Does Exercise-Induced Hypoalgesia Depend on Exercise Duration?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
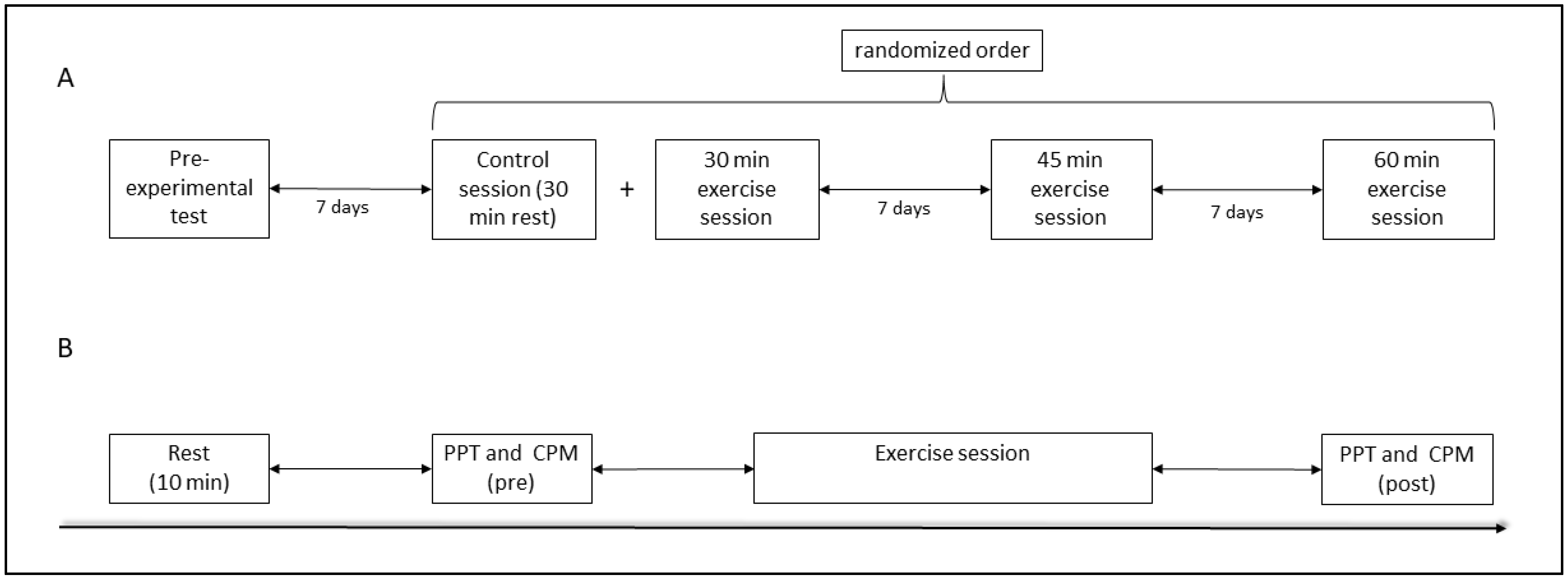
2.1. Study Population and Experimental Design
2.2. Pre-Experimental Test
2.3. Exercise Sessions
2.4. Pressure Pain Threshold Assessment
2.5. Conditioned Pain Modulation Assessment
2.6. Statistics
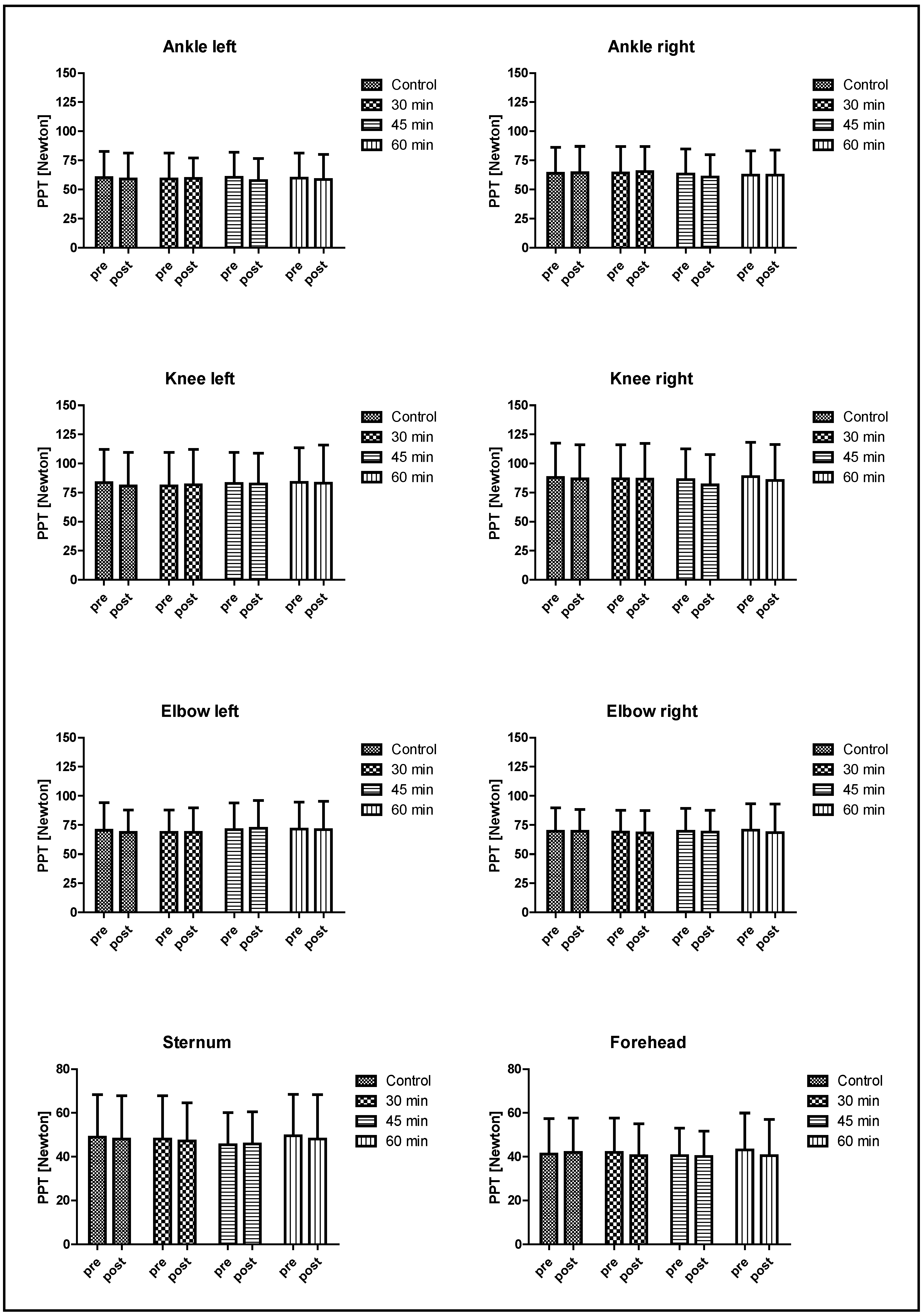
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodges, P.W.; Smeets, R.J. Interaction between pain, movement, and physical activity: Short-term benefits, long-term consequences, and targets for treatment. Clin. J. Pain 2015, 31, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.; Nijs, J.; Kosek, E.; Wideman, T.; Hasenbring, M.I.; Koltyn, K.; Graven-Nielsen, T.; Polli, A. Exercise-Induced Hypoalgesia in Pain-Free and Chronic Pain Populations: State of the Art and Future Directions. J. Pain 2019, 20, 1249–1266. [Google Scholar] [CrossRef]
- Wewege, M.A.; Jones, M.D. Exercise-Induced Hypoalgesia in Healthy Individuals and People With Chronic Musculoskeletal Pain: A Systematic Review and Meta-Analysis. J. Pain 2021, 22, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Vaegter, H.B.; Jones, M.D. Exercise-induced hypoalgesia after acute and regular exercise: Experimental and clinical manifestations and possible mechanisms in individuals with and without pain. Pain Rep. 2020, 5, e823. [Google Scholar] [CrossRef]
- Naugle, K.M.; Fillingim, R.B.; Riley, J.L. A meta-analytic review of the hypoalgesic effects of exercise. J. Pain 2012, 13, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Micalos, P.S.; Arendt-Nielsen, L. Differential pain response at local and remote muscle sites following aerobic cycling exercise at mild and moderate intensity. Springerplus 2016, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Tomschi, F.; Lieverkus, D.; Hilberg, T. Exercise-induced hypoalgesia (EIH) in response to different exercise intensities. Eur. J. Appl. Physiol. 2022, 122, 2213–2222. [Google Scholar] [CrossRef]
- Micalos, P.S. Perspectives on biochemical and neurosensory mechanisms for exercise-induced pain inhibition. Fatigue Biomed. Health Behav. 2014, 2, 219–230. [Google Scholar] [CrossRef]
- Black, J.; Starmer, G.A.; Egger, G. The Painlessness of the Long Distance Runner. Med. J. Aust. 1979, 1, 522–523. [Google Scholar] [CrossRef]
