Effectiveness of Repetitive Transcranial Magnetic Stimulation Combined with Visual Feedback Training in Improving Neuroplasticity and Lower Limb Function after Chronic Stroke: A Pilot Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
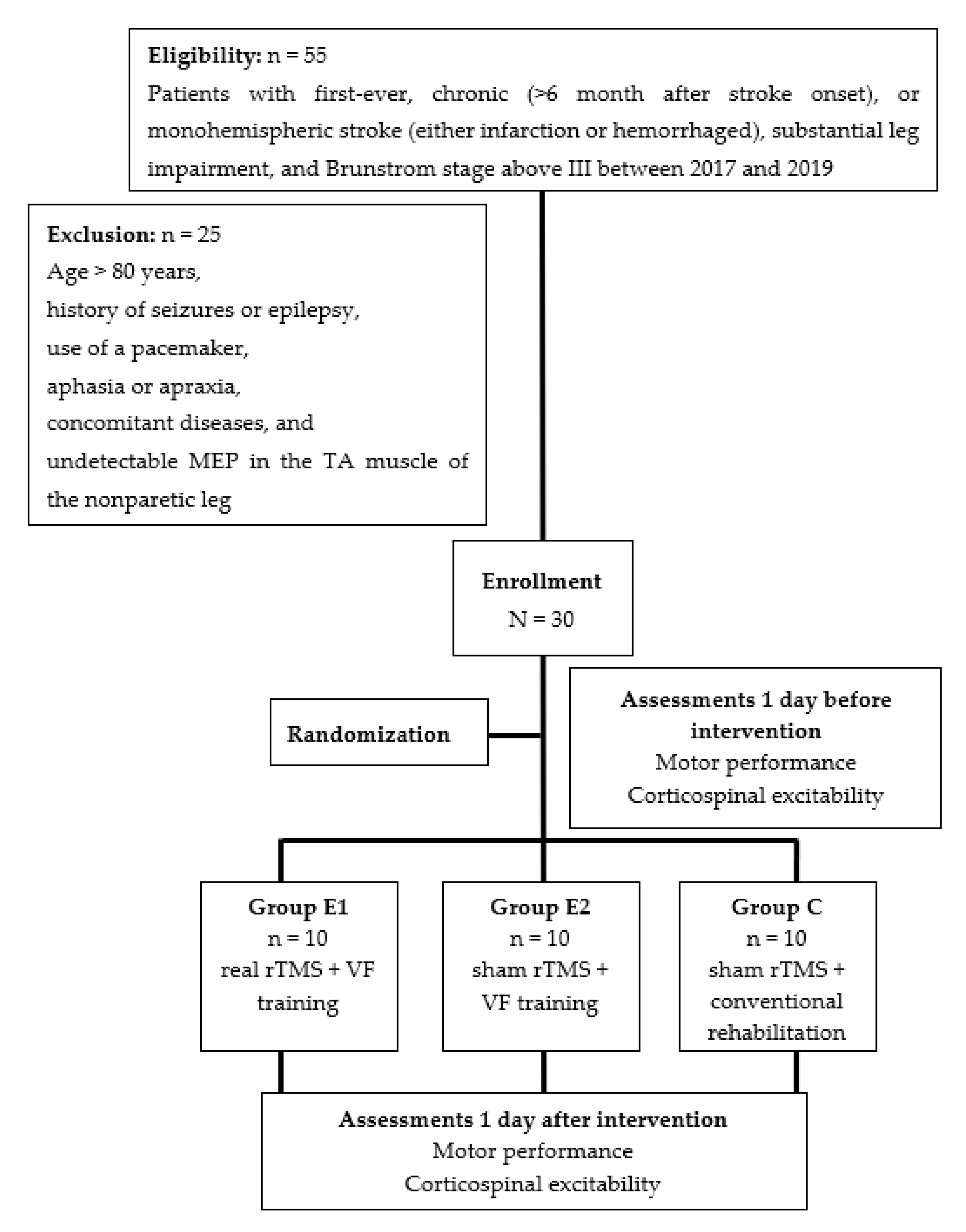
2.1. Participants
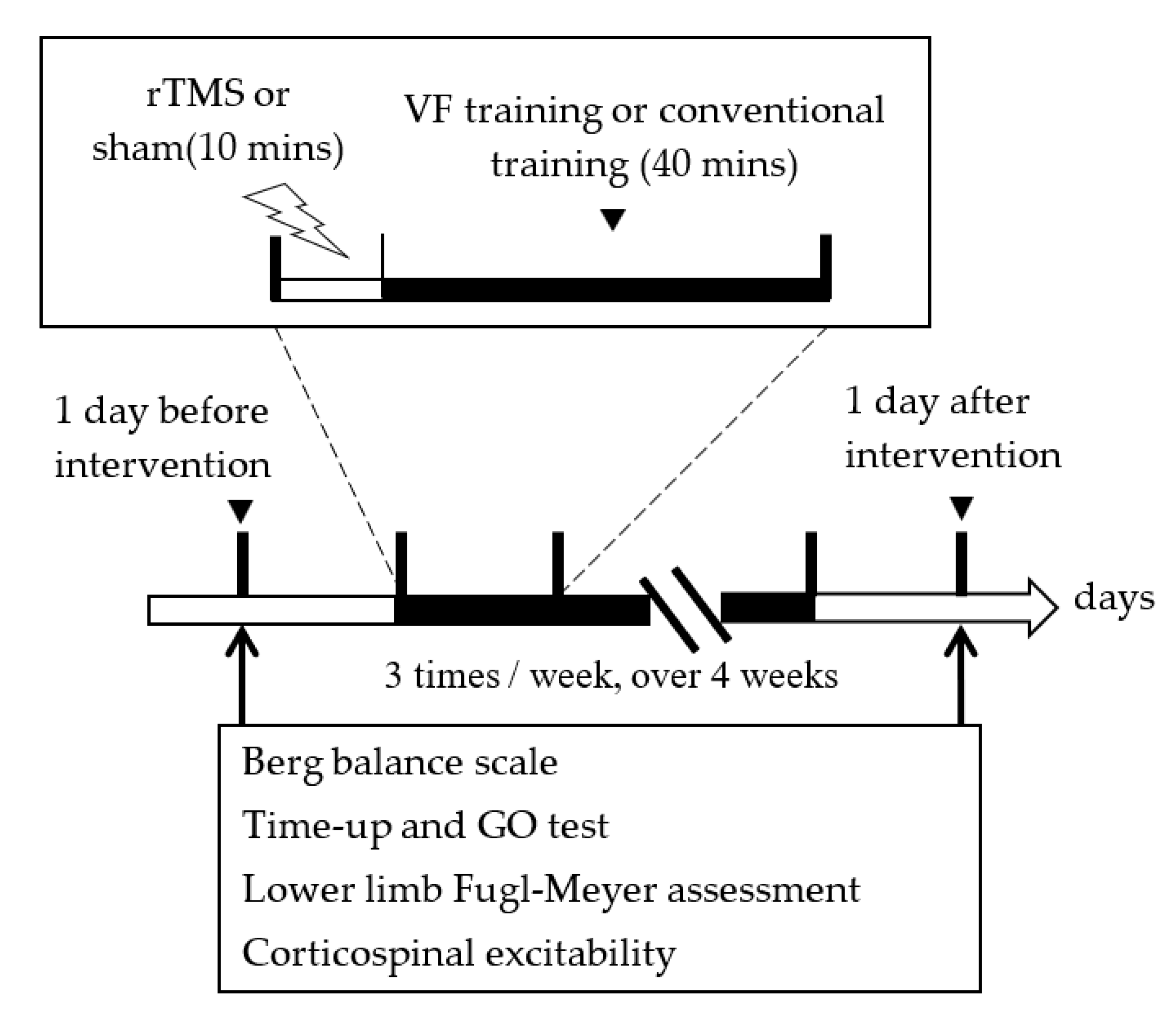
2.2. Study Design
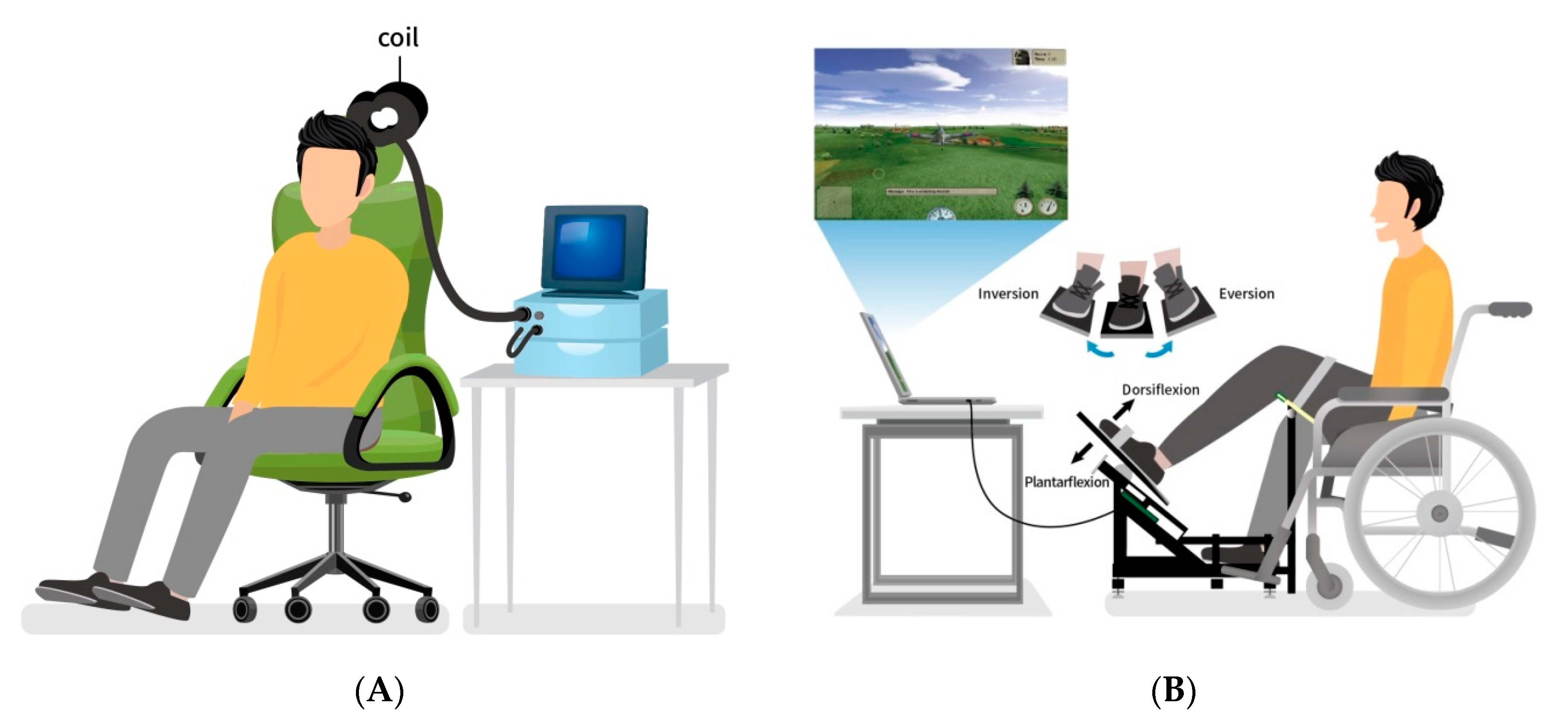
2.3. Transcranial Magnetic Stimulation Procedure
2.4. Individualized Game-Based VF Intervention
2.5. Conventional Training
2.6. Outcome Measurements
2.7. Data Analysis
3. Results
3.1. Participants
3.2. Motor Performance
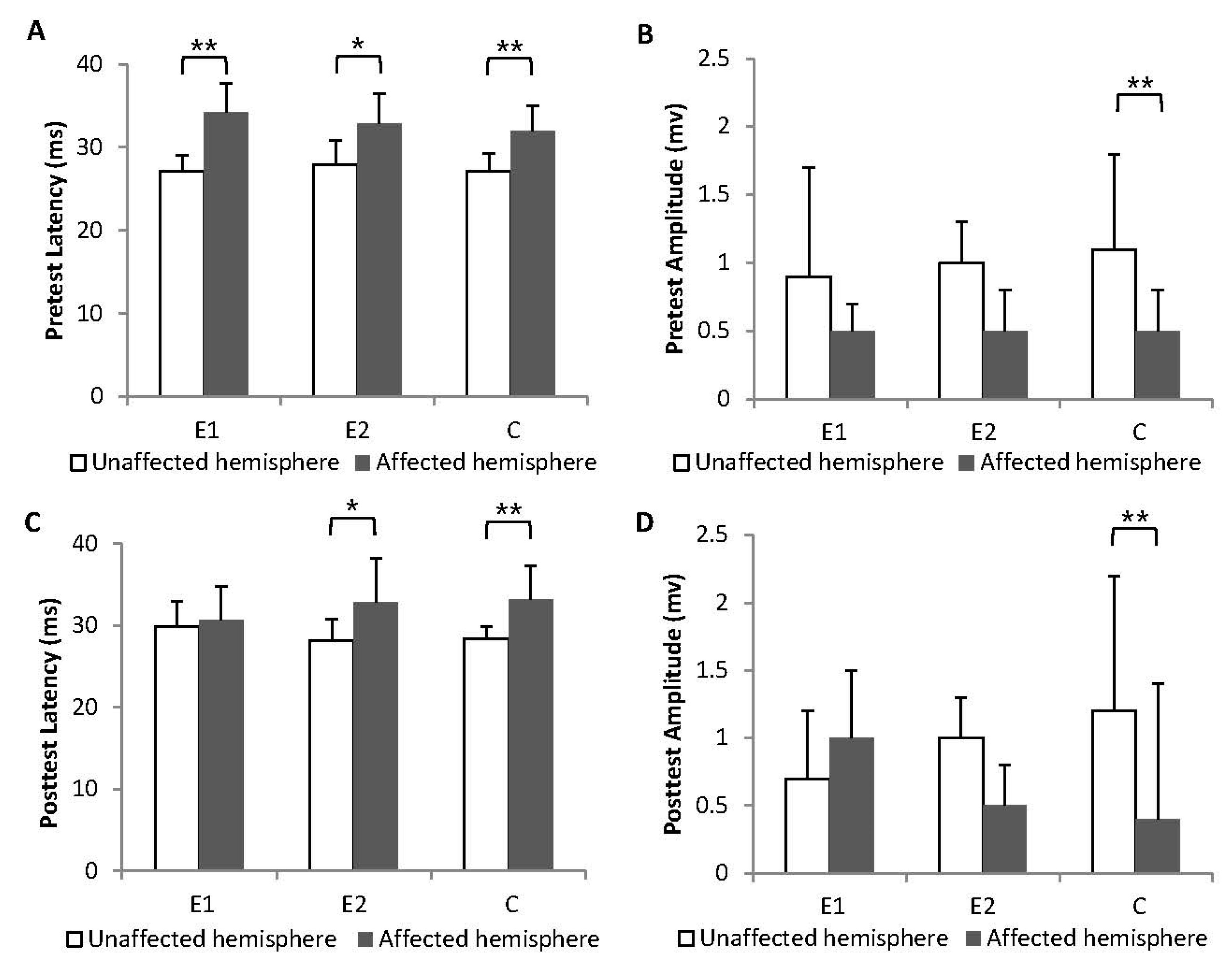
3.3. Corticospinal Excitability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nathan, P.W.; Smith, M.C.; Deacon, P. The corticospinal tracts in man. Course and location of fibres at different segmental levels. Brain 1990, 113 Pt 2, 303–324. [Google Scholar] [CrossRef]
- Jankowska, E.; Edgley, S.A. How can corticospinal tract neurons contribute to ipsilateral movements? A question with implications for recovery of motor functions. Neuroscientist 2006, 12, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; Galvin, R.; Horgan, N.F. Fall-related experiences of stroke survivors: A meta-ethnography. Disabil. Rehabil. 2017, 39, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, H.M.; de Oliveira, L.C.; Bonifacio, S.R.; Brandao, T.C.P.; Silva, W.P.; Pereira, G.S.; Silva, S.M. Use of the International Classification of Functioning, Disability and Health (ICF) to expand and standardize the assessment of quality-of-life following a stroke: Proposal for the use of codes and qualifiers. Disabil. Rehabil. 2022, 44, 7449–7454. [Google Scholar] [CrossRef] [PubMed]
- Netz, J.; Lammers, T.; Homberg, V. Reorganization of motor output in the non-affected hemisphere after stroke. Brain 1997, 120 Pt 9, 1579–1586. [Google Scholar] [CrossRef]
- Swayne, O.B.; Rothwell, J.C.; Ward, N.S.; Greenwood, R.J. Stages of motor output reorganization after hemispheric stroke suggested by longitudinal studies of cortical physiology. Cereb. Cortex 2008, 18, 1909–1922. [Google Scholar] [CrossRef] [PubMed]
