Therapeutic Delivery of Tumor Suppressor miRNAs for Breast Cancer Treatment
Abstract
Simple Summary
Abstract
1. Introduction
2. Tumor Suppressor miRNAs in BC
2.1. Mir-125 a, b
2.2. Let-7
2.3. miR 31
2.4. miRNA-34a
2.5. miR-200
2.6. miR-145
2.7. miR-335
2.8. miR-203
2.9. miRNA-339-5p
2.10. miRNA-433
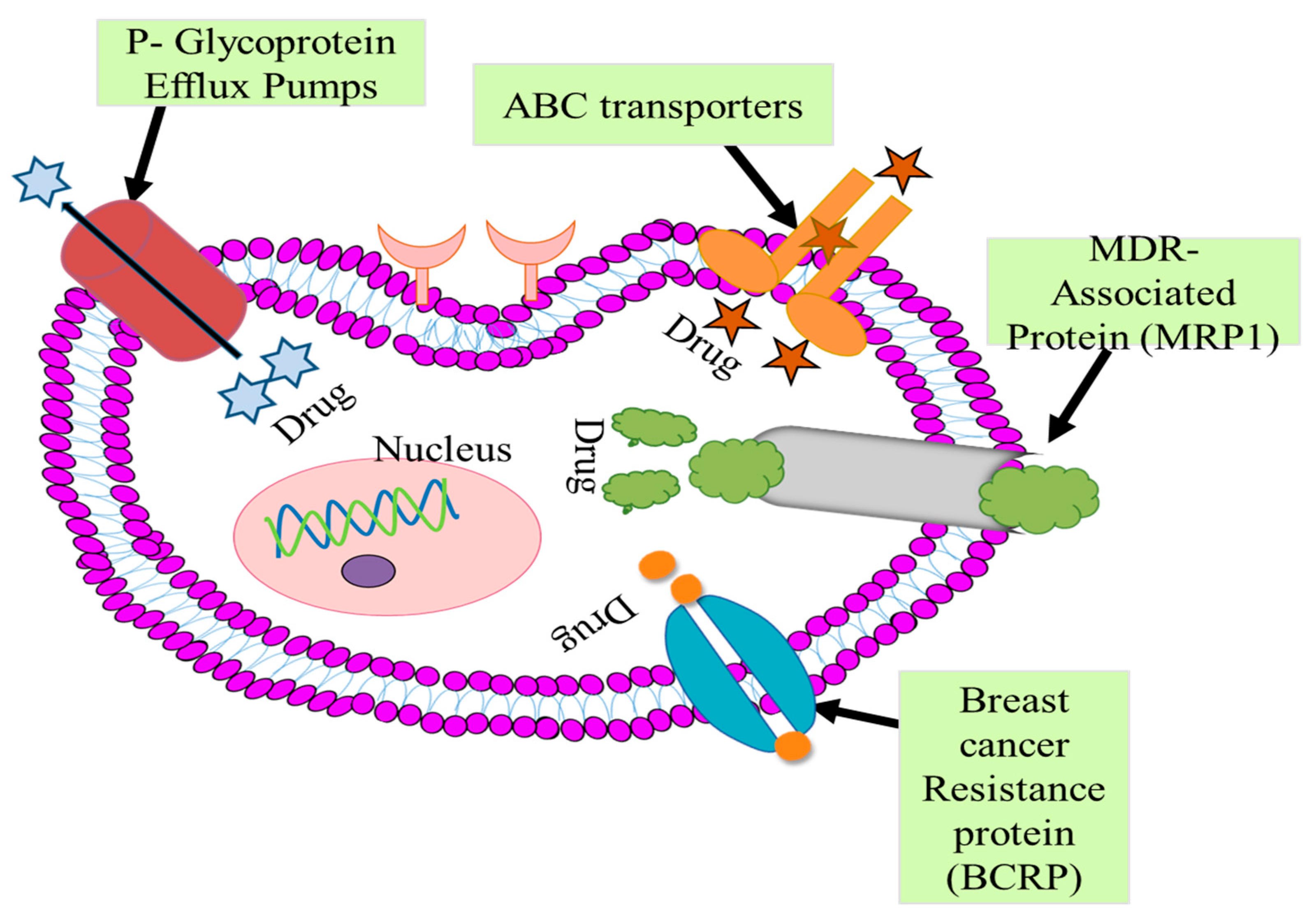
3. Challenges Associated with Traditional BC Therapies
3.1. P-Glycoprotein Efflux Pumps
3.2. ABC-Transporter
3.3. MDR-Associated Protein (MRP1)
Multidrug Resistance
3.4. Breast Cancer Resistance Protein
3.5. Microtubule Alteration
4. In Vivo Studies of Various Delivery Platforms
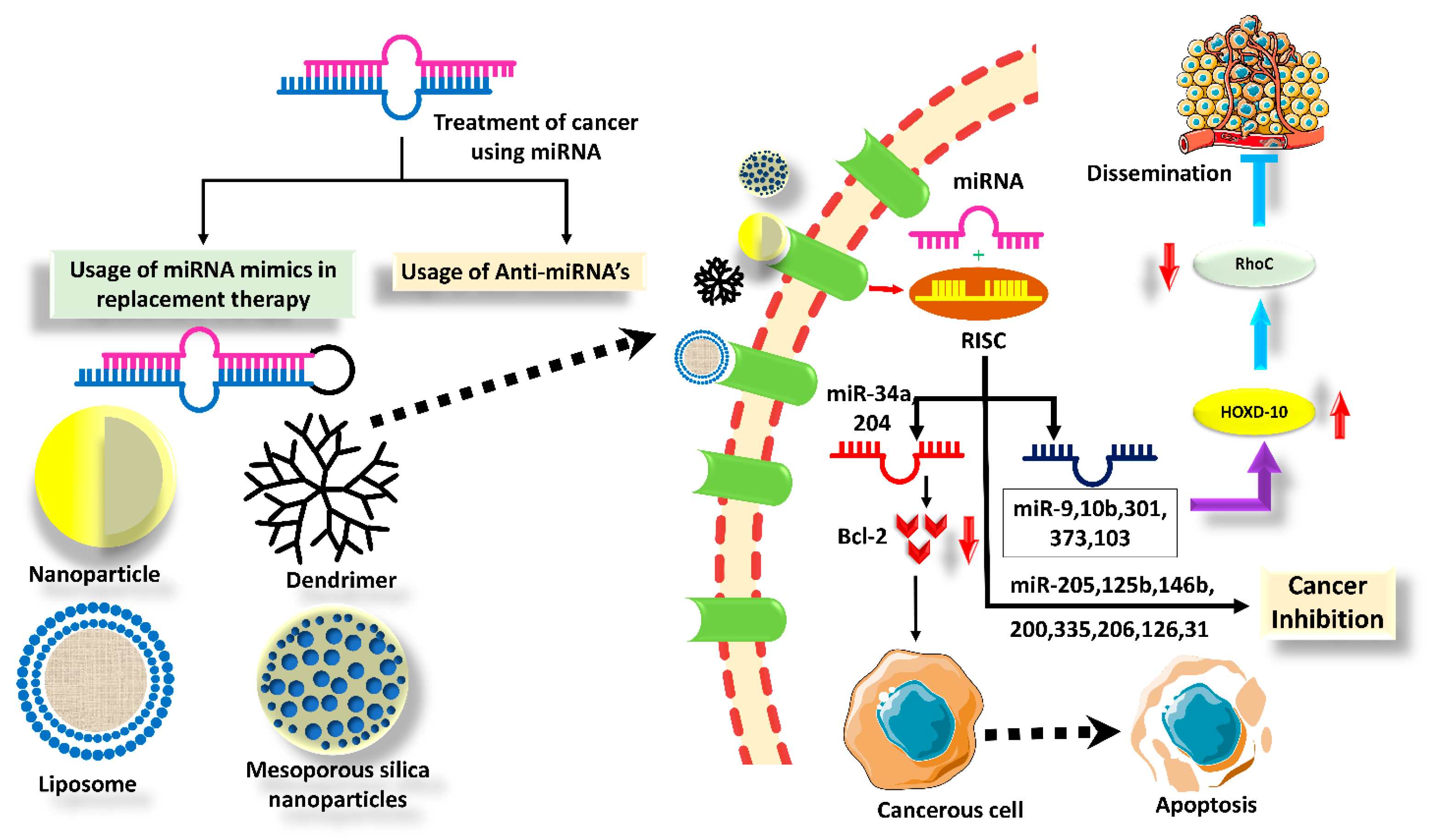
5. Nanotechnology Enabled the Delivery of TS miRNAs in BC
5.1. PLGA Particles
5.2. Dendrimers
5.3. Polyethyleneimine (PEI)
5.4. Liposomes
5.5. Modified Extracellular Vesicles (EVs)
5.6. Challenges in Nano Drug Delivery
6. Advanced Strategies for TS miRNA Delivery in BC
6.1. Viral Delivery
6.2. Self-Assembled RNA-Triple-Helix Hydrogel Drug Delivery System
6.3. Hyaluronic Acid/Protamine Sulfate Interpolyelectrolyte Complexes (HP/IPECs)
7. Discussion
8. Future Prospects
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, O.; Bray, F.; Coleman, M.P.; Vanderpuye, V.; Eniu, A.; Kotha, S.R.; Sarker, M.; Huong, T.T.; Allemani, C.; Dvaladze, A.; et al. The Global Burden of Women’s Cancers: A Grand Challenge in Global Health. Lancet 2017, 389, 847–860. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, X.; Li, X.; Xu, G.; Bai, Y.; Wu, J.; Piao, Y.; Shi, Y.; Xiang, R.; Wang, L. Nucleotide de Novo Synthesis Increases Breast Cancer Stemness and Metastasis via CGMP-PKG-MAPK Signaling Pathway. PLoS Biol. 2020, 18, e3000872. [Google Scholar] [CrossRef]
- Mohankumar, K.M.; Currle, D.S.; White, E.; Boulos, N.; Dapper, J.; Eden, C.; Nimmervoll, B.; Thiruvenkatam, R.; Connelly, M.; Kranenburg, T.A.; et al. An in Vivo Screen Identifies Ependymoma Oncogenes and Tumor-Suppressor Genes. Nat. Genet. 2015, 47, 878–887. [Google Scholar] [CrossRef]
- Garreffa, E.; Arora, D. Breast Cancer in the Elderly, in Men and during Pregnancy. Available online: https://agegap.shef.ac.uk (accessed on 12 January 2023).
- Ying, S.Y.; Chang, D.C.; Lin, S.L. The MicroRNA (MiRNA): Overview of the RNA Genes That Modulate Gene Function. Mol. Biotechnol. 2008, 38, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, F.; Tadayyon Tabrizi, A.; Hashemian, P.; Alijannejad, S.; Rahdar, H.A.; Ferns, G.A.; Hassanian, S.M.; Shahidsales, S.; Avan, A. Role of Regulatory MiRNAs of the Wnt/β-Catenin Signaling Pathway in Tumorigenesis of Breast Cancer. Gene 2020, 754, 144892. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Zhou, D. MicroRNAs in Colorectal Carcinoma—From Pathogenesis to Therapy. J. Exp. Clin. Cancer Res. 2016, 35, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- De Rie, D.; Abugessaisa, I.; Alam, T.; Arner, E.; Arner, P.; Ashoor, H.; Åström, G.; Babina, M.; Bertin, N.; Burroughs, A.M.; et al. An Integrated Expression Atlas of MiRNAs and Their Promoters in Human and Mouse. Nat. Biotechnol. 2017, 35, 872–878. [Google Scholar] [CrossRef]