- Janal, M.N.; Colt, E.W.D.; Clark, C.W.; Glusman, M. Pain sensitivity, mood and plasma endocrine levels in man following long-distance running: Effects of naloxone. Pain 1984, 19, 13–25. [Google Scholar] [CrossRef]
- Tesarz, J.; Schuster, A.K.; Hartmann, M.; Gerhardt, A.; Eich, W. Pain perception in athletes compared to normally active controls: A systematic review with meta-analysis. Pain 2012, 153, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Jackek, L.; Sliz, D.; Szczepan, W.; Szymon, P.; Arkadiusz, B.; Mamcarz, A. How to calculate a maximum heart rate correctly? Folia Cardiol. 2022, 17, 289–292. [Google Scholar] [CrossRef]
- Triantafyllidi, H.; Birmpa, D.; Benas, D.; Trivilou, P.; Fambri, A.; Iliodromitis, E.K. Cardiopulmonary Exercise Testing: The ABC for the Clinical Cardiologist. Cardiology 2022, 147, 62–71. [Google Scholar] [CrossRef]
- Bizjak, D.A.; Tomschi, F.; Bales, G.; Nader, E.; Romana, M.; Connes, P.; Bloch, W.; Grau, M. Does endurance training improve red blood cell aging and hemorheology in moderate-trained healthy individuals? J. Sport Health Sci. 2020, 9, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.; Petersen, K.K.; Sloth, E.; Manum, L.A.; McDonald, A.K.; Andersen, P.G.; Vaegter, H.B. Hypoalgesia after exercises with painful vs. non-painful muscles in healthy subjects—A randomized cross-over study. Scand. J. Pain 2022, 22, 614–621. [Google Scholar] [CrossRef]
- Katz-Betzalel, N.; Weissman-Fogel, I.; Kodesh, E. Aerobic Upper-Limb Exercise-Induced Hypoalgesia: Does It Work? Appl. Sci. 2022, 12, 11391. [Google Scholar] [CrossRef]
- Vaegter, H.B.; Lyng, K.D.; Yttereng, F.W.; Christensen, M.H.; Sørensen, M.B.; Graven-Nielsen, T. Exercise-Induced Hypoalgesia After Isometric Wall Squat Exercise: A Test-Retest Reliabilty Study. Pain Med. 2019, 20, 129–137. [Google Scholar] [CrossRef]
- Hilberg, T.; Czepa, D.; Freialdenhoven, D.; Boettger, M.K. Joint pain in people with hemophilia depends on joint status. Pain 2011, 152, 2029–2035. [Google Scholar] [CrossRef]
- Krüger, S.; Khayat, D.; Hoffmeister, M.; Hilberg, T. Pain thresholds following maximal endurance exercise. Eur. J. Appl. Physiol. 2016, 116, 535–540. [Google Scholar] [CrossRef]
- Krüger, S.; Hilberg, T. Understanding the pain profile in patients with haemophilia: Impaired descending pain inhibition as measured by conditioned pain modulation. Haemophilia 2020, 26, 236–242. [Google Scholar] [CrossRef]