- Liepert, J.; Miltner, W.H.; Bauder, H.; Sommer, M.; Dettmers, C.; Taub, E.; Weiller, C. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neurosci. Lett. 1998, 250, 5–8. [Google Scholar] [CrossRef]
- Nudo, R.J. Functional and structural plasticity in motor cortex: Implications for stroke recovery. Phys. Med. Rehabil. Clin. N. Am. 2003, 14, S57–S76. [Google Scholar] [CrossRef]
- Khedr, E.M.; Fetoh, N.A. Short- and long-term effect of rTMS on motor function recovery after ischemic stroke. Restor. Neurol. Neurosci. 2010, 28, 545–559. [Google Scholar] [CrossRef]
- Shimizu, T.; Hosaki, A.; Hino, T.; Sato, M.; Komori, T.; Hirai, S.; Rossini, P.M. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain 2002, 125, 1896–1907. [Google Scholar] [CrossRef]
- Khedr, E.M.; Abdel-Fadeil, M.R.; Farghali, A.; Qaid, M. Role of 1 and 3 Hz repetitive transcranial magnetic stimulation on motor function recovery after acute ischaemic stroke. Eur. J. Neurol. 2009, 16, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Feydy, A.; Carlier, R.; Roby-Brami, A.; Bussel, B.; Cazalis, F.; Pierot, L.; Burnod, Y.; Maier, M.A. Longitudinal study of motor recovery after stroke: Recruitment and focusing of brain activation. Stroke 2002, 33, 1610–1617. [Google Scholar] [CrossRef]
- Johansen-Berg, H.; Dawes, H.; Guy, C.; Smith, S.M.; Wade, D.T.; Matthews, P.M. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain 2002, 125, 2731–2742. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.S.; Brown, M.M.; Thompson, A.J.; Frackowiak, R.S. Neural correlates of motor recovery after stroke: A longitudinal fMRI study. Brain 2003, 126, 2476–2496. [Google Scholar] [CrossRef]
- Hummel, F.C.; Cohen, L.G. Non-invasive brain stimulation: A new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2006, 5, 708–712. [Google Scholar] [CrossRef]
- Lomarev, M.P.; Kim, D.Y.; Richardson, S.P.; Voller, B.; Hallett, M. Safety study of high-frequency transcranial magnetic stimulation in patients with chronic stroke. Clin. Neurophysiol. 2007, 118, 2072–2075. [Google Scholar] [CrossRef]
