Parameter Mapping Sonification of Human Olfactory Thresholds
Abstract
Simple Summary
Abstract
1. Introduction
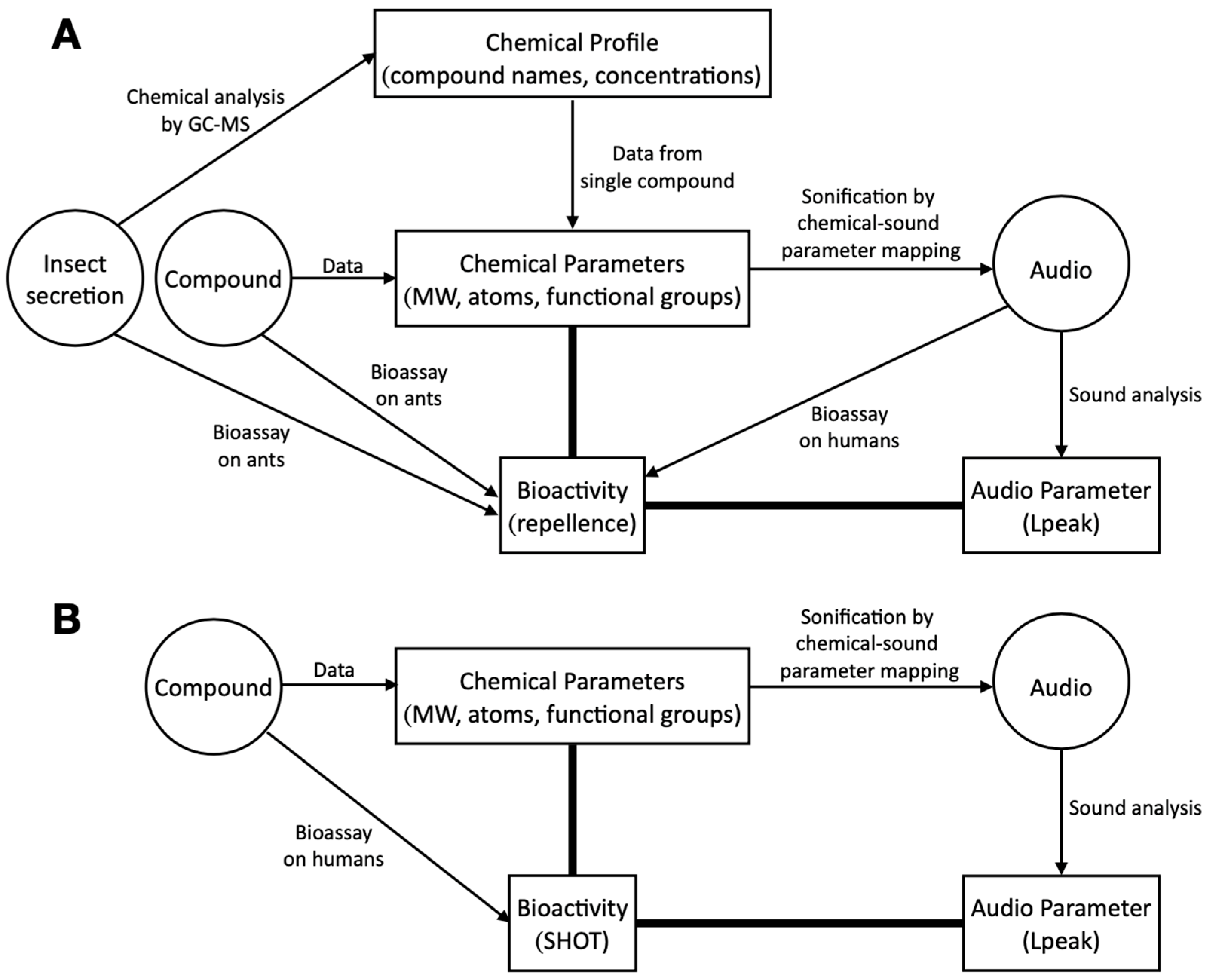
2. Materials and Methods
2.1. Human Olfactory Threshold
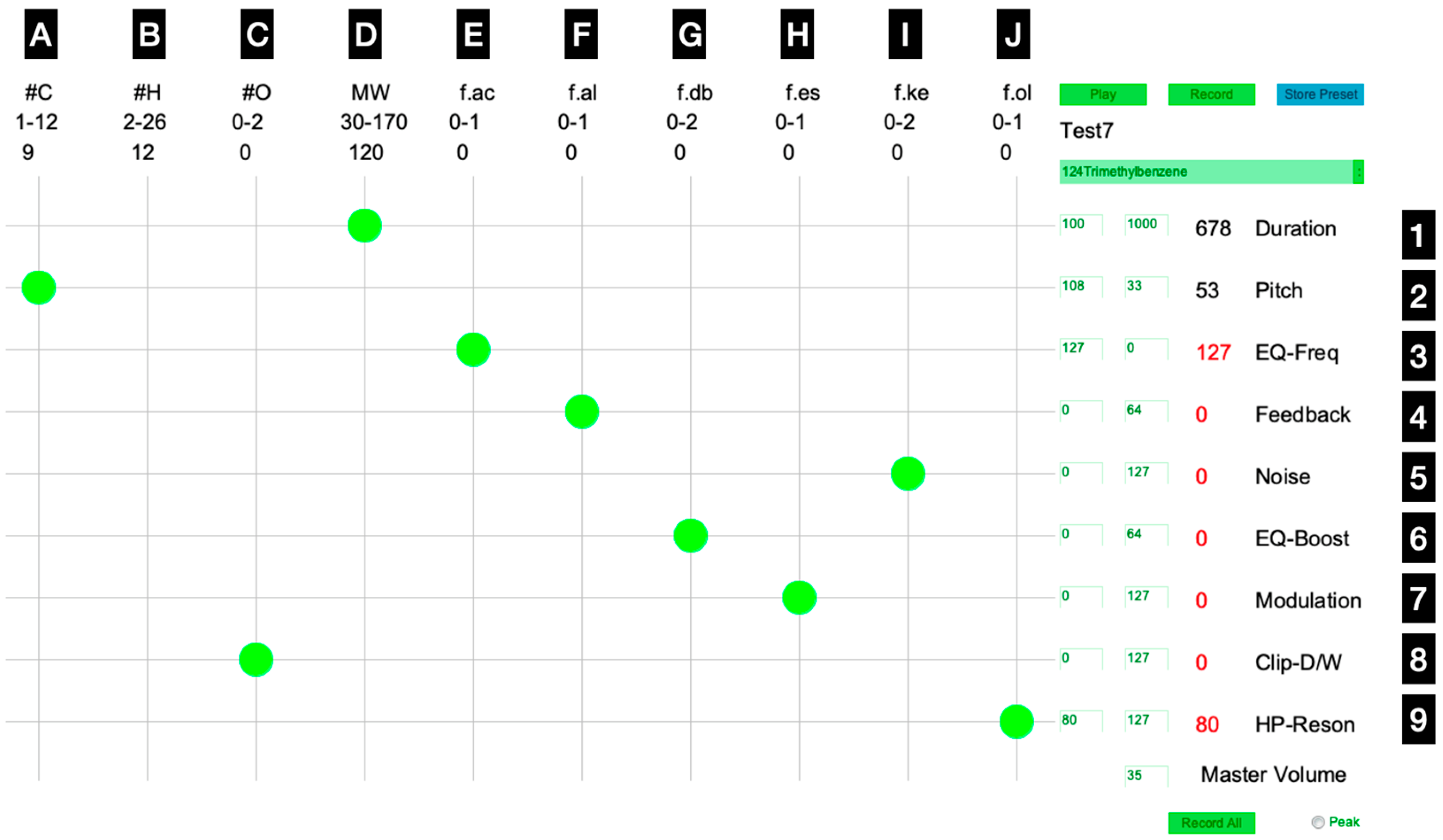
2.2. Sonification
2.3. Statistical Analyses
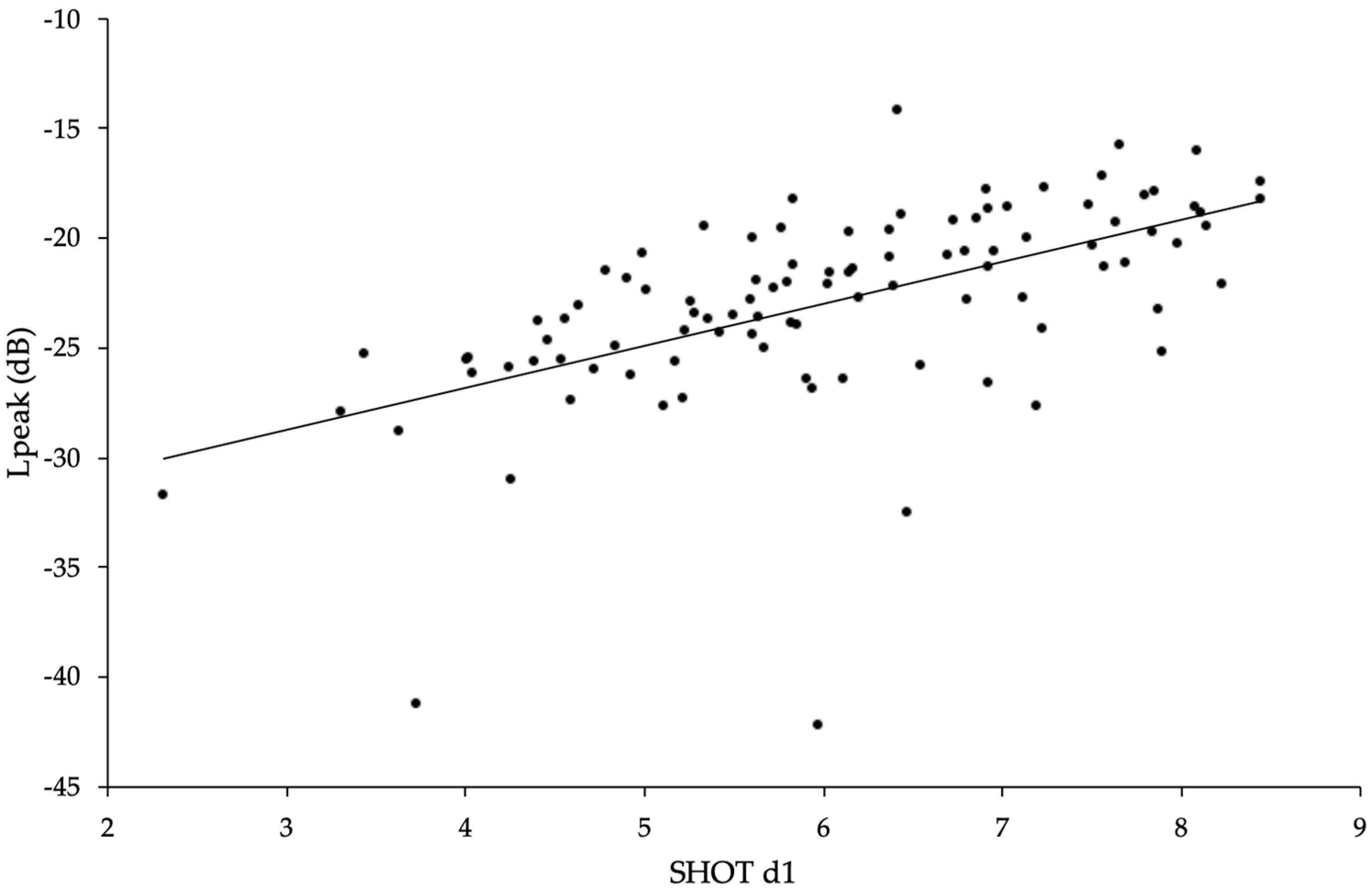
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pasteels, J.M.; Grégoire, J.-C.; Rowell-Rahier, M. The chemical ecology of defense in arthropods. Annu. Rev. Entomol. 1983, 28, 263–289. [Google Scholar] [CrossRef]
- Bhakuni, D.S.; Rawat, D.S. Bioactive Marine Natural Products; Springer & Anamaya: New York, NY, USA; New Delhi, India, 2005; ISBN 978-1-4020-3472-5. [Google Scholar]
- Buckingham, J. Dictionary of Natural Products on CD-ROM, Version 15.1; Chapman & Hall/CRC: Boca Raton, FL, USA, 2007.
- Muller-Schwarze, D. Chemical Ecology of Vertebrates, 1st ed.; Cambridge University Press: Cambridge, UK, 2006; ISBN 978-0-521-36377-8. [Google Scholar]
- Eisner, T.; Eisner, M.; Siegler, M. Secret Weapons: Defenses of Insects, Spiders, Scorpions, and Other Many-Legged Creatures; Harvard University Press: Cambridge, MA, USA, 2005; ISBN 978-0-674-02403-8. [Google Scholar]
- Miller, J.R.; Siegert, P.Y.; Amimo, F.A.; Walker, E.D. Designation of chemicals in terms of the locomotor responses they elicit from insects: An update of Dethier et al. (1960). J. Econ. Entomol. 2009, 102, 2056–2060. [Google Scholar] [CrossRef] [PubMed]
- Ruxton, G.D.; Sherratt, T.N.; Speed, M.P. Avoiding Attack. The Evolutionary Ecology of Crypsis, Warning Signals, and Mimicry; Oxford University Press: Oxford, UK, 2004; ISBN 978-0-19-852860-9. [Google Scholar]
- Whitman, D.W.; Blum, M.R.; Alsop, D.W. Allomones: Chemicals for defense. In Insect Defenses: Adaptive Mechanisms and Strategies of Prey and Predators; Evans, D.L., Schmidt, J.O., Eds.; State University of New York Press: Albany, NY, USA, 1990; pp. 289–351. [Google Scholar]
- Whittaker, R.H.; Feeny, P.P. Allelochemics: Chemical interactions between species. Science 1971, 171, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Barrass, S.; Kramer, G. Using sonification. Multimed. Syst. 1999, 7, 23–31. [Google Scholar] [CrossRef]
