Reciprocal Interactions between Circadian Clocks, Food Intake, and Energy Metabolism
Abstract
Simple Summary
Abstract
1. Circadian Rhythmicity
1.1. The Circadian Multi-Oscillatory System
1.2. Molecular Machinery
1.3. Synchronization of the Master Clock by Light
2. Effects of Circadian Rhythms on Metabolism
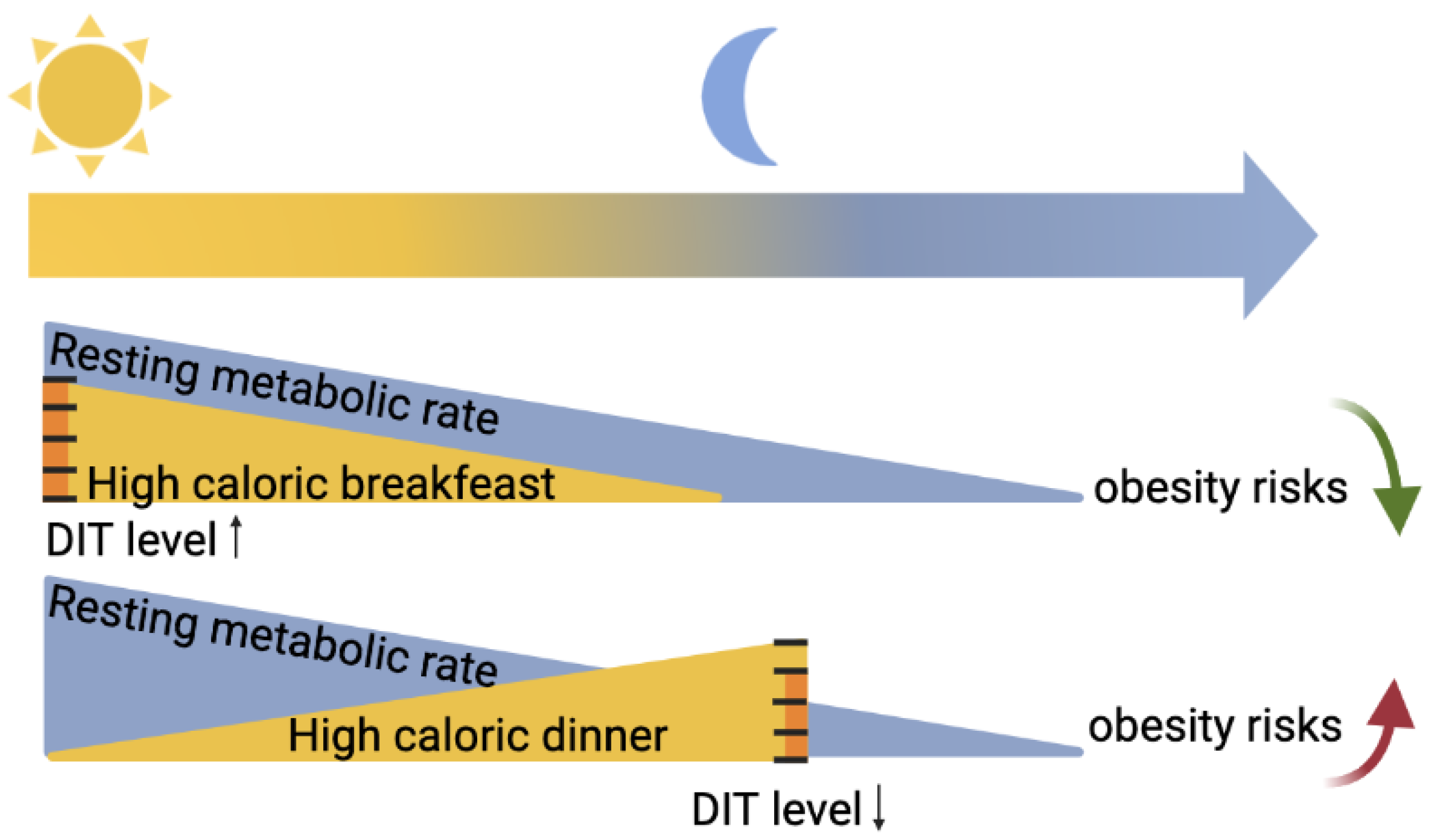
2.1. Circadian Rhythmicity in Energy Metabolism
2.2. Circadian Rhythmicity in Food Intake
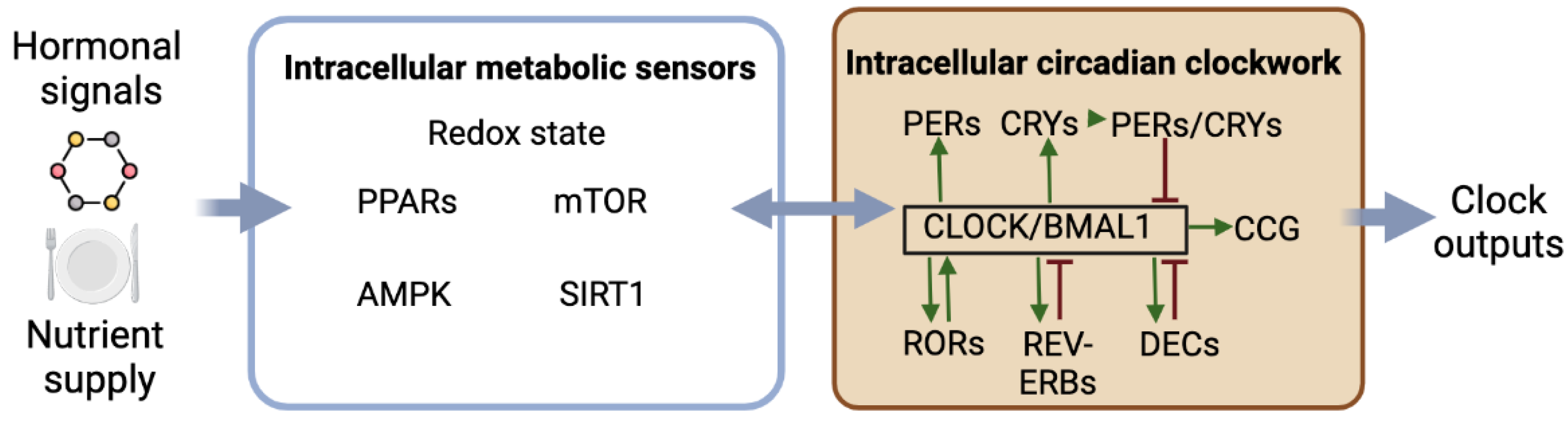
2.3. Interactions between the Circadian Clockwork and Intracellular Metabolism
2.4. Circadian Disruption Affects Metabolism
2.4.1. Genetic Causes
2.4.2. Mistimed Light Exposure
2.4.3. Mistimed Sleep/Wake Cycle
2.4.4. Mistimed Eating
3. Effects of Metabolism on Circadian Rhythms
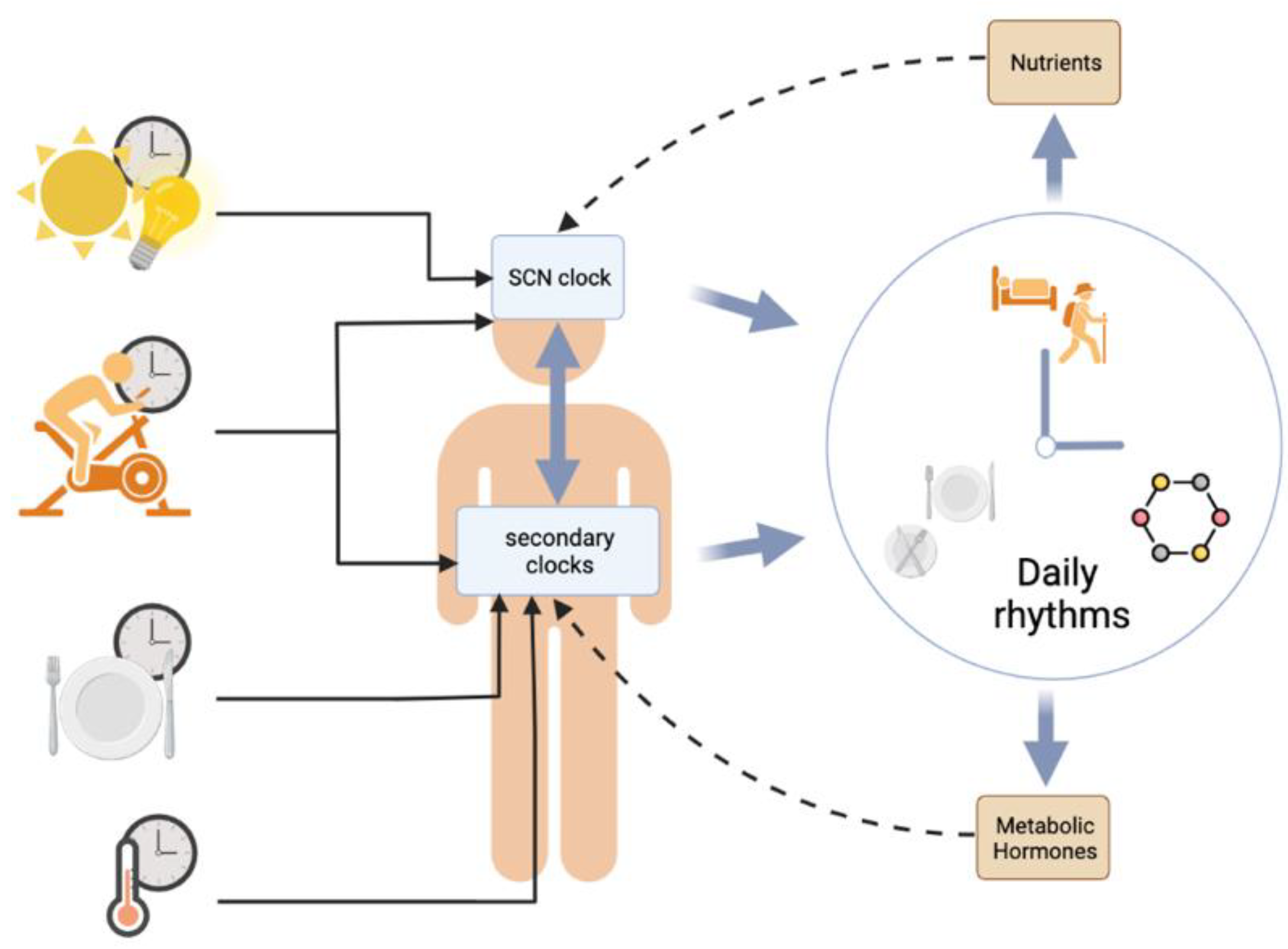
3.1. Synchronization of Circadian Clocks by Metabolic Cues
3.1.1. Synchronization of the SCN Clock
3.1.2. Synchronization of the Peripheral Clocks
3.2. Metabolic Disturbances Affect Circadian Rhythmicity
3.2.1. Calorie Restriction and Negative Energy Balance
3.2.2. Obesity and Positive Energy Balance
3.2.3. Diabetes
4. Chrononutrition and Metabolic Health
4.1. Time Slot and Regularity
4.2. Shortening of the Daily Period of Eating
4.3. Chronotypes
4.4. Sex Differences
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Golombek, D.A.; Rosenstein, R.E. Physiology of circadian entrainment. Physiol. Rev. 2010, 90, 1063–1102. [Google Scholar] [CrossRef] [PubMed]
- Okamura, H.; Yamaguchi, S.; Yagita, K. Molecular machinery of the circadian clock in mammals. Cell Tissue Res. 2002, 309, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Partch, C.L.; Green, C.B.; Takahashi, J.S. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014, 24, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Narasimamurthy, R.; Virshup, D.M. Molecular Mechanisms Regulating Temperature Compensation of the Circadian Clock. Front. Neurol. 2017, 8, 161. [Google Scholar] [CrossRef]
- Kumar Jha, P.; Challet, E.; Kalsbeek, A. Circadian rhythms in glucose and lipid metabolism in nocturnal and diurnal mammals. Mol. Cell Endocrinol. 2015, 418 Pt 1, 74–88. [Google Scholar] [CrossRef]
- Wyse, J.P.; Mercer, T.H.; Gleeson, N.P. Time-of-day dependence of isokinetic leg strength and associated interday variability. Br. J. Sports Med. 1994, 28, 167–170. [Google Scholar] [CrossRef]
- Moussay, S.; Dosseville, F.; Gauthier, A.; Larue, J.; Sesboüe, B.; Davenne, D. Circadian rhythms during cycling exercise and finger-tapping task. Chronobiol. Int. 2002, 19, 1137–1149. [Google Scholar] [CrossRef]
- Souissi, N.; Gauthier, A.; Sesboüé, B.; Larue, J.; Davenne, D. Circadian rhythms in two types of anaerobic cycle leg exercise: Force-velocity and 30-s Wingate tests. Int. J. Sports Med. 2004, 25, 14–19. [Google Scholar]
- Waterhouse, J.; Drust, B.; Weinert, D.; Edwards, B.; Gregson, W.; Atkinson, G.; Kao, S.; Aizawa, S.; Reilly, T. The circadian rhythm of core temperature: Origin and some implications for exercise performance. Chronobiol. Int. 2005, 22, 207–225. [Google Scholar] [CrossRef]
- Gabriel, B.M.; Zierath, J.R. Circadian rhythms and exercise—Re-setting the clock in metabolic disease. Nat. Rev. Endocrinol. 2019, 15, 197–206. [Google Scholar] [CrossRef]
- Aoyama, S.; Shibata, S. Time-of-Day-Dependent Physiological Responses to Meal and Exercise. Front. Nutr. 2020, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Zitting, K.-M.; Vujovic, N.; Yuan, R.K.; Isherwood, C.M.; Medina, J.E.; Wang, W.; Buxton, O.M.; Williams, J.S.; Czeisler, C.A.; Duffy, J.F. Human Resting Energy Expenditure Varies with Circadian Phase. Curr. Biol. 2018, 28, 3685–3690.e3. [Google Scholar] [CrossRef] [PubMed]
- Ruddick-Collins, L.C.; Johnston, J.D.; Morgan, P.J.; Johnstone, A.M. The Big Breakfast Study: Chrono-nutrition influence on energy expenditure and bodyweight. Nutr. Bull. 2018, 43, 174–183. [Google Scholar] [CrossRef]
- Richter, J.; Herzog, N.; Janka, S.; Baumann, T.; Kistenmacher, A.; Oltmanns, K.M. Twice as High Diet-Induced Thermogenesis after Breakfast vs Dinner on High-Calorie as well as Low-Calorie Meals. J. Clin. Endocrinol. Metab. 2020, 105, dgz311. [Google Scholar] [CrossRef] [PubMed]
- Romon, M.; Edme, J.L.; Boulenguez, C.; Lescroart, J.L.; Frimat, P. Circadian variation of diet-induced thermogenesis. Am. J. Clin. Nutr. 1993, 57, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.J.; Garcia, J.I.; Myers, S.; Yang, J.N.; Trienekens, N.; Scheer, F.A.J.L. The Human Circadian System Has a Dominating Role in Causing the Morning/Evening Difference in Diet-Induced Thermogenesis. Obesity 2015, 23, 2053–2058. [Google Scholar] [CrossRef]
- Ruddick-Collins, L.C.; Flanagan, A.; Johnston, J.D.; Morgan, P.J.; Johnstone, A.M. Circadian Rhythms in Resting Metabolic Rate Account for Apparent Daily Rhythms in the Thermic Effect of Food. J. Clin. Endocrinol. Metab. 2022, 107, e708–e715. [Google Scholar] [CrossRef]
- Johnston, J.D.; Ordovás, J.M.; Scheer, F.A.; Turek, F.W. Circadian Rhythms, Metabolism, and Chrononutrition in Rodents and Humans. Adv. Nutr. 2016, 7, 399–406. [Google Scholar] [CrossRef]
- Ruddick-Collins, L.C.; Morgan, P.J.; Johnstone, A.M. Mealtime: A circadian disruptor and determinant of energy balance? J. Neuroendocrinol. 2020, 32, e12886. [Google Scholar] [CrossRef]
