Zingiber officinale Roscoe Rhizome Extract Exerts Senomorphic and Anti-Inflammatory Activities on Human Endothelial Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. BV2 Cells
2.3. Neuroinflammatory Model
2.4. HUVEC Cells
2.5. Characterization of Young and Senescent HUVEC Cells
2.6. Cell Viability Assay
2.7. Zingiber Officinale Roscoe Extract Treatments
2.8. RNA Isolation, mRNA and Mature miRNAs Expression by RT-qPCR
2.9. Western Blot Analysis
2.10. ELISA Assay
2.11. Statistical Analysis
3. Results
3.1. ZTE Is the Main Responsible for ZOE Anti-Inflammatory Activity in the In Vitro Model of Neuroinflammation
3.2. Replicative Senescence in HUVECs
3.3. Zingiber Officinale Extract Effect on HUVEC Viability
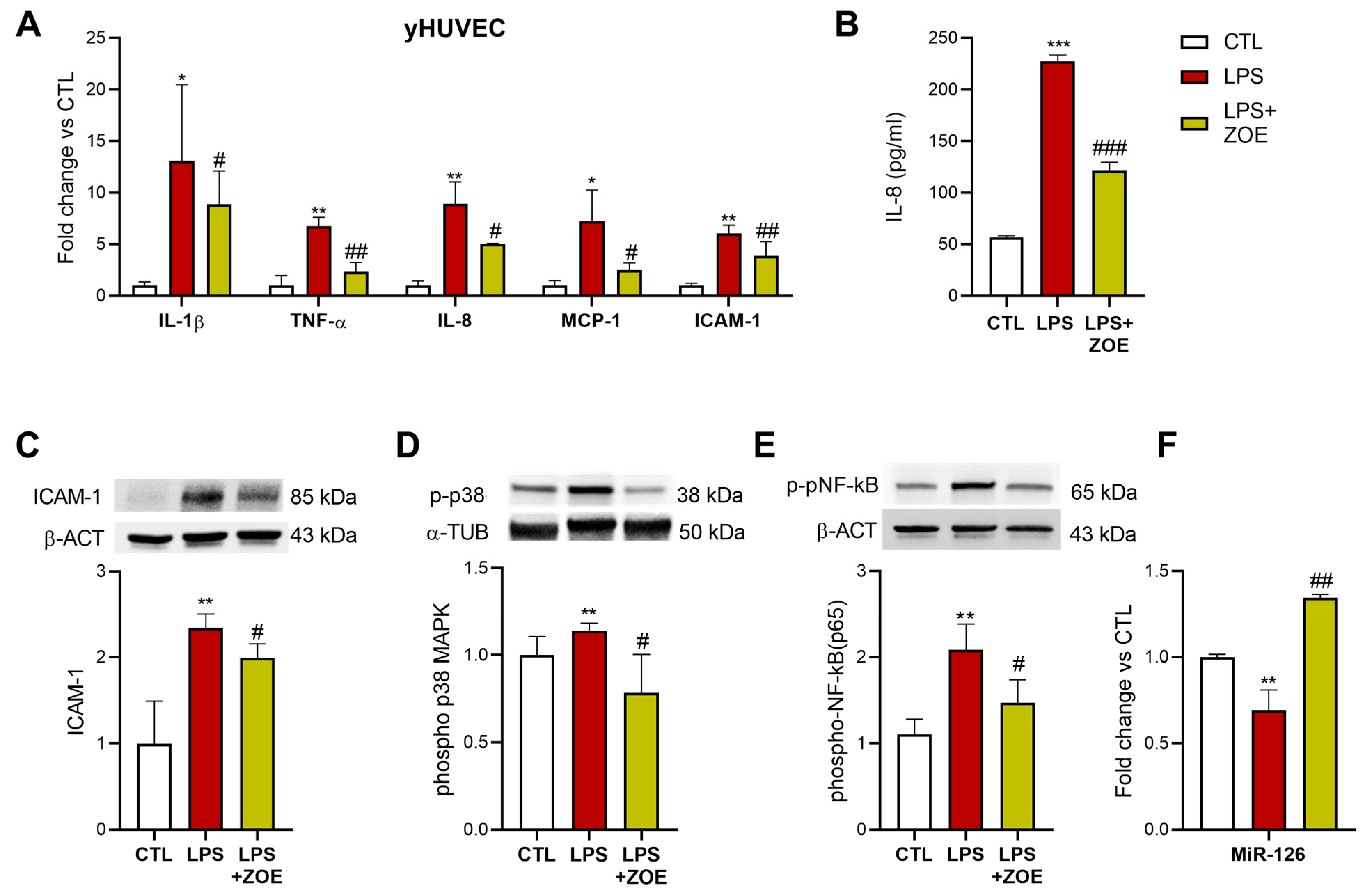
3.4. ZOE Anti-Inflammatory Activity in LPS-Stimulated HUVECs
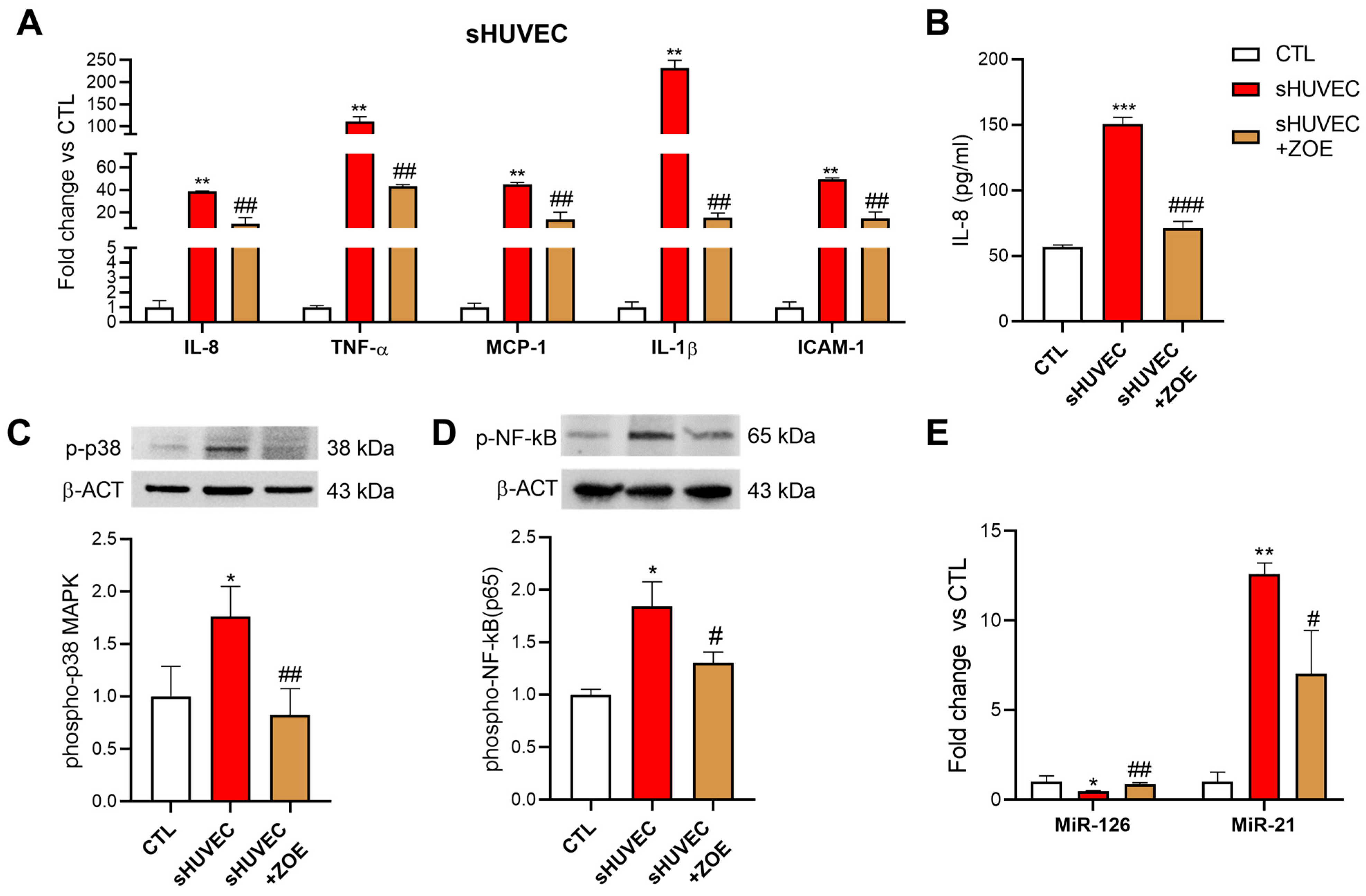
3.5. Senomorphic Effect of ZOE in Senescent HUVECs
3.6. ZTE Biological Activity in yHUVEC and sHUVEC
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Boccardi, V.; Mecocci, P. Senotherapeutics: Targeting senescent cells for the main age-related diseases. Mech. Ageing Dev. 2021, 197, 111526. [Google Scholar] [CrossRef] [PubMed]
- Sanada, F.; Taniyama, Y.; Muratsu, J.; Otsu, R.; Shimizu, H.; Rakugi, H.; Morishita, R. Source of Chronic Inflammation in Aging. Front. Cardiovasc. Med. 2018, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.; Sousa-Victor, P. Regulation of inflammation as an anti-aging intervention. FEBS J. 2020, 287, 43–52. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Birch, J.; Gil, J. Senescence and the SASP: Many therapeutic avenues. Genes Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; van de Sluis, B.; Kirkland, J.L.; van Deursen, J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting cellular senescence with senotherapeutics: Senolytics and senomorphics. FEBS J. 2022, 290, 1362–1383. [Google Scholar] [CrossRef]
- Ozkur, M.; Benlier, N.; Takan, I.; Vasileiou, C.; Georgakilas, A.G.; Pavlopoulou, A.; Cetin, Z.; Saygili, E.I. Ginger for Healthy Ageing: A Systematic Review on Current Evidence of Its Antioxidant, Anti-Inflammatory, and Anticancer Properties. Oxid. Med. Cell. Longev. 2022, 2022, 4748447. [Google Scholar] [CrossRef]
