Mitochondrial Dynamics as Potential Modulators of Hormonal Therapy Effectiveness in Males
Abstract
Simple Summary
Abstract
1. Andrological Diseases Affecting the Fertile Potential
1.1. Childhood and Adolescence
1.2. Adulthood
2. Hormonal Therapy for Andrological Diseases
2.1. hCG and FSH
2.2. Hormonal Therapy for Cryptorchid Patients
2.3. Hormonal Therapy for Infertile Men
2.4. Applications of Hormonal Therapy for Patients with Other Andrological Diseases
3. Luteinizing Hormone Receptor
3.1. LH as Ligand
3.2. hCG as Ligand
3.3. LHCGR Variants
4. Mitochondrial Dynamics
4.1. Mitochondrial Fission
4.2. Mitochondrial Fusion
5. Implications of Mitochondrial Dynamics in Male Infertility
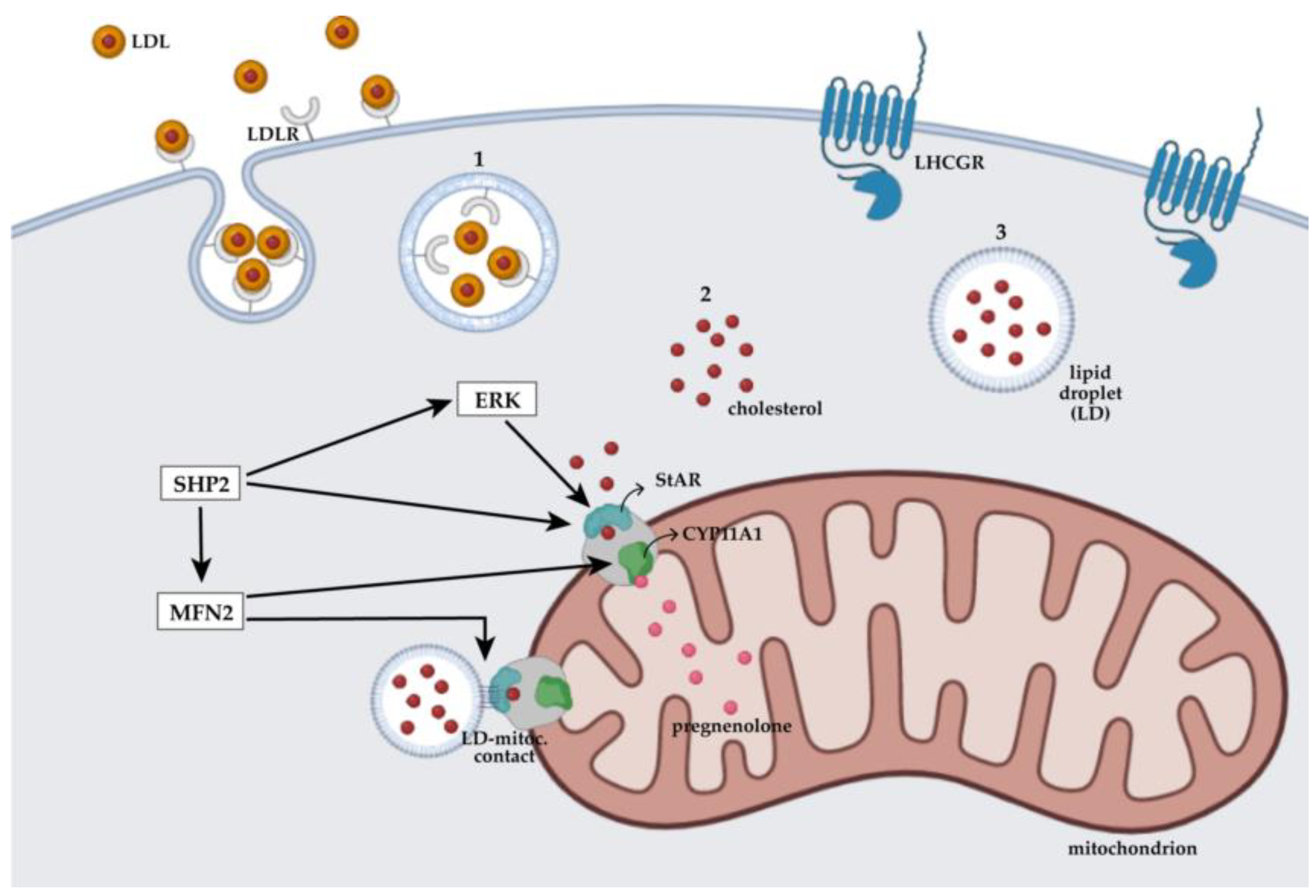
5.1. Mitochondrial Dynamics Regulate Steroidogenesis in Male Cells
5.2. Mitochondrial Dynamics in Spermatogenesis
5.3. The Effect of Hormonal Therapies May Correlate with Mitochondrial Assembly
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABP | androgen binding protein |
| ALF | TF II Aα/β-like factor |
| AP-1 | activator protein 1 |
| AR | androgen receptor |
| C/EBPs | CCAAT/enhancer-binding proteins |
| cAMP | adenosine 3′,5′-cyclic monophosphate |
| CREB | cAMP response element-binding protein |
| CREM | cAMP response element modulator |
| CTP | C-terminal peptide |
| CYP11A1 | cytochrome P450 family 11 subfamily A member 1 |
| CYP17A1 | 17α-Hydroxylase/17,20-lyase P450 |
| DRP1 | dynamin-related protein 1 |
| EGFR | epidermal growth factor receptor |
| ER | endoplasic reticulum |
| ERK1/2 | extracellular signal-regulated kinase 1/2 |
| ERRα | estrogen-related receptor alpha |
| ETC | electron transport chain |
| FIS1 | mitochondrial fission protein 1 |
| FSH | follicle-stimulating hormone |
| FSHR | follicle-stimulating hormone receptor |
| GH | growth hormone |
| GnRH | gonadotropin-releasing hormone |
| GPCRs | guanine nucleotide-binding protein-coupled receptors |
| GPHRs | glycoprotein hormone receptors |
| hCG | human chorionic gonadotropin |
| hLHCGR | human luteinizing hormone/choriogonadotropin hormone receptor |
| HPG | hypothalamic-pituitary-gonadal axis |
| HR1 | heptad repeat domain 1 |
| HR2 | heptad repeat domain 2 |
| 3β-HSD | 3β-hydroxysteroid dehydrogenase |
| 17β-HSD3 | 17β-hydroxysteroid dehydrogenase type 3 |
| IGF-1 | insulin-like growth factor-1 |
| IMM | inner mitochondrial membrane |
| K.O. | knockout |
| LCH | Leydig cell hypoplasia |
| LDL | low-density-lipoprotein |
| LDLR | low-density-lipoprotein receptor |
| LH | luteinizing hormone |
| LHCGR | luteinizing hormone/choriogonadotropin hormone receptor |
| L-OPA1 | optic atrophy 1 fusion protein–long form |
| LRRD | leucine-rich repeat domain |
| MAM | mitochondria-associated membranes |
| MFF | mitochondrial fission factor |
| MFN1 | mitofusin-1 |
| MFN2 | mitofusin-2 |
| MiD49 | mitochondrial dynamics protein of 49 kDa |
| MiD51 | mitochondrial dynamics protein of 51 kDa |
| MPP | male-limited precocious puberty |
| MPP | male-limited precocious puberty |
| mtDNA | mitochondrial DNA |
| OCR | oxygen consumption rate |
| OMM | outer mitochondrial membrane |
| OPA1 | optic atrophy 1 fusion protein |
| OXPHOS | oxidative phosphorylation |
| PBDE | polybrominated diphenyl ethers |
| PGC-1α | peroxisome proliferator-activated receptor-gamma coactivator-1α |
| PGC-1β | peroxisome proliferator-activated receptor-gamma coactivator-1β |
| PKA | protein kinase A |
| PKB/AKT | protein kinase B |
| rFSH | recombinant follicle-stimulating hormone |
| ROS | reactive oxygen species |
| SERM | selective estrogen receptor modulators |
| SF-1 | steroidogenic factor 1 |
| S-OPA1 | optic atrophy 1 fusion protein–short form |
| StAR | steroidogenic acute regulatory protein |
| TT | testosterone |
| TMD | transmembrane domain |
| TRT | testosterone replacement treatment |
| TSH | thyroid-stimulating hormone |
| TSHR | thyroid-stimulating hormone receptor |
References
- Shin, J.; Jeon, G.W. Comparison of Diagnostic and Treatment Guidelines for Undescended Testis. Clin. Exp. Pediatr. 2020, 63, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, H.E.; Bjerknes, R.; Cortes, D.; Jørgensen, N.; Rajpert-De Meyts, E.; Thorsson, A.V.; Thorup, J.; Main, K.M. Cryptorchidism: Classification, Prevalence and Long-Term Consequences. Acta Paediatr. 2007, 96, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Nef, S.; Parada, L.F. Cryptorchidism in Mice Mutant for Insl3. Nat. Genet. 1999, 22, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.S.; Toft, G.; Thulstrup, A.M.; Henriksen, T.B.; Olsen, J.; Christensen, K.; Bonde, J.P. Cryptorchidism Concordance in Monozygotic and Dizygotic Twin Brothers, Full Brothers, and Half-Brothers. Fertil. Steril. 2010, 93, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.; Brock, G.B. Cryptorchidism and Its Impact on Male Fertility: A State of Art Review of Current Literature. Can. Urol. Assoc. J. 2011, 5, 210–214. [Google Scholar] [CrossRef]
- Zampieri, N.; Caridha, D.; Patanè, S.; Bianchi, F.; Vestri, E.; Bruno, C.; Camoglio, F. Elastosonographic Evaluation of the Post-Operative Morpho-Volumetric Recovery of the Gonad in the Cryptorchid Patient. Am. J. Clin. Exp. Urol. 2019, 7, 182–187. [Google Scholar]
- Peretti, M.; Zampieri, N.; Bertozzi, M.; Bianchi, F.; Patanè, S.; Spigo, V.; Camoglio, F.S. Mean Platelet Volume and Testicular Torsion: New Findings. Urol. J. 2019, 16, 83–85. [Google Scholar] [CrossRef]
- Favorito, L.A.; Cavalcante, A.G.; Costa, W.S. Anatomic Aspects of Epididymis and Tunica Vaginalis in Patients with Testicular Torsion. Int. Braz. J. Urol. 2004, 30, 420–424. [Google Scholar] [CrossRef]
- Errico, A.; Camoglio, F.S.; Zampieri, N.; Dando, I. Testicular Torsion: Preliminary Results of In Vitro Cell Stimulation Using Chorionic Gonadotropin. Cells 2022, 11, 450. [Google Scholar] [CrossRef]