- Grond, F.; Berger, J. Parameter mapping sonification. In The Sonification Handbook; Hermann, T., Hunt, A., Neuhoff, J.G., Eds.; Logos Publishing House: Berlin, Germany, 2011; pp. 363–397. ISBN 978-3-8325-2819-5. [Google Scholar]
- Boevé, J.-L.; Giot, R. Chemical composition: Hearing insect defensive volatiles. Patterns 2021, 2, 100352. [Google Scholar] [CrossRef]
- Delatour, T. Molecular music: The acoustic conversion of molecular vibrational spectra. Comput. Music J. 2010, 24, 48–68. [Google Scholar] [CrossRef]
- Yeung, E. Pattern recognition by audio representation of multivariate analytical data. Anal. Chem. 1980, 52, 1120–1123. [Google Scholar] [CrossRef]
- Baier, G.; Hermann, T. Sonification: Listen to brain activity. In Music That Works–Contributions of Biology, Neurophysiology, Psychology, Sociology, Medicine and Musicology; Haas, R., Brandes, V., Eds.; Springer: Wien, Austria; New York, NY, USA, 2009; pp. 11–23. [Google Scholar]
- Bidelman, G.M. Sonification of scalp-recorded frequency-following responses (FFRs) offers improved response detection over conventional statistical metrics. J. Neurosci. Methods 2018, 293, 59–66. [Google Scholar] [CrossRef]
- Buehler, M.J. Nanomechanical sonification of the 2019-nCoV coronavirus spike protein through a materiomusical approach. arXiv 2020, arXiv:2003.14258. [Google Scholar]
- Bywater, R.P.; Middleton, J.N. Melody discrimination and protein fold classification. Heliyon 2016, 2, e00175. [Google Scholar] [CrossRef]
- Carey, J. Musical genes. Proc. Natl. Acad. Sci. USA 2016, 113, 1958–1959. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, M.A.; Gutierrez-Pulido, J.R. An overview of auditory display to assist comprehension of molecular information. Interact. Comput. 2006, 18, 853–868. [Google Scholar] [CrossRef]
- Kather, J.N.; Hermann, T.; Bukschat, Y.; Kramer, T.; Schad, L.R.; Zöllner, F.G. Polyphonic sonification of electrocardiography signals for diagnosis of cardiac pathologies. Sci. Rep. 2017, 7, 44549. [Google Scholar] [CrossRef]