- Jais, A.; Brüning, J.C. Arcuate Nucleus-Dependent Regulation of Metabolism-Pathways to Obesity and Diabetes Mellitus. Endocr. Rev. 2022, 43, 314–328. [Google Scholar] [CrossRef]
- Aschoff, J.; von Goetz, C.; Wildgruber, C.; Wever, R.A. Meal timing in humans during isolation without time cues. J. Biol. Rhythms 1986, 1, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Estrada, D.; Aguilar-Roblero, R.; Alva-Sánchez, C.; Villanueva, I. The homeostatic feeding response to fasting is under chronostatic control. Chronobiol. Int. 2018, 35, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Sargent, C.; Zhou, X.; Matthews, R.W.; Darwent, D.; Roach, G.D. Daily Rhythms of Hunger and Satiety in Healthy Men during One Week of Sleep Restriction and Circadian Misalignment. Int. J. Environ. Res. Public Health 2016, 13, 170. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.J.L.; Morris, C.J.; Shea, S.A. The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity 2013, 21, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Rynders, C.A.; Morton, S.J.; Bessesen, D.H.; Wright, K.P., Jr.; Broussard, J.L. Circadian Rhythm of Substrate Oxidation and Hormonal Regulators of Energy Balance. Obesity 2020, 28, S104–S113. [Google Scholar] [CrossRef]
- Nagai, K.; Nishio, T.; Nakagawa, H.; Nakamura, S.; Fukuda, Y. Effect of bilateral lesions of the suprachiasmatic nuclei on the circadian rhythm of food-intake. Brain Res. 1978, 142, 384–389. [Google Scholar] [CrossRef]
- Challet, E. The circadian regulation of food intake. Nat. Rev. Endocrinol. 2019, 15, 393–405. [Google Scholar] [CrossRef]
- Cedernaes, J.; Huang, W.; Ramsey, K.M.; Waldeck, N.; Cheng, L.; Marcheva, B.; Omura, C.; Kobayashi, Y.; Peek, C.B.; Levine, D.C.; et al. Transcriptional Basis for Rhythmic Control of Hunger and Metabolism within the AgRP Neuron. Cell Metab. 2019, 29, 1078–1091.e5. [Google Scholar] [CrossRef]
- Kim, E.R.; Xu, Y.; Cassidy, R.M.; Lu, Y.; Yang, Y.; Tian, J.; Li, D.-P.; Van Drunen, R.; Ribas-Latre, A.; Cai, Z.-L.; et al. Paraventricular hypothalamus mediates diurnal rhythm of metabolism. Nat. Commun. 2020, 11, 3794. [Google Scholar] [CrossRef]
- Reichelt, A.C.; Killcross, S.; Hambly, L.D.; Morris, M.J.; Westbrook, R.F. Impact of adolescent sucrose access on cognitive control, recognition memory, and parvalbumin immunoreactivity. Learn. Mem. 2015, 22, 215–224. [Google Scholar] [CrossRef]
- Koch, C.E.; Begemann, K.; Kiehn, J.T.; Griewahn, L.; Mauer, J.; Hess, M.E.; Moser, A.; Schmid, S.M.; Brüning, J.C.; Oster, H. Circadian regulation of hedonic appetite in mice by clocks in dopaminergic neurons of the VTA. Nat. Commun. 2020, 11, 3071. [Google Scholar] [CrossRef] [PubMed]
- Raspé, E.; Duez, H.; Gervois, P.; Fiévet, C.; Fruchart, J.C.; Besnard, S.; Mariani, J.; Tedgui, A.; Staels, B. Transcriptional regulation of apolipoprotein C-III gene expression by the orphan nuclear receptor RORalpha. J. Biol. Chem. 2001, 276, 2865–2871. [Google Scholar] [CrossRef] [PubMed]
- Lau, P.; Nixon, S.J.; Parton, R.G.; Muscat, G.E.O. RORalpha regulates the expression of genes involved in lipid homeostasis in skeletal muscle cells: Caveolin-3 and CPT-1 are direct targets of ROR. J. Biol. Chem. 2004, 279, 36828–36840. [Google Scholar] [CrossRef]
- Ramakrishnan, S.N.; Lau, P.; Burke, L.J.; Muscat, G.E.O. Rev-erbbeta regulates the expression of genes involved in lipid absorption in skeletal muscle cells: Evidence for cross-talk between orphan nuclear receptors and myokines. J. Biol. Chem. 2005, 280, 8651–8659. [Google Scholar] [CrossRef]
- Doi, M.; Hirayama, J.; Sassone-Corsi, P. Circadian regulator CLOCK is a histone acetyltransferase. Cell 2006, 125, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Nader, N.; Chrousos, G.P.; Kino, T. Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: Potential physiological implications. FASEB J. 2009, 23, 1572–1583. [Google Scholar] [CrossRef]
- Canaple, L.; Rambaud, J.; Dkhissi-Benyahya, O.; Rayet, B.; Tan, N.S.; Michalik, L.; Delaunay, F.; Wahli, W.; Laudet, V. Reciprocal regulation of brain and muscle Arnt-like protein 1 and peroxisome proliferator-activated receptor alpha defines a novel positive feedback loop in the rodent liver circadian clock. Mol. Endocrinol. 2006, 20, 1715–1727. [Google Scholar] [CrossRef]
- Schmutz, I.; Ripperger, J.A.; Baeriswyl-Aebischer, S.; Albrecht, U. The mammalian clock component PERIOD2 coordinates circadian output by interaction with nuclear receptors. Genes Dev. 2010, 24, 345–357. [Google Scholar] [CrossRef]
- Grimaldi, B.; Bellet, M.M.; Katada, S.; Astarita, G.; Hirayama, J.; Amin, R.H.; Granneman, J.G.; Piomelli, D.; Leff, T.; Sassone-Corsi, P. PER2 controls lipid metabolism by direct regulation of PPARγ. Cell Metab. 2010, 12, 509–520. [Google Scholar] [CrossRef]
- Nakahata, Y.; Kaluzova, M.; Grimaldi, B.; Sahar, S.; Hirayama, J.; Chen, D.; Guarente, L.P.; Sassone-Corsi, P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 2008, 134, 329–340. [Google Scholar] [CrossRef]
- Ramsey, K.M.; Yoshino, J.; Brace, C.S.; Abrassart, D.; Kobayashi, Y.; Marcheva, B.; Hong, H.-K.; Chong, J.L.; Buhr, E.D.; Lee, C.; et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 2009, 324, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.A.; Yu, Y.V.; Govindaiah, G.; Ye, X.; Artinian, L.; Coleman, T.P.; Sweedler, J.V.; Cox, C.L.; Gillette, M.U. Circadian rhythm of redox state regulates excitability in suprachiasmatic nucleus neurons. Science 2012, 337, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Asher, G.; Gatfield, D.; Stratmann, M.; Reinke, H.; Dibner, C.; Kreppel, F.; Mostoslavsky, R.; Alt, F.W.; Schibler, U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 2008, 134, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-C.; Guarente, L. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell 2013, 153, 1448–1460. [Google Scholar] [CrossRef] [PubMed]