- Zampieri, N.; Patanè, S.; Camoglio, F.S. Twenty-year Experience with Macro-area School Screening for Andrological Disease in Paediatric Age. Andrologia 2021, 53, e14209. [Google Scholar] [CrossRef]
- Infertility. Available online: https://www.who.int/health-topics/infertility (accessed on 9 February 2023).
- Sharma, A.; Minhas, S.; Dhillo, W.S.; Jayasena, C.N. Male Infertility Due to Testicular Disorders. J. Clin. Endocrinol. Metab. 2021, 106, e442–e459. [Google Scholar] [CrossRef]
- Jungwirth, A.; Giwercman, A.; Tournaye, H.; Diemer, T.; Kopa, Z.; Dohle, G.; Krausz, C. European Association of Urology Working Group on Male Infertility European Association of Urology Guidelines on Male Infertility: The 2012 Update. Eur. Urol. 2012, 62, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Salonia, A.; Rastrelli, G.; Hackett, G.; Seminara, S.B.; Huhtaniemi, I.T.; Rey, R.A.; Hellstrom, W.J.G.; Palmert, M.R.; Corona, G.; Dohle, G.R.; et al. Paediatric and Adult-Onset Male Hypogonadism. Nat. Rev. Dis. Prim. 2019, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.M.; Lee, S.D. Current Issues in Adolescent Varicocele: Pediatric Urological Perspectives. World J. Mens. Health 2018, 36, 123. [Google Scholar] [CrossRef]
- Johnson, D.; Sandlow, J. Treatment of Varicoceles: Techniques and Outcomes. Fertil. Steril. 2017, 108, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Marmar, J.L.; Agarwal, A.; Prabakaran, S.; Agarwal, R.; Short, R.A.; Benoff, S.; Thomas, A.J. Reassessing the Value of Varicocelectomy as a Treatment for Male Subfertility with a New Meta-Analysis. Fertil. Steril. 2007, 88, 639–648. [Google Scholar] [CrossRef]
- Richardson, I.; Grotas, A.B.; Nagler, H.M. Outcomes of Varicocelectomy Treatment: An Updated Critical Analysis. Urol. Clin. N. Am. 2008, 35, 191–209. [Google Scholar] [CrossRef]
- Zitzmann, M.; Nieschlag, E. Hormone Substitution in Male Hypogonadism. Mol. Cell. Endocrinol. 2000, 161, 73–88. [Google Scholar] [CrossRef]
- Nguyen, C.P.; Hirsch, M.S.; Moeny, D.; Kaul, S.; Mohamoud, M.; Joffe, H.V. Testosterone and “Age-Related Hypogonadism”—FDA Concerns. N. Engl. J. Med. 2015, 373, 689–691. [Google Scholar] [CrossRef]
- Nwabuobi, C.; Arlier, S.; Schatz, F.; Guzeloglu-Kayisli, O.; Lockwood, C.; Kayisli, U. HCG: Biological Functions and Clinical Applications. Int. J. Mol. Sci. 2017, 18, 2037. [Google Scholar] [CrossRef]
- Strott, C.A.; Yoshimi, T.; Ross, G.T.; Lipsett, M.B. Ovarian Physiology: Relationship Between Plasma LH and Steroidogenesis by the Follicle and Corpus Luteum; Effect of HCG. J. Clin. Endocrinol. Metab. 1969, 29, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Stenman, U.-H.; Alfthan, H. Determination of Human Chorionic Gonadotropin. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Casarini, L.; Lispi, M.; Longobardi, S.; Milosa, F.; La Marca, A.; Tagliasacchi, D.; Pignatti, E.; Simoni, M. LH and HCG Action on the Same Receptor Results in Quantitatively and Qualitatively Different Intracellular Signalling. PLoS ONE 2012, 7, e46682. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.A. Biological Functions of HCG and HCG-Related Molecules. Reprod. Biol. Endocrinol. 2010, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.S.D.; Faiman, C.; Hobson, W.C.; Prasad, A.V.; Reyes, F.I. Pituitary-Gonadal Relations in Infancy. I. Patterns of Serum Gonadotropin Concentrations from Birth to Four Years of Age in Man and Chimpanzee. J. Clin. Endocrinol. Metab. 1975, 40, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, O.; Michel Ortega, R.M.; Angeles-Sánchez, J.; Villarreal-Garza, C.; Avilés-Salas, A.; Chanona-Vilchis, J.G.; Aréchaga-Ocampo, E.; Luévano-González, A.; Jiménez, M.A.; Aguilar, J.L. Serum Human Chorionic Gonadotropin Is Associated with Angiogenesis in Germ Cell Testicular Tumors. J. Exp. Clin. Cancer Res. 2009, 28, 120. [Google Scholar] [CrossRef]
- Das, N.; Kumar, T.R. Molecular Regulation of Follicle-Stimulating Hormone Synthesis, Secretion and Action. J. Mol. Endocrinol. 2018, 60, R131–R155. [Google Scholar] [CrossRef]
- Santi, D.; Simoni, M. Biosimilar Recombinant Follicle Stimulating Hormones in Infertility Treatment. Expert Opin. Biol. Ther. 2014, 14, 1399–1409. [Google Scholar] [CrossRef]
- Rajfer, J.; Handelsman, D.J.; Swerdloff, R.S.; Hurwitz, R.; Kaplan, H.; Vandergast, T.; Ehrlich, R.M. Hormonal Therapy of Cryptorchidism. N. Engl. J. Med. 1986, 314, 466–470. [Google Scholar] [CrossRef]
- Matthews, L.A.; Abdul-Karim, F.W.; Elder, J.S. Effect of Preoperative Human Chorionic Gonadotropin on Intra-Abdominal Rat Testes Undergoing Standard and Fowler-Stephens Orchiopexy. J. Urol. 1997, 157, 2315–2317. [Google Scholar] [CrossRef]
- Leslie, S.W.; Sajjad, H.; Villanueva, C.A. Cryptorchidism. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Zampieri, N.; Murri, V.; Camoglio, F.S. Post-Operative Use of Human Chorionic Gonadotrophin (u-HCG) Inpatients Treated for Intrabdominal Unilateral Undescended Testes. Am. J. Clin. Exp. Urol. 2018, 6, 133–137. [Google Scholar] [PubMed]
- Hildorf, S.; Cortes, D.; Clasen-Linde, E.; Fossum, M.; Thorup, J. The Impact of Early and Successful Orchidopexy on Hormonal Follow-up for 208 Boys with Bilateral Non-Syndromic Cryptorchidism. Pediatr. Surg. Int. 2021, 37, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Holtermann Entwistle, O.; Sharma, A.; Jayasena, C.N. What Must Be Considered When Prescribing Hormonal Pharmacotherapy for Male Infertility? Expert Opin. Pharmacother. 2022, 23, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Bayram, F.; Elbuken, G.; Korkmaz, C.; Aydogdu, A.; Karaca, Z.; Cakır, I. The Effects of Gonadotropin Replacement Therapy on Metabolic Parameters and Body Composition in Men with Idiopathic Hypogonadotropic Hypogonadism. Horm. Metab. Res. 2016, 48, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Santi, D.; Casarini, L.; Alviggi, C.; Simoni, M. Efficacy of Follicle-Stimulating Hormone (FSH) Alone, FSH + Luteinizing Hormone, Human Menopausal Gonadotropin or FSH + Human Chorionic Gonadotropin on Assisted Reproductive Technology Outcomes in the “Personalized” Medicine Era: A Meta-Analysis. Front. Endocrinol. 2017, 8, 114. [Google Scholar] [CrossRef]