- Wang, J.; Samuels, D.C.; Zhao, S.; Xiang, Y.; Zhao, Y.Y.; Guo, Y. Current Research on Non-Coding Ribonucleic Acid (RNA). Genes 2017, 8, 366. [Google Scholar] [CrossRef]
- Blenkiron, C.; Miska, E.A. MiRNAs in Cancer: Approaches, Aetiology, Diagnostics and Therapy. Hum. Mol. Genet. 2007, 16, R106–R113. [Google Scholar] [CrossRef]
- He, Y.; Lin, J.; Kong, D.; Huang, M.; Xu, C.; Kim, T.K.; Etheridge, A.; Luo, Y.; Ding, Y.; Wang, K. Current State of Circulating MicroRNAs as Cancer Biomarkers. Clin. Chem. 2015, 61, 1138–1155. [Google Scholar] [CrossRef]
- Liu, Q.; Peng, F.; Chen, J. The Role of Exosomal Micrornas in the Tumor Microenvironment of Breast Cancer. Int. J. Mol. Sci. 2019, 20, 3884. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Z. The Emerging Role of MicroRNAs in Breast Cancer. J. Oncol. 2020, 2020, 9160905. [Google Scholar] [CrossRef]
- Nurzadeh, M.; Naemi, M.; Sheikh Hasani, S. A Comprehensive Review on Oncogenic MiRNAs in Breast Cancer. J. Genet. 2021, 100, 1–21. [Google Scholar] [CrossRef]
- Grimaldi, A.M.; Salvatore, M.; Incoronato, M. MiRNA-Based Therapeutics in Breast Cancer: A Systematic Review. Front. Oncol. 2021, 11, 668464. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, M.; Coppola, V.; Addario, A.; Patrizii, M.; Maugeri-Saccá, M.; Memeo, L.; Colarossi, C.; Francescangeli, F.; Biffoni, M.; Collura, D.; et al. Control of Tumor and Microenvironment Cross-Talk by MiR-15a and MiR-16 in Prostate Cancer. Oncogene 2011, 30, 4231–4242. [Google Scholar] [CrossRef]
- Baselga, J.; Swain, S.M. Novel Anticancer Targets: Revisiting ERBB2 and Discovering ERBB3. Nat. Rev. Cancer 2009, 9, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Mattie, M.D.; Benz, C.C.; Bowers, J.; Sensinger, K.; Wong, L.; Scott, G.K.; Fedele, V.; Ginzinger, D.; Getts, R.; Haqq, C. Optimized High-Throughput MicroRNA Expression Profiling Provides Novel Biomarker Assessment of Clinical Prostate and Breast Cancer Biopsies. Mol. Cancer 2006, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.K.; Goga, A.; Bhaumik, D.; Berger, C.E.; Sullivan, C.S.; Benz, C.C. Coordinate Suppression of ERBB2 and ERBB3 by Enforced Expression of Micro-RNA MiR-125a or MiR-125b. J. Biol. Chem. 2007, 282, 1479–1486. [Google Scholar] [CrossRef]
- Yu, F.; Yao, H.; Zhu, P.; Zhang, X.; Pan, Q.; Gong, C.; Huang, Y.; Hu, X.; Su, F.; Lieberman, J.; et al. Let-7 Regulates Self Renewal and Tumorigenicity of Breast Cancer Cells. Cell 2007, 131, 1109–1123. [Google Scholar] [CrossRef]