- Chieffo, R.; De Prezzo, S.; Houdayer, E.; Nuara, A.; Di Maggio, G.; Coppi, E.; Ferrari, L.; Straffi, L.; Spagnolo, F.; Velikova, S.; et al. Deep repetitive transcranial magnetic stimulation with H-coil on lower limb motor function in chronic stroke: A pilot study. Arch. Phys. Med. Rehabil. 2014, 95, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.N.; Hu, C.J.; Chi, J.Y.; Lin, L.F.; Yen, T.H.; Lin, Y.K.; Liou, T.H. Effects of repetitive transcranial magnetic stimulation of the unaffected hemisphere leg motor area in patients with subacute stroke and substantial leg impairment: A pilot study. J. Rehabil. Med. 2015, 47, 305–310. [Google Scholar] [CrossRef]
- Rastgoo, M.; Naghdi, S.; Nakhostin Ansari, N.; Olyaei, G.; Jalaei, S.; Forogh, B.; Najari, H. Effects of repetitive transcranial magnetic stimulation on lower extremity spasticity and motor function in stroke patients. Disabil. Rehabil. 2016, 38, 1918–1926. [Google Scholar] [CrossRef]
- Wang, R.Y.; Tseng, H.Y.; Liao, K.K.; Wang, C.J.; Lai, K.L.; Yang, Y.R. rTMS combined with task-oriented training to improve symmetry of interhemispheric corticomotor excitability and gait performance after stroke: A randomized trial. Neurorehabil. Neural Repair 2012, 26, 222–230. [Google Scholar] [CrossRef]
- Gustavsson, M.; Kjork, E.K.; Erhardsson, M.; Alt Murphy, M. Virtual reality gaming in rehabilitation after stroke—User experiences and perceptions. Disabil. Rehabil. 2022, 44, 6759–6765. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Brito, F.; Fialho, M.; Virgolino, A.; Neves, I.; Miranda, A.C.; Sousa-Santos, N.; Caneiras, C.; Carrico, L.; Verdelho, A.; Santos, O. Game-based interventions for neuropsychological assessment, training and rehabilitation: Which game-elements to use? A systematic review. J. Biomed. Inform. 2019, 98, 103287. [Google Scholar] [CrossRef] [PubMed]