- Hall, S.H.; Conti, M.; French, F.S.; Joseph, D.R. Follicle-Stimulating Hormone Regulation of Androgen-Binding Protein Messenger RNA in Sertoli Cell Cultures. Mol. Endocrinol. 1990, 4, 349–355. [Google Scholar] [CrossRef]
- Cannarella, R.; La Vignera, S.; Condorelli, R.A.; Mongioì, L.M.; Calogero, A.E. FSH Dosage Effect on Conventional Sperm Parameters: A Meta-Analysis of Randomized Controlled Studies. Asian J. Androl. 2020, 22, 309–316. [Google Scholar] [CrossRef]
- Zambrano, F.; Carrau, T.; Gärtner, U.; Seipp, A.; Taubert, A.; Felmer, R.; Sanchez, R.; Hermosilla, C. Leukocytes Coincubated with Human Sperm Trigger Classic Neutrophil Extracellular Traps Formation, Reducing Sperm Motility. Fertil. Steril. 2016, 106, 1053–1060.e1. [Google Scholar] [CrossRef]
- Liu, P.Y.; Baker, H.W.G.; Jayadev, V.; Zacharin, M.; Conway, A.J.; Handelsman, D.J. Induction of Spermatogenesis and Fertility during Gonadotropin Treatment of Gonadotropin-Deficient Infertile Men: Predictors of Fertility Outcome. J. Clin. Endocrinol. Metab. 2009, 94, 801–808. [Google Scholar] [CrossRef]
- Vicari, E.; Mongioì, A.; Calogero, A.E.; Moncada, M.L.; Sidoti, G.; Polosa, P.; D’Agata, R. Therapy with Human Chorionic Gonadotrophin Alone Induces Spermatogenesis in Men with Isolated Hypogonadotrophic Hypogonadism-Long-Term Follow-Up. Int. J. Androl. 1992, 15, 320–329. [Google Scholar] [CrossRef]
- Bahadir, G.B.; Gollu, G.; Ilkay, H.; Bagriacik, U.; Hasirci, N.; Bingol-Kologlu, M. LOCAL-IGF-1 and GH Application IMPROVES Germ Cell Histology, Spermatogenesis and Fertility after Experimental Testicular Torsion and Detorsion. J. Pediatr. Urol. 2022, 18, e1–e410. [Google Scholar] [CrossRef] [PubMed]
- Goodman, H.M. Discovery of the Luteinizing Hormone of the Anterior Pituitary Gland. Am. J. Physiol.-Endocrinol. Metab. 2004, 287, E818–E819. [Google Scholar] [CrossRef]
- Ezcurra, D.; Humaidan, P. A Review of Luteinising Hormone and Human Chorionic Gonadotropin When Used in Assisted Reproductive Technology. Reprod. Biol. Endocrinol. 2014, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Ilahi, S.; Ilahi, T.B. Anatomy, Adenohypophysis (Pars Anterior, Anterior Pituitary). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Tremblay, J.J. Molecular Regulation of Steroidogenesis in Endocrine Leydig Cells. Steroids 2015, 103, 3–10. [Google Scholar] [CrossRef]
- Stojkov, N.J.; Janjic, M.M.; Baburski, A.Z.; Mihajlovic, A.I.; Drljaca, D.M.; Sokanovic, S.J.; Bjelic, M.M.; Kostic, T.S.; Andric, S.A. Sustained in Vivo Blockade of α 1 -Adrenergic Receptors Prevented Some of Stress-Triggered Effects on Steroidogenic Machinery in Leydig Cells. Am. J. Physiol.-Endocrinol. Metab. 2013, 305, E194–E204. [Google Scholar] [CrossRef] [PubMed]
- Rey, R.A. Biomarkers of Male Hypogonadism in Childhood and Adolescence. Adv. Lab. Med. Av. En Med. Lab. 2020, 1, 1–13. [Google Scholar] [CrossRef]
- Bjerner, J.; Biernat, D.; Fosså, S.D.; Bjøro, T. Reference Intervals for Serum Testosterone, SHBG, LH and FSH in Males from the NORIP Project. Scand. J. Clin. Lab. Investig. 2009, 69, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, L.; Cofini, M.; Leonardi, A.; Penta, L.; Esposito, S. Up-To-Date Review About Minipuberty and Overview on Hypothalamic-Pituitary-Gonadal Axis Activation in Fetal and Neonatal Life. Front. Endocrinol. 2018, 9, 410. [Google Scholar] [CrossRef]
- Fowler, P.A.; Bhattacharya, S.; Gromoll, J.; Monteiro, A.; O’Shaughnessy, P.J. Maternal Smoking and Developmental Changes in Luteinizing Hormone (LH) and the LH Receptor in the Fetal Testis. J. Clin. Endocrinol. Metab. 2009, 94, 4688–4695. [Google Scholar] [CrossRef]
- Bergadá, I.; Milani, C.; Bedecarrás, P.; Andreone, L.; Ropelato, M.G.; Gottlieb, S.; Bergadá, C.; Campo, S.; Rey, R.A. Time Course of the Serum Gonadotropin Surge, Inhibins, and Anti-Müllerian Hormone in Normal Newborn Males during the First Month of Life. J. Clin. Endocrinol. Metab. 2006, 91, 4092–4098. [Google Scholar] [CrossRef]
- Howard, S.R. Interpretation of Reproductive Hormones before, during and after the Pubertal Transition—Identifying Health and Disordered Puberty. Clin. Endocrinol. 2021, 95, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.E.; Nayini, K.P.; Mills, W.E.; Lockwood, G.M.; Banerjee, S. Circulating LH/HCG Receptor (LHCGR) May Identify Pre-Treatment IVF Patients at Risk of OHSS and Poor Implantation. Reprod. Biol. Endocrinol. 2011, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Riccetti, L.; De Pascali, F.; Gilioli, L.; Potì, F.; Giva, L.B.; Marino, M.; Tagliavini, S.; Trenti, T.; Fanelli, F.; Mezzullo, M.; et al. Human LH and HCG Stimulate Differently the Early Signalling Pathways but Result in Equal Testosterone Synthesis in Mouse Leydig Cells in Vitro. Reprod. Biol. Endocrinol. 2017, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, F.; Ye, L.; Zirkin, B.; Chen, H. Steroidogenesis in Leydig Cells: Effects of Aging and Environmental Factors. Reproduction 2017, 154, R111–R122. [Google Scholar] [CrossRef] [PubMed]
- Riccetti, L.; Yvinec, R.; Klett, D.; Gallay, N.; Combarnous, Y.; Reiter, E.; Simoni, M.; Casarini, L.; Ayoub, M.A. Human Luteinizing Hormone and Chorionic Gonadotropin Display Biased Agonism at the LH and LH/CG Receptors. Sci. Rep. 2017, 7, 940. [Google Scholar] [CrossRef] [PubMed]
