wan, S. Long-Term Daytime Warming Rather Than Nighttime Warming Alters Soil Microbial Composition in a Semi-Arid Grassland. Biology 2023, 12, 699. https://doi.org/10.3390/biology12050699
Feng J, Ru J, Song J, Qiu X, Wan S. Long-Term Daytime Warming Rather Than Nighttime Warming Alters Soil Microbial Composition in a Semi-Arid Grassland. Biology. 2023; 12(5):699. https://doi.org/10.3390/biology12050699
Chicago/Turabian StyleFeng, Jiayin, Jingyi Ru, Jian Song, Xueli Qiu, and Shiqiang Wan. 2023. "Long-Term Daytime Warming Rather Than Nighttime Warming Alters Soil Microbial Composition in a Semi-Arid Grassland" Biology 12, no. 5: 699. https://doi.org/10.3390/biology12050699
APA StyleFeng, J., Ru, J., Song, J., Qiu, X., & Wan, S. (2023). Long-Term Daytime Warming Rather Than Nighttime Warming Alters Soil Microbial Composition in a Semi-Arid Grassland. Biology, 12(5), 699. https://doi.org/10.3390/biology12050699