- Pak, N.W.; Lee, J.H. Effects of visual feedback training and visual targets on muscle activation, balancing, and walking ability in adults after hemiplegic stroke: A preliminary, randomized, controlled study. Int. J. Rehabil. Res. 2020, 43, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Sutbeyaz, S.; Yavuzer, G.; Sezer, N.; Koseoglu, B.F. Mirror therapy enhances lower-extremity motor recovery and motor functioning after stroke: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2007, 88, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Archer, D.B.; Kang, N.; Misra, G.; Marble, S.; Patten, C.; Coombes, S.A. Visual feedback alters force control and functional activity in the visuomotor network after stroke. Neuroimage Clin. 2018, 17, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Sarlegna, F.R.; Mutha, P.K. The influence of visual target information on the online control of movements. Vis. Res. 2015, 110, 144–154. [Google Scholar] [CrossRef]
- Yarossi, M.; Manuweera, T.; Adamovich, S.V.; Tunik, E. The Effects of Mirror Feedback during Target Directed Movements on Ipsilateral Corticospinal Excitability. Front. Hum. Neurosci. 2017, 11, 242. [Google Scholar] [CrossRef]
- Lin, C.H.; Chiang, S.L.; Lu, L.H.; Wei, S.H.; Sung, W.H. Validity of an ankle joint motion and position sense measurement system and its application in healthy subjects and patients with ankle sprain. Comput. Methods Programs Biomed. 2016, 131, 89–96. [Google Scholar] [CrossRef]
- Chen, S.C.; Lin, C.H.; Su, S.W.; Chang, Y.T.; Lai, C.H. Feasibility and effect of interactive telerehabilitation on balance in individuals with chronic stroke: A pilot study. J. Neuroeng. Rehabil. 2021, 18, 71. [Google Scholar] [CrossRef]
- Hung, E.S.; Chen, S.C.; Chang, F.C.; Shiao, Y.; Peng, C.W.; Lai, C.H. Effects of Interactive Video Game-Based Exercise on Balance in Diabetic Patients with Peripheral Neuropathy: An Open-Level, Crossover Pilot Study. Evid. Based Complement. Alternat Med. 2019, 2019, 4540709. [Google Scholar] [CrossRef]
- Yuan, R.Y.; Chen, S.C.; Peng, C.W.; Lin, Y.N.; Chang, Y.T.; Lai, C.H. Effects of interactive video-game-based exercise on balance in older adults with mild-to-moderate Parkinson’s disease. J. Neuroeng. Rehabil. 2020, 17, 91. [Google Scholar] [CrossRef]