- Kossack, N.; Troppmann, B.; Richter-Unruh, A.; Kleinau, G.; Gromoll, J. Aberrant Transcription of the LHCGR Gene Caused by a Mutation in Exon 6A Leads to Leydig Cell Hypoplasia Type II. Mol. Cell. Endocrinol. 2013, 366, 59–67. [Google Scholar] [CrossRef]
- Gromoll, J.; Wistuba, J.; Terwort, N.; Godmann, M.; Müller, T.; Simoni, M. A New Subclass of the Luteinizing Hormone/Chorionic Gonadotropin Receptor Lacking Exon 10 Messenger RNA in the New World Monkey (Platyrrhini) Lineage1. Biol. Reprod. 2003, 69, 75–80. [Google Scholar] [CrossRef]
- Ascoli, M.; Fanelli, F.; Segaloff, D.L. The Lutropin/Choriogonadotropin Receptor, A 2002 Perspective. Endocr. Rev. 2002, 23, 141–174. [Google Scholar] [CrossRef]
- Breen, S.M.; Andric, N.; Ping, T.; Xie, F.; Offermans, S.; Gossen, J.A.; Ascoli, M. Ovulation Involves the Luteinizing Hormone-Dependent Activation of Gq/11 in Granulosa Cells. Mol. Endocrinol. 2013, 27, 1483–1491. [Google Scholar] [CrossRef]
- Jonas, K.C.; Chen, S.; Virta, M.; Mora, J.; Franks, S.; Huhtaniemi, I.; Hanyaloglu, A.C. Temporal Reprogramming of Calcium Signalling via Crosstalk of Gonadotrophin Receptors That Associate as Functionally Asymmetric Heteromers. Sci. Rep. 2018, 8, 2239. [Google Scholar] [CrossRef]
- Zhang, M.; Guan, R.; Segaloff, D.L. Revisiting and Questioning Functional Rescue between Dimerized LH Receptor Mutants. Mol. Endocrinol. 2012, 26, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Jeoung, M.; Lee, C.; Ji, I.; Ji, T.H. Trans-Activation, Cis-Activation and Signal Selection of Gonadotropin Receptors. Mol. Cell. Endocrinol. 2007, 260–262, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.-X.; Johnson, N.B.; Segaloff, D.L. Constitutive and Agonist-Dependent Self-Association of the Cell Surface Human Lutropin Receptor. J. Biol. Chem. 2004, 279, 5904–5914. [Google Scholar] [CrossRef]
- Kossack, N.; Simoni, M.; Richter-Unruh, A.; Themmen, A.P.N.; Gromoll, J. Mutations in a Novel, Cryptic Exon of the Luteinizing Hormone/Chorionic Gonadotropin Receptor Gene Cause Male Pseudohermaphroditism. PLoS Med. 2008, 5, e88. [Google Scholar] [CrossRef] [PubMed]
- Troppmann, B.; Kleinau, G.; Krause, G.; Gromoll, J. Structural and Functional Plasticity of the Luteinizing Hormone/Choriogonadotrophin Receptor. Hum. Reprod. Update 2013, 19, 583–602. [Google Scholar] [CrossRef]
- Liu, W.; Han, B.; Zhu, W.; Cheng, T.; Fan, M.; Wu, J.; Yang, Y.; Zhu, H.; Si, J.; Lyu, Q.; et al. Polymorphism in the Alternative Donor Site of the Cryptic Exon of LHCGR: Functional Consequences and Associations with Testosterone Level. Sci. Rep. 2017, 7, 45699. [Google Scholar] [CrossRef]
- HGMD® Home Page. Available online: https://www.hgmd.cf.ac.uk/ac/index.php (accessed on 10 February 2023).
- Jahan, S.; Abul Hasanat, M.; Alam, F.; Fariduddin, M.; Tofail, T. Leydig Cell Hypoplasia: A Unique Paradox in the Diagnosis of 46, XY Disorders of Sex Development. AACE Clin. Case Rep. 2020, 6, e117–e122. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Wang, N.; Zhu, H.; Han, B.; Sun, F.; Yao, H.; Zhang, Q.; Zhu, W.; Cheng, T.; et al. Next-Generation Sequencing Reveals Genetic Landscape in 46, XY Disorders of Sexual Development Patients with Variable Phenotypes. Hum. Genet. 2018, 137, 265–277. [Google Scholar] [CrossRef]
- Vezzoli, V.; Duminuco, P.; Vottero, A.; Kleinau, G.; Schülein, R.; Minari, R.; Bassi, I.; Bernasconi, S.; Persani, L.; Bonomi, M. A New Variant in Signal Peptide of the Human Luteinizing Hormone Receptor (LHCGR) Affects Receptor Biogenesis Causing Leydig Cell Hypoplasia. Hum. Mol. Genet. 2015, 24, 6003–6012. [Google Scholar] [CrossRef]
- Potorac, I.; Trehan, A.; Szymańska, K.; Fudvoye, J.; Thiry, A.; Huhtaniemi, I.; Daly, A.F.; Beckers, A.; Parent, A.-S.; Rivero-Müller, A. Compound Heterozygous Mutations in the Luteinizing Hormone Receptor Signal Peptide Causing 46,XY Disorder of Sex Development. Eur. J. Endocrinol. 2019, 181, K11–K20. [Google Scholar] [CrossRef]
- Newton, C.L.; Anderson, R.C.; Katz, A.A.; Millar, R.P. Loss-of-Function Mutations in the Human Luteinizing Hormone Receptor Predominantly Cause Intracellular Retention. Endocrinology 2016, 157, 4364–4377. [Google Scholar] [CrossRef] [PubMed]
- Gromoll, J.; Eiholzer, U.; Nieschlag, E.; Simoni, M. Male Hypogonadism Caused by Homozygous Deletion of Exon 10 of the Luteinizing Hormone (LH) Receptor: Differential Action of Human Chorionic Gonadotropin and LH. J. Clin. Endocrinol. Metab. 2000, 85, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Gurnurkar, S.; DiLillo, E.; Carakushansky, M. A Case of Familial Male-Limited Precocious Puberty with a Novel Mutation. J. Clin. Res. Pediatr. Endocrinol. 2021, 13, 239–244. [Google Scholar] [CrossRef]
- Boot, A.M.; Lumbroso, S.; Verhoef-Post, M.; Richter-Unruh, A.; Looijenga, L.H.J.; Funaro, A.; Beishuizen, A.; van Marle, A.; Drop, S.L.S.; Themmen, A.P.N. Mutation Analysis of the LH Receptor Gene in Leydig Cell Adenoma and Hyperplasia and Functional and Biochemical Studies of Activating Mutations of the LH Receptor Gene. J. Clin. Endocrinol. Metab. 2011, 96, E1197–E1205. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Saji, M.; Hidaka, A.; Moriya, N.; Okuno, A.; Kohn, L.D.; Cutler, G.B. A New Constitutively Activating Point Mutation in the Luteinizing Hormone/Choriogonadotropin Receptor Gene in Cases of Male-Limited Precocious Puberty. J. Clin. Endocrinol. Metab. 1995, 80, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-M.; Leschek, E.W.; Brain, C.; Chan, W.-Y. A Novel Luteinizing Hormone Receptor Mutation in a Patient with Familial Male-Limited Precocious Puberty: Effect of the Size of a Critical Amino Acid on Receptor Activity. Mol. Genet. Metab. 1999, 66, 68–73. [Google Scholar] [CrossRef]
- Byambaragchaa, M.; Seong, H.-K.; Choi, S.-H.; Kim, D.-J.; Kang, M.-H.; Min, K.-S. Constitutively Activating Mutants of Equine LH/CGR Constitutively Induce Signal Transduction and Inactivating Mutations Impair Biological Activity and Cell-Surface Receptor Loss In Vitro. Int. J. Mol. Sci. 2021, 22, 10723. [Google Scholar] [CrossRef]
