Therapeutic Potential of a Senolytic Approach in a Murine Model of Chronic GVHD
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
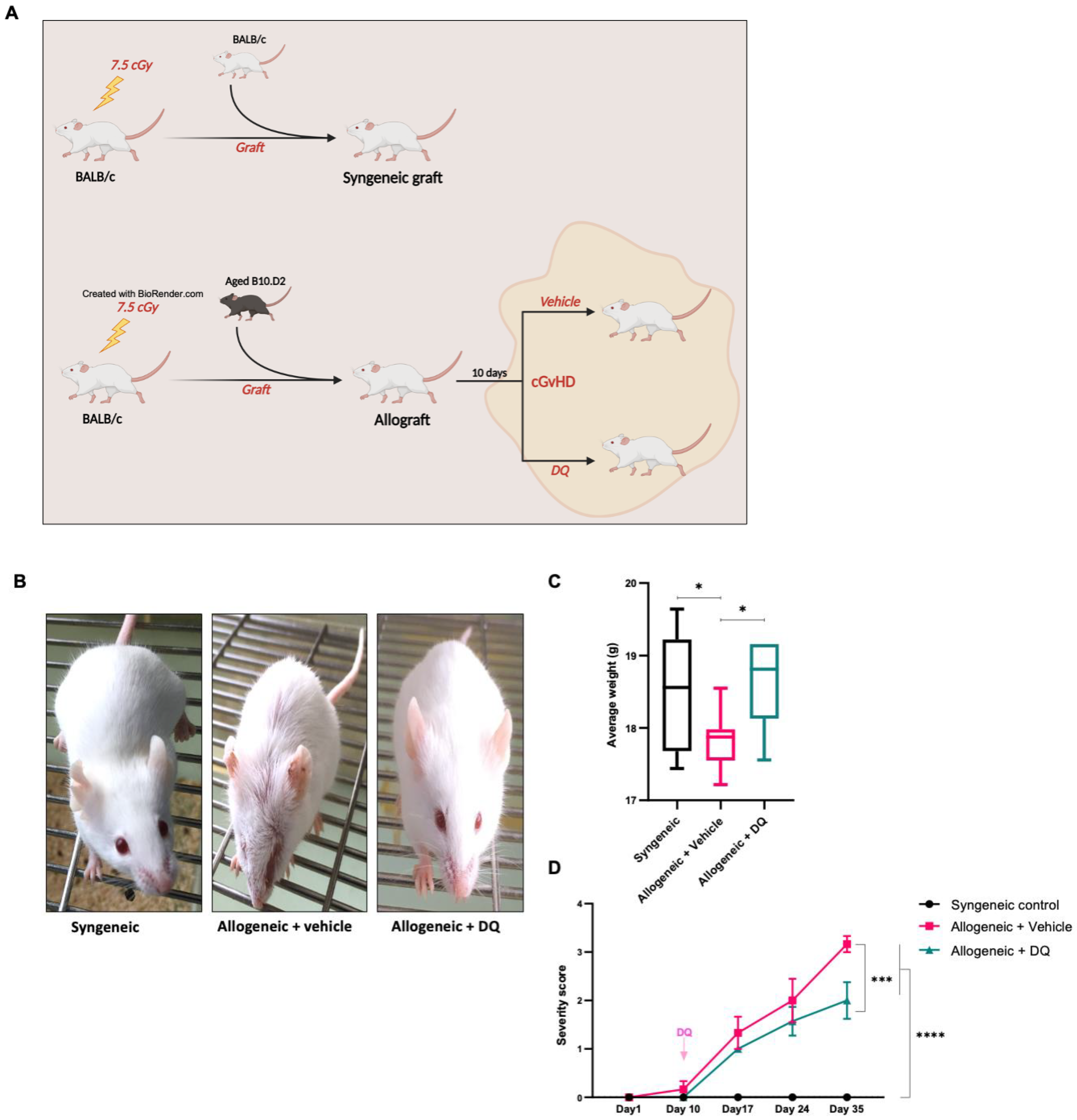
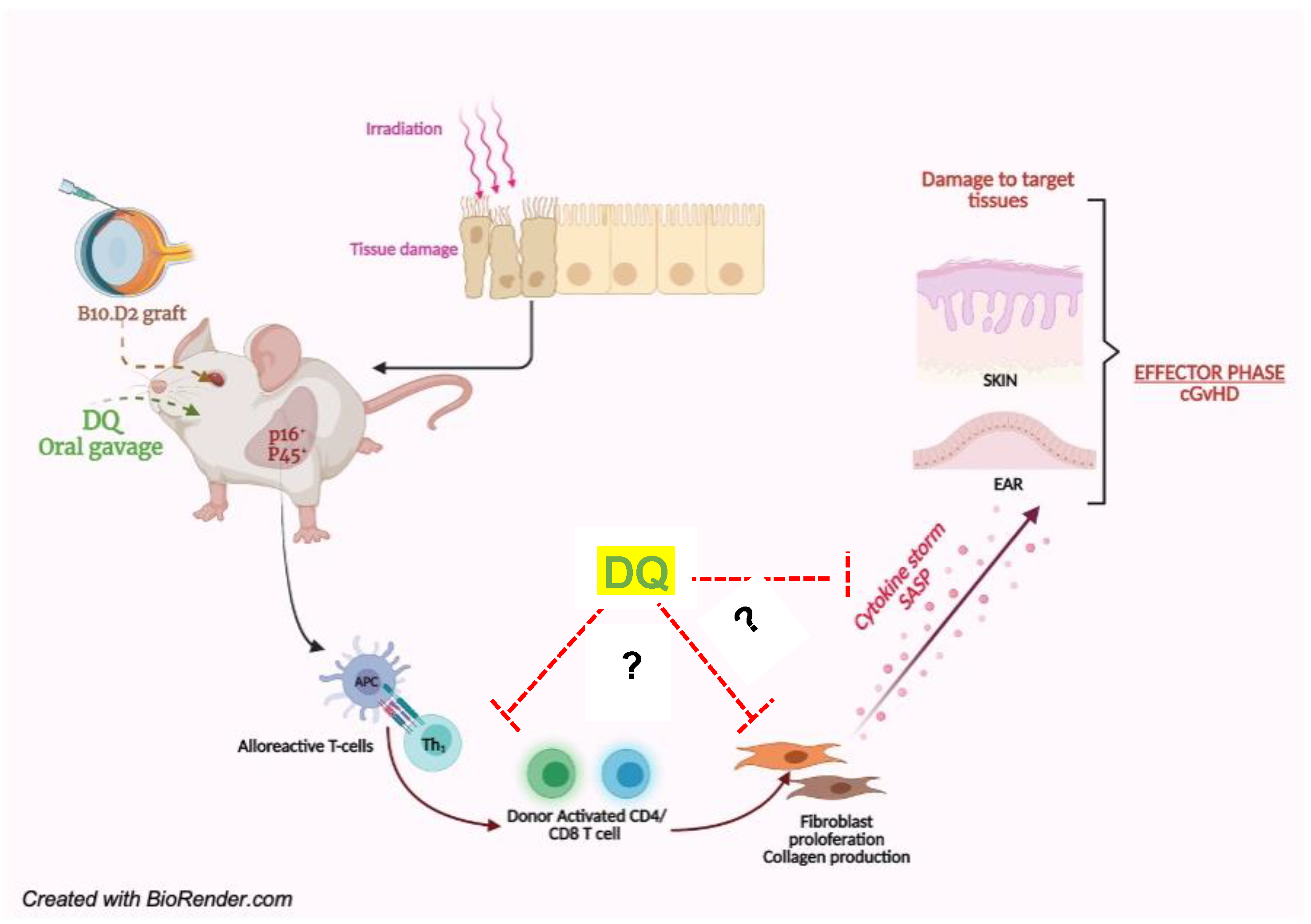
2.1. Establishing a Murine Model of cGVHD
2.2. Disease Severity Index
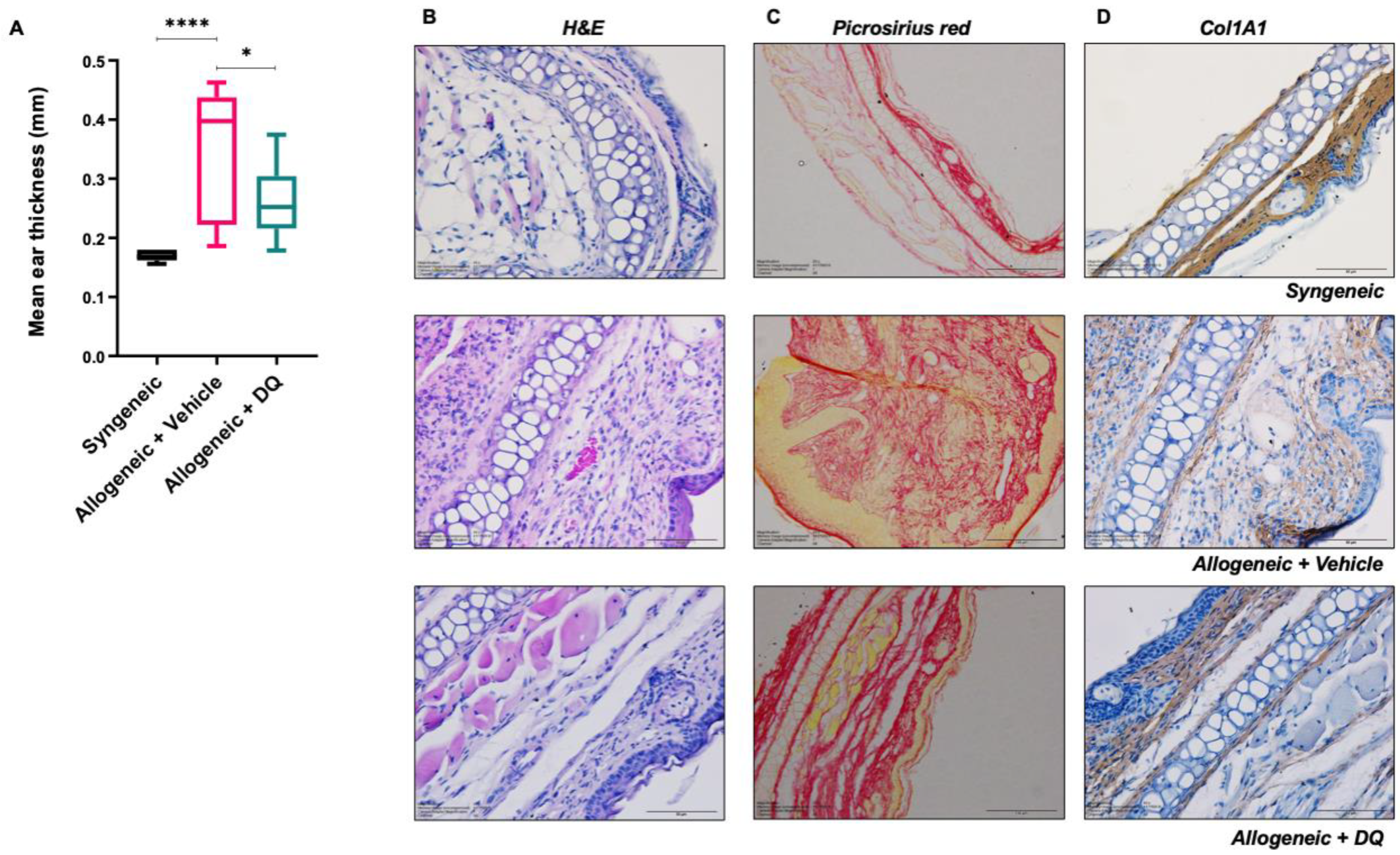
2.3. Earlobe Thickness
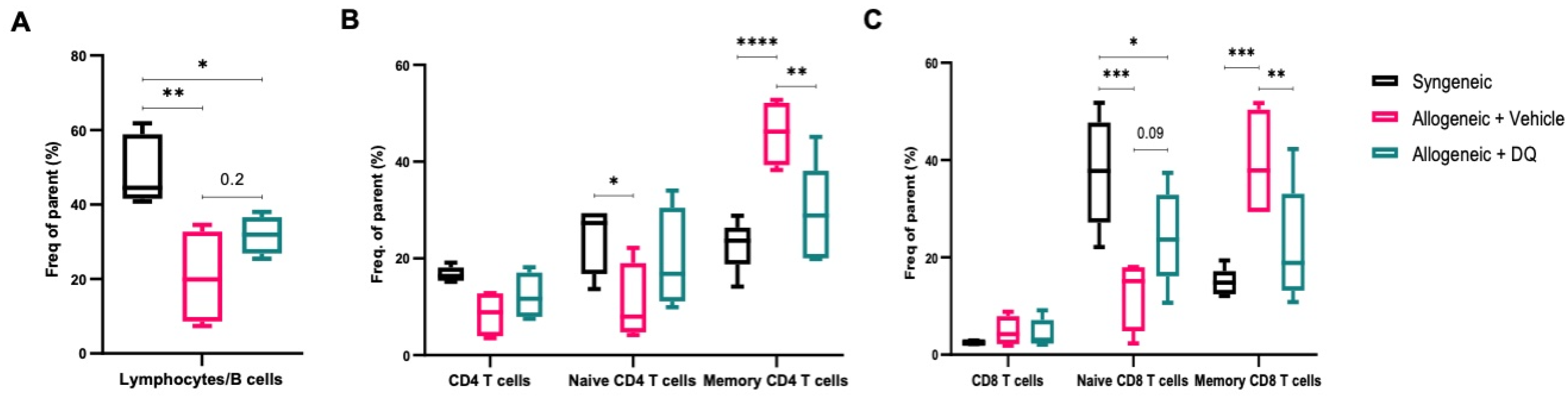
2.4. Flow Cytometric Analysis of Spleen Cell Subsets
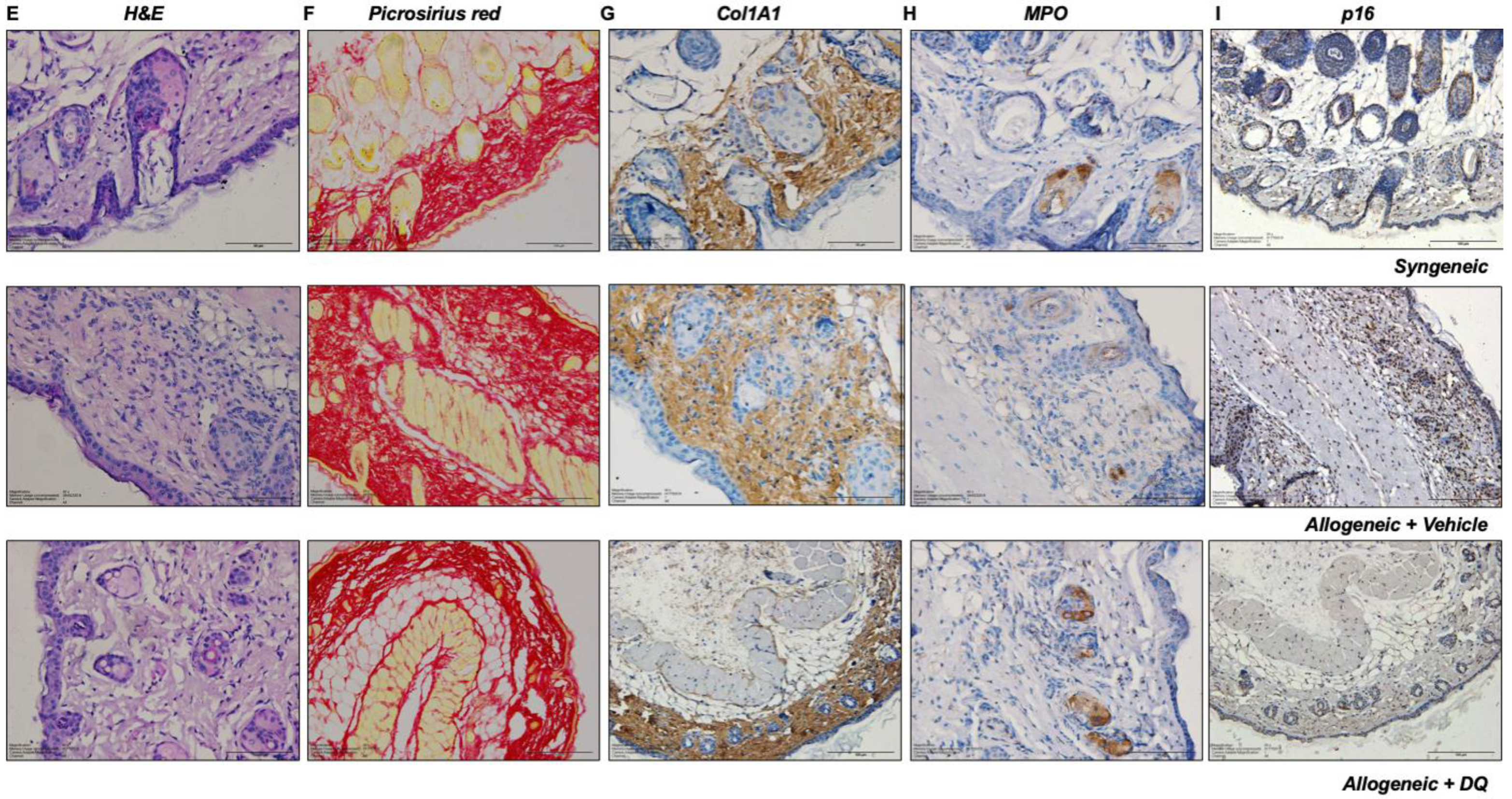
2.5. Histopathological and Immunohistochemistry Analyses
2.6. Multiplex Protein ELISA
2.7. cDNA Synthesis and qPCR
2.8. Statistical Analysis
3. Results
3.1. DQ Alleviates Adverse Effects of Allogeneic Graft on the General Well-Being of the Recipient
3.2. DQ Mitigates Allograft Associated Skin Fibrosis
3.3. DQ Significantly Inhibits Allograft Associated Increase in Circulating Memory T Cells without Significantly Affecting the Decrease in B Cell Subsets
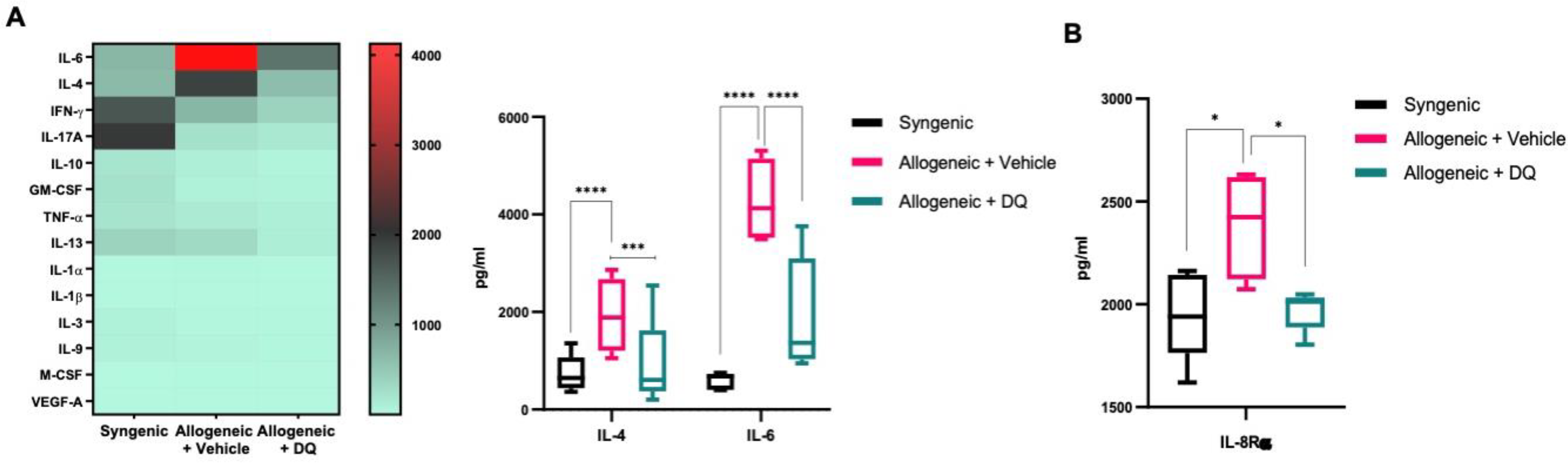
3.4. DQ Mitigates the Increase in IL-4, IL-6 and IL-8Rα upon Allografting
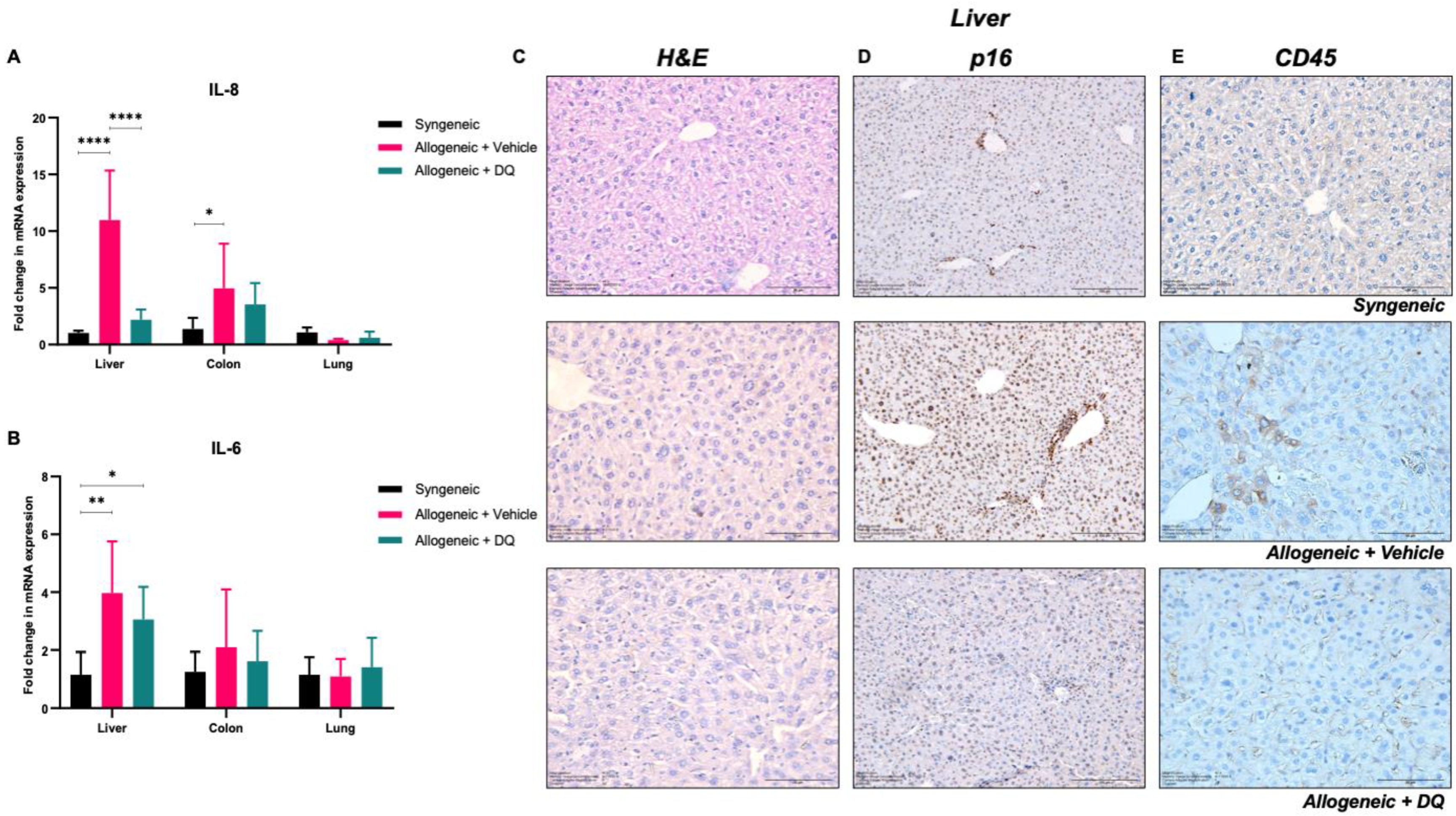
3.5. DQ Targets Allograft-Associated Senescent Cell Pool
4. Discussion
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooke, K.R.; Luznik, L.; Sarantopoulos, S.; Hakim, F.T.; Jagasia, M.; Fowler, D.H.; van den Brink, M.R.M.; Hansen, J.A.; Parkman, R.; Miklos, D.B.; et al. The Biology of Chronic Graft-versus-Host Disease: A Task Force Report from the National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2017, 23, 211–234. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Flowers, M.E.D. Recognizing and Managing Chronic Graft-Versus-Host Disease. Hematology 2008, 2008, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Presland, R.B. Biology of chronic graft-vs-host disease: Immune mechanisms and progress in biomarker discovery. World J. Transpl. 2016, 6, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro-Vornhagen, A.; Hallek, M.J.; Storb, R.F.; von Bergwelt-Baildon, M.S. The role of B cells in the pathogenesis of graft-versus-host disease. Blood 2009, 114, 4919–4927. [Google Scholar] [CrossRef]
