The Effects of Galactic Cosmic Rays on the Central Nervous System: From Negative to Unexpectedly Positive Effects That Astronauts May Encounter
Abstract
Simple Summary
Abstract
1. Introduction
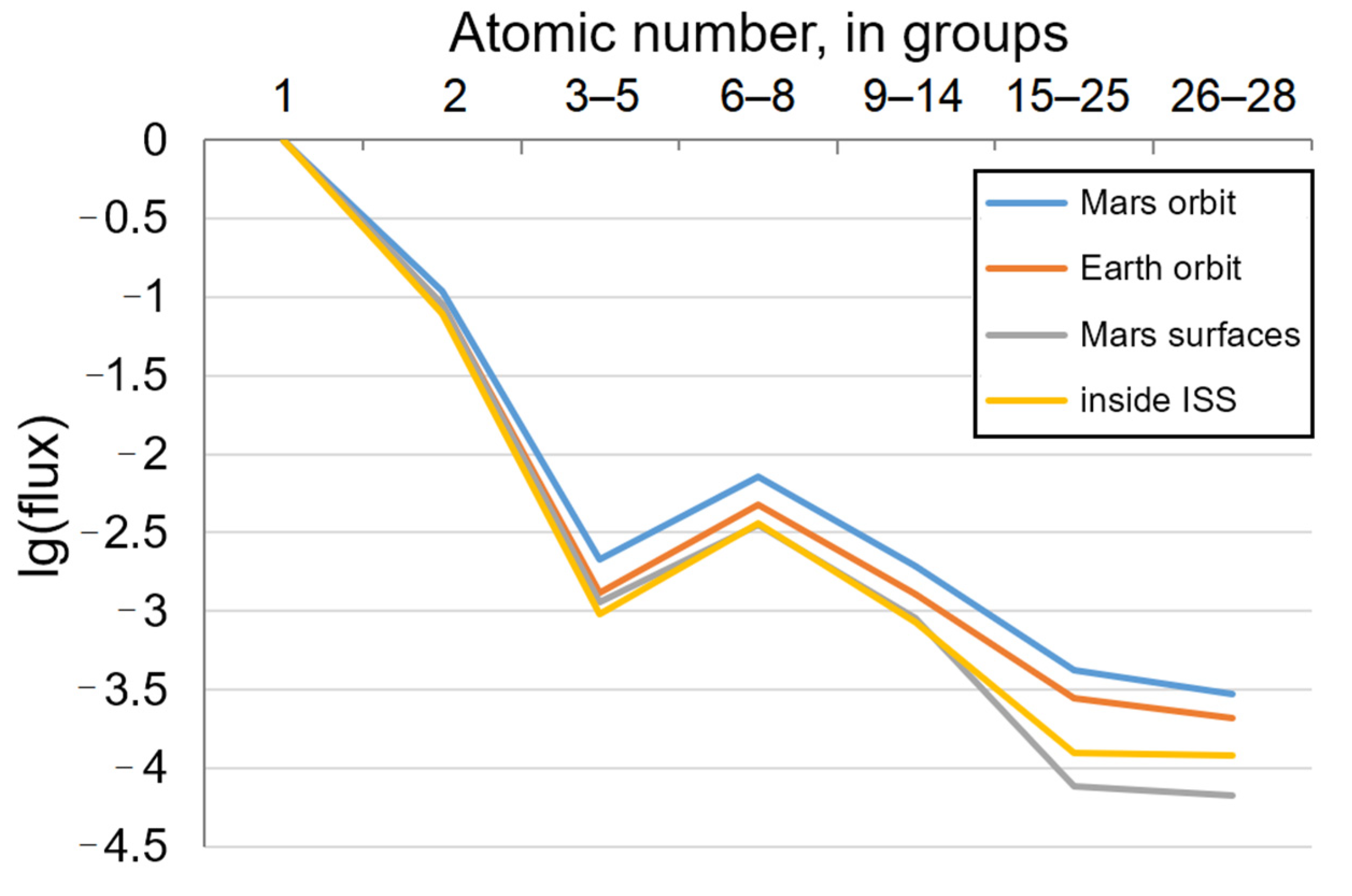
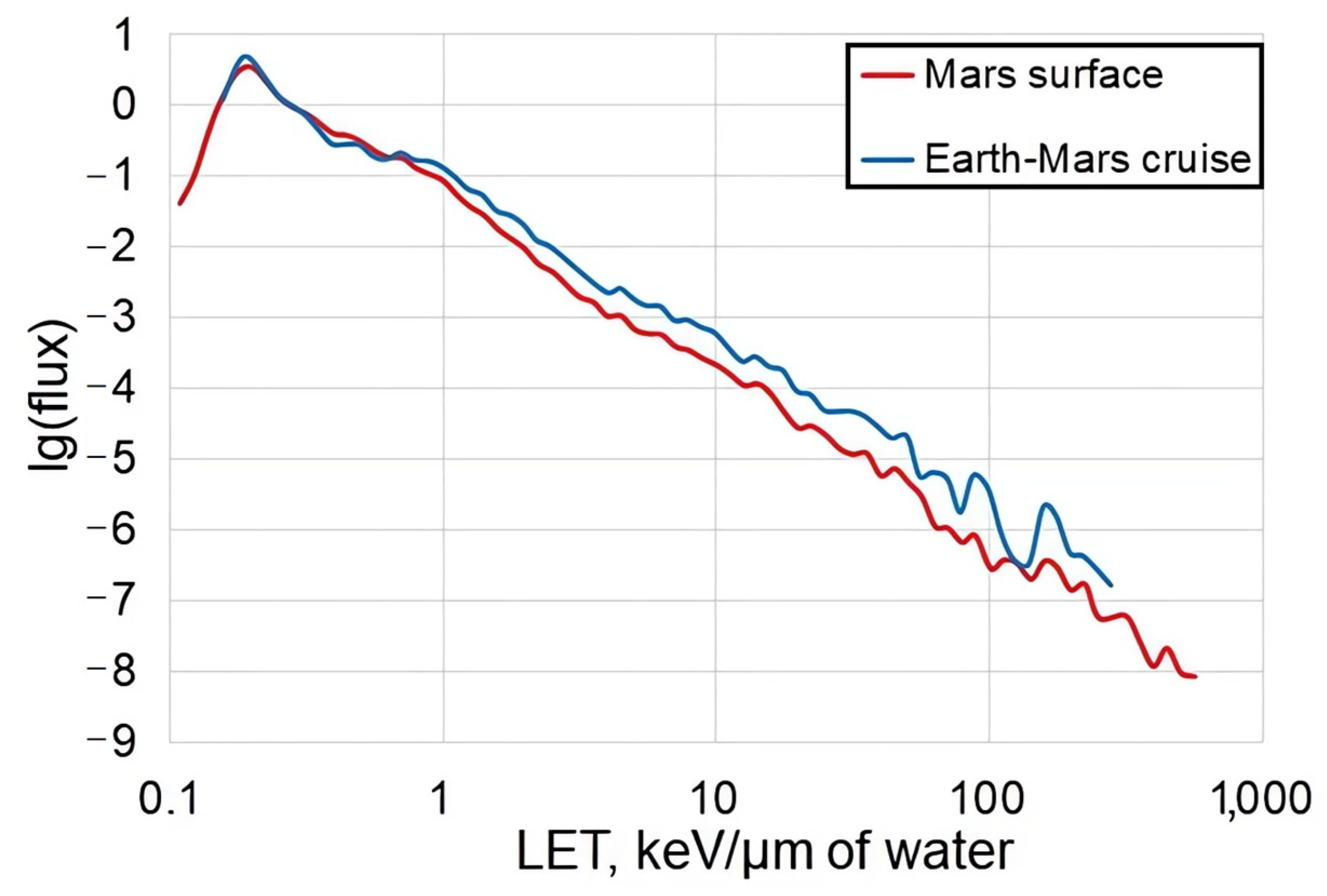
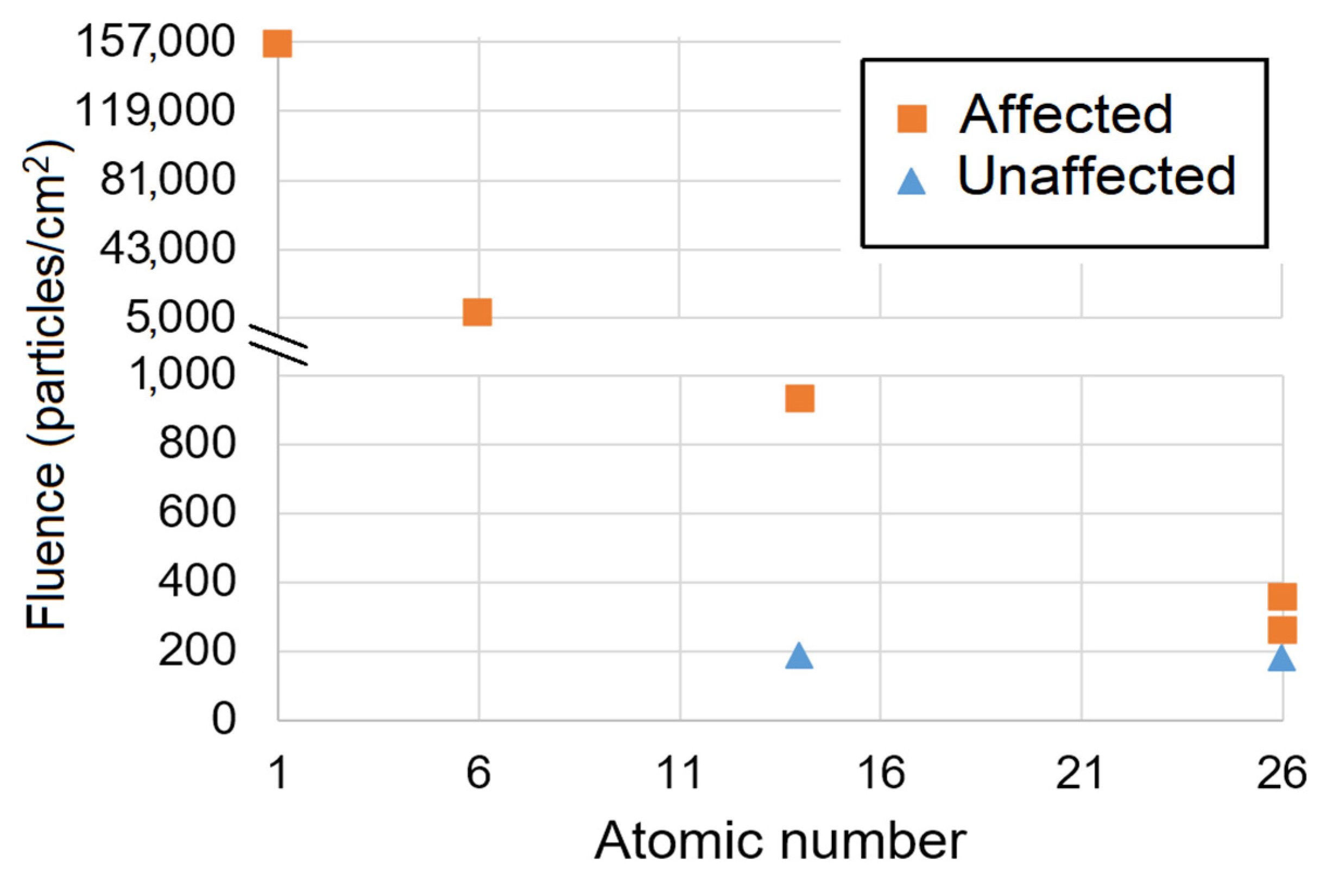
2. Galactic Cosmic Rays
3. Well-Being, Weight, Locomotor Abilities, and IR
4. Mental Health and Ionizing Radiation
4.1. Anxiety and Ionizing Radiation
4.2. Depressive-like Behavior and IR
4.3. Protons and HZE Stimulate Habituation, Orientation and Exploratory Behavior
5. Cognition and Ionizing Radiation
5.1. Primate Studies
5.2. Fear and Contextual Memory, High-Level Cognitive Tasks
5.3. Spatial Memory and Learning
5.4. Recognition Memory
5.5. Limitation of Some Cognitive Studies and Summarizing
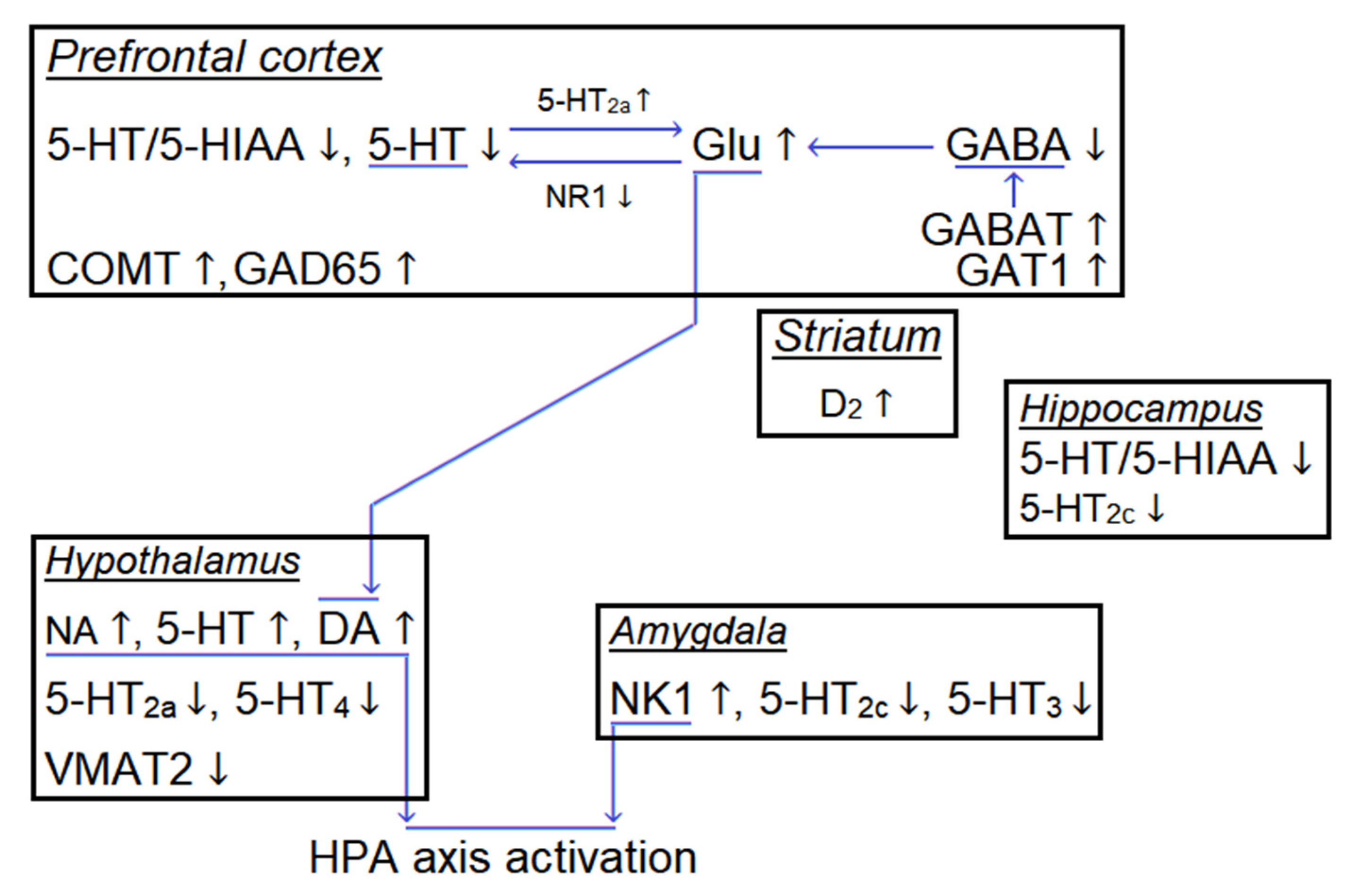
6. Direct and Indirect Effects of Irradiation: Nature of IR Positive Effect on Cognitive Abilities
7. Combined Effects of Hypogravity and IR and Their Mechanisms
8. IR Role in Neurogenesis: A Double-Edged Sword
9. Neurodegenerative Processes in Light of Radiation
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orosei, R.; Lauro, S.E.; Pettinelli, E.; Cicchetti, A.; Coradini, M.; Cosciotti, B.; Di Paolo, F.; Flamini, E.; Mattei, E.; Pajola, M.; et al. Radar evidence of subglacial liquid water on Mars. Science 2018, 361, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lucey, P.G.; Milliken, R.E.; Hayne, P.O.; Fisher, E.; Williams, J.P.; Hurley, D.M.; Elphic, R.C. Direct evidence of surface exposed water ice in the lunar polar regions. Proc. Natl. Acad. Sci. USA 2018, 115, 8907–8912. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Li, S.; Xu, R.; Liu, Y.; Wu, X.; Yang, W.; Wei, Y.; Lin, Y.; He, Z.; Hui, H.; et al. In situ detection of water on the Moon by the Chang’E-5 lander. Sci. Adv. 2022, 8, eabl9174. [Google Scholar] [CrossRef]
- Kokhan, V.S.; Matveeva, M.I.; Mukhametov, A.; Shtemberg, A.S. Risk of defeats in the central nervous system during deep space missions. Neurosci. Biobehav. Rev. 2016, 71, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Swenberg, C.E.; Horneck, G.; Stassinopoulos, E.G. (Eds.) Biological Effects and Physics of Solar and Galactic Cosmic Radiation; Plenum Press: New York, NY, USA, 1993. [Google Scholar]
- Cucinotta, F.A.; Alp, M.; Sulzman, F.M.; Wang, M. Space radiation risks to the central nervous system. Life Sci. Space Res. 2014, 2, 54–69. [Google Scholar] [CrossRef]
- Cekanaviciute, E.; Rosi, S.; Costes, S.V. Central Nervous System Responses to Simulated Galactic Cosmic Rays. Int. J. Mol. Sci. 2018, 19, 3669. [Google Scholar] [CrossRef]
- Kiffer, F.; Boerma, M.; Allen, A. Behavioral effects of space radiation: A comprehensive review of animal studies. Life Sci. Space Res. Amst. 2019, 21, 1–21. [Google Scholar] [CrossRef]
- Kokhan, V.S.; Anokhin, P.K.; Belov, O.V.; Gulyaev, M.V. Cortical Glutamate/GABA Imbalance after Combined Radiation Exposure: Relevance to Human Deep-Space Missions. Neuroscience 2019, 416, 295–308. [Google Scholar] [CrossRef]
- Whoolery, C.W.; Yun, S.; Reynolds, R.P.; Lucero, M.J.; Soler, I.; Tran, F.H.; Ito, N.; Redfield, R.L.; Richardson, D.R.; Shih, H.Y.; et al. Multi-domain cognitive assessment of male mice shows space radiation is not harmful to high-level cognition and actually improves pattern separation. Sci. Rep. 2020, 10, 2737. [Google Scholar] [CrossRef]
- Belyaeva, A.G.; Shtemberg, A.S.; Nosovskii, A.M.; Vasil’eva, O.N.; Gordeev, Y.V.; Kudrin, V.S.c.; Narkevich, V.B.; Krasavin, E.A.; Timoshenko, G.N.; Lapin, B.A.; et al. The Effects of High-Energy Protons and Carbon Ions (12C) on the Cognitive Function and the Content of Monoamines and Their Metabolites in Peripheral Blood in Monkeys. Neurochem. J. 2017, 11, 168–175. [Google Scholar] [CrossRef]