- Siviero-Miachon, A.A.; Kizys, M.M.L.; Ribeiro, M.M.; Garcia, F.E.; Spinola-Castro, A.M.; Dias da Silva, M.R. Cosegregation of a Novel Mutation in the Sixth Transmembrane Segment of the Luteinizing/Choriogonadotropin Hormone Receptor with Two Brazilian Siblings with Severe Testotoxicosis. Endocr. Res. 2017, 42, 117–124. [Google Scholar] [CrossRef]
- Hirakawa, T.; Ascoli, M. A Constitutively Active Somatic Mutation of the Human Lutropin Receptor Found in Leydig Cell Tumors Activates the Same Families of G Proteins as Germ Line Mutations Associated with Leydig Cell Hyperplasia. Endocrinology 2003, 144, 3872–3878. [Google Scholar] [CrossRef]
- Latronico, A.C.; Shinozaki, H.; Guerra, G., Jr.; Pereira, M.A.A.; Lemos Marini, S.H.V.; Baptista, M.T.M.; Arnhold, I.J.P.; Fanelli, F.; Mendonca, B.B.; Segaloff, D.L. Gonadotropin-Independent Precocious Puberty Due to Luteinizing Hormone Receptor Mutations in Brazilian Boys: A Novel Constitutively Activating Mutation in the First Transmembrane Helix. J. Clin. Endocrinol. Metab. 2000, 85, 4799–4805. [Google Scholar] [CrossRef]
- Latronico, A.C.; Anasti, J.; Arnhold, I.J.; Mendonça, B.B.; Domenice, S.; Albano, M.C.; Zachman, K.; Wajchenberg, B.L.; Tsigos, C. A Novel Mutation of the Luteinizing Hormone Receptor Gene Causing Male Gonadotropin-Independent Precocious Puberty. J. Clin. Endocrinol. Metab. 1995, 80, 2490–2494. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wang, H.; Li, J.; Zhou, Z.; Du, Y.; Lin, M.; Sha, J. Involvement of ALF in Human Spermatogenesis and Male Infertility. Int. J. Mol. Med. 2006, 17, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Simoni, M.; Tüttelmann, F.; Michel, C.; Böckenfeld, Y.; Nieschlag, E.; Gromoll, J. Polymorphisms of the Luteinizing Hormone/Chorionic Gonadotropin Receptor Gene: Association with Maldescended Testes and Male Infertility. Pharm. Genom. 2008, 18, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, S.S.; Zhu, Y.-S.; Cai, L.-Q.; Katz, M.D.; Herrera, C.; DeFillo-Ricart, M.; Imperato-McGinley, J. A Novel Mutation of the Human Luteinizing Hormone Receptor in 46XY and 46XX Sisters. J. Clin. Endocrinol. Metab. 1998, 83, 2091–2098. [Google Scholar] [CrossRef]
- Latronico, A.C.; Chai, Y.; Arnhold, I.J.P.; Liu, X.; Mendonca, B.B.; Segaloff, D.L. A Homozygous Microdeletion in Helix 7 of the Luteinizing Hormone Receptor Associated with Familial Testicular and Ovarian Resistance Is Due to Both Decreased Cell Surface Expression and Impaired Effector Activation by the Cell Surface Receptor. Mol. Endocrinol. 1998, 12, 442–450. [Google Scholar] [CrossRef]
- Yariz, K.O.; Walsh, T.; Uzak, A.; Spiliopoulos, M.; Duman, D.; Onalan, G.; King, M.-C.; Tekin, M. Inherited Mutation of the Luteinizing Hormone/Choriogonadotropin Receptor (LHCGR) in Empty Follicle Syndrome. Fertil. Steril. 2011, 96, e125–e130. [Google Scholar] [CrossRef]
- Capalbo, A.; Sagnella, F.; Apa, R.; Fulghesu, A.M.; Lanzone, A.; Morciano, A.; Farcomeni, A.; Gangale, M.F.; Moro, F.; Martinez, D.; et al. The 312N Variant of the Luteinizing Hormone/Choriogonadotropin Receptor Gene (LHCGR) Confers up to 2·7-Fold Increased Risk of Polycystic Ovary Syndrome in a Sardinian Population. Clin. Endocrinol. 2012, 77, 113–119. [Google Scholar] [CrossRef]
- Piersma, D.; Berns, E.M.J.J.; Verhoef-Post, M.; Uitterlinden, A.G.; Braakman, I.; Pols, H.A.P.; Themmen, A.P.N. A Common Polymorphism Renders the Luteinizing Hormone Receptor Protein More Active by Improving Signal Peptide Function and Predicts Adverse Outcome in Breast Cancer Patients. J. Clin. Endocrinol. Metab. 2006, 91, 1470–1476. [Google Scholar] [CrossRef]
- Giacomello, M.; Pyakurel, A.; Glytsou, C.; Scorrano, L. The Cell Biology of Mitochondrial Membrane Dynamics. Nat. Rev. Mol. Cell Biol. 2020, 21, 204–224. [Google Scholar] [CrossRef]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial Fission, Fusion, and Stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef]
- Duarte, A.; Poderoso, C.; Cooke, M.; Soria, G.; Cornejo Maciel, F.; Gottifredi, V.; Podestá, E.J. Mitochondrial Fusion Is Essential for Steroid Biosynthesis. PLoS ONE 2012, 7, e45829. [Google Scholar] [CrossRef] [PubMed]
- Zacharioudakis, E.; Gavathiotis, E. Mitochondrial Dynamics Proteins as Emerging Drug Targets. Trends Pharmacol. Sci. 2023, 44, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Zhou, D.; Wang, M.; Li, E.; Hou, C.; Su, Y.; Zou, Q.; Zhou, P.; Liu, X. RACGAP1 Modulates ECT2-Dependent Mitochondrial Quality Control to Drive Breast Cancer Metastasis. Exp. Cell Res. 2021, 400, 112493. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Xi, S. The Role of Mitochondrial Fission Proteins in Mitochondrial Dynamics in Kidney Disease. Int. J. Mol. Sci. 2022, 23, 14725. [Google Scholar] [CrossRef]
- Lewis, S.C.; Uchiyama, L.F.; Nunnari, J. ER-Mitochondria Contacts Couple MtDNA Synthesis with Mitochondrial Division in Human Cells. Science 2016, 353, aaf5549. [Google Scholar] [CrossRef] [PubMed]
- Sabouny, R.; Shutt, T.E. Reciprocal Regulation of Mitochondrial Fission and Fusion. Trends Biochem. Sci. 2020, 45, 564–577. [Google Scholar] [CrossRef]
- Kalia, R.; Wang, R.Y.-R.; Yusuf, A.; Thomas, P.V.; Agard, D.A.; Shaw, J.M.; Frost, A. Structural Basis of Mitochondrial Receptor Binding and Constriction by DRP1. Nature 2018, 558, 401–405. [Google Scholar] [CrossRef]
- Chang, Y.; Song, Z.; Chen, C. FAK Regulates Cardiomyocyte Mitochondrial Fission and Function through Drp1. FEBS J. 2022, 289, 1897–1910. [Google Scholar] [CrossRef]
- Ren, L.; Chen, X.; Chen, X.; Li, J.; Cheng, B.; Xia, J. Mitochondrial Dynamics: Fission and Fusion in Fate Determination of Mesenchymal Stem Cells. Front. Cell Dev. Biol. 2020, 8, 580070. [Google Scholar] [CrossRef]