- Um, J.H.; Yang, S.; Yamazaki, S.; Kang, H.; Viollet, B.; Foretz, M.; Chung, J.H. Activation of 5′-AMP-activated kinase with diabetes drug metformin induces casein kinase Iepsilon (CKIepsilon)-dependent degradation of clock protein mPer2. J. Biol. Chem. 2007, 282, 20794–20798. [Google Scholar] [CrossRef]
- Lamia, K.A.; Sachdeva, U.M.; DiTacchio, L.; Williams, E.C.; Alvarez, J.G.; Egan, D.F.; Vasquez, D.S.; Juguilon, H.; Panda, S.; Shaw, R.J.; et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 2009, 326, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, C.; Kathale, N.D.; Liu, D.; Lee, C.; Freeman, D.A.; Hogenesch, J.B.; Cao, R.; Liu, A.C. mTOR signaling regulates central and peripheral circadian clock function. PLoS Genet. 2018, 14, e1007369. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Li, T.; Han, J.; Wang, Y. The tight junction protein TJP1 regulates the feeding-modulated hepatic circadian clock. Nat. Commun. 2020, 11, 589. [Google Scholar] [CrossRef]
- Martino, T.A.; Oudit, G.Y.; Herzenberg, A.M.; Tata, N.; Koletar, M.M.; Kabir, G.M.; Belsham, D.D.; Backx, P.H.; Ralph, M.R.; Sole, M.J. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1675–R1683. [Google Scholar] [CrossRef]
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005, 308, 1043–1045. [Google Scholar] [CrossRef]
- Delezie, J.; Dumont, S.; Dardente, H.; Oudart, H.; Gréchez-Cassiau, A.; Klosen, P.; Teboul, M.; Delaunay, F.; Pévet, P.; Challet, E. The nuclear receptor REV-ERBα is required for the daily balance of carbohydrate and lipid metabolism. FASEB J. 2012, 26, 3321–3335. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Bradfield, C.A.; Hussain, M.M. Global and hepatocyte-specific ablation of Bmal1 induces hyperlipidaemia and enhances atherosclerosis. Nat. Commun. 2016, 7, 13011. [Google Scholar] [CrossRef] [PubMed]
- Lamia, K.A.; Storch, K.-F.; Weitz, C.J. Physiological significance of a peripheral tissue circadian clock. Proc. Natl. Acad. Sci. USA 2008, 105, 15172–15177. [Google Scholar] [CrossRef]
- Marcheva, B.; Ramsey, K.M.; Buhr, E.D.; Kobayashi, Y.; Su, H.; Ko, C.H.; Ivanova, G.; Omura, C.; Mo, S.; Vitaterna, M.H.; et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 2010, 466, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Woon, P.Y.; Kaisaki, P.J.; Bragança, J.; Bihoreau, M.-T.; Levy, J.C.; Farrall, M.; Gauguier, D. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc. Natl. Acad. Sci. USA 2007, 104, 14412–14417. [Google Scholar] [CrossRef]
- Garaulet, M.; Lee, Y.-C.; Shen, J.; Parnell, L.D.; Arnett, D.K.; Tsai, M.Y.; Lai, C.-Q.; Ordovas, J.M. CLOCK genetic variation and metabolic syndrome risk: Modulation by monounsaturated fatty acids. Am. J. Clin. Nutr. 2009, 90, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Ruano, E.G.; Canivell, S.; Vieira, E. REV-ERB ALPHA polymorphism is associated with obesity in the Spanish obese male population. PLoS ONE 2014, 9, e104065. [Google Scholar] [CrossRef]
- Kovanen, L.; Donner, K.; Kaunisto, M.; Partonen, T. CRY1, CRY2 and PRKCDBP genetic variants in metabolic syndrome. Hypertens Res. 2015, 38, 186–192. [Google Scholar] [CrossRef]
- Škrlec, I.; Talapko, J.; Džijan, S.; Cesar, V.; Lazić, N.; Lepeduš, H. The Association between Circadian Clock Gene Polymorphisms and Metabolic Syndrome: A Systematic Review and Meta-Analysis. Biology 2021, 11, 20. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, L.; Gao, Y.; Jiang, L.; Yuan, L.; Wang, P.; Cao, Y.; Song, X.; Ge, L.; Ding, G. Association between shift work or long working hours with metabolic syndrome: A systematic review and dose-response meta-analysis of observational studies. Chronobiol. Int. 2021, 38, 318–333. [Google Scholar] [CrossRef]
- Khosravipour, M.; Khanlari, P.; Khazaie, S.; Khosravipour, H.; Khazaie, H. A systematic review and meta-analysis of the association between shift work and metabolic syndrome: The roles of sleep, gender, and type of shift work. Sleep Med. Rev. 2021, 57, 101427. [Google Scholar] [CrossRef] [PubMed]
- Sooriyaarachchi, P.; Jayawardena, R.; Pavey, T.; King, N.A. Shift work and the risk for metabolic syndrome among healthcare workers: A systematic review and meta-analysis. Obes. Rev. 2022, 23, e13489. [Google Scholar] [CrossRef] [PubMed]
- Bartol-Munier, I.; Gourmelen, S.; Pevet, P.; Challet, E. Combined effects of high-fat feeding and circadian desynchronization. Int. J. Obes. 2006, 30, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.-L.; Tsai, Y.-C.; Hwang, K.; Huang, Y.-W.; Tzeng, J.-E. Repeated light-dark shifts speed up body weight gain in male F344 rats. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E212–E217. [Google Scholar] [CrossRef] [PubMed]
- Grosbellet, E.; Zahn, S.; Arrivé, M.; Dumont, S.; Gourmelen, S.; Pévet, P.; Challet, E.; Criscuolo, F. Circadian desynchronization triggers premature cellular aging in a diurnal rodent. FASEB J. 2015, 29, 4794–4803. [Google Scholar] [CrossRef]