- Perez, R.E.; Younger, S.; Bertheau, E.; Fallgren, C.M.; Weil, M.M.; Raber, J. Effects of chronic exposure to a mixed field of neutrons and photons on behavioral and cognitive performance in mice. Behav. Brain Res. 2020, 379, 112377. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Singla, N.; Dhawan, D.K. Low dose X-irradiation mitigates diazepam induced depression in rat brain. Regul. Toxicol Pharm. 2016, 80, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Kokhan, V.S.; Matveeva, M.I.; Bazyan, A.S.; Kudrin, V.S.; Mukhametov, A.; Shtemberg, A.S. Combined effects of antiorthostatic suspension and ionizing radiation on the behaviour and neurotransmitters changes in different brain structures of rats. Behav. Brain Res. 2017, 320, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Chicheva, M.M.; Mal’tsev, A.V.; Kokhan, V.S.; Bachurin, S.O. The Effect of Ionizing Radiation on Cognitive Functions in Mouse Models of Alzheimer’s Disease. Dokl. Biol. Sci. Proc. Acad. Sci. USSR Biol. Sci. Sect./Transl. Russ. 2020, 494, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Hinshaw, R.G.; Le, K.X.; Park, M.A.; Wang, S.; Belanger, A.P.; Dubey, S.; Frost, J.L.; Shi, Q.; Holton, P.; et al. Space-like 56Fe irradiation manifests mild, early sex-specific behavioral and neuropathological changes in wildtype and Alzheimer’s-like transgenic mice. Sci. Rep. 2019, 9, 12118. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, J.; Grantham, V. Radiation Hormesis: Historical and Current Perspectives. J. Nucl. Med. Technol. 2015, 43, 242–246. [Google Scholar] [CrossRef]
- Chancellor, J.C.; Blue, R.S.; Cengel, K.A.; Aunon-Chancellor, S.M.; Rubins, K.H.; Katzgraber, H.G.; Kennedy, A.R. Limitations in predicting the space radiation health risk for exploration astronauts. NPJ Microgravity 2018, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, F.A.; Cacao, E. Non-Targeted Effects Models Predict Significantly Higher Mars Mission Cancer Risk than Targeted Effects Models. Sci. Rep. 2017, 7, 1832. [Google Scholar] [CrossRef]
- Hassler, D.M.; Zeitlin, C.; Wimmer-Schweingruber, R.F.; Ehresmann, B.; Rafkin, S.; Eigenbrode, J.L.; Brinza, D.E.; Weigle, G.; Bottcher, S.; Bohm, E.; et al. Mars’ surface radiation environment measured with the Mars Science Laboratory’s Curiosity rover. Science 2014, 343, 1244797. [Google Scholar] [CrossRef]
- Nelson, G.A. Fundamental space radiobiology. Gravit. Space Biol. Bull. Publ. Am. Soc. Gravit. Space Biol. 2003, 16, 29–36. [Google Scholar]
- Reitz, G.; Berger, T.; Matthiae, D. Radiation exposure in the moon environment. Planet. Space Sci. 2012, 74, 78–83. [Google Scholar] [CrossRef]
- Simpson, J.A. Elemental and Isotopic Composition of the Galactic Cosmic Rays. Annu. Rev. Nucl. Part Sci. 1983, 33, 323–382. [Google Scholar] [CrossRef]
- Kohler, J.; Ehresmann, B.; Zeitlin, C.; Wimmer-Schweingruber, R.F.; Hassler, D.M.; Reitz, G.; Brinza, D.E.; Appel, J.; Bottcher, S.; Bohm, E.; et al. Measurements of the neutron spectrum in transit to Mars on the Mars Science Laboratory. Life Sci. Space Res. Amst. 2015, 5, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Straume, T.; Slaba, T.C.; Bhattacharya, S.; Braby, L.A. Cosmic-ray interaction data for designing biological experiments in space. Life Sci. Space Res. Amst. 2017, 13, 51–59. [Google Scholar] [CrossRef]
- Matthiä, D.; Ehresmann, B.; Lohf, H.; Köhler, J.; Zeitlin, C.; Appel, J.; Sato, T.; Slaba, T.; Martin, C.; Berger, T.; et al. The Martian surface radiation environment—A comparison of models and MSL/RAD measurements. J. Space Weather Space Clim. 2016, 6, A13. [Google Scholar] [CrossRef]