- Cutler, C.; Miklos, D.; Kim, H.T.; Treister, N.; Woo, S.B.; Bienfang, D.; Klickstein, L.B.; Levin, J.; Miller, K.; Reynolds, C.; et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood 2006, 108, 756–762. [Google Scholar] [CrossRef]
- Fujii, H.; Cuvelier, G.; She, K.; Aslanian, S.; Shimizu, H.; Kariminia, A.; Krailo, M.; Chen, Z.; McMaster, R.; Bergman, A.; et al. Biomarkers in newly diagnosed pediatric-extensive chronic graft-versus-host disease: A report from the Children’s Oncology Group. Blood 2008, 111, 3276–3285. [Google Scholar] [CrossRef]
- Yamane, M.; Sato, S.; Shimizu, E.; Shibata, S.; Hayano, M.; Yaguchi, T.; Kamijuku, H.; Ogawa, M.; Suzuki, T.; Mukai, S.; et al. Senescence-associated secretory phenotype promotes chronic ocular graft-vs-host disease in mice and humans. FASEB J. 2020, 34, 10778–10800. [Google Scholar] [CrossRef] [PubMed]
- Ngoi, N.Y.; Liew, A.Q.; Chong, S.J.F.; Davids, M.S.; Clement, M.V.; Pervaiz, S. The redox-senescence axis and its therapeutic targeting. Redox Biol. 2021, 45, 102032. [Google Scholar] [CrossRef]
- Jung, S.H.; Hwang, H.J.; Kang, D.; Park, H.A.; Lee, H.C.; Jeong, D.; Lee, K.; Park, H.J.; Ko, Y.G.; Lee, J.S. mTOR kinase leads to PTEN-loss-induced cellular senescence by phosphorylating p53. Oncogene 2019, 38, 1639–1650. [Google Scholar] [CrossRef]
- Milanovic, M.; Fan, D.N.Y.; Belenki, D.; Däbritz, J.H.M.; Zhao, Z.; Yu, Y.; Dörr, J.R.; Dimitrova, L.; Lenze, D.; Monteiro Barbosa, I.A.; et al. Senescence-associated reprogramming promotes cancer stemness. Nature 2018, 553, 96–100. [Google Scholar] [CrossRef]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.M.; Marquess, D.; Dananberg, J.; van Deursen, J.M. Senescent cells: An emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef]
- Ramu, D.; Shan, T.W.; Hirpara, J.L.; Pervaiz, S. Cellular senescence: Silent operator and therapeutic target in cancer. IUBMB Life 2021, 73, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.W.; Lasitschka, F.; Andrulis, M.; et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Strobl, J.; Krausgruber, T.; Kleißl, L.; Reininger, B.; Herac, M.; Bayer, N.; Krall, C.; Wohlfarth, P.; Mitterbauer, M.; Kalhs, P.; et al. Anti-Apoptotic Molecule BCL2 Is a Therapeutic Target in Steroid-Refractory Graft-Versus-Host Disease. J. Investig. Dermatol. 