- Opperhuizen, A.-L.; Stenvers, D.J.; Jansen, R.D.; Foppen, E.; Fliers, E.; Kalsbeek, A. Light at night acutely impairs glucose tolerance in a time-, intensity- and wavelength-dependent manner in rats. Diabetologia 2017, 60, 1333–1343. [Google Scholar] [CrossRef]
- Okuliarova, M.; Rumanova, V.S.; Stebelova, K.; Zeman, M. Dim Light at Night Disturbs Molecular Pathways of Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6919. [Google Scholar] [CrossRef]
- Rumanova, V.S.; Okuliarova, M.; Foppen, E.; Kalsbeek, A.; Zeman, M. Exposure to dim light at night alters daily rhythms of glucose and lipid metabolism in rats. Front. Physiol. 2022, 13, 973461. [Google Scholar] [CrossRef]
- Gutiérrez-Pérez, M.; González-González, S.; Estrada-Rodriguez, K.P.; Espítia-Bautista, E.; Guzmán-Ruiz, M.A.; Escalona, R.; Escobar, C.; Guerrero-Vargas, N.N. Dim Light at Night Promotes Circadian Disruption in Female Rats, at the Metabolic, Reproductive, and Behavioral Level. Adv. Biol. 2023, e2200289. [Google Scholar] [CrossRef]
- Obayashi, K.; Yamagami, Y.; Tatsumi, S.; Kurumatani, N.; Saeki, K. Indoor light pollution and progression of carotid atherosclerosis: A longitudinal study of the HEIJO-KYO cohort. Environ. Int. 2019, 133, 105184. [Google Scholar] [CrossRef]
- Morris, C.J.; Purvis, T.E.; Hu, K.; Scheer, F.A.J.L. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc. Natl. Acad. Sci. USA 2016, 113, E1402–E1411. [Google Scholar] [CrossRef]
- Leproult, R.; Holmbäck, U.; Van Cauter, E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 2014, 63, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.J.L.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Delgado, R.; Angeles-Castellanos, M.; Buijs, M.R.; Escobar, C. Internal desynchronization in a model of night-work by forced activity in rats. Neuroscience 2008, 154, 922–931. [Google Scholar] [CrossRef]
- Roenneberg, T.; Allebrandt, K.V.; Merrow, M.; Vetter, C. Social jetlag and obesity. Curr. Biol. 2012, 22, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.C.; Silva, C.M.; Balieiro, L.C.T.; Gonçalves, B.F.; Fahmy, W.M.; Crispim, C.A. Association between social jetlag food consumption and meal times in patients with obesity-related chronic diseases. PLoS ONE 2019, 14, e0212126. [Google Scholar] [CrossRef] [PubMed]
- Bray, M.S.; Ratcliffe, W.F.; Grenett, M.H.; Brewer, R.A.; Gamble, K.L.; Young, M.E. Quantitative analysis of light-phase restricted feeding reveals metabolic dyssynchrony in mice. Int. J. Obes. 2013, 37, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, Y.; Hashimoto, C.; Nakao, R.; Yamazaki, H.; Hiroyama, H.; Nemoto, T.; Yamamoto, S.; Sakurai, M.; Oike, H.; Wada, N.; et al. Short-term feeding at the wrong time is sufficient to desynchronize peripheral clocks and induce obesity with hyperphagia, physical inactivity and metabolic disorders in mice. Metabolism 2016, 65, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, A.; Aoki, N.; Ohtsu, T.; Ikeda, Y.; Tahara, Y.; Shibata, S. Controlling access time to a high-fat diet during the inactive period protects against obesity in mice. Chronobiol. Int. 2014, 31, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Arble, D.M.; Bass, J.; Laposky, A.D.; Vitaterna, M.H.; Turek, F.W. Circadian timing of food intake contributes to weight gain. Obesity 2009, 17, 2100–2102. [Google Scholar] [CrossRef]
- Oosterman, J.E.; Foppen, E.; van der Spek, R.; Fliers, E.; Kalsbeek, A.; la Fleur, S.E. Timing of fat and liquid sugar intake alters substrate oxidation and food efficiency in male Wistar rats. Chronobiol. Int. 2015, 32, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Panda, S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab. 2015, 22, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Itani, O.; Jike, M.; Watanabe, N.; Kaneita, Y. Short sleep duration and health outcomes: A systematic review, meta-analysis, and meta-regression. Sleep Med. 2017, 32, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Dashti, H.S.; Gómez-Abellán, P.; Qian, J.; Esteban, A.; Morales, E.; Scheer, F.A.J.L.; Garaulet, M. Late eating is associated with cardiometabolic risk traits, obesogenic behaviors, and impaired weight loss. Am. J. Clin. Nutr. 2021, 113, 154–161. [Google Scholar] [CrossRef]
- McHill, A.W.; Phillips, A.J.; Czeisler, C.A.; Keating, L.; Yee, K.; Barger, L.K.; Garaulet, M.; Scheer, F.A.; Klerman, E.B. Later circadian timing of food intake is associated with increased body fat. Am. J. Clin. Nutr. 2017, 106, 1213–1219. [Google Scholar] [CrossRef]
- Vujović, N.; Piron, M.J.; Qian, J.; Chellappa, S.L.; Nedeltcheva, A.; Barr, D.; Heng, S.W.; Kerlin, K.; Srivastav, S.; Wang, W.; et al. Late isocaloric eating increases hunger, decreases energy expenditure, and modifies metabolic pathways in adults with overweight and obesity. Cell Metab. 2022, 34, 1486–1498.e7. [Google Scholar] [CrossRef]
- Kelly, K.P.; McGuinness, O.P.; Buchowski, M.; Hughey, J.J.; Chen, H.; Powers, J.; Page, T.; Johnson, C.H. Eating breakfast and avoiding late-evening snacking sustains lipid oxidation. PLoS Biol. 2020, 18, e3000622. [Google Scholar] [CrossRef]
- Bonnet, J.P.; Cardel, M.I.; Cellini, J.; Hu, F.B.; Guasch-Ferré, M. Breakfast Skipping, Body Composition, and Cardiometabolic Risk: A Systematic Review and Meta-Analysis of Randomized Trials. Obesity 2020, 28, 1098–1109. [Google Scholar] [CrossRef]