- Gomolka, S.; Vaegter, H.B.; Nijs, J.; Meeus, M.; Gajsar, H.; Hasenbring, M.I.; Titze, C. Assessing Endogenous Pain Inhibition: Test-Retest Reliability of Exercise-Induced Hypoalgesia in Local and Remote Body Parts After Aerobic Cycling. Pain Med. 2019, 20, 2272–2282. [Google Scholar] [CrossRef] [PubMed]
- Böing-Meßing, D.M.; Tomschi, F.; Cegla, T.; Hilberg, T. The eEgg: Evaluation of a New Device to Measure Pain. Front. Physiol. 2022, 13, 832172. [Google Scholar] [CrossRef] [PubMed]
- Hakansson, S.; Jones, M.D.; Ristov, M.; Marcos, L.; Clark, T.; Ram, A.; Morey, R.; Franklin, A.; McCarthy, C.; Carli, L.D.; et al. Intensity-dependent effects of aerobic training on pressure pain threshold in overweight men: A randomized trial. Eur. J. Pain 2018, 22, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Kodesh, E.; Weissman-Fogel, I. Exercise-induced hypoalgesia—Interval versus continuous mode. Appl. Physiol. Nutr. Metab. 2014, 39, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Monnier-Benoit, P.; Groslambert, A. Effects of steady-state exercise on perceived pain: Comparison of sedentary students and cyclists. Percept. Mot. Skills 2006, 103, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.D.; Shepanski, M.A.; Ruble, S.B.; Valic, Z.; Buckwalter, J.B.; Clifford, P.S. Intensity and duration threshold for aerobic exercise-induced analgesia to pressure pain. Arch. Phys. Med. Rehabil. 2004, 85, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- Naugle, K.M.; Naugle, K.E.; Fillingim, R.B.; Samuels, B.; Riley, J.L. Intensity thresholds for aerobic exercise-induced hypoalgesia. Med. Sci. Sports Exerc. 2014, 46, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Vaegter, H.B.; Bjerregaard, L.K.; Redin, M.-M.; Rasmussen, S.H.; Graven-Nielsen, T. Hypoalgesia after bicycling at lactate threshold is reliable between sessions. Eur. J. Appl. Physiol. 2019, 119, 91–102. [Google Scholar] [CrossRef]
- Schoenfeld, T.J.; Swanson, C. A Runner’s High for New Neurons? Potential Role for Endorphins in Exercise Effects on Adult Neurogenesis. Biomolecules 2021, 11, 1077. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, L.; Kindermann, W. Beta-endorphin, adrenocorticotropic hormone, cortisol and catecholamines during aerobic and anaerobic exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 61, 165–171. [Google Scholar] [CrossRef]