- Rabin, B.M.; Carrihill-Knoll, K.L.; Shukitt-Hale, B. Operant responding following exposure to HZE particles and its relationship to particle energy and linear energy transfer. Adv. Space Res. 2011, 48, 370–377. [Google Scholar] [CrossRef]
- Britten, R.A.; Jewell, J.S.; Duncan, V.D.; Hadley, M.M.; Macadat, E.; Musto, A.E.; Tessa, C. Impaired Attentional Set-Shifting Performance after Exposure to 5 cGy of 600 MeV/n (28)Si Particles. Radiat. Res. 2018, 189, 273–282. [Google Scholar] [CrossRef]
- Saganti, P.B.; Cucinotta, F.A.; Wilson, J.W.; Cleghorn, T.F.; Zeitlin, C.J. Model calculations of the particle spectrum of the galactic cosmic ray (GCR) environment: Assessment with ACE/CRIS and MARIE measurements. Radiat. Meas. 2006, 41, 1152–1157. [Google Scholar] [CrossRef]
- Mewaldt, R.A. Galactic cosmic ray composition and energy spectra. Adv. Space Res. Off. J. Comm. Space Res. 1994, 14, 737–747. [Google Scholar] [CrossRef]
- Ehresmann, B.; Zeitlin, C.; Hassler, D.M.; Wimmer-Schweingruber, R.F.; Böhm, E.; Böttcher, S.; Brinza, D.E.; Burmeister, S.; Guo, J.; Köhler, J.; et al. Charged particle spectra obtained with the Mars Science Laboratory Radiation Assessment Detector (MSL/RAD) on the surface of Mars. J. Geophys. Res. Planets 2014, 19, 468–479. [Google Scholar] [CrossRef]
- Dobynde, M.I.; Effenberger, F.; Kartashov, D.A.; Shprits, Y.Y.; Shurshakov, V.A. Ray-tracing simulation of the radiation dose distribution on the surface of the spherical phantom of the MATROSHKA-R experiment onboard the ISS. Life Sci. Space Res. Amst. 2019, 21, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Zeitlin, C.; Hassler, D.M.; Cucinotta, F.A.; Ehresmann, B.; Wimmer-Schweingruber, R.F.; Brinza, D.E.; Kang, S.; Weigle, G.; Bottcher, S.; Bohm, E.; et al. Measurements of energetic particle radiation in transit to Mars on the Mars Science Laboratory. Science 2013, 340, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Narici, L.; Casolino, M.; Fino, L.D.; Larosa, M.; Picozza, P.; Zaconte, V. Radiation survey in the International Space Station. J. Space Weather Space Clim. 2015, 5, A37. [Google Scholar] [CrossRef]
- Friedberg, W.; Copeland, K.; Duke, F.E.; O’Brien, K.; Darden, E.B., Jr. Health aspects of radiation exposure on a simulated mission to Mars. Radioact. Environ. 2005, 7, 894–901. [Google Scholar] [CrossRef]
- Berger, T. Radiation dosimetry onboard the International Space Station ISS. Z. Med. Phys. 2008, 18, 265–275. [Google Scholar] [CrossRef]
- Kiffer, F.C.; Luitel, K.; Tran, F.H.; Patel, R.A.; Guzman, C.S.; Soler, I.; Xiao, R.; Shay, J.W.; Yun, S.; Eisch, A.J. Effects of a 33-ion sequential beam galactic cosmic ray analog on male mouse behavior and evaluation of CDDO-EA as a radiation countermeasure. Behav. Brain Res. 2022, 419, 113677. [Google Scholar] [CrossRef]
- Rivera, P.D.; Shih, H.Y.; Leblanc, J.A.; Cole, M.G.; Amaral, W.Z.; Mukherjee, S.; Zhang, S.; Lucero, M.J.; Decarolis, N.A.; Chen, B.P.; et al. Acute and fractionated exposure to high-LET 56Fe HZE-particle radiation both result in similar long-term deficits in adult hippocampal neurogenesis. Radiat. Res. 2013, 180, 658–667. [Google Scholar] [CrossRef]
- Kokhan, V.S.; Lebedeva-Georgievskaya, K.B.; Kudrin, V.S.; Bazyan, A.S.; Maltsev, A.V.; Shtemberg, A.S. An investigation of the single and combined effects of hypogravity and ionizing radiation on brain monoamine metabolism and rats’ behavior. Life Sci. Space Res. 2019, 20, 12–19. [Google Scholar] [CrossRef]