- Wicherski, J.; Schlesinger, S.; Fischer, F. Association between Breakfast Skipping and Body Weight-A Systematic Review and Meta-Analysis of Observational Longitudinal Studies. Nutrients 2021, 13, 272. [Google Scholar] [CrossRef]
- Ricotti, R.; Caputo, M.; Monzani, A.; Pigni, S.; Antoniotti, V.; Bellone, S.; Prodam, F. Breakfast Skipping, Weight, Cardiometabolic Risk, and Nutrition Quality in Children and Adolescents: A Systematic Review of Randomized Controlled and Intervention Longitudinal Trials. Nutrients 2021, 13, 3331. [Google Scholar] [CrossRef]
- Zerón-Rugerio, M.F.; Hernáez, Á.; Porras-Loaiza, A.P.; Cambras, T.; Izquierdo-Pulido, M. Eating Jet Lag: A Marker of the Variability in Meal Timing and Its Association with Body Mass Index. Nutrients 2019, 11, 2980. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Raingard, H.; Dumont, S.; Kalsbeek, A.; Vuillez, P.; Challet, E. Ultradian feeding in mice not only affects the peripheral clock in the liver, but also the master clock in the brain. Chronobiol. Int. 2017, 34, 17–36. [Google Scholar] [CrossRef] [PubMed]
- de Goede, P.; Sen, S.; Su, Y.; Foppen, E.; Poirel, V.-J.; Challet, E.; Kalsbeek, A. An Ultradian Feeding Schedule in Rats Affects Metabolic Gene Expression in Liver, Brown Adipose Tissue and Skeletal Muscle with Only Mild Effects on Circadian Clocks. Int. J. Mol. Sci. 2018, 19, 3171. [Google Scholar] [CrossRef] [PubMed]
- Greenwell, B.J.; Trott, A.J.; Beytebiere, J.R.; Pao, S.; Bosley, A.; Beach, E.; Finegan, P.; Hernandez, C.; Menet, J.S. Rhythmic Food Intake Drives Rhythmic Gene Expression More Potently than the Hepatic Circadian Clock in Mice. Cell Rep. 2019, 27, 649–657.e5. [Google Scholar] [CrossRef]
- Jha, P.K.; Bouâouda, H.; Kalsbeek, A.; Challet, E. Distinct feedback actions of behavioural arousal to the master circadian clock in nocturnal and diurnal mammals. Neurosci. Biobehav. Rev. 2021, 123, 48–60. [Google Scholar] [CrossRef]
- Hughes, A.T.L.; Piggins, H.D. Feedback actions of locomotor activity to the circadian clock. Prog. Brain Res. 2012, 199, 305–336. [Google Scholar] [PubMed]
- Buhr, E.D.; Yoo, S.-H.; Takahashi, J.S. Temperature as a universal resetting cue for mammalian circadian oscillators. Science 2010, 330, 379–385. [Google Scholar] [CrossRef]
- Farsi, H.; Achaâban, M.R.; Piro, M.; Bothorel, B.; Ouassat, M.; Challet, E.; Pévet, P.; El Allali, K. Entrainment of circadian rhythms of locomotor activity by ambient temperature cycles in the dromedary camel. Sci. Rep. 2020, 10, 19515. [Google Scholar] [CrossRef]
- Farsi, H.; Harti, D.; Achaâban, M.R.; Piro, M.; Raverot, V.; Bothorel, B.; Ouassat, M.; Challet, E.; Pévet, P.; El Allali, K. Melatonin rhythm and other outputs of the master circadian clock in the desert goat (Capra hircus) are entrained by daily cycles of ambient temperature. J. Pineal. Res. 2020, 68, e12634. [Google Scholar] [CrossRef]
- Damiola, F.; Le Minh, N.; Preitner, N.; Kornmann, B.; Fleury-Olela, F.; Schibler, U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000, 14, 2950–2961. [Google Scholar] [CrossRef]
- Stokkan, K.A.; Yamazaki, S.; Tei, H.; Sakaki, Y.; Menaker, M. Entrainment of the circadian clock in the liver by feeding. Science 2001, 291, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Kida, M.; Tsuji, K.; Mano, T. Feeding cycles entrain circadian rhythms of locomotor activity in CS mice but not in C57BL/6J mice. Physiol. Behav. 1989, 45, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Mr, C.; Kj, H.; Rj, T.; Dm, G.; Bult-Ito, A. Entrainment of the master circadian clock by scheduled feeding. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R551–R555. [Google Scholar]
- Holmes, M.M.; Mistlberger, R.E. Food anticipatory activity and photic entrainment in food-restricted BALB/c mice. Physiol. Behav. 2000, 68, 655–666. [Google Scholar] [CrossRef]
- Webb, A.B.; Angelo, N.; Huettner, J.E.; Herzog, E.D. Intrinsic, nondeterministic circadian rhythm generation in identified mammalian neurons. Proc. Natl. Acad. Sci. USA 2009, 106, 16493–16498. [Google Scholar] [CrossRef]
- Guo, H.; Brewer, J.M.; Champhekar, A.; Harris, R.B.S.; Bittman, E.L. Differential control of peripheral circadian rhythms by suprachiasmatic-dependent neural signals. Proc. Natl. Acad. Sci. USA 2005, 102, 3111–3116. [Google Scholar] [CrossRef]
- Challet, E. Keeping circadian time with hormones. Diabetes Obes. Metab. 2015, 17 (Suppl. S1), 76–83. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Verhagen, L.A.W.; Schalij, I.; Foppen, E.; Saboureau, M.; Bothorel, B.; Buijs, R.M.; Pévet, P. Opposite actions of hypothalamic vasopressin on circadian corticosterone rhythm in nocturnal versus diurnal species. Eur. J. Neurosci. 2008, 27, 818–827. [Google Scholar] [CrossRef]
- Husse, J.; Leliavski, A.; Tsang, A.H.; Oster, H.; Eichele, G. The light-dark cycle controls peripheral rhythmicity in mice with a genetically ablated suprachiasmatic nucleus clock. FASEB J. 2014, 28, 4950–4960. [Google Scholar] [CrossRef]
- Redlin, U. Neural basis and biological function of masking by light in mammals: Suppression of melatonin and locomotor activity. Chronobiol. Int. 2001, 18, 737–758. [Google Scholar] [CrossRef]
- Ishida, A.; Mutoh, T.; Ueyama, T.; Bando, H.; Masubuchi, S.; Nakahara, D.; Tsujimoto, G.; Okamura, H. Light activates the adrenal gland: Timing of gene expression and glucocorticoid release. Cell Metab. 2005, 2, 297–307. [Google Scholar] [CrossRef]