- Staege, M.S. A short treatise concerning a musical approach for the interpretation of gene expression data. Sci. Rep. 2015, 5, 15281. [Google Scholar] [CrossRef] [PubMed]
- Temple, M.D. An auditory display tool for DNA sequence analysis. BMC Bioinform. 2017, 18, e221. [Google Scholar] [CrossRef]
- Hegg, J.C.; Middleton, J.; Robertson, B.L.; Kennedy, B.P. The sound of migration: Exploring data sonification as a means of interpreting multivariate salmon movement datasets. Heliyon 2018, 4, e00532. [Google Scholar] [CrossRef] [PubMed]
- Schito, J.; Fabrikant, S.I. Exploring maps by sounds: Using parameter mapping sonification to make digital elevation models audible. Int. J. Geogr. Inf. Sci. 2018, 32, 874–906. [Google Scholar] [CrossRef]
- George, S.S.; Crawford, D.; Reubold, T.; Giorgi, E. Making climate data sing: Using music-like sonifications to convey a key climate record. Bull. Am. Meteorol. Soc. 2017, 98, 23–27. [Google Scholar] [CrossRef]
- Avanzo, S.; Barbera, R.; De Mattia, F.; La Rocca, G.; Sorrentino, M.; Vicinanza, D. Data sonification of volcano seismograms and sound/timbre reconstruction of ancient musical instruments with grid infrastructures. Procedia Comput. Sci. 2010, 1, 397–406. [Google Scholar] [CrossRef]
- Paté, A.; Boschi, L.; Carrou, J.-L.L.; Holtzman, B. Categorization of seismic sources by auditory display: A blind test. Int. J. Hum.-Comput. Stud. 2016, 85, 57–67. [Google Scholar] [CrossRef]
- Kadkhodaie, A.; Rezaee, R. Have you ever heard the sound of well logs or reservoir data? J. Pet. Sci. Eng. 2017, 156, 340–347. [Google Scholar] [CrossRef]
- Misdariis, N.; Özcan, E.; Grassi, M.; Pauletto, S.; Barrass, S.; Bresin, R.; Susini, P. Sound experts’ perspectives on astronomy sonification projects. Nat. Astron. 2022, 6, 1249–1255. [Google Scholar] [CrossRef]
- Cullen, C. The Sonic Representation of Mathematical Data. Ph.D. Thesis, Dublin Institute of Technology, Dublin, Ireland, 2005. [Google Scholar]
- Axon, L.; Happa, J.; Goldsmith, M.; Creese, S. Hearing attacks in network data: An effectiveness study. Comput. Secur. 2019, 83, 367–388. [Google Scholar] [CrossRef]