- Liepert, J.; Hamzei, F.; Weiller, C. Motor cortex disinhibition of the unaffected hemisphere after acute stroke. Muscle Nerve 2000, 23, 1761–1763. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Wood-Dauphinee, S.; Williams, J.I. The Balance Scale: Reliability assessment with elderly residents and patients with an acute stroke. Scand. J. Rehabil. Med. 1995, 27, 27–36. [Google Scholar] [PubMed]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Fugl-Meyer, A.R.; Jaasko, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [PubMed]
- Zheng, C.J.; Liao, W.J.; Xia, W.G. Effect of combined low-frequency repetitive transcranial magnetic stimulation and virtual reality training on upper limb function in subacute stroke: A double-blind randomized controlled trail. J. Huazhong Univ. Sci. Technol. Med. Sci. 2015, 35, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.N.; Carey, J.; Edelman, B.J.; Doud, A.; Grande, A.; Lakshminarayan, K.; He, B. Combined rTMS and virtual reality brain-computer interface training for motor recovery after stroke. J. Neural Eng. 2018, 15, 016009. [Google Scholar] [CrossRef]
- Bestmann, S.; Krakauer, J.W. The uses and interpretations of the motor-evoked potential for understanding behaviour. Exp. Brain Res. 2015, 233, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Mansur, C.G.; Fregni, F.; Boggio, P.S.; Riberto, M.; Gallucci-Neto, J.; Santos, C.M.; Wagner, T.; Rigonatti, S.P.; Marcolin, M.A.; Pascual-Leone, A. A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology 2005, 64, 1802–1804. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, N.; Tada, T.; Toshima, M.; Chuma, T.; Matsuo, Y.; Ikoma, K. Inhibition of the unaffected motor cortex by 1 Hz repetitive transcranical magnetic stimulation enhances motor performance and training effect of the paretic hand in patients with chronic stroke. J. Rehabil. Med. 2008, 40, 298–303. [Google Scholar] [CrossRef]