2020, 140, 2188–2198. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, M.B.; Saccon, T.D.; Nunes, A.D.C.; Kirkland, J.L.; Tchkonia, T.; Schneider, A.; Masternak, M.M. Dasatinib plus quercetin prevents uterine age-related dysfunction and fibrosis in mice. Aging 2020, 12, 2711–2722. [Google Scholar] [CrossRef] [PubMed]
- Ellison-Hughes, G.M. First evidence that senolytics are effective at decreasing senescent cells in humans. EBioMedicine 2020, 56, 102473. [Google Scholar] [CrossRef]
- Iske, J.; Seyda, M.; Heinbokel, T.; Maenosono, R.; Minami, K.; Nian, Y.; Quante, M.; Falk, C.S.; Azuma, H.; Martin, F.; et al. Senolytics prevent mt-DNA-induced inflammation and promote the survival of aged organs following transplantation. Nat. Commun. 2020, 11, 4289. [Google Scholar] [CrossRef]
- Fan, T.; Du, Y.; Zhang, M.; Zhu, A.R..; Zhang, J.A. Senolytics cocktail dasatinib and quercetin alleviate human umbilical vein endothelial cell senescence via the TRAF6-MAPK-NF-kappaB Axis in a YTHDF2-dependent manner. Gerontolology 2022, 68, 920–934. [Google Scholar] [CrossRef]
- Hickson, L.J.; Langhi Prata, L.G.P.; Bobart, S.A.; Evans, T.K.; Giorgadze, N.; Hashmi, S.K.; Herrmann, S.M.; Jensen, M.D.; Jia, Q.; Jordan, K.L.; et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 2019, 47, 446–456. [Google Scholar] [CrossRef]
- Morin, F.; Kavian, N.; Marut, W.; Chéreau, C.; Cerles, O.; Grange, P.; Weill, B.; Nicco, C.; Batteux, F. Inhibition of EGFR Tyrosine Kinase by Erlotinib Prevents Sclerodermatous Graft-Versus-Host Disease in a Mouse Model. J. Investig. Dermatol. 2015, 135, 2385–2393. [Google Scholar] [CrossRef]
- Morin, F.; Kavian, N.; Nicco, C.; Cerles, O.; Chéreau, C.; Batteux, F. Improvement of Sclerodermatous Graft-Versus-Host Disease in Mice by Niclosamide. J. Investig. Dermatol. 2016, 136, 2158–2167. [Google Scholar] [CrossRef] [PubMed]
- Kavian, N.; Marut, W.; Servettaz, A.; Laude, H.; Nicco, C.; Chéreau, C.; Weill, B.; Batteux, F. Arsenic Trioxide Prevents Murine Sclerodermatous Graft-versus-Host Disease. J. Immunol. 2012, 188, 5142. [Google Scholar] [CrossRef]