- Worrall, D. Using sound to identify correlations in market data. In Proceedings of the CMMR/ICAD 2009, Copenhagen, Denmark, 18–22 May 2009; Ystad, S., Aramaki, M., Kronland-Martinet, R., Jensen, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 5954 LNCS, pp. 202–218. [Google Scholar]
- Geronazzo, M.; Bedin, A.; Brayda, L.; Campus, C.; Avanzini, F. Interactive spatial sonification for non-visual exploration of virtual maps. Int. J. Hum. Comput. Stud. 2016, 85, 4–15. [Google Scholar] [CrossRef]
- Martínez, B.D.; Villegas, O.O.; Sánchez, V.G.; de Jesús Ochoa Domínguez, H.; Maynez, L.O. Visual perception substitution by the auditory sense. In Computational Science and Its Applications; Springer: Berlin/Heidelberg, Germany, 2011; pp. 522–533. [Google Scholar]
- Heinsohn, R.J.; Cimbala, J.M.; Heinsohn, R.J. Indoor Air Quality Engineering: Environmental Health and Control of Indoor Pollutants; Marcel Dekker: New York, NY, USA, 2003; ISBN 978-0-8247-4061-0. [Google Scholar]
- Maroni, M.; Seifert, B.; Lindvall, T. (Eds.) Indoor Air Quality: A Comprehensive Reference Book; Air Quality Monographs; Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 1995; ISBN 978-0-444-81642-9. [Google Scholar]
- Salthammer, T.; Uhde, E. (Eds.) Organic Indoor Air Pollutants: Occurrence, Measurement, Evaluation; Wiley-VCH: Weinheim, Germany; New York, NY, USA, 1999; ISBN 978-3-527-29622-4. [Google Scholar]
- Abraham, M.H.; Sánchez-Moreno, R.; Gil-Lostes, J.; Acree, W.E., Jr.; Cometto-Muñiz, J.E.; Cain, W.S. The biological and toxicological activity of gases and vapors. Toxicol. Vitro 2010, 24, 357–362. [Google Scholar] [CrossRef]
- Abraham, M.H.; Sánchez-Moreno, R.; Cometto-Muñiz, J.E.; Cain, W.S. An algorithm for 353 odor detection thresholds in humans. Chem. Senses 2012, 37, 207–218. [Google Scholar] [CrossRef]
- Devos, M.; Patte, F.; Rouault, J.; Laffort, P. Standardized Human Olfactory Thresholds; Oxford University Press: Oxford, UK, 2011; ISBN 978-0-19-963146-9. [Google Scholar]
- van Gemert, L.J. Odour threshold values in air. In Odour Thresholds. Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter & Partners BV: Utrecht, The Netherlands, 2011; pp. 11–206. ISBN 978-90-810894-0-1. [Google Scholar]