- Caparelli, E.; Backus, W.; Telang, F.; Wang, G.; Maloney, T.; Goldstein, R.; Henn, F. Is 1 Hz rTMS Always Inhibitory in Healthy Individuals? Open. Neuroimag. J. 2012, 6, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Betti, S.; Fedele, M.; Castiello, U.; Sartori, L.; Budisavljevic, S. Corticospinal excitability and conductivity are related to the anatomy of the corticospinal tract. Brain Struct. Funct. 2022, 227, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Pazzaglia, C.; Imbimbo, I.; Tranchita, E.; Minganti, C.; Ricciardi, D.; Lo Monaco, R.; Parisi, A.; Padua, L. Comparison of virtual reality rehabilitation and conventional rehabilitation in Parkinson’s disease: A randomised controlled trial. Physiotherapy 2020, 106, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Levac, D.E.; Huber, M.E.; Sternad, D. Learning and transfer of complex motor skills in virtual reality: A perspective review. J. Neuroeng. Rehabil. 2019, 16, 121. [Google Scholar] [CrossRef]
- Levin, M.F.; Demers, M. Motor learning in neurological rehabilitation. Disabil. Rehabil. 2021, 43, 3445–3453. [Google Scholar] [CrossRef]
- Zelik, K.E.; Honert, E.C. Ankle and foot power in gait analysis: Implications for science, technology and clinical assessment. J. Biomech. 2018, 75, 1–12. [Google Scholar] [CrossRef]
- Li, S. Ankle and Foot Spasticity Patterns in Chronic Stroke Survivors with Abnormal Gait. Toxins 2020, 12, 646. [Google Scholar] [CrossRef] [PubMed]
- Bensoussan, L.; Mesure, S.; Viton, J.M.; Delarque, A. Kinematic and kinetic asymmetries in hemiplegic patients’ gait initiation patterns. J. Rehabil. Med. 2006, 38, 287–294. [Google Scholar] [CrossRef]
- Daly, J.J.; Zimbelman, J.; Roenigk, K.L.; McCabe, J.P.; Rogers, J.M.; Butler, K.; Burdsall, R.; Holcomb, J.P.; Marsolais, E.B.; Ruff, R.L. Recovery of coordinated gait: Randomized controlled stroke trial of functional electrical stimulation (FES) versus no FES, with weight-supported treadmill and over-ground training. Neurorehabil. Neural Repair 2011, 25, 588–596. [Google Scholar] [CrossRef]
- Laver, K.E.; Lange, B.; George, S.; Deutsch, J.E.; Saposnik, G.; Crotty, M. Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2017, 11, CD008349. [Google Scholar] [CrossRef]