- Saccon, T.D.; Nagpal, R.; Yadav, H.; Cavalcante, M.B.; Nunes, A.D.D.C.; Schneider, A.; Gesing, A.; Hughes, B.; Yousefzadeh, M.; Tchkonia, T.; et al. Senolytic Combination of Dasatinib and Quercetin Alleviates Intestinal Senescence and Inflammation and Modulates the Gut Microbiome in Aged Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1895–1905. [Google Scholar] [CrossRef] [PubMed]
- Khoder, A.; Alsuliman, A.; Basar, R.; Sobieski, C.; Kondo, K.; Alousi, A.M.; Szydlo, R.; Muftuoglu, M.; Shaim, H.; Apperley, J.F.; et al. Evidence for B Cell Exhaustion in Chronic Graft-versus-Host Disease. Front. Immunol. 2018, 8, 1937. [Google Scholar] [CrossRef] [PubMed]
- van der Maas, N.G.; Berghuis, D.; van der Burg, M.; Lankester, A.C. B Cell Reconstitution and Influencing Factors after Hematopoietic Stem Cell Transplantation in Children. Front. Immunol. 2019, 10, 782. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Ma, X.; Cui, C.; Deenik, P.R.; Henderson, P.K.P.; Sigler, A.L.; Cui, L. Real-time imaging of senescence in tumors with DNA damage. Sci. Rep. 2019, 9, 2102. [Google Scholar] [CrossRef] [PubMed]
- Omori, S.; Wang, T.W.; Johmura, Y.; Kanai, T.; Nakano, Y.; Kido, T.; Susaki, E.A.; Nakajima, T.; Shichino, S.; Ueha, S.; et al. Generation of a p16 Reporter Mouse and Its Use to Characterize and Target p16(high) Cells In Vivo. Cell Metab. 2020, 32, 814–828.e6. [Google Scholar] [CrossRef]
- Blazar, B.R.; Murphy, W.J.; Abedi, M. Advances in graft-versus-host disease biology and therapy. Nat. Rev. Immunol. 2012, 12, 443–458. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, K.P.A.; Blazar, B.R.; Hill, G.R. Cytokine mediators of chronic graft-versus-host disease. J. Clin. Investig. 2017, 127, 2452–2463. [Google Scholar] [CrossRef]
- Alexander, K.A.; Flynn, R.; Lineburg, K.E.; Kuns, R.D.; Teal, B.E.; Olver, S.D.; Lor, M.; Raffelt, N.C.; Koyama, M.; Leveque, L.; et al. CSF-1-dependant donor-derived macrophages mediate chronic graft-versus-host disease. J. Clin. Investig. 2014, 124, 4266–4280. [Google Scholar] [CrossRef]