- Wang, H.; van Spyk, E.; Liu, Q.; Geyfman, M.; Salmans, M.L.; Kumar, V.; Ihler, A.; Li, N.; Takahashi, J.S.; Andersen, B. Time-Restricted Feeding Shifts the Skin Circadian Clock and Alters UVB-Induced DNA Damage. Cell Rep. 2017, 20, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Bur, I.M.; Zouaoui, S.; Fontanaud, P.; Coutry, N.; Molino, F.; Martin, A.O.; Mollard, P.; Bonnefont, X. The comparison between circadian oscillators in mouse liver and pituitary gland reveals different integration of feeding and light schedules. PLoS ONE 2010, 5, e15316. [Google Scholar] [CrossRef] [PubMed]
- Wehrens, S.M.T.; Christou, S.; Isherwood, C.; Middleton, B.; Gibbs, M.A.; Archer, S.N.; Skene, D.J.; Johnston, J.D. Meal Timing Regulates the Human Circadian System. Curr. Biol. 2017, 27, 1768–1775.e3. [Google Scholar] [CrossRef] [PubMed]
- Kräuchi, K.; Cajochen, C.; Werth, E.; Wirz-Justice, A. Alteration of internal circadian phase relationships after morning versus evening carbohydrate-rich meals in humans. J. Biol. Rhythms 2002, 17, 364–376. [Google Scholar] [CrossRef]
- Hirota, T.; Okano, T.; Kokame, K.; Shirotani-Ikejima, H.; Miyata, T.; Fukada, Y. Glucose down-regulates Per1 and Per2 mRNA levels and induces circadian gene expression in cultured Rat-1 fibroblasts. J. Biol. Chem. 2002, 277, 44244–44251. [Google Scholar] [CrossRef] [PubMed]
- Oosterman, J.E.; Belsham, D.D. Glucose Alters Per2 Rhythmicity Independent of AMPK, Whereas AMPK Inhibitor Compound C Causes Profound Repression of Clock Genes and AgRP in mHypoE-37 Hypothalamic Neurons. PLoS ONE 2016, 11, e0146969. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Otsuka, M.; Fuse, Y.; Hirao, A.; Shibata, S. Refeeding after fasting elicits insulin-dependent regulation of Per2 and Rev-erbα with shifts in the liver clock. J. Biol. Rhythms 2011, 26, 230–240. [Google Scholar] [CrossRef]
- Crosby, P.; Hamnett, R.; Putker, M.; Hoyle, N.P.; Reed, M.; Karam, C.J.; Maywood, E.S.; Stangherlin, A.; Chesham, J.E.; Hayter, E.A.; et al. Insulin/IGF-1 Drives PERIOD Synthesis to Entrain Circadian Rhythms with Feeding Time. Cell 2019, 177, 896–909.e20. [Google Scholar] [CrossRef]
- Ando, H.; Ushijima, K.; Fujimura, A. Indirect effects of glucagon-like peptide-1 receptor agonist exendin-4 on the peripheral circadian clocks in mice. PLoS ONE 2013, 8, e81119. [Google Scholar] [CrossRef]
- Landgraf, D.; Tsang, A.H.; Leliavski, A.; Koch, C.E.; Barclay, J.L.; Drucker, D.J.; Oster, H. Oxyntomodulin regulates resetting of the liver circadian clock by food. eLife 2015, 4, e06253. [Google Scholar] [CrossRef] [PubMed]
- Mukherji, A.; Kobiita, A.; Chambon, P. Shifting the feeding of mice to the rest phase creates metabolic alterations, which, on their own, shift the peripheral circadian clocks by 12 hours. Proc. Natl. Acad. Sci. USA 2015, 112, E6683–E6690. [Google Scholar] [CrossRef]
- Ikeda, Y.; Kamagata, M.; Hirao, M.; Yasuda, S.; Iwami, S.; Sasaki, H.; Tsubosaka, M.; Hattori, Y.; Todoh, A.; Tamura, K.; et al. Glucagon and/or IGF-1 Production Regulates Resetting of the Liver Circadian Clock in Response to a Protein or Amino Acid-only Diet. EBioMedicine 2018, 28, 210–224. [Google Scholar] [CrossRef]
- Sasaki, H.; Hattori, Y.; Ikeda, Y.; Kamagata, M.; Iwami, S.; Yasuda, S.; Tahara, Y.; Shibata, S. Forced rather than voluntary exercise entrains peripheral clocks via a corticosterone/noradrenaline increase in PER2::LUC mice. Sci. Rep. 2016, 6, 27607. [Google Scholar] [CrossRef] [PubMed]
- Kon, N.; Hirota, T.; Kawamoto, T.; Kato, Y.; Tsubota, T.; Fukada, Y. Activation of TGF-beta/activin signalling resets the circadian clock through rapid induction of Dec1 transcripts. Nat. Cell Biol. 2008, 10, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Yoshitane, H.; Asano, Y.; Sagami, A.; Sakai, S.; Suzuki, Y.; Okamura, H.; Iwasaki, W.; Ozaki, H.; Fukada, Y. Functional D-box sequences reset the circadian clock and drive mRNA rhythms. Commun. Biol. 2019, 2, 300. [Google Scholar] [CrossRef]
- Brown, S.A.; Zumbrunn, G.; Fleury-Olela, F.; Preitner, N.; Schibler, U. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr. Biol. 2002, 12, 1574–1583. [Google Scholar] [CrossRef]
- Ohnishi, N.; Tahara, Y.; Kuriki, D.; Haraguchi, A.; Shibata, S. Warm water bath stimulates phase-shifts of the peripheral circadian clocks in PER2::LUCIFERASE mouse. PLoS ONE 2014, 9, e100272. [Google Scholar] [CrossRef]
- Reinke, H.; Saini, C.; Fleury-Olela, F.; Dibner, C.; Benjamin, I.J.; Schibler, U. Differential display of DNA-binding proteins reveals heat-shock factor 1 as a circadian transcription factor. Genes Dev. 2008, 22, 331–345. [Google Scholar] [CrossRef]
- Challet, E. Interactions between light, mealtime and calorie restriction to control daily timing in mammals. J. Comp. Physiol. B 2010, 180, 631–644. [Google Scholar] [CrossRef]
- Mendoza, J.; Graff, C.; Dardente, H.; Pevet, P.; Challet, E. Feeding cues alter clock gene oscillations and photic responses in the suprachiasmatic nuclei of mice exposed to a light/dark cycle. J. Neurosci. 2005, 25, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Challet, E.; Losee-Olson, S.; Turek, F.W. Reduced glucose availability attenuates circadian responses to light in mice. Am. J. Physiol. 1999, 276, R1063–R1070. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-J.; Cheng, R.-C.; Cheng, P.-C.; Wang, Y.-C.; Huang, R.-C. KATP Channels Mediate Differential Metabolic Responses to Glucose Shortage of the Dorsomedial and Ventrolateral Oscillators in the Central Clock. Sci. Rep. 2017, 7, 640. [Google Scholar] [CrossRef] [PubMed]