- Zhou, G.; Bennett, P.V.; Cutter, N.C.; Sutherland, B.M. Proton-HZE-particle sequential dual-beam exposures increase anchorage-independent growth frequencies in primary human fibroblasts. Radiat. Res. 2006, 166, 488–494. [Google Scholar] [CrossRef]
- Demizu, Y.; Kagawa, K.; Ejima, Y.; Nishimura, H.; Sasaki, R.; Soejima, T.; Yanou, T.; Shimizu, M.; Furusawa, Y.; Hishikawa, Y.; et al. Cell biological basis for combination radiotherapy using heavy-ion beams and high-energy X-rays. Radiother. Oncol. 2004, 71, 207–211. [Google Scholar] [CrossRef]
- Higgins, P.D.; DeLuca, P.M., Jr.; Pearson, D.W.; Gould, M.N. V79 survival following simultaneous or sequential irradiation by 15-MeV neutrons and 60Co photons. Radiat. Res. 1983, 95, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Butenko, A.V.; Gordeev, I.S.; Kovalenko, A.D.; Paraipan, M.; Syresin, E.M.; Timoshenko, G.N. Prediction of radiation environment around NICA complex. Phys. Part. Nucl. Lett. 2022, 19, 123–128. [Google Scholar] [CrossRef]
- Acharya, M.M.; Baulch, J.E.; Klein, P.M.; Baddour, A.A.D.; Apodaca, L.A.; Kramar, E.A.; Alikhani, L.; Garcia, C., Jr.; Angulo, M.C.; Batra, R.S.; et al. New Concerns for Neurocognitive Function during Deep Space Exposures to Chronic, Low Dose-Rate, Neutron Radiation. eNeuro 2019, 6. [Google Scholar] [CrossRef]
- Bevelacqua, J.J.; Welsh, J.; Mortazavi, S.M.J. Comments on “New Concerns for Neurocognitive Function during Deep Space Exposures to Chronic, Low Dose Rate, Neutron Radiation”. eNeuro 2020, 7. [Google Scholar] [CrossRef]
- Thomson, J.F.; Williamson, F.S.; Grahn, D. Life shortening in mice exposed to fission neutrons and gamma rays. V. Further studies with single low doses. Radiat. Res. 1985, 104, 420–428. [Google Scholar] [CrossRef]
- Caratero, A.; Courtade, M.; Bonnet, L.; Planel, H.; Caratero, C. Effect of a continuous gamma irradiation at a very low dose on the life span of mice. Gerontology 1998, 44, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Whoolery, C.W.; Walker, A.K.; Richardson, D.R.; Lucero, M.J.; Reynolds, R.P.; Beddow, D.H.; Clark, K.L.; Shih, H.Y.; LeBlanc, J.A.; Cole, M.G.; et al. Whole-Body Exposure to (28)Si-Radiation Dose-Dependently Disrupts Dentate Gyrus Neurogenesis and Proliferation in the Short Term and New Neuron Survival and Contextual Fear Conditioning in the Long Term. Radiat. Res. 2017, 188, 532–551. [Google Scholar] [CrossRef]
- Wang, B.; Tanaka, K.; Ji, B.; Ono, M.; Fang, Y.; Ninomiya, Y.; Maruyama, K.; Izumi-Nakajima, N.; Begum, N.; Higuchi, M.; et al. Low-dose total-body carbon-ion irradiations induce early transcriptional alteration without late Alzheimer’s disease-like pathogenesis and memory impairment in mice. J. Neurosci. Res. 2014, 92, 915–926. [Google Scholar] [CrossRef]
- Pecaut, M.J.; Haerich, P.; Zuccarelli, C.N.; Smith, A.L.; Zendejas, E.D.; Nelson, G.A. Behavioral consequences of radiation exposure to simulated space radiation in the C57BL/6 mouse: Open field, rotorod, and acoustic startle. Cogn. Affect Behav. Neurosci. 2002, 2, 329–340. [Google Scholar] [CrossRef]
- Ye, F.; Zhao, T.; Liu, X.; Jin, X.; Liu, X.; Wang, T.; Li, Q. Long-term Autophagy and Nrf2 Signaling in the Hippocampi of Developing Mice after Carbon Ion Exposure. Sci. Rep. 2015, 5, 18636. [Google Scholar] [CrossRef]
- Pecaut, M.J.; Haerich, P.; Miller, C.N.; Smith, A.L.; Zendejas, E.D.; Nelson, G.A. The effects of low-dose, high-LET radiation exposure on three models of behavior in C57BL/6 mice. Radiat. Res. 2004, 162, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.R.; Raber, J.; Chakraborti, A.; Sharma, S.; Fike, J.R. 56Fe Irradiation Alters Spine Density and Dendritic Complexity in the Mouse Hippocampus. Radiat. Res. 2015, 184, 586–594. [Google Scholar] [CrossRef]