- Melino, M.; Gadd, V.L.; Alexander, K.A.; Beattie, L.; Lineburg, K.E.; Martinez, M.; Teal, B.; Le Texier, L.; Irvine, K.M.; Miller, G.C.; et al. Spatiotemporal Characterization of the Cellular and Molecular Contributors to Liver Fibrosis in a Murine Hepatotoxic-Injury Model. Am. J. Pathol. 2016, 186, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.C.; Garcia, C.C.; Teixeira, M.M.; Amaral, F.A. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev. Clin. Immunol. 2014, 10, 593–619. [Google Scholar] [CrossRef] [PubMed]
- Gourh, P.; Arnett, F.C.; Assassi, S.; Tan, F.K.; Huang, M.; Diekman, L.; Mayes, M.D.; Reveille, J.D.; Agarwal, S.K. Plasma cytokine profiles in systemic sclerosis: Associations with autoantibody subsets and clinical manifestations. Arthritis Res. Ther. 2009, 11, R147. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.R.; Kunkel, S.L.; Todd, R.F., 3rd; Weiss, S.J. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science 1991, 254, 99–102. [Google Scholar] [CrossRef]
- Brennan, K.; Zheng, J. Interleukin 8. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2007; pp. 1–4. [Google Scholar]
- Zhang, Y.; Joe, G.; Hexner, E.; Zhu, J.; Emerson, S.G. Alloreactive Memory T Cells Are Responsible for the Persistence of Graft-versus-Host Disease. J. Immunol. 2005, 174, 3051. [Google Scholar] [CrossRef]
- Loschi, M.; Porcher, R.; Peffault de Latour, R.; Vanneaux, V.; Robin, M.; Xhaard, A.; Sicre de Fontebrune, F.; Larghero, J.; Socie, G. High Number of Memory T Cells Is Associated with Higher Risk of Acute Graft-versus-Host Disease after Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2015, 21, 569–574. [Google Scholar] [CrossRef]
- Buxbaum, N.P.; Farthing, D.E.; Maglakelidze, N.; Lizak, M.; Merkle, H.; Carpenter, A.C.; Oliver, B.U.; Kapoor, V.; Castro, E.; Swan, G.A.; et al. In vivo kinetics and nonradioactive imaging of rapidly proliferating cells in graft-versus-host disease. JCI Insight 2017, 2, e92851. [Google Scholar] [CrossRef] [PubMed]
- Bohmann, E.M.; Fehn, U.; Holler, B.; Weber, D.; Holler, E.; Herr, W.; Hoffmann, P.; Edinger, M.; Wolff, D. Altered immune reconstitution of B and T cells precedes the onset of clinical symptoms of chronic graft-versus-host disease and is influenced by the type of onset. Ann. Hematol. 2017, 96, 299–310. [Google Scholar] [CrossRef]