- Liu, Y.; Merrill, R.A.; Strack, S. A-Kinase Anchoring Protein 1: Emerging Roles in Regulating Mitochondrial Form and Function in Health and Disease. Cells 2020, 9, 298. [Google Scholar] [CrossRef]
- Gandre-Babbe, S.; van der Bliek, A.M. The Novel Tail-Anchored Membrane Protein Mff Controls Mitochondrial and Peroxisomal Fission in Mammalian Cells. Mol. Biol. Cell 2008, 19, 2402–2412. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Jin, S.; Lendahl, U.; Nistér, M.; Zhao, J. Human Fis1 Regulates Mitochondrial Dynamics through Inhibition of the Fusion Machinery. EMBO J. 2019, 38, e99748. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, D.D.L.; Garcia, A.A.; Lee, L.; Mochly-Rosen, D.; Ferreira, J.C.B. Mitochondrial Fusion, Fission, and Mitophagy in Cardiac Diseases: Challenges and Therapeutic Opportunities. Antioxid. Redox Signal. 2022, 36, 844–863. [Google Scholar] [CrossRef]
- Kleele, T.; Rey, T.; Winter, J.; Zaganelli, S.; Mahecic, D.; Perreten Lambert, H.; Ruberto, F.P.; Nemir, M.; Wai, T.; Pedrazzini, T.; et al. Distinct Fission Signatures Predict Mitochondrial Degradation or Biogenesis. Nature 2021, 593, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, R.; Higgs, H.N. Revolutionary View of Two Ways to Split a Mitochondrion. Nature 2021, 593, 346–347. [Google Scholar] [CrossRef] [PubMed]
- Nagdas, S.; Kashatus, D. The Interplay between Oncogenic Signaling Networks and Mitochondrial Dynamics. Antioxidants 2017, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, H.; Li, R.; Mui, D.; Toan, S.; Chang, X.; Zhou, H. DNA-PKcs Interacts with and Phosphorylates Fis1 to Induce Mitochondrial Fragmentation in Tubular Cells during Acute Kidney Injury. Sci. Signal. 2022, 15, eabh1121. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Liao, J.; Bai, T.; Wang, B.; Yangzom, C.; Ahmed, Z.; Mehmood, K.; Abbas, R.Z.; Li, Y.; Tang, Z.; et al. Battery Wastewater Induces Nephrotoxicity via Disordering the Mitochondrial Dynamics. Chemosphere 2022, 303, 135018. [Google Scholar] [CrossRef]
- Kato, M.; Abdollahi, M.; Tunduguru, R.; Tsark, W.; Chen, Z.; Wu, X.; Wang, J.; Chen, Z.B.; Lin, F.-M.; Lanting, L.; et al. MiR-379 Deletion Ameliorates Features of Diabetic Kidney Disease by Enhancing Adaptive Mitophagy via FIS1. Commun. Biol. 2021, 4, 30. [Google Scholar] [CrossRef]
- Qin, X.; Zhao, Y.; Gong, J.; Huang, W.; Su, H.; Yuan, F.; Fang, K.; Wang, D.; Li, J.; Zou, X.; et al. Berberine Protects Glomerular Podocytes via Inhibiting Drp1-Mediated Mitochondrial Fission and Dysfunction. Theranostics 2019, 9, 1698–1713. [Google Scholar] [CrossRef]
- Ferreira-da-Silva, A.; Valacca, C.; Rios, E.; Pópulo, H.; Soares, P.; Sobrinho-Simões, M.; Scorrano, L.; Máximo, V.; Campello, S. Mitochondrial Dynamics Protein Drp1 Is Overexpressed in Oncocytic Thyroid Tumors and Regulates Cancer Cell Migration. PLoS ONE 2015, 10, e0122308. [Google Scholar] [CrossRef] [PubMed]
- Tsuyoshi, H.; Orisaka, M.; Fujita, Y.; Asare-Werehene, M.; Tsang, B.K.; Yoshida, Y. Prognostic Impact of Dynamin Related Protein 1 (Drp1) in Epithelial Ovarian Cancer. BMC Cancer 2020, 20, 467. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.M.; Min, K.; Kwon, T.K. Inhibition of Drp1 Sensitizes Cancer Cells to Cisplatin-Induced Apoptosis through Transcriptional Inhibition of c-FLIP Expression. Molecules 2020, 25, 5793. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Yang, M.; Wu, G.; Mo, S.; Wu, X.; Zhang, S.; Yu, R.; Hu, Y.; Xu, Y.; Li, Z.; et al. Epigenetic Induction of Mitochondrial Fission Is Required for Maintenance of Liver Cancer–Initiating Cells. Cancer Res. 2021, 81, 3835–3848. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; McIntyre, R.L.; Janssens, G.E.; Houtkooper, R.H. Mitochondrial Fission and Fusion: A Dynamic Role in Aging and Potential Target for Age-Related Disease. Mech. Ageing Dev. 2020, 186, 111212. [Google Scholar] [CrossRef]
- Chen, H.; Detmer, S.A.; Ewald, A.J.; Griffin, E.E.; Fraser, S.E.; Chan, D.C. Mitofusins Mfn1 and Mfn2 Coordinately Regulate Mitochondrial Fusion and Are Essential for Embryonic Development. J. Cell Biol. 2003, 160, 189–200. [Google Scholar] [CrossRef]
- Sebastián, D.; Hernández-Alvarez, M.I.; Segalés, J.; Sorianello, E.; Muñoz, J.P.; Sala, D.; Waget, A.; Liesa, M.; Paz, J.C.; Gopalacharyulu, P.; et al. Mitofusin 2 (Mfn2) Links Mitochondrial and Endoplasmic Reticulum Function with Insulin Signaling and Is Essential for Normal Glucose Homeostasis. Proc. Natl. Acad. Sci. USA 2012, 109, 5523–5528. [Google Scholar] [CrossRef]
- Chen, H.; McCaffery, J.M.; Chan, D.C. Mitochondrial Fusion Protects against Neurodegeneration in the Cerebellum. Cell 2007, 130, 548–562. [Google Scholar] [CrossRef]
- Zhao, T.; Huang, X.; Han, L.; Wang, X.; Cheng, H.; Zhao, Y.; Chen, Q.; Chen, J.; Cheng, H.; Xiao, R.; et al. Central Role of Mitofusin 2 in Autophagosome-Lysosome Fusion in Cardiomyocytes. J. Biol. Chem. 2012, 287, 23615–23625. [Google Scholar] [CrossRef]
- Mourier, A.; Motori, E.; Brandt, T.; Lagouge, M.; Atanassov, I.; Galinier, A.; Rappl, G.; Brodesser, S.; Hultenby, K.; Dieterich, C.; et al. Mitofusin 2 Is Required to Maintain Mitochondrial Coenzyme Q Levels. J. Cell Biol. 2015, 208, 429–442. [Google Scholar] [CrossRef]
- Liesa, M.; Borda-d’Água, B.; Medina-Gómez, G.; Lelliott, C.J.; Paz, J.C.; Rojo, M.; Palacín, M.; Vidal-Puig, A.; Zorzano, A. Mitochondrial Fusion Is Increased by the Nuclear Coactivator PGC-1β. PLoS ONE 2008, 3, e3613. [Google Scholar] [CrossRef] [PubMed]