- Kokhan, V.S.; Shakhbazian, E.V.; Markova, N.A. Psycho-emotional status but not cognition are changed under the combined effect of ionizing radiations at doses related to deep space missions. Behav. Brain Res. 2019, 362, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Raber, J.; Yamazaki, J.; Torres, E.R.S.; Kirchoff, N.; Stagaman, K.; Sharpton, T.; Turker, M.S.; Kronenberg, A. Combined Effects of Three High-Energy Charged Particle Beams Important for Space Flight on Brain, Behavioral and Cognitive Endpoints in B6D2F1 Female and Male Mice. Front. Physiol. 2019, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Rice, O.V.; Grande, A.V.; Dehktyar, N.; Bruneus, M.; Robinson, J.K.; Gatley, S.J. Long-term effects of irradiation with iron-56 particles on the nigrostriatal dopamine system. Radiat. Environ. Biophys. 2009, 48, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Vichaya, E.G.; Malik, S.; Sominsky, L.; Ford, B.G.; Spencer, S.J.; Dantzer, R. Microglia depletion fails to abrogate inflammation-induced sickness in mice and rats. J. Neuroinflamm. 2020, 17, 172. [Google Scholar] [CrossRef]
- Parihar, V.K.; Allen, B.D.; Caressi, C.; Kwok, S.; Chu, E.; Tran, K.K.; Chmielewski, N.N.; Giedzinski, E.; Acharya, M.M.; Britten, R.A.; et al. Cosmic radiation exposure and persistent cognitive dysfunction. Sci. Rep. 2016, 6, 34774. [Google Scholar] [CrossRef]
- Rola, R.; Fishman, K.; Baure, J.; Rosi, S.; Lamborn, K.R.; Obenaus, A.; Nelson, G.A.; Fike, J.R. Hippocampal neurogenesis and neuroinflammation after cranial irradiation with 56Fe particles. Radiat. Res. 2008, 169, 626–632. [Google Scholar] [CrossRef]
- Matveeva, M.I.; Shtemberg, A.S.; Timoshenko, G.N.; Krasavin, E.A.; Narkevich, V.B.; Klodt, P.M.; Kudrin, V.S.; Bazyan, A.S. The effects of irradiation by 12C carbon ions on monoamine exchange in several rat brain structures. Neurochem. J. 2013, 7, 303–307. [Google Scholar] [CrossRef]
- Jones, G.H.; Robbins, T.W. Differential effects of mesocortical, mesolimbic, and mesostriatal dopamine depletion on spontaneous, conditioned, and drug-induced locomotor activity. Pharmacol. Biochem. Behav. 1992, 43, 887–895. [Google Scholar] [CrossRef]
- Suedfeld, P. Invulnerability, coping, salutogenesis, integration: Four phases of space psychology. Aviat. Space Environ. Med. 2005, 76, B61–B66. [Google Scholar]
- Palinkas, L.A. Psychosocial issues in long-term space flight: Overview. Gravit. Space Biol. Bull. Publ. Am. Soc. Gravit. Space Biol. 2001, 14, 25–33. [Google Scholar]
- LaMontagne, A.D.; Martin, A.; Page, K.M.; Reavley, N.J.; Noblet, A.J.; Milner, A.J.; Keegel, T.; Smith, P.M. Workplace mental health: Developing an integrated intervention approach. BMC Psychiatry 2014, 14, 131. [Google Scholar] [CrossRef] [PubMed]
- Kanas, N.; Manzey, D. Space Psychology and Psychiatry, 2nd ed.; Microcosm Press: El Segundo, CA, USA; Springer: Dordrecht, The Netherlands, 2008; Volume xv, 240p. [Google Scholar]
- Kanas, N.; Salnitskiy, V.; Gushin, V.; Weiss, D.S.; Grund, E.M.; Flynn, C.; Kozerenko, O.; Sled, A.; Marmar, C.R. Asthenia--does it exist in space? Psychosom. Med. 2001, 63, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Ball, J.; Evans, C.H. Safe Passage: Astronaut Care for Exploration Missions; National Academy Press: Washington, DC, USA, 2001; p. 291. [Google Scholar]