- Alarie, Y.; Schaper, M.; Nielsen, G.D.; Abraham, M.H. Structure-activity relationships of volatile organic chemicals as sensory irritants. Arch. Toxicol. 1998, 72, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.H.; Sanchez-Moreno, R.; Gil-Lostes, J.; Cometto-Muñiz, J.E.; Cain, W.S. Physicochemical modeling of sensory irritation in humans and experimental animals. In Toxicology of the Nose and Upper Airways; Morris, J.B., Shusterman, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 390–403. ISBN 978-0-429-14066-2. [Google Scholar]
- Cometto-Muñiz, J.E.; Abraham, M.H. Odor Detection by Humans of Lineal Aliphatic Aldehydes and Helional as Gauged by Dose–Response Functions. Chem. Senses 2010, 35, 289–299. [Google Scholar] [CrossRef]
- Cometto-Muñiz, J.E.; Abraham, M.H. Structure–activity relationships on the odor detectability of homologous carboxylic acids by humans. Exp. Brain Res. 2010, 207, 75–84. [Google Scholar] [CrossRef]
- Abraham, M.H.; Acree, W.E.; Cometto-Muñiz, J.E. Descriptors for terpene esters from chromatographic and partition measurements: Estimation of human odor detection thresholds. J. Chromatogr. A 2020, 1609, 460428. [Google Scholar] [CrossRef]
- Aakash, A.; Nabi, D. Reliable prediction of sensory irritation threshold values of organic compounds using new models based on linear free energy relationships and GC × GC retention parameters. Chemosphere 2023, 313, 137339. [Google Scholar] [CrossRef] [PubMed]
- Cometto-Muñiz, J.E.; Cain, W.S. Thresholds for odor and nasal pungency. Physiol. Behav. 1990, 48, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Cometto-Muñiz, J.E.; Cain, W.S. Nasal pungency, odor, and eye irritation thresholds for homologous acetates. Pharmacol. Biochem. Behav. 1991, 39, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Lide, D.R. Physical constants of organic compounds. In CRC Handbook of Chemistry and Physics, 89th ed.; (Internet Version 2009); CRC Press: Taylor, TX, USA; Francis, UT, USA; Boca Raton, FL, USA; pp. 3:1–523. ISBN 978-1-4200-6679-1.