- Soriano, F.X.; Liesa, M.; Bach, D.; Chan, D.C.; Palacín, M.; Zorzano, A. Evidence for a Mitochondrial Regulatory Pathway Defined by Peroxisome Proliferator–Activated Receptor-γ Coactivator-1α, Estrogen-Related Receptor-α, and Mitofusin 2. Diabetes 2006, 55, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Olichon, A.; ElAchouri, G.; Baricault, L.; Delettre, C.; Belenguer, P.; Lenaers, G. OPA1 Alternate Splicing Uncouples an Evolutionary Conserved Function in Mitochondrial Fusion from a Vertebrate Restricted Function in Apoptosis. Cell Death Differ. 2007, 14, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Gilkerson, R.; De La Torre, P.; St. Vallier, S. Mitochondrial OMA1 and OPA1 as Gatekeepers of Organellar Structure/Function and Cellular Stress Response. Front. Cell Dev. Biol. 2021, 9, 626117. [Google Scholar] [CrossRef]
- Ban, T.; Ishihara, T.; Kohno, H.; Saita, S.; Ichimura, A.; Maenaka, K.; Oka, T.; Mihara, K.; Ishihara, N. Molecular Basis of Selective Mitochondrial Fusion by Heterotypic Action between OPA1 and Cardiolipin. Nat. Cell Biol. 2017, 19, 856–863. [Google Scholar] [CrossRef]
- Stephan, T.; Brüser, C.; Deckers, M.; Steyer, A.M.; Balzarotti, F.; Barbot, M.; Behr, T.S.; Heim, G.; Hübner, W.; Ilgen, P.; et al. MICOS Assembly Controls Mitochondrial Inner Membrane Remodeling and Crista Junction Redistribution to Mediate Cristae Formation. EMBO J. 2020, 39, e104105. [Google Scholar] [CrossRef]
- Zhao, S.; Heng, N.; Wang, H.; Wang, H.; Zhang, H.; Gong, J.; Hu, Z.; Zhu, H. Mitofusins: From Mitochondria to Fertility. Cell Mol. Life Sci. 2022, 79, 370. [Google Scholar] [CrossRef]
- Sèdes, L.; Thirouard, L.; Maqdasy, S.; Garcia, M.; Caira, F.; Lobaccaro, J.-M.A.; Beaudoin, C.; Volle, D.H. Cholesterol: A Gatekeeper of Male Fertility? Front. Endocrinol. 2018, 9, 369. [Google Scholar] [CrossRef]
- Steinfeld, K.; Beyer, D.; Mühlfeld, C.; Mietens, A.; Eichner, G.; Altinkilic, B.; Kampschulte, M.; Jiang, Q.; Krombach, G.A.; Linn, T.; et al. Low Testosterone in ApoE/LDL Receptor Double-Knockout Mice Is Associated with Rarefied Testicular Capillaries Together with Fewer and Smaller Leydig Cells. Sci. Rep. 2018, 8, 5424. [Google Scholar] [CrossRef]
- Wasilewski, M.; Semenzato, M.; Rafelski, S.M.; Robbins, J.; Bakardjiev, A.I.; Scorrano, L. Optic Atrophy 1-Dependent Mitochondrial Remodeling Controls Steroidogenesis in Trophoblasts. Curr. Biol. 2012, 22, 1228–1234. [Google Scholar] [CrossRef]
- Rone, M.B.; Midzak, A.S.; Issop, L.; Rammouz, G.; Jagannathan, S.; Fan, J.; Ye, X.; Blonder, J.; Veenstra, T.; Papadopoulos, V. Identification of a Dynamic Mitochondrial Protein Complex Driving Cholesterol Import, Trafficking, and Metabolism to Steroid Hormones. Mol. Endocrinol. 2012, 26, 1868–1882. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, Y.; Zhao, C.; Miao, Y.; Cai, J.; Song, L.; Wei, J.; Chakraborty, T.; Wu, L.; Wang, D.; et al. The Role of StAR2 Gene in Testicular Differentiation and Spermatogenesis in Nile Tilapia (Oreochromis niloticus). J. Steroid Biochem. Mol. Biol. 2021, 214, 105974. [Google Scholar] [CrossRef] [PubMed]
- Galano, M.; Papadopoulos, V. Role of Constitutive STAR in Mitochondrial Structure and Function in MA-10 Leydig Cells. Endocrinology 2022, 163, bqac091. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.; Orlando, U.; Maloberti, P.; Podestá, E.J.; Cornejo Maciel, F. Tyrosine Phosphatase SHP2 Regulates the Expression of Acyl-CoA Synthetase ACSL4. J. Lipid Res. 2011, 52, 1936–1948. [Google Scholar] [CrossRef]
- Duarte, A.; Castillo, A.F.; Podestá, E.J.; Poderoso, C. Mitochondrial Fusion and ERK Activity Regulate Steroidogenic Acute Regulatory Protein Localization in Mitochondria. PLoS ONE 2014, 9, e100387. [Google Scholar] [CrossRef]
- Medar, M.L.J.; Marinkovic, D.Z.; Kojic, Z.; Becin, A.P.; Starovlah, I.M.; Kravic-Stevovic, T.; Andric, S.A.; Kostic, T.S. Dependence of Leydig Cell’s Mitochondrial Physiology on Luteinizing Hormone Signaling. Life 2020, 11, 19. [Google Scholar] [CrossRef]
- Shan, A.; Li, M.; Li, X.; Li, Y.; Yan, M.; Xian, P.; Chang, Y.; Chen, X.; Tang, N.-J. BDE-47 Decreases Progesterone Levels in BeWo Cells by Interfering with Mitochondrial Functions and Genes Related to Cholesterol Transport. Chem. Res. Toxicol. 2019, 32, 621–628. [Google Scholar] [CrossRef]
- Han, X.; Tang, R.; Chen, X.; Xu, B.; Qin, Y.; Wu, W.; Hu, Y.; Xu, B.; Song, L.; Xia, Y.; et al. 2,2’,4,4’-Tetrabromodiphenyl Ether (BDE-47) Decreases Progesterone Synthesis through CAMP-PKA Pathway and P450scc Downregulation in Mouse Leydig Tumor Cells. Toxicology 2012, 302, 44–50. [Google Scholar] [CrossRef]
- Brzoskwinia, M.; Pardyak, L.; Kaminska, A.; Tworzydlo, W.; Hejmej, A.; Marek, S.; Bilinski, S.M.; Bilinska, B. Flutamide Treatment Reveals a Relationship between Steroidogenic Activity of Leydig Cells and Ultrastructure of Their Mitochondria. Sci. Rep. 2021, 11, 13772. [Google Scholar] [CrossRef]
- Wang, X.; Wen, Y.; Zhang, J.; Swanson, G.; Guo, S.; Cao, C.; Krawetz, S.A.; Zhang, Z.; Yuan, S. MFN2 Interacts with Nuage-Associated Proteins and Is Essential for Male Germ Cell Development by Controlling MRNA Fate during Spermatogenesis. Development 2021, 148, dev196295. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Wang, M.; Jiang, M.; Wang, Y.; Sun, Y.; Wang, J.; Xie, T.; Tang, C.; Tang, N.; et al. GASZ and Mitofusin-Mediated Mitochondrial Functions Are Crucial for Spermatogenesis. EMBO Rep. 2016, 17, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Sun, Y.; Sun, Q.; Zhang, J.; Jiang, M.; Chang, C.; Huang, X.; Wang, C.; Wang, P.; Zhang, Z.; et al. MFN2 Plays a Distinct Role from MFN1 in Regulating Spermatogonial Differentiation. Stem Cell Rep. 2020, 14, 803–817. [Google Scholar] [CrossRef] [PubMed]
- Varuzhanyan, G.; Rojansky, R.; Sweredoski, M.J.; Graham, R.L.J.; Hess, S.; Ladinsky, M.S.; Chan, D.C. Mitochondrial Fusion Is Required for Spermatogonial Differentiation and Meiosis. Elife 2019, 8, e51601. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Chen, W.; Wang, P.; Zhang, X.; Wang, L.; Wang, S.; Wang, Y. MFN1 and MFN2 Are Dispensable for Sperm Development and Functions in Mice. Int. J. Mol. Sci. 2021, 22, 13507. [Google Scholar] [CrossRef] [PubMed]