- Garrett-Bakelman, F.E.; Darshi, M.; Green, S.J.; Gur, R.C.; Lin, L.; Macias, B.R.; McKenna, M.J.; Meydan, C.; Mishra, T.; Nasrini, J.; et al. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 2019, 364, eaau8650. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Kimeldorf, D.J.; Hunt, E.L. Spatial avoidance in the rat as a result of exposure to ionizing radiation. Br. J. Radiol. 1957, 30, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Kimeldorf, D.J. Some factors which influence radiation-conditioned behavior of rats. Radiat. Res. 1960, 12, 719–727. [Google Scholar] [CrossRef]
- Kimeldorf, D.J.; Garcia, J.; Rubadeau, D.O. Radiation-induced conditioned avoidance behavior in rats, mice, and cats. Radiat. Res. 1960, 12, 710–718. [Google Scholar] [CrossRef]
- Arbit, J. Emotionality and avoidance conditioning to x radiation. J. Comp. Physiol. Psychol. 1961, 54, 653–657. [Google Scholar] [CrossRef]
- Casadesus, G.; Shukitt-Hale, B.; Cantuti-Castelvetri, I.; Rabin, B.M.; Joseph, J.A. The effects of heavy particle irradiation on exploration and response to environmental change. Adv. Space Res. Off. J. Comm. Space Res. 2004, 33, 1340–1346. [Google Scholar] [CrossRef]
- Rabin, B.M.; Carrihill-Knoll, K.L.; Shukitt-Hale, B. Comparison of the Effectiveness of Exposure to Low-LET Helium Particles ((4)He) and Gamma Rays ((137)Cs) on the Disruption of Cognitive Performance. Radiat. Res. 2015, 184, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Kiffer, F.; Howe, A.K.; Carr, H.; Wang, J.; Alexander, T.; Anderson, J.E.; Groves, T.; Seawright, J.W.; Sridharan, V.; Carter, G.; et al. Late effects of (1)H irradiation on hippocampal physiology. Life Sci. Space Res. Amst. 2018, 17, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Rabin, B.M.; Carrihill-Knoll, K.L.; Miller, M.G.; Shukitt-Hale, B. Age as a factor in the responsiveness of the organism to the disruption of cognitive performance by exposure to HZE particles differing in linear energy transfer. Life Sci. Space Res. Amst. 2018, 16, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Lillie, L.E.; Temple, N.J.; Florence, L.Z. Reference values for young normal Sprague-Dawley rats: Weight gain, hematology and clinical chemistry. Hum. Exp. Toxicol. 1996, 15, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Borodovitsyna, O.; Flamini, M.D.; Chandler, D.J. Acute Stress Persistently Alters Locus Coeruleus Function and Anxiety-like Behavior in Adolescent Rats. Neuroscience 2018, 373, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A. Distributed circuits underlying anxiety. Front. Behav. Neurosci. 2014, 8, 112. [Google Scholar] [CrossRef]
- Fuss, J.; Ben Abdallah, N.M.; Vogt, M.A.; Touma, C.; Pacifici, P.G.; Palme, R.; Witzemann, V.; Hellweg, R.; Gass, P. Voluntary exercise induces anxiety-like behavior in adult C57BL/6J mice correlating with hippocampal neurogenesis. Hippocampus 2010, 20, 364–376. [Google Scholar] [CrossRef]
- Fuss, J.; Ben Abdallah, N.M.; Hensley, F.W.; Weber, K.J.; Hellweg, R.; Gass, P. Deletion of running-induced hippocampal neurogenesis by irradiation prevents development of an anxious phenotype in mice. PLoS ONE 2010, 5, e0012769. [Google Scholar] [CrossRef]
- Kokhan, V.S.; Mariasina, S.; Pikalov, V.A.; Abaimov, D.A.; Somasundaram, S.G.; Kirkland, C.E.; Aliev, G. Neurokinin-1 Receptor Antagonist Reverses Functional CNS Alteration Caused by Combined gamma-rays and Carbon Nuclei Irradiation. CNS Neurol. Disord. Drug Targets 2022, 21, 278–289. [Google Scholar] [CrossRef]
- Kokhan, V.S. Glutamate/GABA disbalance in comparative analysis of radiation and traumatic injury of the brain cortex. Aviakosmicheskaia I Ekol. Meditsina=Aerosp. Environ. Med. 2019, 53, 5–10. [Google Scholar] [CrossRef]
- Krukowski, K.; Feng, X.; Paladini, M.S.; Chou, A.; Sacramento, K.; Grue, K.; Riparip, L.K.; Jones, T.; Campbell-Beachler, M.; Nelson, G.; et al. Temporary microglia-depletion after cosmic radiation modifies phagocytic activity and prevents cognitive deficits. Sci. Rep. 2018, 8, 7857. [Google Scholar] [CrossRef]