- Challet, E.; Denis, I.; Rochet, V.; Aïoun, J.; Gourmelen, S.; Lacroix, H.; Goustard-Langelier, B.; Papillon, C.; Alessandri, J.-M.; Lavialle, M. The role of PPARβ/δ in the regulation of glutamatergic signaling in the hamster suprachiasmatic nucleus. Cell Mol. Life Sci. 2013, 70, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.-X.; Challet, E.; Pévet, P.; Kalsbeek, A.; Escobar, C.; Buijs, R.M. A circulating ghrelin mimetic attenuates light-induced phase delay of mice and light-induced Fos expression in the suprachiasmatic nucleus of rats. Eur. J. Neurosci. 2008, 27, 1965–1972. [Google Scholar] [CrossRef]
- Grosbellet, E.; Gourmelen, S.; Pévet, P.; Criscuolo, F.; Challet, E. Leptin normalizes photic synchronization in male ob/ob mice, via indirect effects on the suprachiasmatic nucleus. Endocrinology 2015, 156, 1080–1090. [Google Scholar] [CrossRef]
- Acosta-Rodríguez, V.; Rijo-Ferreira, F.; Izumo, M.; Xu, P.; Wight-Carter, M.; Green, C.B.; Takahashi, J.S. Circadian alignment of early onset caloric restriction promotes longevity in male C57BL/6J mice. Science 2022, 376, 1192–1202. [Google Scholar] [CrossRef]
- Grosbellet, E.; Dumont, S.; Schuster-Klein, C.; Guardiola-Lemaitre, B.; Pevet, P.; Criscuolo, F.; Challet, E. Leptin modulates the daily rhythmicity of blood glucose. Chronobiol. Int. 2015, 32, 637–649. [Google Scholar] [CrossRef]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef]
- Mendoza, J.; Pévet, P.; Challet, E. High-fat feeding alters the clock synchronization to light. J. Physiol. 2008, 586, 5901–5910. [Google Scholar] [CrossRef]
- Corbalán-Tutau, M.D.; Madrid, J.A.; Ordovás, J.M.; Smith, C.E.; Nicolás, F.; Garaulet, M. Differences in daily rhythms of wrist temperature between obese and normal-weight women: Associations with metabolic syndrome features. Chronobiol. Int. 2011, 28, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Mäntele, S.; Otway, D.T.; Middleton, B.; Bretschneider, S.; Wright, J.; Robertson, M.D.; Skene, D.J.; Johnston, J.D. Daily rhythms of plasma melatonin, but not plasma leptin or leptin mRNA, vary between lean, obese and type 2 diabetic men. PLoS ONE 2012, 7, e37123. [Google Scholar] [CrossRef] [PubMed]
- Corbalán-Tutau, D.; Madrid, J.A.; Nicolás, F.; Garaulet, M. Daily profile in two circadian markers “melatonin and cortisol” and associations with metabolic syndrome components. Physiol. Behav. 2014, 123, 231–235. [Google Scholar] [CrossRef]
- McHill, A.W.; Czeisler, C.A.; Phillips, A.J.K.; Keating, L.; Barger, L.K.; Garaulet, M.; Scheer, F.A.J.L.; Klerman, E.B. Caloric and Macronutrient Intake Differ with Circadian Phase and between Lean and Overweight Young Adults. Nutrients 2019, 11, 587. [Google Scholar] [CrossRef] [PubMed]
- Kudo, T.; Akiyama, M.; Kuriyama, K.; Sudo, M.; Moriya, T.; Shibata, S. Night-time restricted feeding normalises clock genes and Pai-1 gene expression in the db/db mouse liver. Diabetologia 2004, 47, 1425–1436. [Google Scholar] [CrossRef] [PubMed]
- Grosbellet, E.; Dumont, S.; Schuster-Klein, C.; Guardiola-Lemaitre, B.; Pevet, P.; Criscuolo, F.; Challet, E. Circadian phenotyping of obese and diabetic db/db mice. Biochimie 2016, 124, 198–206. [Google Scholar] [CrossRef]
- Gubin, D.G.; Nelaeva, A.A.; Uzhakova, A.E.; Hasanova, Y.V.; Cornelissen, G.; Weinert, D. Disrupted circadian rhythms of body temperature, heart rate and fasting blood glucose in prediabetes and type 2 diabetes mellitus. Chronobiol. Int. 2017, 34, 1136–1148. [Google Scholar] [CrossRef]
- Stenvers, D.J.; Jongejan, A.; Atiqi, S.; Vreijling, J.P.; Limonard, E.J.; Endert, E.; Baas, F.; Moerland, P.D.; Fliers, E.; Kalsbeek, A.; et al. Diurnal rhythms in the white adipose tissue transcriptome are disturbed in obese individuals with type 2 diabetes compared with lean control individuals. Diabetologia 2019, 62, 704–716. [Google Scholar] [CrossRef]
- Kaur, M.; Bala, R. Chronotherapy: A review. Int. J. Pharm. Sci. Res. 2013, 4, 90–102. [Google Scholar]
- Pickel, L.; Sung, H.-K. Feeding Rhythms and the Circadian Regulation of Metabolism. Front. Nutr. 2020, 7, 39. [Google Scholar] [CrossRef]
- Van Cauter, E.; Polonsky, K.S.; Scheen, A.J. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr. Rev. 1997, 18, 716–738. [Google Scholar] [PubMed]
- Hess, J.M.; Jonnalagadda, S.S.; Slavin, J.L. What Is a Snack, Why Do We Snack, and How Can We Choose Better Snacks? A Review of the Definitions of Snacking, Motivations to Snack, Contributions to Dietary Intake, and Recommendations for Improvement. Adv. Nutr. 2016, 7, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Martini, D.; Scaglioni, S.; Sculati, M.; Donini, L.M.; Leonardi, F.; Agostoni, C.; Castelnuovo, G.; Ferrara, N.; Ghiselli, A.; et al. Snacking in nutrition and health. Int. J. Food Sci. Nutr. 2019, 70, 909–923. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Lloren, J.I.; Mashchak, A.; Hill, M.; Fraser, G.E. Meal Frequency and Timing Are Associated with Changes in Body Mass Index in Adventist Health Study 2. J. Nutr. 2017, 147, 1722–1728. [Google Scholar] [CrossRef]
- Manoogian, E.N.C.; Chaix, A.; Panda, S. When to Eat: The Importance of Eating Patterns in Health and Disease. J. Biol. Rhythms 2019, 34, 579–581. [Google Scholar] [CrossRef]