- Dangi-Garimella, S.; Yun, J.; Eves, E.M.; Newman, M.; Erkeland, S.J.; Hammond, S.M.; Minn, A.J.; Rosner, M.R. Raf Kinase Inhibitory Protein Suppresses a Metastasis Signalling Cascade Involving LIN28 and Let-7. EMBO J. 2009, 28, 347–358. [Google Scholar] [CrossRef]
- Sarkadi, B.; Homolya, L.; Szakács, G.; Váradi, A. Human Multidrug Resistance ABCB and ABCG Transporters: Participation in a Chemoimmunity Defense System. Physiol. Rev. 2006, 86, 1179–1236. [Google Scholar] [CrossRef] [PubMed]
- Barrette-Lee, P. A Pleiotropically Acting MicroRNA, MiR-31, Inhibits Breast Cancer Metastasis. Adv. Breast Cancer 2009, 6, 24–25. [Google Scholar]
- Yamakuchi, M.; Ferlito, M.; Lowenstein, C.J. MiR-34a Repression of SIRT1 Regulates Apoptosis. 2008. Available online: www.pnas.org/cgi/content/full/ (accessed on 1 January 2023).
- Asghari, F.; Haghnavaz, N.; Baradaran, B.; Hemmatzadeh, M.; Kazemi, T. Tumor Suppressor MicroRNAs: Targeted Molecules and Signaling Pathways in Breast Cancer. Biomed. Pharmacother. 2016, 81, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Das, P.K.; Siddika, M.A.; Asha, S.Y.; Aktar, S.; Rakib, M.A.; Khanam, J.A.; Pillai, S.; Islam, F. MicroRNAs, a Promising Target for Breast Cancer Stem Cells. Mol. Diagn. Ther. 2020, 24, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M.; Mo, Y.Y. MicroRNA-145 Suppresses Cell Invasion and Metastasis by Directly Targeting Mucin 1. Cancer Res. 2010, 70, 378–387. [Google Scholar] [CrossRef]
- Tavazoie, S.F.; Alarcón, C.; Oskarsson, T.; Padua, D.; Wang, Q.; Bos, P.D.; Gerald, W.L.; Massagué, J. Endogenous Human MicroRNAs That Suppress Breast Cancer Metastasis. Nature 2008, 451, 147–152. [Google Scholar] [CrossRef]
- Heyn, H.; Engelmann, M.; Schreek, S.; Ahrens, P.; Lehmann, U.; Kreipe, H.; Schlegelberger, B.; Beger, C. MicroRNA MiR-335 Is Crucial for the BRCA1 Regulatory Cascade in Breast Cancer Development. Int. J. Cancer 2011, 129, 2797–2806. [Google Scholar] [CrossRef]
- Palareti, G.; Legnani, C.; Cosmi, B.; Antonucci, E.; Erba, N.; Poli, D.; Testa, S.; Tosetto, A. Comparison between Different D-Dimer Cutoff Values to Assess the Individual Risk of Recurrent Venous Thromboembolism: Analysis of Results Obtained in the DULCIS Study. Int. J. Lab. Hematol. 2016, 38, 42–49. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, X.; Dhakal, I.B.; Beggs, M.; Kadlubar, S.; Luo, D. Induction of Cell Proliferation and Survival Genes by Estradiol-Repressed MicroRNAs in Breast Cancer Cells. BMC Cancer 2012, 12, 29. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Wu, Q.; Wang, C.-Q.; Wang, X.-N.; Wang, Y.; Zhao, J.-J.; Mao, S.-S.; Zhang, G.-H.; Zhang, N.; Xu, X.-C. MiR-339-5p Inhibits Breast Cancer Cell Migration and Invasion In Vitro and May Be a Potential Biomarker for Breast Cancer Prognosis. 2010. Available online: http://www.biomedcentral.com/1471-2407/10/542 (accessed on 10 January 2023).