- Shtemberg, A.S.; Bazian, A.S.; Lebedeva-Georgievskaya, K.B.; Matveeva, M.I.; Kudrin, V.S.; Narkevich, V.B.; Klodt, P.M.; Kokhan, V.S. Effects of exposure to high-energy protons on rat’s behavior and underlying neurochemical mechanisms. Aviakosmicheskaia I Ekol. Meditsina=Aerosp. Environ. Med. 2013, 47, 54–60. [Google Scholar]
- Wang, Q.; Timberlake, M.A., 2nd; Prall, K.; Dwivedi, Y. The recent progress in animal models of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 77, 99–109. [Google Scholar] [CrossRef]
- Kolesnikova, I.A.; Budennay, N.N.; Severiukhin, Y.S.; Lyakhova, K.N.; Utina, D.M. Analysis of morphofunctional state of experimental animals brain fields under proton irradiation over the long period. J. New Med. Technol. 2018, 25, 177–181. [Google Scholar]
- Campos, A.C.; Fogaca, M.V.; Aguiar, D.C.; Guimaraes, F.S. Animal models of anxiety disorders and stress. Rev. Bras. De Psiquiatr. 2013, 35 (Suppl. S2), S101–S111. [Google Scholar] [CrossRef]
- Ruff, H.A.; Saltarelli, L.M. Exploratory play with objects: Basic cognitive processes and individual differences. New Dir. Child Dev. 1993, 1993, 5–16. [Google Scholar] [CrossRef]
- Shtemberg, A.S.; Lebedeva-Georgievskaia, K.V.; Matveeva, M.I.; Kudrin, V.S.; Narkevich, V.B.; Klodt, P.M.; Bazian, A.S. Effect of space flight factors simulated in ground-based experiments on the behavior, discriminant learning, and exchange of monoamines in different brain structures of rats. Izv. Akad. Nauk. Seriia Biol./Ross. Akad. Nauk 2014, 168–175. [Google Scholar] [CrossRef]
- Forss, S.I.F.; Motes-Rodrigo, A.; Dongre, P.; Mohr, T.; van de Waal, E. Captivity and habituation to humans raise curiosity in vervet monkeys. Anim. Cogn. 2022, 25, 671–682. [Google Scholar] [CrossRef]
- Walsh, J.; Desbonnet, L.; Clarke, N.; Waddington, J.L.; O’Tuathaigh, C.M. Disruption of exploratory and habituation behavior in mice with mutation of DISC1: An ethologically based analysis. J. Neurosci. Res. 2012, 90, 1445–1453. [Google Scholar] [CrossRef]
- Salomons, A.R.; Arndt, S.S.; Ohl, F. Impact of anxiety profiles on cognitive performance in BALB/c and 129P2 mice. Cogn. Affect. Behav. Neurosci. 2012, 12, 794–803. [Google Scholar] [CrossRef]
- Typlt, M.; Mirkowski, M.; Azzopardi, E.; Ruth, P.; Pilz, P.K.; Schmid, S. Habituation of reflexive and motivated behavior in mice with deficient BK channel function. Front. Integr. Neurosci. 2013, 7, 79. [Google Scholar] [CrossRef]
- Al-Amri, A.H.; Armstrong, P.; Amici, M.; Ligneul, C.; Rouse, J.; El-Asrag, M.E.; Pantiru, A.; Vancollie, V.E.; Ng, H.W.Y.; Ogbeta, J.A.; et al. PDZD8 Disruption Causes Cognitive Impairment in Humans, Mice, and Fruit Flies. Biol. Psychiatry 2022, 92, 323–334. [Google Scholar] [CrossRef]
- Kavšek, M. Predicting later IQ from infant visual habituation and dishabituation: A meta-analysis. J. Appl. Dev. Psychol. 2004, 25, 369–393. [Google Scholar] [CrossRef]
- Rabin, B.M.; Heroux, N.A.; Shukitt-Hale, B.; Carrihill-Knoll, K.L.; Beck, Z.; Baxter, C. Lack of reliability in the disruption of cognitive performance following exposure to protons. Radiat. Environ. Biophys. 2015, 54, 285–295. [Google Scholar] [CrossRef]
- Ades, H.W.; Grodsky, M.A.; Riopelle, A.J. Learned performance of monkeys after single and repeated x irradiations. J. Comp. Physiol. Psychol. 1956, 49, 521–524. [Google Scholar] [CrossRef]
- Harlow, H.F.; Schrier, A.M.; Simons, D.G. Exposure of primates to cosmic radiation above 90,000 feet. J. Comp. Physiol. Psychol. 1956, 49, 195–200. [Google Scholar] [CrossRef]