- Schwarz, L.; Kindermann, W. Beta-endorphin, catecholamines, and cortisol during exhaustive endurance exercise. Int. J. Sports Med. 1989, 10, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Sprouse-Blum, A.S.; Smith, G.; Sugai, D.; Parsa, F.D. Understanding endorphins and their importance in pain management. Hawaii Med. J. 2010, 69, 70–71. [Google Scholar] [PubMed]
- Jones, M.D.; Valenzuela, T.; Booth, J.; Taylor, J.L.; Barry, B.K. Explicit Education About Exercise-Induced Hypoalgesia Influences Pain Responses to Acute Exercise in Healthy Adults: A Randomized Controlled Trial. J. Pain 2017, 18, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Oostinga, D.; Steverink, J.G.; van Wijck, A.J.M.; Verlaan, J.-J. An understanding of bone pain: A narrative review. Bone 2020, 134, 115272. [Google Scholar] [CrossRef]
- Maeda, L.; Ono, M.; Koyama, T.; Oshiro, Y.; Sumitani, M.; Mashimo, T.; Shibata, M. Human brain activity associated with painful mechanical stimulation to muscle and bone. J. Anesth. 2011, 25, 523–530. [Google Scholar] [CrossRef]
- Hviid, J.-C.T.; Thorlund, J.B.; Vaegter, H.B. Walking increases pain tolerance in humans: An experimental cross-over study. Scand. J. Pain 2019, 19, 813–822. [Google Scholar] [CrossRef]
- Vaegter, H.B.; Dørge, D.B.; Schmidt, K.S.; Jensen, A.H.; Graven-Nielsen, T. Test-Retest Reliabilty of Exercise-Induced Hypoalgesia After Aerobic Exercise. Pain Med. 2018, 19, 2212–2222. [Google Scholar] [CrossRef]
- Ellingson, L.D.; Koltyn, K.F.; Kim, J.-S.; Cook, D.B. Does exercise induce hypoalgesia through conditioned pain modulation? Psychophysiology 2014, 51, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Hackett, J.; Naugle, K.E.; Naugle, K.M. The Decline of Endogenous Pain Modulation With Aging: A Meta-Analysis of Temporal Summation and Conditioned Pain Modulation. J. Pain 2020, 21, 514–528. [Google Scholar] [CrossRef]
- Iacovides, S.; Avidon, I.; Baker, F.C. Does pain vary across the menstrual cycle? A review. Eur. J. Pain 2015, 19, 1389–1405. [Google Scholar] [CrossRef]
| Parameter | Subjects (n = 36) |
|---|---|
| Gender [male/female] | 18/18 |
| Age [years] | 26.6 ± 3.2 |
| Height [m] | 1.8 ± 0.1 |
| Weight [kg] | 73.3 ± 11.1 |
| VO2max [mL/min/kg] | 42.5 ± 8.0 |
| 75% of VO2max [mL/min/kg] | 31.8 ± 6.0 |
| Max. heart rate [1/min] | 184.2 ± 9.0 |
| Peak Power [W] | 259.7 ± 57.1 |
| Max. lactate [mmol/L] | 11.3 ± 2.3 |
| Power at 75% of VO2max [W] | 172.2 ± 39.1 |
| Parameter | Time | 30 Min Exercise Session | 45 Min Exercise Session | 60 Min Exercise Session |
|---|---|---|---|---|
| RPE (6–20) | 10 Min | 13.4 ± 1.8 | 12.9 ± 1.6 | 12.9 ± 1.7 |