- Yamashita, K.; Choi, U.; Woltz, P.C.; Foster, S.F.; Sneller, M.C.; Hakim, F.T.; Fowler, D.H.; Bishop, M.R.; Pavletic, S.Z.; Tamari, M.; et al. Severe chronic graft-versus-host disease is characterized by a preponderance of CD4+ effector memory cells relative to central memory cells. Blood 2004, 103, 3986–3988. [Google Scholar] [CrossRef]
- Paldor, M.; Levkovitch-Siany, O.; Eidelshtein, D.; Adar, R.; Enk, C.D.; Marmary, Y.; Elgavish, S.; Nevo, Y.; Benyamini, H.; Plaschkes, I.; et al. Single-cell transcriptomics reveals a senescence-associated IL-6/CCR6 axis driving radiodermatitis. EMBO Mol. Med. 2022, 14, e15653. [Google Scholar] [CrossRef]
- Kuba, A.; Raida, L.; Mrazek, F.; Schneiderova, P.; Kriegova, E.; Langova, K.; Furst, T.; Furstova, J.; Faber, E.; Papajik, T. NFKB1 gene single-nucleotide polymorphisms: Implications for graft-versus-host disease in allogeneic hematopoietic stem cell transplantation. Ann. Hematol. 2020, 99, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hu, X.; Hu, G.; Xu, C.; Jiang, H. Curcumin Attenuates Hydrogen Peroxide-Induced Premature Senescence via the Activation of SIRT1 in Human Umbilical Vein Endothelial Cells. Biol. Pharm. Bull. 2015, 38, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; He, Y.; Zhang, R.; Zheng, G.; Zhou, D. The curcumin analog EF24 is a novel senolytic agent. Aging 2019, 11, 771–782. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, D.; Guo, Q.; Shan, W.; Yang, J.; Lin, T.; Ye, S.; Zhou, X.; Ge, Y.; Bi, S.; et al. Theranostic Quercetin Nanoparticle for Treatment of Hepatic Fibrosis. Bioconjugate Chem. 2019, 30, 2939–2946. [Google Scholar] [CrossRef]
- Ren, K.-W.; Li, Y.-H.; Wu, G.; Ren, J.-Z.; Lu, H.-B.; Li, Z.-M.; Han, X.W. Quercetin nanoparticles display antitumor activity via proliferation inhibition and apoptosis induction in liver cancer cells. Int. J. Oncol. 2017, 50, 1299–1311. [Google Scholar] [CrossRef]
- Han, Q.; Wang, X.; Cai, S.; Liu, X.; Zhang, Y.; Yang, L.; Wang, C.; Yang, R. Quercetin nanoparticles with enhanced bioavailability as multifunctional agents toward amyloid induced neurotoxicity. J. Mater. Chem. B 2018, 6, 1387–1393. [Google Scholar] [CrossRef]