- Forouzanfar, F.; Hosseinzadeh, H. Medicinal herbs in the treatment of neuropathic pain: A review. Iran. J. Basic Med. Sci. 2018, 21, 347–358. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Ballester, P.; Cerda, B.; Arcusa, R.; Marhuenda, J.; Yamedjeu, K.; Zafrilla, P. Effect of Ginger on Inflammatory Diseases. Molecules 2022, 27, 7223. [Google Scholar] [CrossRef]
- Borgonetti, V.; Governa, P.; Biagi, M.; Pellati, F.; Galeotti, N. Zingiber officinale Roscoe rhizome extract alleviates neuropathic pain by inhibiting neuroinflammation in mice. Phytomedicine 2020, 78, 153307. [Google Scholar] [CrossRef]
- Moaddel, R.; Rossi, M.; Rodriguez, S.; Munk, R.; Khadeer, M.; Abdelmohsen, K.; Gorospe, M.; Ferrucci, L. Identification of gingerenone A as a novel senolytic compound. PLoS ONE 2022, 17, e0266135. [Google Scholar] [CrossRef] [PubMed]
- Borgonetti, V.; Benatti, C.; Governa, P.; Isoldi, G.; Pellati, F.; Alboni, S.; Tascedda, F.; Montopoli, M.; Galeotti, N.; Manetti, F.; et al. Non-psychotropic Cannabis sativa L. phytocomplex modulates microglial inflammatory response through CB2 receptors-, endocannabinoids-, and NF-kappaB-mediated signaling. Phytother. Res. 2022, 36, 2246–2263. [Google Scholar] [CrossRef]
- Matacchione, G.; Perugini, J.; Di Mercurio, E.; Sabbatinelli, J.; Prattichizzo, F.; Senzacqua, M.; Storci, G.; Dani, C.; Lezoche, G.; Guerrieri, M.; et al. Senescent macrophages in the human adipose tissue as a source of inflammaging. Geroscience 2022, 44, 1941–1960. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal. Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Solt, L.A.; May, M.J. The IkappaB kinase complex: Master regulator of NF-kappaB signaling. Immunol. Res. 2008, 42, 3–18. [Google Scholar] [CrossRef]
- Alique, M.; Bodega, G.; Giannarelli, C.; Carracedo, J.; Ramirez, R. MicroRNA-126 regulates Hypoxia-Inducible Factor-1alpha which inhibited migration, proliferation, and angiogenesis in replicative endothelial senescence. Sci. Rep. 2019, 9, 7381. [Google Scholar] [CrossRef]