| Group E1 (n = 10) | Group E2 (n = 10) | Group C (n = 10) | F (r) | p | |
|---|---|---|---|---|---|
| Age, year | 62.3 ± 15.3 | 56.4 ± 17.5 | 61.1 ± 13.2 | 0.406 | 0.670 |
| Sex, male/female | 6/4 | 6/4 | 7/3 | (0.287) | 0.866 |
| Hemispheric side, left/right | 6/4 | 5/5 | 4/6 | (0.800) | 0.670 |
| Modified Ashworth Scale, MAS | 0.8 ± 0.9 | 0.9 ± 0.8 | 1.2 ± 1.0 | 0.485 | 0.621 |
| Mini-Mental State Examination | 29.9 ± 0.3 | 30 ± 0.1 | 29.6 ± 0.9 | 1.258 | 0.300 * |
| Time poststroke, months | 29.8 ± 20.9 | 31.6 ± 23.8 | 48.0 ± 29.0 | 1.635 | 0.214 * |
| Br. Stage 1 of lower extremity | 3.8 ± 0.8 | 3.7 ± 0.5 | 3.5 ± 0.8 | 0.444 | 0.646 |
| Pretest | Posttest | Change | Pa for Intragroup Difference | Pb for Intergroup Difference | |
|---|---|---|---|---|---|
| Fugl–Meyer Assessment of Lower Extremity (FMA-LE) | |||||
| Group E1 | 25.1 ± 9.2 | 25.6 ± 8.9 | 0.5 ± 0.8 | 0.102 | 0.441 |
| Group E2 | 19.7 ± 8.7 | 20.6 ± 8.9 | 0.9 ± 2.1 | 0.102 | |
| Group C | 24.9 ± 7.1 | 25.0 ± 7.0 | 0.1 ± 0.3 | 0.317 | |
| Berg Balance Scale (BBS) | |||||
| Group E1 | 41.7 ± 11.5 | 43.6 ± 10.9 | 1.9 ± 1.5 | 0.011 * | 0.167 |
| Group E2 | 34.7 ± 13.8 | 37.5 ± 14.9 | 2.8 ± 4.6 | 0.066 | |
| Group C | 40.3 ± 18.2 | 41.4 ± 18.5 | 1.1 ± 2.5 | 0.109 | |
| Time Up and Go (TUG) | |||||
| Group E1 | 39.3 ± 32.2 | 29.8 ± 17.2 | −9.4 ± 17.8 | 0.008 * | 0.052 |
| Group E2 | 51.4 ± 40.2 | 49.1 ± 40.2 | −2.2 ± 4.3 | 0.093 | |
| Group C | 30.8 ± 23.9 | 30.5 ± 39.4 | −0.2 ± 1.6 | 0.541 | |
| Pretest | Posttest | Change | Pa for Intragroup Difference | Pb for Intergroup Difference | |
|---|---|---|---|---|---|
| MEP latency UH, ms | |||||
| Group E1 | 27.1 ± 1.9, n = 10 | 29.8 ± 3.1, n = 10 | 2.6 ± 3.5 | 0.011 * (−2.547) | 0.092 |
| Group E2 | 27.9 ± 2.9, n = 10 | 28.1 ± 2.7, n = 10 | 0.2 ± 3.0 | 0.799 (−0.255) | |
| Group C | 27.1 ± 2.2, n = 10 | 28.4 ± 1.5, n = 10 | 1.2 ± 1.9 | 0.093 (−1.682) | |
| MEP amplitude UH, mV | |||||
| Group E1 | 0.9 ± 0.8, n = 10 | 0.7 ± 0.5, n = 10 | −0.1 ± 0.3 | 0.205 (−1.268) | 0.546 |