- Jansson, M.D.; Damas, N.D.; Lees, M.; Jacobsen, A.; Lund, A.H. MiR-339-5p Regulates the P53 Tumor-Suppressor Pathway by Targeting MDM2. Oncogene 2014, 34, 1908–1918. [Google Scholar] [CrossRef]
- Tang, J.; Chen, J.; Wang, Y.; Zhou, S. The Role of MiRNA-433 in Malignant Tumors of Digestive Tract as Tumor Suppressor. Cancer Rep. 2022, 5, e1694. [Google Scholar] [CrossRef]
- Lou, W.; Liu, J.; Ding, B.; Xu, L.; Fan, W. Identification of Chemoresistance-Associated MiRNAs in Breast Cancer. Cancer Manag. Res. 2018, 10, 4747–4757. [Google Scholar] [CrossRef] [PubMed]
- Griñán-Lisón, C.; Olivares-Urbano, M.A.; Jiménez, G.; López-Ruiz, E.; del Val, C.; Morata-Tarifa, C.; Entrena, J.M.; González-Ramírez, A.R.; Boulaiz, H.; Zurita Herrera, M.; et al. MiRNAs as Radio-Response Biomarkers for Breast Cancer Stem Cells. Mol. Oncol. 2020, 14, 556–570. [Google Scholar] [CrossRef] [PubMed]
- Muluhngwi, P.; Klinge, C.M. Roles for MiRNAs in Endocrine Resistance in Breast Cancer. Endocr.-Relat. Cancer 2015, 22, R279–R300. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.Z.; Tan, C.L.; He, Y.J.; Zhang, G.Q.; Xu, Y.; Tang, J.H. Functional MiRNAs in Breast Cancer Drug Resistance. OncoTargets Ther. 2018, 11, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, L.; Wu, H. Nanomaterials for Cancer Therapies. Nanotechnol. Rev. 2017, 6, 473–496. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for Cancer Therapy: Current Progress and Perspectives. J. Hematol. Oncol. 2021, 14, 1–27. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, D.Y.; Huang, L. In Vivo Delivery of MiRNAs for Cancer Therapy: Challenges and Strategies. Adv. Drug Deliv. Rev. 2015, 81, 128–141. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Cheng, R.; Yang, Z.; Tian, Z.M. Nanotechnology for Cancer Therapy Based on Chemotherapy. Molecules 2018, 23, 826. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Lungu, I.I.; Grumezescu, A.M.; Volceanov, A.; Andronescu, E. Nanobiomaterials Used in Cancer Therapy: An up-to-Date Overview. Molecules 2019, 24, 3547. [Google Scholar] [CrossRef]
- Chang, X.B. A Molecular Understanding of ATP-Dependent Solute Transport by Multidrug Resistance-Associated Protein MRP1. Cancer Metastasis Rev. 2007, 26, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Zhou, S.; Schuetz, J.D.; Bunting, K.D.; Colapietro, A.-M.; Sampath, J.; Morris, J.J.; Lagutina, I.; Grosveld, G.C.; Osawa, M.; Nakauchi, H.; et al. The ABC Transporter Bcrp1/ABCG2 Is Expressed in a Wide Variety of Stem Cells and Is a Molecular Determinant of the Side-Population Phenotype. 2001, Volume 7. Available online: http://medicine.nature.com (accessed on 17 January 2023).
- Rees, D.C.; Johnson, E.; Lewinson, O. ABC Transporters: The Power to Change. Nat. Rev. Mol. Cell Biol. 2009, 10, 218–227. [Google Scholar] [CrossRef]
- Pan, Y.Z.; Morris, M.E.; Yu, A.M. MicroRNA-328 Negatively Regulates the Expression of Breast Cancer Resistance Protein (BCRP/ABCG2) in Human Cancer Cells. Mol. Pharmacol. 2009, 75, 1374–1379. [Google Scholar] [CrossRef]
- Niu, J.; Xue, A.; Chi, Y.; Xue, J.; Wang, W.; Zhao, Z.; Fan, M.; Yang, C.H.; Shao, Z.M.; Pfeffer, L.M.; et al. Induction of MiRNA-181a by Genotoxic Treatments Promotes Chemotherapeutic Resistance and Metastasis in Breast Cancer. Oncogene 2016, 35, 1302–1313. [Google Scholar] [CrossRef]
- Perez, E.A. Impact, Mechanisms, and Novel Chemotherapy Strategies for Overcoming Resistance to Anthracyclines and Taxanes in Metastatic Breast Cancer. Breast Cancer Res. Treat. 2009, 114, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Zaman, G.J.R.; Flenst, M.J.; Van Leusden, M.R.; De Haas, M.; Mulder, H.S.; Lankelma, J.; Pinedo, H.M.; Scheper, R.J.; Baas, F.; Broxterman, H.J.; et al. The Human Multidrug Resistance-Associated Protein MRP Is a Plasma Membrane Drug-Efflux Pump. 1994, Volume 91. Available online: https://www.pnas.org (accessed on 21 January 2023).
- Robey, R.W.; Polgar, O.; Deeken, J.; To, K.W.; Bates, S.E. ABCG2: Determining Its Relevance in Clinical Drug Resistance. Cancer Metastasis Rev. 2007, 26, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Kamath, K.; Wilson, L.; Cabral, F.; Jordan, M.A. ΒIII-Tubulin Induces Paclitaxel Resistance in Association with Reduced Effects on Microtubule Dynamic Instability. J. Biol. Chem. 2005, 280, 12902–12907. [Google Scholar] [CrossRef]
- Berrieman, H.K.; Lind, M.J.; Cawkwell, L. Do β-Tubulin Mutations Have a Role in Resistance to Chemotherapy? Lancet Oncol. 2004, 5, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, X.; Chu, Q. Bio-Based Nanomaterials for Cancer Therapy. Nano Today 2021, 38, 101134. [Google Scholar] [CrossRef]