- Nagata, Y. Measurement of odor threshold by triangle odor bag method. In Odor Measurement Review; Ministry of the Environment, Government of Japan: Tokyo, Japan, 2003; pp. 118–127. [Google Scholar]
- Roth, D. BlackHole. Available online: https://existential.audio/blackhole/2021 (accessed on 14 January 2022).
- Boulanger, R.; Lazzarini, V. The Audio Programming Book; MIT Press: Cambridge, MA, USA, 2010; ISBN 978-0-262-01446-5. [Google Scholar]
- Lowry, R. VassarStats: Website for Statistical Computation. Available online: http://vassarstats.net/ (accessed on 21 January 2023).
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Lee, B.K.; Mayhew, E.J.; Sanchez-Lengeling, B.; Wei, J.N.; Qian, W.W.; Little, K.; Andres, M.; Nguyen, B.B.; Moloy, T.; Parker, J.K.; et al. A Principal Odor Map Unifies Diverse Tasks in Human Olfactory Perception. BioRxiv 2022, 1–15. [Google Scholar] [CrossRef]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Boevé, J.-L.; Pasteels, J.M. Modes of defense in nematine sawfly larvae. Efficiency against ants and birds. J. Chem. Ecol. 1985, 11, 1019–1036. [Google Scholar] [CrossRef]
- Belkin, K.; Martin, R.; Kemp, S.E.; Gilbert, A.N. Auditory pitch as a perceptual analogue to odor quality. Psychol. Sci. 1997, 8, 340–342. [Google Scholar] [CrossRef]
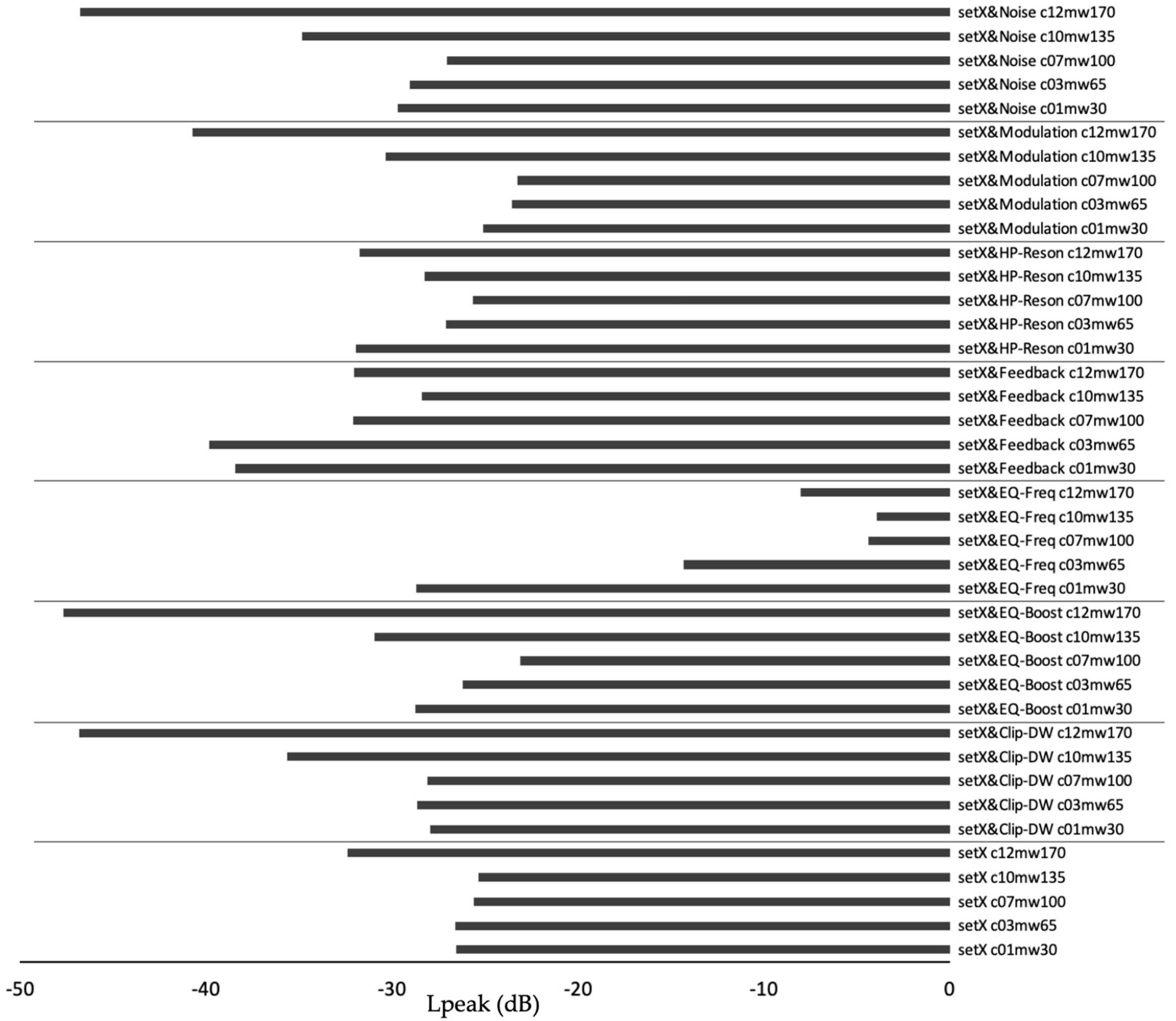
| Set | Mapping Condition | Mapping Goal | rS | t | p |
|---|---|---|---|---|---|
| set01 | A2-D1--C3-E7-F4-G5-H8-I6-J9 | random C E F G H I J | 0.1529 | 1.53 | 0.129 |
| set02 | A2-D1--C5-E3-F6-G7-H9-I4-J8 | random C E F G H I J | 0.465 | 5.2 | <0.001 |
| set03 | A2-D1--C6-E3-F8-G4-H7-I5-J9 | random C E F G H I J | 0.4738 | 5.33 | <0.001 |
| set04 | A2-D1--C4-E9-F6-G5-H3-I7-J8 | random C E F G H I J | 0.3194 | 3.34 | 0.001 |
| set05 | A2-D1--C4-E5-F8-G6-H7-I9-J3 | random C E F G H I J | 0.2514 | 2.57 | 0.012 |
| set06 | A2-D1--C8-E3-F6-G7-H9-I4-J5 | random C E F G H I J | 0.3572 | 3.79 | <0.001 |
| set07 | A2-D1--C6-E3-F4-G7-H5-I8-J9 | random C E F G H I J | 0.46 | 5.13 | <0.001 |
| set08 | A2-D1--C5-E6-F3-G7-H9-I8-J4 | random C E F G H I J | 0.2048 | 2.07 | 0.041 |
| set09 | A2-D1--C7-E3-F4-G9-H8-I5-J6 | random C E F G H I J | 0.5212 | 6.04 | <0.001 |