- Chen, B.; Sun, Y.; Zhang, J.; Zhu, Q.; Yang, Y.; Niu, X.; Deng, Z.; Li, Q.; Wang, Y. Human embryonic stem cell-derived exosomes promote pressure ulcer healing in aged mice by rejuvenating senescent endothelial cells. Stem Cell Res. Ther. 2019, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, Y.; Shao, L.; Laberge, R.M.; Demaria, M.; Campisi, J.; Janakiraman, K.; Sharpless, N.E.; Ding, S.; Feng, W.; et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 2016, 22, 78–83. [Google Scholar] [CrossRef]
- Zhu, Y.; Doornebal, E.J.; Pirtskhalava, T.; Giorgadze, N.; Wentworth, M.; Fuhrmann-Stroissnigg, H.; Niedernhofer, L.J.; Robbins, P.D.; Tchkonia, T.; Kirkland, J.L. New agents that target senescent cells: The flavone, fisetin, and the BCL-X(L) inhibitors, A1331852 and A1155463. Aging 2017, 9, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tchkonia, T.; Fuhrmann-Stroissnigg, H.; Dai, H.M.; Ling, Y.Y.; Stout, M.B.; Pirtskhalava, T.; Giorgadze, N.; Johnson, K.O.; Giles, C.B.; et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 2016, 15, 428–435. [Google Scholar] [CrossRef]
- Khoury, M.; Escriou, V.; Courties, G.; Galy, A.; Yao, R.; Largeau, C.; Scherman, D.; Jorgensen, C.; Apparailly, F. Efficient suppression of murine arthritis by combined anticytokine small interfering RNA lipoplexes. Arthritis Rheum. 2008, 58, 2356–2367. [Google Scholar] [CrossRef] [PubMed]
- Yeste, A.; Takenaka, M.C.; Mascanfroni, I.D.; Nadeau, M.; Kenison, J.E.; Patel, B.; Tukpah, A.M.; Babon, J.A.; DeNicola, M.; Kent, S.C.; et al. Tolerogenic nanoparticles inhibit T cell-mediated autoimmunity through SOCS2. Sci. Signal. 2016, 9, ra61. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Kawakami, Y.; Tsubota, K. Cascade of inflammatory, fibrotic process, and stress-induced senescence in chronic GVHD-related dry eye disease. Int. J. Mol. Sci. 2021, 22, 6114. [Google Scholar] [CrossRef]
- Jiao, Y.; Davis, J.E.; Rautela, J.; Carrington, E.M.; Ludford-Menting, M.J.; Goh, W.; Delconte, R.B.; Souza-Fonseca-Guimaraes, F.; Koldej, R.; Gray, D.; et al. Recipient BCL2 inhibition and NK cell ablation form part of a reduced intensity conditioning regime that improves allo-bone marrow transplantation outcomes. Cell Death Differ. 2019, 26, 1516–1530. [Google Scholar] [CrossRef] [PubMed]