| 20 Min | 14.8 ± 1.8 | 14.5 ± 1.5 | 14.2 ± 1.6 | |
| 30 Min | 15.6 ± 2.0 | 15.2 ± 1.6 | 15.2 ± 1.7 | |
| 40 Min | X | 16.1 ± 1.7 | 15.7 ± 1.7 | |
| 45 Min | X | 16.5 ± 1.7 * | X | |
| 50 Min | X | X | 16.3 ± 1.8 | |
| 60 Min | X | X | 16.7 ± 1.9 * | |
| HR (1/Min) | 0 Min | 64.5 ± 11.0 | 68.3 ± 11.8 | 68.8 ± 14.2 |
| 10 Min | 151.2 ± 13.9 | 150.2 ± 14.3 | 151.4 ± 13.6 | |
| 20 Min | 159.2 ± 13.2 | 157.9 ± 13.2 | 158.4 ± 10.8 | |
| 30 Min | 162.5 ± 12.9 | 162.2 ± 12.5 | 163.1 ± 9.3 | |
| 40 Min | X | 164.8 ± 12.6 | 164.8 ± 9.3 | |
| 45 Min | X | 165.8 ± 12.4 * | X | |
| 50 Min | X | X | 166.5 ± 8.8 | |
| 60 Min | X | X | 167.8 ± 9.4 * | |
| Lactate (mmoL/L) | Pre | 0.9 ± 0.3 | 0.9 ± 0.2 | 1.0 ± 0.3 |
| Post | 4.2 ± 1.9 | 3.4 ± 1.5 * | 3.0 ± 1.7 * |
| Time Point | Control Session | 30 Min Exercise Session | 45 Min Exercise Session | 60 Min Exercise Session | |
|---|---|---|---|---|---|
| Stand-alone conditioning test stimulus (saCS) (NRS points) | pre post | 57.6 (19.6) # 59.6 (20.0) * | 59.6 (20.1) 55.0 (19.4) * | 57.6 (21.3) 58.0 (22.0) | 56.1 (21.4) 57.6 (20.6) |
| Stand-alone test stimulus (saTS) (NRS points) | pre post | 52.4 (12.1) 52.1 (11.7) | 52.0 (12.3) 47.2 (13.3) | 52.5 (14.0) 46.4 (15.0) * | 52.8 (12.3) 46.0 (13.7) * |
| Test stimulus Intensity under conditioning influence (ciTS) (NRS points) | pre post | 27.2 (18.0) 27.5 (19.1) | 27.6 (19.3) 23.9 (17.9) | 26.6 (17.1) 23.8 (16.7) | 29.7 (18.2) 22.9 (17.2) |
| CPM response (CPMre) (NRS points) | pre post | −25.2 (17.8) −24.6 (16.1) | −24.4 (16.9) −23.3 (16.4) | −26.0 (17.5) −22.6 (16.7) | −23.1 (16.9) −23.1 (17.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomschi, F.; Kieckbusch, L.; Zachow, J.; Hilberg, T. Does Exercise-Induced Hypoalgesia Depend on Exercise Duration? Biology 2023, 12, 222. https://doi.org/10.3390/biology12020222
Tomschi F, Kieckbusch L, Zachow J, Hilberg T. Does Exercise-Induced Hypoalgesia Depend on Exercise Duration? Biology. 2023; 12(2):222. https://doi.org/10.3390/biology12020222
Chicago/Turabian StyleTomschi, Fabian, Luisa Kieckbusch, Julius Zachow, and Thomas Hilberg. 2023. "Does Exercise-Induced Hypoalgesia Depend on Exercise Duration?" Biology 12, no. 2: 222. https://doi.org/10.3390/biology12020222
APA StyleTomschi, F., Kieckbusch, L., Zachow, J., & Hilberg, T. (2023). Does Exercise-Induced Hypoalgesia Depend on Exercise Duration? Biology, 12(2), 222. https://doi.org/10.3390/biology12020222