- Olivieri, F.; Prattichizzo, F.; Grillari, J.; Balistreri, C.R. Cellular Senescence and Inflammaging in Age-Related Diseases. Mediat. Inflamm. 2018, 2018, 9076485. [Google Scholar] [CrossRef]
- Lasry, A.; Ben-Neriah, Y. Senescence-associated inflammatory responses: Aging and cancer perspectives. Trends Immunol. 2015, 36, 217–228. [Google Scholar] [CrossRef]
- Zammel, N.; Saeed, M.; Bouali, N.; Elkahoui, S.; Alam, J.M.; Rebai, T.; Kausar, M.A.; Adnan, M.; Siddiqui, A.J.; Badraoui, R. Antioxidant and Anti-Inflammatory Effects of Zingiber officinale roscoe and Allium subhirsutum: In Silico, Biochemical and Histological Study. Foods 2021, 10, 1383. [Google Scholar] [CrossRef]
- Han, Y.A.; Song, C.W.; Koh, W.S.; Yon, G.H.; Kim, Y.S.; Ryu, S.Y.; Kwon, H.J.; Lee, K.H. Anti-inflammatory effects of the Zingiber officinale roscoe constituent 12-dehydrogingerdione in lipopolysaccharide-stimulated Raw 264.7 cells. Phytother. Res. 2013, 27, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Mohd Sahardi, N.F.N.; Jaafar, F.; Mad Nordin, M.F.; Makpol, S. Zingiber Officinale Roscoe Prevents Cellular Senescence of Myoblasts in Culture and Promotes Muscle Regeneration. Evid. Based Complement. Alternat. Med. 2020, 2020, 1787342. [Google Scholar] [CrossRef]
- Yu, T.J.; Tang, J.Y.; Shiau, J.P.; Hou, M.F.; Yen, C.H.; Ou-Yang, F.; Chen, C.Y.; Chang, H.W. Gingerenone A Induces Antiproliferation and Senescence of Breast Cancer Cells. Antioxidants 2022, 11, 587. [Google Scholar] [CrossRef] [PubMed]
- Callcott, E.T.; Blanchard, C.L.; Oli, P.; Santhakumar, A.B. Pigmented Rice-Derived Phenolic Compounds Reduce Biomarkers of Oxidative Stress and Inflammation in Human Umbilical Vein Endothelial Cells. Mol. Nutr. Food Res. 2018, 62, e1800840. [Google Scholar] [CrossRef]
- Mobasheri, L.; Khorashadizadeh, M.; Safarpour, H.; Mohammadi, M.; Anani Sarab, G.; Askari, V.R. Anti-Inflammatory Activity of Ferula assafoetida Oleo-Gum-Resin (Asafoetida) against TNF-alpha-Stimulated Human Umbilical Vein Endothelial Cells (HUVECs). Mediat. Inflamm. 2022, 2022, 5171525. [Google Scholar] [CrossRef] [PubMed]