- Pecot, C.V.; Rupaimoole, R.; Yang, D.; Akbani, R.; Ivan, C.; Lu, C.; Wu, S.; Han, H.D.; Shah, M.Y.; Rodriguez-Aguayo, C.; et al. Tumour Angiogenesis Regulation by the MiR-200 Family. Nat. Commun. 2013, 4, 2427. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Majeti, B.K.; Acevedo, L.M.; Murphy, E.A.; Mukthavaram, R.; Scheppke, L.; Huang, M.; Shields, D.J.; Lindquist, J.N.; Lapinski, P.E.; et al. MicroRNA-132-Mediated Loss of P120RasGAP Activates the Endothelium to Facilitate Pathological Angiogenesis. Nat. Med. 2010, 16, 909–914. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Wang, Y.; Chen, M. Hyaluronic Acid-Coated PEI-PLGA Nanoparticles Mediated Co-Delivery of Doxorubicin and MiR-542-3p for Triple Negative Breast Cancer Therapy. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 411–420. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, L.; Du, P.; Ma, L.; Zhang, W.; Zheng, L.; Lan, B.; Zhang, B.; Ma, F.; Xu, B.; et al. Transcriptional Downregulation of MiR-4306 Serves as a New Therapeutic Target for Triple Negative Breast Cancer. Theranostics 2019, 9, 1401–1416. [Google Scholar] [CrossRef]
- Kong, W.; He, L.; Richards, E.J.; Challa, S.; Xu, C.X.; Permuth-Wey, J.; Lancaster, J.M.; Coppola, D.; Sellers, T.A.; Djeu, J.Y.; et al. Upregulation of MiRNA-155 Promotes Tumour Angiogenesis by Targeting VHL and Is Associated with Poor Prognosis and Triple-Negative Breast Cancer. Oncogene 2014, 33, 679–689. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, X.; Zhang, X.; Liu, B.; Huang, L. Nanoparticles Modified with Tumor-Targeting ScFv Deliver SiRNA and MiRNA for Cancer Therapy. Mol. Ther. 2010, 18, 1650–1656. [Google Scholar] [CrossRef]
- Ahir, M.; Upadhyay, P.; Ghosh, A.; Sarker, S.; Bhattacharya, S.; Gupta, P.; Ghosh, S.; Chattopadhyay, S.; Adhikary, A. Delivery of Dual MiRNA through CD44-Targeted Mesoporous Silica Nanoparticles for Enhanced and Effective Triple-Negative Breast Cancer Therapy. Biomater. Sci. 2020, 8, 2939–2954. [Google Scholar] [CrossRef]
- Ghosh, R.; Singh, L.C.; Shohet, J.M.; Gunaratne, P.H. A Gold Nanoparticle Platform for the Delivery of Functional MicroRNAs into Cancer Cells. Biomaterials 2013, 34, 807–816. [Google Scholar] [CrossRef]
- van Dongen, S.; Abreu-Goodger, C.; Enright, A.J. Detecting MicroRNA Binding and SiRNA Off-Target Effects from Expression Data. Nat. Methods 2008, 5, 1023–1025. [Google Scholar] [CrossRef]
- Loinger, A.; Shemla, Y.; Simon, I.; Margalit, H.; Biham, O. Competition between Small RNAs: A Quantitative View. Biophys. J. 2012, 102, 1712–1721. [Google Scholar] [CrossRef]
- Yu, B.; Zhao, X.; Lee, J.L.; Lee, R.J. Targeted Delivery Systems for Oligonucleotide Therapeutics. AAPS J. 2009, 11, 195–203. [Google Scholar] [CrossRef]
- Velpurisiva, P.; Gad, A.; Piel, B.; Jadia, R.; Rai, P. Nanoparticle Design Strategies for Effective Cancer Immunotherapy. J. Biomed. 2017, 2, 64–77. [Google Scholar] [CrossRef]
- Cun, D.; Jensen, D.K.; Maltesen, M.J.; Bunker, M.; Whiteside, P.; Scurr, D.; Foged, C.; Nielsen, H.M. High Loading Efficiency and Sustained Release of SiRNA Encapsulated in PLGA Nanoparticles: Quality by Design Optimization and Characterization. Eur. J. Pharm. Biopharm. 2011, 77, 26–35. [Google Scholar] [CrossRef]
- O’Neill, C.P.; Dwyer, R.M. Nanoparticle-Based Delivery of Tumor Suppressor MicroRNA for Cancer Therapy. Cells 2020, 9, 521. [Google Scholar] [CrossRef]
- Valcourt, D.M.; Day, E.S. Dual Regulation of MiR-34a and Notch Signaling in Triple-Negative Breast Cancer by Antibody/MiRNA Nanocarriers. Mol. Ther.-Nucleic Acids 2020, 21, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, Y.; Kilchrist, K.V.; Li, J.; Duvall, C.L.; Oupický, D. Endosomolytic and Tumor-Penetrating Mesoporous Silica Nanoparticles for SiRNA/MiRNA Combination Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 4308–4322. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Cao, M.; Zhang, J.; Hu, K.; Yin, Z.; Zhou, Z.; Xiao, X.; Yang, Y.; Sheng, W.; Wu, Y.; et al. Hyaluronic Acid-Chitosan Nanoparticles for Co-Delivery of MiR-34a and Doxorubicin in Therapy against Triple Negative Breast Cancer. Biomaterials 2014, 35, 4333–4344. [Google Scholar] [CrossRef] [PubMed]
- Alhasan, A.H.; Patel, P.C.; Choi, C.H.J.; Mirkin, C.A. Exosome Encased Spherical Nucleic Acid Gold Nanoparticle Conjugates as Potent MicroRNA Regulation Agents. Small 2014, 10, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Trendel, J.; Schwarzl, T.; Horos, R.; Prakash, A.; Bateman, A.; Hentze, M.W.; Krijgsveld, J. The Human RNA-Binding Proteome and Its Dynamics during Translational Arrest. Cell 2019, 176, 391–403.e19. [Google Scholar] [CrossRef]
- Cheng, R.F.; Wang, J.; Zhang, J.Y.; Sun, L.; Zhao, Y.R.; Qiu, Z.Q.; Sun, B.C.; Sun, Y. MicroRNA-506 Is up-Regulated in the Development of Pancreatic Ductal Adenocarcinoma and Is Associated with Attenuated Disease Progression. Chin. J. Cancer 2016, 35, 64. [Google Scholar] [CrossRef]
- Xue, W.; Dahlman, J.E.; Tammela, T.; Khan, O.F.; Sood, S.; Dave, A.; Cai, W.; Chirino, L.M.; Yang, G.R.; Bronson, R.; et al. Small RNA Combination Therapy for Lung Cancer. Proc. Natl. Acad. Sci. USA 2014, 111, E3553–E3561. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, G.; Su, Z.; Jiang, Z.; Chen, L.; Wang, J.; Yu, S.; Liu, Z. Poly(Amido Amine) Is an Ideal Carrier of MiR-7 for Enhancing Gene Silencing Effects on the EGFR Pathway in U251 Glioma Cells. Oncol. Rep. 2013, 29, 1387–1394. [Google Scholar] [CrossRef]