| set10 | A2-D1--C3-E7-F6-G4-H5-I8-J9 | random C E F G H I J | 0.0917 | 0.91 | 0.365 |
| set11 | A2-D1--C9-E3-F8-G7-H6-I5-J4 | random C E F G H I J | 0.2718 | 2.8 | 0.006 |
| set12 | A2-D1--C7-E8-F5-G3-H4-I6-J9 | random C E F G H I J | 0.3874 | 4.16 | <0.001 |
| set13 | A2-D1--C4-E7-F3-G8-H9-I5-J6 | random C E F G H I J | 0.2424 | 2.47 | 0.015 |
| set14 | A2-D1--C4-E3-F5-G8-H6-I7-J9 | random C E F G H I J | 0.4246 | 4.64 | <0.001 |
| set15 | A2-D1--C3-E7-F8-G4-H9-I5-J6 | random C E F G H I J | −0.0325 | −0.32 | 0.750 |
| set16 | A2-D1--C9-E4-F3-G5-H6-I8-J7 | random C E F G H I J | 0.2613 | 2.68 | 0.009 |
| set17 | A2-D1--C8-E7-F9-G3-H4-I5-J6 | random C E F G H I J | 0.186 | 1.87 | 0.064 |
| set18 | A2-D1--C5-E9-F4-G6-H7-I8-J3 | random C E F G H I J | 0.3953 | 4.26 | <0.001 |
| set19 | A2-D1--C7-E3-F9-G6-H4-I5-J8 | random C E F G H I J | 0.2825 | 2.92 | 0.004 |
| set20 | A2-D1--C9-E4-F3-G5-H6-I7-J8 | random C E F G H I J | 0.3283 | 3.44 | 0.001 |
| set21 | A2-D1--C3-E7-F4-G6-H5-I8-J9 | random C E F G H I J | 0.2306 | 2.35 | 0.021 |
| set22 | A2-D1--C6-E4-F8-G7-H9-I5-J3 | random C E F G H I J | 0.4456 | 4.93 | <0.001 |
| set23 | A2-D1--C5-E3-F8-G6-H7-I9-J4 | random C E F G H I J | 0.4013 | 4.34 | <0.001 |
| set24 | A2-D1--C9-E3-F4-G6-H5-I8-J7 | random C E F G H I J | 0.5892 | 7.22 | <0.001 |
| set26 | A2-D1--C9-E3-F4-G7-H5-I8-J6 | random C G H I J | 0.5789 | 7.03 | <0.001 |
| set27 | A2-D1--C5-E3-F4-G7-H8-I6-J9 | random C G H I J | 0.6049 | 7.52 | <0.001 |
| set28 | A2-D1--C6-E3-F4-G5-H7-I8-J9 | random C G H I J | 0.5681 | 6.83 | <0.001 |
| set29 | A2-D1--C5-E3-F4-G7-H9-I6-J8 | random C G H I J | 0.663 | 8.77 | <0.001 |
| set30 | A2-D1--C5-E3-F4-G9-H6-I8-J7 | random C G H I J | 0.5095 | 5.86 | <0.001 |
| set54 | A2-D1--C9-E3-F4-G7-H6-I5-J8 | random C G H I J | 0.5951 | 7.33 | <0.001 |
| set55 | A2-D1--C7-E3-F4-G5-H6-I8-J9 | random C G H I J | 0.5711 | 6.89 | <0.001 |
| set56 | A2-D1--C9-E3-F4-G6-H7-I8-J5 | random C G H I J | 0.6461 | 8.38 | <0.001 |
| set57 | A2-D1--C8-E3-F4-G6-H7-I5-J9 | random C G H I J | 0.7138 | 10.09 | <0.001 |
| set58 | A2-D1--C7-E3-F4-G8-H9-I5-J6 | random C G H I J | 0.5539 | 6.59 | <0.001 |
| set59 | A2-D1--C6-E3-F4-G8-H7-I9-J5 | random C G H I J | 0.4435 | 4.9 | <0.001 |