- Saji, N.; Francis, N.; Blanchard, C.L.; Schwarz, L.J.; Santhakumar, A.B. Rice Bran Phenolic Compounds Regulate Genes Associated with Antioxidant and Anti-Inflammatory Activity in Human Umbilical Vein Endothelial Cells with Induced Oxidative Stress. Int. J. Mol. Sci. 2019, 20, 4715. [Google Scholar] [CrossRef]
- Matacchione, G.; Valli, D.; Silvestrini, A.; Giuliani, A.; Sabbatinelli, J.; Giordani, C.; Coppari, S.; Rippo, M.R.; Albertini, M.C.; Olivieri, F. Curcumin, Polydatin and Quercetin Synergistic Activity Protects from High-Glucose-Induced Inflammation and Oxidative Stress. Antioxidants 2022, 11, 1037. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, S.C.; Yu, T.; Yi, Y.S.; Rhee, M.H.; Sung, G.H.; Yoo, B.C.; Cho, J.Y. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediat. Inflamm. 2014, 2014, 352371. [Google Scholar] [CrossRef]
- Park, M.H.; Hong, J.T. Roles of NF-kappaB in Cancer and Inflammatory Diseases and Their Therapeutic Approaches. Cells 2016, 5, 15. [Google Scholar] [CrossRef]
- Salminen, A.; Huuskonen, J.; Ojala, J.; Kauppinen, A.; Kaarniranta, K.; Suuronen, T. Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Res. Rev. 2008, 7, 83–105. [Google Scholar] [CrossRef] [PubMed]
- Mahluji, S.; Ostadrahimi, A.; Mobasseri, M.; Ebrahimzade Attari, V.; Payahoo, L. Anti-inflammatory effects of zingiber officinale in type 2 diabetic patients. Adv. Pharm. Bull. 2013, 3, 273–276. [Google Scholar] [CrossRef]
- Mozaffari-Khosravi, H.; Naderi, Z.; Dehghan, A.; Nadjarzadeh, A.; Fallah Huseini, H. Effect of Ginger Supplementation on Proinflammatory Cytokines in Older Patients with Osteoarthritis: Outcomes of a Randomized Controlled Clinical Trial. J. Nutr. Gerontol. Geriatr. 2016, 35, 209–218. [Google Scholar] [CrossRef]
- Olivieri, F.; Prattichizzo, F.; Giuliani, A.; Matacchione, G.; Rippo, M.R.; Sabbatinelli, J.; Bonafe, M. miR-21 and miR-146a: The microRNAs of inflammaging and age-related diseases. Ageing Res. Rev. 2021, 70, 101374. [Google Scholar] [CrossRef]
- Olivieri, F.; Bonafe, M.; Spazzafumo, L.; Gobbi, M.; Prattichizzo, F.; Recchioni, R.; Marcheselli, F.; La Sala, L.; Galeazzi, R.; Rippo, M.R.; et al. Age- and glycemia-related miR-126-3p levels in plasma and endothelial cells. Aging 2014, 6, 771–787. [Google Scholar] [CrossRef]