- Chung, W.M.; Chang, W.C.; Chen, L.; Chang, Y.Y.; Shyr, C.R.; Hung, Y.C.; Ma, W.L. MicroRNA-21 Promotes the Ovarian Teratocarcinoma PA1 Cell Line by Sustaining Cancer Stem/Progenitor Populations in Vitro. Stem Cell Res. Ther. 2013, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chitkara, D.; Singh, S.; Mittal, A. Nanocarrier-Based Co-Delivery of Small Molecules and SiRNA/MiRNA for Treatment of Cancer. Ther. Deliv. 2016, 7, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Cao, N.; Chen, J.; Yu, X.; Shuai, X. Multifunctional Nanocarrier Mediated Co-Delivery of Doxorubicin and SiRNA for Synergistic Enhancement of Glioma Apoptosis in Rat. Biomaterials 2012, 33, 1170–1179. [Google Scholar] [CrossRef]
- Malik, S.; Lim, J.; Slack, F.J.; Braddock, D.T.; Bahal, R. Next Generation MiRNA Inhibition Using Short Anti-Seed PNAs Encapsulated in PLGA Nanoparticles. J. Control. Release 2020, 327, 406–419. [Google Scholar] [CrossRef]
- Kapadia, C.H.; Ioele, S.A.; Day, E.S. Layer-by-Layer Assembled PLGA Nanoparticles Carrying MiR-34a Cargo Inhibit the Proliferation and Cell Cycle Progression of Triple-Negative Breast Cancer Cells. J. Biomed. Mater. Res.-Part A 2020, 108, 601–613. [Google Scholar] [CrossRef]
- Bhargava-Shah, A.; Foygel, K.; Devulapally, R.; Paulmurugan, R. Orlistat and Antisense-MiRNA-Loaded PLGA-PEG Nanoparticles for Enhanced Triple Negative Breast Cancer Therapy. Nanomedicine 2016, 11, 235–247. [Google Scholar] [CrossRef]
- Chen, G.Q.; Zhao, Z.W.; Zhou, H.Y.; Liu, Y.J.; Yang, H.J. Systematic Analysis of MicroRNA Involved in Resistance of the MCF-7 Human Breast Cancer Cell to Doxorubicin. Med. Oncol. 2010, 27, 406–415. [Google Scholar] [CrossRef]
- Kovalchuk, O.; Filkowski, J.; Meservy, J.; Ilnytskyy, Y.; Tryndyak, V.P.; Chekhun, V.F.; Pogribny, I.P. Involvement of MicroRNA-451 in Resistance of the MCF-7 Breast Cancer Cells to Chemotherapeutic Drug Doxorubicin. Mol. Cancer Ther. 2008, 7, 2152–2159. [Google Scholar] [CrossRef]
- Trucillo, P. Drug Carriers: Classification, Administration, Release Profiles, and Industrial Approach. Processes 2021, 9, 470. [Google Scholar] [CrossRef]
- Dzmitruk, V.; Apartsin, E.; Ihnatsyeu-Kachan, A.; Abashkin, V.; Shcharbin, D.; Bryszewska, M. Dendrimers Show Promise for SiRNA and Microrna Therapeutics. Pharmaceutics 2018, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Xiao, Y.; Ouyang, Z.; Shen, M.; Shi, X. Efficient Co-Delivery of MicroRNA 21 Inhibitor and Doxorubicin to Cancer Cells Using Core-Shell Tecto Dendrimers Formed via Supramolecular Host-Guest Assembly. J. Mater. Chem. B 2020, 8, 2768–2774. [Google Scholar] [CrossRef]
- Xiong, H.; Liu, S.; Wei, T.; Cheng, Q.; Siegwart, D.J. Theranostic Dendrimer-Based Lipid Nanoparticles Containing PEGylated BODIPY Dyes for Tumor Imaging and Systemic MRNA Delivery in Vivo. J. Control. Release 2020, 325, 198–205. [Google Scholar] [CrossRef]
- Liu, L.; Kuang, Y.; Yang, H.; Chen, Y. An Amplification Strategy Using DNA-Peptide Dendrimer Probe and Mass Spectrometry for Sensitive MicroRNA Detection in Breast Cancer. Anal. Chim. Acta 2019, 1069, 73–81. [Google Scholar] [CrossRef]
- Goyal, R.; Tripathi, S.K.; Tyagi, S.; Sharma, A.; Ram, K.R.; Chowdhuri, D.K.; Shukla, Y.; Kumar, P.; Gupta, K.C. Linear PEI Nanoparticles: Efficient PDNA/SiRNA Carriers in Vitro and in Vivo. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Jia, J.; Cong, X.; Liu, Z.; Li, Q. N-Isopropylacrylamide-Modified Polyethylenimine-Mediated MiR-29a Delivery to Inhibit the Proliferation and Migration of Lung Cancer Cells. Colloids Surf. B Biointerfaces 2021, 198, 111463. [Google Scholar] [CrossRef]
- Brunot, C.; Ponsonnet, L.; Lagneau, C.; Farge, P.; Picart, C.; Grosgogeat, B. Cytotoxicity of Polyethyleneimine (PEI), Precursor Base Layer of Polyelectrolyte Multilayer Films. Biomaterials 2007, 28, 632–640. [Google Scholar] [CrossRef]
- Tian, H.; Li, F.; Chen, J.; Huang, Y.; Chen, X. N-Isopropylacrylamide-Modified Polyethylenimines as Effective Gene Carriers. Macromol. Biosci. 2012, 12, 1680–1688. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, D.; Xing, Z.; Liang, S.; Han, H.; Shi, H.; Zhang, Y.; Yang, Y.; Li, Q. N-Isopropylacrylamide-Modified Polyethylenimine-Mediated P53 Gene Delivery to Prevent the Proliferation of Cancer Cells. Colloids Surf. B Biointerfaces 2015, 129, 54–62. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, X. MicroRNA Delivery with Bioreducible Polyethylenimine as a Non-Viral Vector for Breast Cancer Gene Therapy. Macromol. Biosci. 2019, 19, 1800445. [Google Scholar] [CrossRef]
- Sharma, B.; Attri, S.; Syal, J.; Sharma, U. Liposomal Nanoparticles: A Viable Nanoscale Drug Carriers for the Treatment of Cancer. In Liposomes; Intech Open: London, UK, 2023. [Google Scholar]
- Paliwal, S.R.; Paliwal, R.; Agrawal, G.P.; Vyas, S.P. Liposomal Nanomedicine for Breast Cancer Therapy. Nanomedicine 2011, 6, 1085–1100. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Song, K.; Lu, X.; Feng, W.; Di, W. Liposomal Delivery of MicroRNA-7 Targeting EGFR to Inhibit the Growth, Invasion, and Migration of Ovarian Cancer. ACS Omega 2021, 6, 11669–11678. [Google Scholar] [CrossRef]