| set60 | A2-D1--C5-E3-F4-G6-H7-I8-J9 | random C G H I J | 0.6306 | 8.04 | <0.001 |
| set61 | A2-D1--C6-E3-F4-G5-H7-I9-J8 | random C G H I J | 0.5282 | 6.16 | <0.001 |
| set62 | A2-D1--C5-E3-F4-G8-H9-I7-J6 | random C G H I J | 0.5202 | 6.03 | <0.001 |
| set63 | A2-D1--C7-E3-F4-G8-H6-I9-J5 | random C G H I J | 0.4822 | 5.45 | <0.001 |
| set64 | A2-D1--C9-E3-F4-G7-H5-I6-J8 | random C G H I J | 0.648 | 8.42 | <0.001 |
| set65 | A2-D1--C5-E3-F4-G9-H6-I7-J8 | random C G H I J | 0.5155 | 5.96 | <0.001 |
| set66 | A2-D1--C5-E3-F4-G6-H7-I9-J8 | random C G H I J | 0.6062 | 7.55 | <0.001 |
| set67 | A2-D1--C5-E3-F4-G7-H6-I9-J8 | random C G H I J | 0.4492 | 4.98 | <0.001 |
| set68 | A2-D1--C6-E3-F4-G5-H9-I7-J8 | random C G H I J | 0.5061 | 5.81 | <0.001 |
| set69 | A2-D1--C9-E3-F4-G6-H5-I7-J8 | random C G H I J | 0.6192 | 7.81 | <0.001 |
| set70 | A2-D1--C6-E3-F4-G8-H9-I7-J5 | random C G H I J | 0.3521 | 3.72 | <0.001 |
| set71 | A2-D1--C8-E3-F4-G5-H7-I6-J9 | random C G H I J | 0.7172 | 10.19 | <0.001 |
| set72 | A2-D1--C7-E3-F4-G5-H8-I9-J6 | random C G H I J | 0.502 | 5.75 | <0.001 |
| set57b | A2-D1--C8-E3-F4-G6-H7-I5-J9 | set57 but Feedback 0–64 | 0.6479 | 8.42 | <0.001 |
| set73 | A2-D1--C8-E3-F4-G5-H7-I6-J9 | set71 but Feedback 0–64 | 0.5136 | 5.93 | <0.001 |
| Variable Comparison | n | rS | t | df | p |
|---|---|---|---|---|---|
| SHOT d1 vs. SHOT d2 | 272 | 0.9858 | 96.62 | 270 | 5 × 10−7 |
| SHOT d1 vs. Lpeak | 100 | 0.6479 | 8.42 | 98 | <1 × 10−6 |
| Log(1/ODT) vs. Lpeak | 100 | 0.6837 | 9.27 | 98 | <1 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boevé, J.-L.; Giot, R. Parameter Mapping Sonification of Human Olfactory Thresholds. Biology 2023, 12, 670. https://doi.org/10.3390/biology12050670
Boevé J-L, Giot R. Parameter Mapping Sonification of Human Olfactory Thresholds. Biology. 2023; 12(5):670. https://doi.org/10.3390/biology12050670
Chicago/Turabian StyleBoevé, Jean-Luc, and Rudi Giot. 2023. "Parameter Mapping Sonification of Human Olfactory Thresholds" Biology 12, no. 5: 670. https://doi.org/10.3390/biology12050670
APA StyleBoevé, J.-L., & Giot, R. (2023). Parameter Mapping Sonification of Human Olfactory Thresholds. Biology, 12(5), 670. https://doi.org/10.3390/biology12050670