- Jeena, K.; Liju, V.B.; Kuttan, R. Antioxidant, anti-inflammatory and antinociceptive activities of essential oil from ginger. Indian J. Physiol. Pharmacol. 2013, 57, 51–62. [Google Scholar]
- Li, J.; Thangaiyan, R.; Govindasamy, K.; Wei, J. Anti-inflammatory and anti-apoptotic effect of zingiberene on isoproterenol-induced myocardial infarction in experimental animals. Hum. Exp. Toxicol. 2021, 40, 915–927. [Google Scholar] [CrossRef]
- Chen, H.; Tang, X.; Liu, T.; Jing, L.; Wu, J. Zingiberene inhibits in vitro and in vivo human colon cancer cell growth via autophagy induction, suppression of PI3K/AKT/mTOR Pathway and caspase 2 deactivation. J. BUON 2019, 24, 1470–1475. [Google Scholar]
- Borgonetti, V.; Governa, P.; Manetti, F.; Galeotti, N. Zingiberene, a non-zinc-binding class I HDAC inhibitor: A novel strategy for the management of neuropathic pain. Phytomedicine 2023, 111, 154670. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matacchione, G.; Borgonetti, V.; Ramini, D.; Silvestrini, A.; Ojetti, M.; Galeotti, N.; Olivieri, F. Zingiber officinale Roscoe Rhizome Extract Exerts Senomorphic and Anti-Inflammatory Activities on Human Endothelial Cells. Biology 2023, 12, 438. https://doi.org/10.3390/biology12030438
Matacchione G, Borgonetti V, Ramini D, Silvestrini A, Ojetti M, Galeotti N, Olivieri F. Zingiber officinale Roscoe Rhizome Extract Exerts Senomorphic and Anti-Inflammatory Activities on Human Endothelial Cells. Biology. 2023; 12(3):438. https://doi.org/10.3390/biology12030438
Chicago/Turabian StyleMatacchione, Giulia, Vittoria Borgonetti, Deborah Ramini, Andrea Silvestrini, Marta Ojetti, Nicoletta Galeotti, and Fabiola Olivieri. 2023. "Zingiber officinale Roscoe Rhizome Extract Exerts Senomorphic and Anti-Inflammatory Activities on Human Endothelial Cells" Biology 12, no. 3: 438. https://doi.org/10.3390/biology12030438
APA StyleMatacchione, G., Borgonetti, V., Ramini, D., Silvestrini, A., Ojetti, M., Galeotti, N., & Olivieri, F. (2023). Zingiber officinale Roscoe Rhizome Extract Exerts Senomorphic and Anti-Inflammatory Activities on Human Endothelial Cells. Biology, 12(3), 438. https://doi.org/10.3390/biology12030438