- Justice, J.N.; Nambiar, A.M.; Tchkonia, T.; LeBrasseur, N.K.; Pascual, R.; Hashmi, S.K.; Prata, L.; Masternak, M.M.; Kritchevsky, S.B.; Musi, N.; et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 2019, 40, 554–563. [Google Scholar] [CrossRef]
- Lehmann, M.; Korfei, M.; Mutze, K.; Klee, S.; Skronska-Wasek, W.; Alsafadi, H.N.; Ota, C.; Costa, R.; Schiller, H.B.; Lindner, M.; et al. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur. Respir. J. 2017, 50, 1602367. [Google Scholar] [CrossRef]
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J.; Oberg, A.L.; Birch, J.; Salmonowicz, H.; Zhu, Y.; et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017, 8, 14532. [Google Scholar] [CrossRef]
| Syngeneic | Allogeneic + Vehicle | Allogeneic + DQ | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 10 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Day 17 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Day 24 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 3 | 1 | 1 | 1 | 1 | 3 | 1 | 2 | 2 | 1 | 1 |
| Day 35 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 4 | 3 | 3 | 3 | 1 | 3 | 1 | 3 | 3 | 1 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raman, D.; Chêne, C.; Nicco, C.; Jeljeli, M.; Eu, J.Q.; Clément, M.-V.; Batteux, F.; Pervaiz, S. Therapeutic Potential of a Senolytic Approach in a Murine Model of Chronic GVHD. Biology 2023, 12, 647. https://doi.org/10.3390/biology12050647
Raman D, Chêne C, Nicco C, Jeljeli M, Eu JQ, Clément M-V, Batteux F, Pervaiz S. Therapeutic Potential of a Senolytic Approach in a Murine Model of Chronic GVHD. Biology. 2023; 12(5):647. https://doi.org/10.3390/biology12050647
Chicago/Turabian StyleRaman, Deepika, Charlotte Chêne, Carole Nicco, Mohamed Jeljeli, Jie Qing Eu, Marie-Véronique Clément, Frédéric Batteux, and Shazib Pervaiz. 2023. "Therapeutic Potential of a Senolytic Approach in a Murine Model of Chronic GVHD" Biology 12, no. 5: 647. https://doi.org/10.3390/biology12050647
APA StyleRaman, D., Chêne, C., Nicco, C., Jeljeli, M., Eu, J. Q., Clément, M.-V., Batteux, F., & Pervaiz, S. (2023). Therapeutic Potential of a Senolytic Approach in a Murine Model of Chronic GVHD. Biology, 12(5), 647. https://doi.org/10.3390/biology12050647