- Sênos Demarco, R.; Jones, D.L. Mitochondrial Fission Regulates Germ Cell Differentiation by Suppressing ROS-Mediated Activation of Epidermal Growth Factor Signaling in the Drosophila Larval Testis. Sci. Rep. 2019, 9, 19695. [Google Scholar] [CrossRef]
- Varuzhanyan, G.; Ladinsky, M.S.; Yamashita, S.-I.; Abe, M.; Sakimura, K.; Kanki, T.; Chan, D.C. Fis1 Ablation in the Male Germline Disrupts Mitochondrial Morphology and Mitophagy, and Arrests Spermatid Maturation. Development 2021, 148, dev199686. [Google Scholar] [CrossRef] [PubMed]
- Varuzhanyan, G.; Chen, H.; Rojansky, R.; Ladinsky, M.S.; McCaffery, J.M.; Chan, D.C. Mitochondrial Fission Factor (Mff) Is Required for Organization of the Mitochondrial Sheath in Spermatids. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129845. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xiao, Y.; Hu, Z.; Gu, J.; Hua, R.; Hai, Z.; Chen, X.; Zhang, J.V.; Yu, Z.; Wu, T.; et al. MFN2 Deficiency Impairs Mitochondrial Functions and PPAR Pathway During Spermatogenesis and Meiosis in Mice. Front. Cell Dev. Biol. 2022, 10, 862506. [Google Scholar] [CrossRef]
- Miret-Casals, L.; Sebastián, D.; Brea, J.; Rico-Leo, E.M.; Palacín, M.; Fernández-Salguero, P.M.; Loza, M.I.; Albericio, F.; Zorzano, A. Identification of New Activators of Mitochondrial Fusion Reveals a Link between Mitochondrial Morphology and Pyrimidine Metabolism. Cell Chem. Biol. 2018, 25, 268–278.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dang, X.; Franco, A.; Zhao, H.; Dorn, G.W. Piperine Derivatives Enhance Fusion and Axonal Transport of Mitochondria by Activating Mitofusins. Chemistry 2022, 4, 655–668. [Google Scholar] [CrossRef]
- Franco, A.; Dang, X.; Zhang, L.; Molinoff, P.B.; Dorn, G.W. Mitochondrial Dysfunction and Pharmacodynamics of Mitofusin Activation in Murine Charcot-Marie-Tooth Disease Type 2A. J. Pharmacol. Exp. Ther. 2022, 383, 137–148. [Google Scholar] [CrossRef] [PubMed]

| Period of Life | hGC Serum Levels (UI/L) | References | |
|---|---|---|---|
| Gestation | 0–1 week | 0–50 | [25] |
| 1–2 weeks | 40–300 | ||
| 3–4 weeks | 500–600 | ||
| 1–2 months | 5000–200,000 | ||
| 2–3 months | 10,000–100,000 | ||
| second trimester | 3000–50,000 | ||
| third trimester | 1000–50,000 |
| Period of Life | LH Serum Levels (UI/L) | References | |
|---|---|---|---|
| Gestation | 0–1 weeks | no detectable | [51] |
| 1–2 weeks | |||
| 3–4 weeks | |||
| 1–2 months | |||
| 2–3 months | 20 | [52] | |
| second trimester | 26.1 | ||
| third trimester | <second trimester | ||
| Birth | |||
| Infancy “mini-puberty” | after 2 days of birth | 0.21 | [53] |
| after 7 days of birth | 3.94 | ||
| after 10 days of birth | 4.81 | ||
| after 20 days of birth | 2.67 | ||
| Childhood | 1–10 years of life | no detectable | [49] |
| Puberty | Tanner stage I | 0.02–0.42 | [54] |
| Tanner stage II | 0.26–4.84 | ||
| Tanner stage III | 0.64–3.74 | ||
| Tanner stage IV | 0.55–7.15 | ||
| Tanner stage V | 1.7–8.6 | ||
| Adulthood | 18–30 years of life | 1.8–8.6 | [50] |
| 50 years of life | 2.1–10.4 | ||
| 70 years of life | 2.22–11.2 |
| Protein | Biological Effect | Physiological Effect | Model/Cell Type | References |
|---|---|---|---|---|
| DRP1 | Maintenance of spermatogonia | spermatogenesis | Spermatogonial cells (Drosophila) | [149] |
| FIS1 | Regulation of spermatid maturation | spermatogenesis | Conditional mouse model | [150] |
| MFF | Determination of mitochondrial sheath | spermatogenesis | Mutant mouse model | [151] |
| MNF1 | Regulation of mitochondrial fusion in neonatal pro-spermatogonia | spermatogenesis | K.O. mouse | [146] |
| MNF1-MNF2 interaction | Support of mitochondrial distribution in the testes | spermatogenesis | Postnatal male germ cells (mouse) | [144] |
| MNF2 | Support of lipid droplet-mitochondria contact | steroidogenesis | BeWo cells (human) | [134] |
| Increment of progesterone production and CYP11A1 expression | steroidogenesis | BeWo (human), mLTC-1 (mouse) cells | [141,142] | |
| Support of spermatogenesis and filling of seminiferous tubes | spermatogenesis | Conditional K.O. mouse model | [152] | |
| Regulation of mitochondrial fusion late steps of spermatogenesis | spermatogenesis | K.O. mouse | [146] | |
| SHP2 | Support of mitochondrial fusion | steroidogenesis | MA-10 cells (mouse) | [138] |
| Regulation of ERK1/2 localization in the mitochondria | steroidogenesis | MA-10 cells (mouse) | [95] | |
| StAR | Conferment of mitochondria and cristae proper conformation | steroidogenesis | MA-10 cells (mouse) | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Errico, A.; Vinco, S.; Ambrosini, G.; Dalla Pozza, E.; Marroncelli, N.; Zampieri, N.; Dando, I. Mitochondrial Dynamics as Potential Modulators of Hormonal Therapy Effectiveness in Males. Biology 2023, 12, 547. https://doi.org/10.3390/biology12040547
Errico A, Vinco S, Ambrosini G, Dalla Pozza E, Marroncelli N, Zampieri N, Dando I. Mitochondrial Dynamics as Potential Modulators of Hormonal Therapy Effectiveness in Males. Biology. 2023; 12(4):547. https://doi.org/10.3390/biology12040547
Chicago/Turabian StyleErrico, Andrea, Sara Vinco, Giulia Ambrosini, Elisa Dalla Pozza, Nunzio Marroncelli, Nicola Zampieri, and Ilaria Dando. 2023. "Mitochondrial Dynamics as Potential Modulators of Hormonal Therapy Effectiveness in Males" Biology 12, no. 4: 547. https://doi.org/10.3390/biology12040547
APA StyleErrico, A., Vinco, S., Ambrosini, G., Dalla Pozza, E., Marroncelli, N., Zampieri, N., & Dando, I. (2023). Mitochondrial Dynamics as Potential Modulators of Hormonal Therapy Effectiveness in Males. Biology, 12(4), 547. https://doi.org/10.3390/biology12040547