- Bavelloni, A.; Ramazzotti, G.; Poli, A.; Piazzi, M.; Focaccia, E.; Blalock, W.; Faenza, I. Mirna-210: A Current Overview. Anticancer Res. 2017, 37, 6511–6521. [Google Scholar] [CrossRef]
- Baroni, S.; Romero-Cordoba, S.; Plantamura, I.; Dugo, M.; D’Ippolito, E.; Cataldo, A.; Cosentino, G.; Angeloni, V.; Rossini, A.; Daidone, M.G.; et al. Exosome-Mediated Delivery of MiR-9 Induces Cancer-Associated Fibroblast-like Properties in Human Breast Fibroblasts. Cell Death Dis. 2016, 7, e2312. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, X.Q.; Duan, J.L.; Bao, C.J.; Cui, Y.N.; Su, Z.B.; Xu, J.R.; Luo, Q.; Chen, M.; Xie, Y.; et al. Nanosized Functional MiRNA Liposomes and Application in the Treatment of TNBC by Silencing Slug Gene. Int. J. Nanomed. 2019, 14, 3645–3667. [Google Scholar] [CrossRef]
- de Miguel Pérez, D.; Rodriguez Martínez, A.; Ortigosa Palomo, A.; Delgado Ureña, M.; Garcia Puche, J.L.; Robles Remacho, A.; Exposito Hernandez, J.; Lorente Acosta, J.A.; Ortega Sánchez, F.G.; Serrano, M.J. Extracellular Vesicle-MiRNAs as Liquid Biopsy Biomarkers for Disease Identification and Prognosis in Metastatic Colorectal Cancer Patients. Sci. Rep. 2020, 10, 3974. [Google Scholar] [CrossRef] [PubMed]
- Battistelli, M.; Falcieri, E. Apoptotic Bodies: Particular Extracellular Vesicles Involved in Intercellular Communication. Biology 2020, 9, 21. [Google Scholar] [CrossRef]
- Hu, Q.L.; Jiang, Q.Y.; Jin, X.; Shen, J.; Wang, K.; Li, Y.B.; Xu, F.J.; Tang, G.P.; Li, Z.H. Cationic MicroRNA-Delivering Nanovectors with Bifunctional Peptides for Efficient Treatment of PANC-1 Xenograft Model. Biomaterials 2013, 34, 2265–2276. [Google Scholar] [CrossRef]
- Unal, O.; Akkoc, Y.; Kocak, M.; Nalbat, E.; Dogan-Ekici, A.I.; Yagci Acar, H.; Gozuacik, D. Treatment of Breast Cancer with Autophagy Inhibitory MicroRNAs Carried by AGO2-Conjugated Nanoparticles. J. Nanobiotechnol. 2020, 18, 1–18. [Google Scholar] [CrossRef]
- Bayraktar, R.; Pichler, M.; Kanlikilicer, P.; Ivan, C.; Bayraktar, E.; Kahraman, N.; Aslan, B.; Oguztuzun, S.; Ulasli, M.; Arslan, A.; et al. MicroRNA 603 Acts as a Tumor Suppressor and Inhibits Triple-Negative Breast Cancer Tumorigenesis by Targeting Elongation Factor 2 Kinase. Available online: www.impactjournals.com/oncotarget (accessed on 1 February 2023).
- Yin, H.; Xiong, G.; Guo, S.; Xu, C.; Xu, R.; Guo, P.; Shu, D. Delivery of Anti-MiRNA for Triple-Negative Breast Cancer Therapy Using RNA Nanoparticles Targeting Stem Cell Marker CD133. Mol. Ther. 2019, 27, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Z.; Chen, C.; Liu, Y.; Si, Q.; Chuang, T.H.; Li, N.; Gomez-Cabrero, A.; Reisfeld, R.A.; Xiang, R.; et al. MicroRNA-19a-3p Inhibits Breast Cancer Progression and Metastasis by Inducing Macrophage Polarization through Downregulated Expression of Fra-1 Proto-Oncogene. Oncogene 2014, 33, 3014–3023. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Qiu, J.; Lu, X.; Li, G. C-MYC Inhibited Ferroptosis and Promoted Immune Evasion in Ovarian Cancer Cells through NCOA4 Mediated Ferritin Autophagy. Cells 2022, 11, 4127. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, N.; Arora, S.; Deshmukh, S.K.; Singh, S.; Marimuthu, S.; Singh, A.P. Exploiting Nanotechnology for the Development of MicroRNA-Based Cancer Therapeutics. J. Biomed. Nanotechnol. 2016, 12, 28–42. [Google Scholar] [CrossRef]
- Polo, E.; Puertas, S.; Moros, M.; Batalla, P.; Guisán, J.M.; De La Fuente, J.M.; Grazú, V. Tips for the Functionalization of Nanoparticles with Antibodies. Methods Mol. Biol. 2013, 1051, 149–163. [Google Scholar] [CrossRef]
- Herrera-Carrillo, E.; Liu, Y.P.; Berkhout, B. Improving MiRNA Delivery by Optimizing MiRNA Expression Cassettes in Diverse Virus Vectors. Hum. Gene Ther. Methods 2017, 28, 177–190. [Google Scholar] [CrossRef]
- Kasar, S.; Salerno, E.; Yuan, Y.; Underbayev, C.; Vollenweider, D.; Laurindo, M.F.; Fernandes, H.; Bonci, D.; Addario, A.; Mazzella, F.; et al. Systemic in Vivo Lentiviral Delivery of MiR-15a/16 Reduces Malignancy in the NZB de Novo Mouse Model of Chronic Lymphocytic Leukemia. Genes Immun. 2012, 13, 109–119. [Google Scholar] [CrossRef]
- Liu, Y.; Lai, L.; Chen, Q.; Song, Y.; Xu, S.; Ma, F.; Wang, X.; Wang, J.; Yu, H.; Cao, X.; et al. MicroRNA-494 Is Required for the Accumulation and Functions of Tumor-Expanded Myeloid-Derived Suppressor Cells via Targeting of PTEN. J. Immunol. 2012, 188, 5500–5510. [Google Scholar] [CrossRef]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-Viral Vectors for Gene-Based Therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Forterre, A.; Komuro, H.; Aminova, S.; Harada, M. A Comprehensive Review of Cancer MicroRNA Therapeutic Delivery Strategies. Cancers 2020, 12, 1852. [Google Scholar] [CrossRef]
- Ding, L.; Li, J.; Wu, C.; Yan, F.; Li, X.; Zhang, S. A Self-Assembled RNA-Triple Helix Hydrogel Drug Delivery System Targeting Triple-Negative Breast Cancer. J. Mater. Chem. B 2023, 8, 3527–3533. [Google Scholar] [CrossRef]