| Group E2 | 1.0 ± 0.3, n = 10 | 1.0 ± 0.3, n = 10 | 0.02 ± 0.5 | 0.758 (−0.308) | |
| Group C | 1.1 ± 0.7, n = 10 | 1.3 ± 1.0, n = 10 | 0.1 ± 0.7 | 0.989 (−0.001) | |
| MEP latency AH, ms | |||||
| Group E1 | 34.2 ± 3.5, n = 6 | 30.7 ± 4.1, n = 7 | 0.9 ± 12.8 | 0.116 (−1.572) | 0.413 |
| Group E2 | 32.9 ± 3.6, n = 6 | 32.8 ± 5.4, n = 6 | −0.03 ± 4.8 | 0.917 (−0.105) | |
| Group C | 32.0 ± 3.0, n = 7 | 33.2 ± 4.1, n = 7 | 0.8 ± 3.9 | 0.674 (−0.420) | |
| MEP amplitude AH, mV | |||||
| Group E1 | 0.5 ± 0.2, n = 6 | 1.0 ± 0.7, n = 7 | 0.3 ± 0.5 | 0.027 * (−2.207) | 0.006 * |
| Group E2 | 0.5 ± 0.2, n = 6 | 0.5 ± 0.5, n = 6 | −0.03 ± 0.2 | 0.581 (−0.552) | |
| Group C | 0.5 ± 0.3, n = 7 | 0.4 ± 0.3, n = 7 | −0.06 ± 0.2 | 0.343 (−0.948) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.-L.; Lin, C.-H.; Tseng, S.-H.; Peng, C.-W.; Lai, C.-H. Effectiveness of Repetitive Transcranial Magnetic Stimulation Combined with Visual Feedback Training in Improving Neuroplasticity and Lower Limb Function after Chronic Stroke: A Pilot Study. Biology 2023, 12, 515. https://doi.org/10.3390/biology12040515
Cheng H-L, Lin C-H, Tseng S-H, Peng C-W, Lai C-H. Effectiveness of Repetitive Transcranial Magnetic Stimulation Combined with Visual Feedback Training in Improving Neuroplasticity and Lower Limb Function after Chronic Stroke: A Pilot Study. Biology. 2023; 12(4):515. https://doi.org/10.3390/biology12040515
Chicago/Turabian StyleCheng, Hsien-Lin, Chueh-Ho Lin, Sung-Hui Tseng, Chih-Wei Peng, and Chien-Hung Lai. 2023. "Effectiveness of Repetitive Transcranial Magnetic Stimulation Combined with Visual Feedback Training in Improving Neuroplasticity and Lower Limb Function after Chronic Stroke: A Pilot Study" Biology 12, no. 4: 515. https://doi.org/10.3390/biology12040515
APA StyleCheng, H.-L., Lin, C.-H., Tseng, S.-H., Peng, C.-W., & Lai, C.-H. (2023). Effectiveness of Repetitive Transcranial Magnetic Stimulation Combined with Visual Feedback Training in Improving Neuroplasticity and Lower Limb Function after Chronic Stroke: A Pilot Study. Biology, 12(4), 515. https://doi.org/10.3390/biology12040515