- Mistlberger, R.E.; Lukman, H.; Nadeau, B.G. Circadian rhythms in the Zucker obese rat: Assessment and intervention. Appetite 1998, 30, 255–267. [Google Scholar] [CrossRef]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.J.; et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef]
- Wilkinson, M.J.; Manoogian, E.N.C.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab. 2020, 31, 92–104.e5. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e3. [Google Scholar] [CrossRef]
- Moon, S.; Kang, J.; Kim, S.H.; Chung, H.S.; Kim, Y.J.; Yu, J.M.; Cho, S.T.; Oh, C.-M.; Kim, T. Beneficial Effects of Time-Restricted Eating on Metabolic Diseases: A Systemic Review and Meta-Analysis. Nutrients 2020, 12, 1267. [Google Scholar] [CrossRef] [PubMed]
- Regmi, P.; Heilbronn, L.K. Time-Restricted Eating: Benefits, Mechanisms, and Challenges in Translation. iScience 2020, 23, 101161. [Google Scholar] [CrossRef]
- Tippairote, T.; Janssen, S.; Chunhabundit, R. Restoration of metabolic tempo through time-restricted eating (TRE) as the preventive measure for metabolic diseases. Crit. Rev. Food Sci. Nutr. 2021, 61, 2444–2453. [Google Scholar] [CrossRef] [PubMed]
- Arora, T.; Taheri, S. Associations among late chronotype, body mass index and dietary behaviors in young adolescents. Int. J. Obes. 2015, 39, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Molina-Montes, E.; Rodríguez-Barranco, M.; Ching-López, A.; Artacho, R.; Huerta, J.M.; Amiano, P.; Lasheras, C.; Moreno-Iribas, C.; Jimenez-Zabala, A.; Chirlaque, M.-D.; et al. Circadian clock gene variants and their link with chronotype, chrononutrition, sleeping patterns and obesity in the European prospective investigation into cancer and nutrition (EPIC) study. Clin. Nutr. 2022, 41, 1977–1990. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Minguez, J.; Gómez-Abellán, P.; Garaulet, M. Timing of Breakfast, Lunch, and Dinner. Effects on Obesity and Metabolic Risk. Nutrients 2019, 11, 2624. [Google Scholar] [CrossRef]
- Wohlgemuth, K.J.; Arieta, L.R.; Brewer, G.J.; Hoselton, A.L.; Gould, L.M.; Smith-Ryan, A.E. Sex differences and considerations for female specific nutritional strategies: A narrative review. J. Int. Soc. Sport. Nutr. 2021, 18, 27. [Google Scholar] [CrossRef]
- Isacco, L.; Duché, P.; Boisseau, N. Influence of Hormonal Status on Substrate Utilization at Rest and during Exercise in the Female Population. Sport. Med. 2012, 42, 327–342. [Google Scholar] [CrossRef]
- Bisdee, J.T.; James, W.P.; Shaw, M.A. Changes in energy expenditure during the menstrual cycle. Br. J. Nutr. 1989, 61, 187–199. [Google Scholar] [CrossRef]
- Draper, C.F.; Duisters, K.; Weger, B.; Chakrabarti, A.; Harms, A.C.; Brennan, L.; Hankemeier, T.; Goulet, L.; Konz, T.; Martin, F.P.; et al. Menstrual cycle rhythmicity: Metabolic patterns in healthy women. Sci. Rep. 2018, 8, 14568. [Google Scholar] [CrossRef]
- Marlatt, K.L.; Pitynski-Miller, D.R.; Gavin, K.M.; Moreau, K.L.; Melanson, E.L.; Santoro, N.; Kohrt, W.M. Body composition and cardiometabolic health across the menopause transition. Obesity 2022, 30, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Van Pelt, R.E.; Gavin, K.M.; Kohrt, W.M. Regulation of Body Composition and Bioenergetics by Estrogens. Endocrinol. Metab. Clin. N. Am. 2015, 44, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Bur, I.M.; Cohen-Solal, A.M.; Carmignac, D.; Abecassis, P.-Y.; Chauvet, N.; Martin, A.O.; van der Horst, G.T.J.; Robinson, I.C.A.F.; Maurel, P.; Mollard, P.; et al. The circadian clock components CRY1 and CRY2 are necessary to sustain sex dimorphism in mouse liver metabolism. J. Biol. Chem. 2009, 284, 9066–9073. [Google Scholar] [CrossRef] [PubMed]
- Weger, B.D.; Gobet, C.; Yeung, J.; Martin, E.; Jimenez, S.; Betrisey, B.; Foata, F.; Berger, B.; Balvay, A.; Foussier, A.; et al. The Mouse Microbiome Is Required for Sex-Specific Diurnal Rhythms of Gene Expression and Metabolism. Cell Metab. 2019, 29, 362–382.e8. [Google Scholar] [CrossRef]
- Duffy, J.F.; Cain, S.W.; Chang, A.-M.; Phillips, A.J.K.; Münch, M.Y.; Gronfier, C.; Wyatt, J.K.; Dijk, D.-J.; Wright, K.P.; Czeisler, C.A. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S3), 15602–15608. [Google Scholar] [CrossRef]
- Qian, J.; Morris, C.J.; Caputo, R.; Wang, W.; Garaulet, M.; Scheer, F.A.J.L. Sex differences in the circadian misalignment effects on energy regulation. Proc. Natl. Acad. Sci. USA 2019, 116, 23806–23812. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grosjean, E.; Simonneaux, V.; Challet, E. Reciprocal Interactions between Circadian Clocks, Food Intake, and Energy Metabolism. Biology 2023, 12, 539. https://doi.org/10.3390/biology12040539
Grosjean E, Simonneaux V, Challet E. Reciprocal Interactions between Circadian Clocks, Food Intake, and Energy Metabolism. Biology. 2023; 12(4):539. https://doi.org/10.3390/biology12040539
Chicago/Turabian StyleGrosjean, Emma, Valérie Simonneaux, and Etienne Challet. 2023. "Reciprocal Interactions between Circadian Clocks, Food Intake, and Energy Metabolism" Biology 12, no. 4: 539. https://doi.org/10.3390/biology12040539
APA StyleGrosjean, E., Simonneaux, V., & Challet, E. (2023). Reciprocal Interactions between Circadian Clocks, Food Intake, and Energy Metabolism. Biology, 12(4), 539. https://doi.org/10.3390/biology12040539