- Wang, S.; Cao, M.; Deng, X.; Xiao, X.; Yin, Z.; Hu, Q.; Zhou, Z.; Zhang, F.; Zhang, R.; Wu, Y.; et al. Degradable Hyaluronic Acid/Protamine Sulfate Interpolyelectrolyte Complexes as MiRNA-Delivery Nanocapsules for Triple-Negative Breast Cancer Therapy. Adv. Healthc. Mater. 2015, 4, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.A.; Ahmed, S.; Jan, B.; Bender, O.; Al Hagbani, T.; Alqarni, A.; Anwar, S. Drugs Repurposed: An Advanced Step towards the Treatment of Breast Cancer and Associated Challenges. Biomed. Pharmacother. 2022, 145, 112375. [Google Scholar] [CrossRef]
- Alamri, A.; Rauf, A.; Khalil, A.A.; Alghamdi, A.; Alafnan, A.; Alshammari, A.; Alshammari, F.; Malik, J.A.; Anwar, S. In Silico Screening of Marine Compounds as an Emerging and Promising Approach against Estrogen Receptor Alpha-Positive Breast Cancer. BioMed Res. Int. 2021, 2021, 1–7. [Google Scholar] [CrossRef]
- Malik, J.A.; Ahmed, S.; Momin, S.S.; Shaikh, S.; Alafnan, A.; Alanazi, J.; Said Almermesh, M.H.; Anwar, S. Drug Repurposing: A New Hope in Drug Discovery for Prostate Cancer. ACS Omega 2022, 8, 56–73. [Google Scholar] [CrossRef]
- Anwar, S.; Malik, J.A.; Ahmed, S.; Kameshwar, V.A.; Alanazi, J.; Alamri, A.; Ahemad, N. Can Natural Products Targeting EMT Serve as the Future Anticancer Therapeutics? Molecules 2022, 27, 7668. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Saleem, H.; Khurshid, U.; Ansari, Y.A.; Alghamdi, S.; Al-Khulaidi, A.A.; Malik, J.A.; Ahemad, N.; Ali, N.A. Comparative phytochemical composition, oleuropein quantification, antioxidant and cytotoxic properties of Olea europaea L. leaves. Nat. Prod. Res 2023, 37, 1023–1029. [Google Scholar] [CrossRef]
- Bender, O.; Shoman, M.E.; Ali, T.F.S.; Dogan, R.; Celik, I.; Mollica, A.; Hamed, M.I.A.; Aly, O.; Alamri, J.A.; Ahemad, N.; et al. Discovery of oxindole-based FLT3 inhibitors as a promising therapeutic lead for acute myeloid leukemia carrying the oncogenic ITD mutation. Arch. Pharm. Res. 2022, 2022, e2200407. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.A.; Jan, R.; Ahmed, S.; Anwar, S. Breast cancer drug repurposing a tool for challenging disease. In Drug Repurposing: Molecular Aspect and Therapeutic Applications; Intech Open: London, UK, 2022; pp. 121–130. [Google Scholar]

| Sr. No. | miRNA | Delivery Systems | Cell Lines | Delivery Route In Vivo | Targeted Gene and Molecular Pathway | Ref. |
|---|---|---|---|---|---|---|
| 1. | miR-200 | (DOPC) nanoliposome | MCF 7, MB231 | Inoculated subcutaneously | IL8, CL1XC | [65] |
| 2. | miR-132 | cRGD | HUVEC, MDA-MB-231, RCP30 | Inoculated subcutaneously | p120RasGAP | [66] |
| 3. | miR-542-3p | PEI-PLGA | MDA-MB-231, MCF 7 | - | CD44, P53 | [67] |
| 4. | miR-4306 | Lentivirus | MCF-7, T47D, ZR-75–1, SK-BR-3, HeLa, HCC1937, MDA- MB-468 | Inoculated subcutaneously | VEGFA SIX1, Cdc42 | [68] |
| 5. | miR-155 | PLGA | HCC1937, HIF1 RCP30 | Inoculated subcutaneously | VHL | [69] |
| 6. | miR-21, miR-145 | Magnetic nanoparticles | MCF-7, HBL100 | - | P53 | [48] |
| S. No | Therapeutics miRNA | Mechanism/Receptor Targeted | Nano-Vehicles Manufacturing Materials | Ref. |
|---|---|---|---|---|
| 1. | miRNA-376b | Blocked autophagy | Superparamagnetic iron oxide nanoparticles | [110] |
| 2. | miRNA-34a | Reduces BC cell migration, tumor growth, and proliferation | Polymers, gold, silica, liposomes | [111] |
| 3. | miRNA-603 | Reduction in angiogenesis and cell migration, proliferation, invasion, and tumor growth | Liposomes | [112] |
| 4. | miRNA21 | Decrease in tumor growth, cell proliferation, and migration | Graphene/polymer hybrids, chitosomes, polymers, | [113] |
| 6. | miRNA-22-3p | Reduces invasion, tumor growth, colony formation, cell proliferation, | Lipids | [114] |
| 7. | miRNA-200c | Reduction in the invasion, multidrug resistance EMT, cell motility | Polymer hybrids/peptide/lipid | [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinde, S.S.; Ahmed, S.; Malik, J.A.; Hani, U.; Khanam, A.; Ashraf Bhat, F.; Ahmad Mir, S.; Ghazwani, M.; Wahab, S.; Haider, N.; et al. Therapeutic Delivery of Tumor Suppressor miRNAs for Breast Cancer Treatment. Biology 2023, 12, 467. https://doi.org/10.3390/biology12030467
Shinde SS, Ahmed S, Malik JA, Hani U, Khanam A, Ashraf Bhat F, Ahmad Mir S, Ghazwani M, Wahab S, Haider N, et al. Therapeutic Delivery of Tumor Suppressor miRNAs for Breast Cancer Treatment. Biology. 2023; 12(3):467. https://doi.org/10.3390/biology12030467
Chicago/Turabian StyleShinde, Sonali S., Sakeel Ahmed, Jonaid Ahmad Malik, Umme Hani, Afreen Khanam, Faisal Ashraf Bhat, Suhail Ahmad Mir, Mohammed Ghazwani, Shadma Wahab, Nazima Haider, and et al. 2023. "Therapeutic Delivery of Tumor Suppressor miRNAs for Breast Cancer Treatment" Biology 12, no. 3: 467. https://doi.org/10.3390/biology12030467
APA StyleShinde, S. S., Ahmed, S., Malik, J. A., Hani, U., Khanam, A., Ashraf Bhat, F., Ahmad Mir, S., Ghazwani, M., Wahab, S., Haider, N., & Almehizia, A. A. (2023). Therapeutic Delivery of Tumor Suppressor miRNAs for Breast Cancer Treatment. Biology, 12(3), 467. https://doi.org/10.3390/biology12030467