- Belyaeva, A.G.; Kudrin, V.S.; Koshlan, I.V.; Koshlan, N.A.; Isakova, M.D.; Bogdanova, Y.V.; Timoshenko, G.N.; Krasavin, E.A.; Blokhina, T.M.; Yashkina, E.I.; et al. Effects of combined exposure to modeled radiation and gravitation factors of the interplanetary flight: Monkeys’ cognitive functions and the content of monoamines and their metabolites; cytogenetic changes in peripheral blood lymphocytes. Life Sci. Space Res. Amst. 2021, 30, 45–54. [Google Scholar] [CrossRef]
- Blair, W.C. The effects of cranial x radiation on maze acquisition in rats. J. Comp. Physiol. Psychol. 1958, 51, 175–177. [Google Scholar] [CrossRef]
- Villasana, L.; Rosenberg, J.; Raber, J. Sex-dependent effects of 56Fe irradiation on contextual fear conditioning in C57BL/6J mice. Hippocampus 2010, 20, 19–23. [Google Scholar] [CrossRef]
- Raber, J.; Marzulla, T.; Kronenberg, A.; Turker, M.S. (16)Oxygen irradiation enhances cued fear memory in B6D2F1 mice. Life Sci. Space Res. Amst. 2015, 7, 61–65. [Google Scholar] [CrossRef]
- Raber, J.; Allen, A.R.; Sharma, S.; Allen, B.; Rosi, S.; Olsen, R.H.; Davis, M.J.; Eiwaz, M.; Fike, J.R.; Nelson, G.A. Effects of Proton and Combined Proton and 56Fe Radiation on the Hippocampus. Radiat. Res. 2016, 185, 20–30. [Google Scholar] [CrossRef]
- Shtemberg, A.S.; Kokhan, V.S.; Matveeva, M.I.; Lebedeva-Georgievskaia, K.V.; Timoshenko, G.N.; Molokanov, A.G.; Krasavin, E.A.; Narkevich, V.B.; Klodt, P.M.; Bazian, A.S. The effect of high-energy protons in the Bragg Peak on the behavior of rats and the exchange of monoamines in some brain structures. Neurochem. J. 2015, 9, 66–72. [Google Scholar] [CrossRef]
- Matzel, L.D.; Han, Y.R.; Grossman, H.; Karnik, M.S.; Patel, D.; Scott, N.; Specht, S.M.; Gandhi, C.C. Individual differences in the expression of a "general" learning ability in mice. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 6423–6433. [Google Scholar] [CrossRef] [PubMed]
- Shukitt-Hale, B.; Szprengiel, A.; Pluhar, J.; Rabin, B.M.; Joseph, J.A. The effects of proton exposure on neurochemistry and behavior. Adv. Space Res. Off. J. Comm. Space Res. 2004, 33, 1334–1339. [Google Scholar] [CrossRef]
- Impey, S.; Jopson, T.; Pelz, C.; Tafessu, A.; Fareh, F.; Zuloaga, D.; Marzulla, T.; Riparip, L.K.; Stewart, B.; Rosi, S.; et al. Bi-directional and shared epigenomic signatures following proton and 56Fe irradiation. Sci. Rep. 2017, 7, 10227. [Google Scholar] [CrossRef]
- Haley, G.E.; Yeiser, L.; Olsen, R.H.; Davis, M.J.; Johnson, L.A.; Raber, J. Early effects of whole-body 56Fe irradiation on hippocampal function in C57BL/6J mice. Radiat. Res. 2013, 179, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Haley, G.E.; Villasana, L.; Dayger, C.; Davis, M.J.; Raber, J. Apolipoprotein e genotype-dependent paradoxical short-term effects of 56fe irradiation on the brain. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 793–799. [Google Scholar] [CrossRef]
- Howe, A.; Kiffer, F.; Alexander, T.C.; Sridharan, V.; Wang, J.; Ntagwabira, F.; Rodriguez, A.; Boerma, M.; Allen, A.R. Long-Term Changes in Cognition and Physiology after Low-Dose (16)O Irradiation. Int. J. Mol. Sci. 2019, 20, 188. [Google Scholar] [CrossRef]
- Kiffer, F.; Alexander, T.; Anderson, J.E.; Groves, T.; Wang, J.; Sridharan, V.; Boerma, M.; Allen, A.R. Late Effects of (16)O-Particle Radiation on Female Social and Cognitive Behavior and Hippocampal Physiology. Radiat. Res. 2019, 191, 278–294. [Google Scholar] [CrossRef]
- Carr, H.; Alexander, T.C.; Groves, T.; Kiffer, F.; Wang, J.; Price, E.; Boerma, M.; Allen, A.R. Early effects of (16)O radiation on neuronal morphology and cognition in a murine model. Life Sci. Space Res. Amst. 2018, 17, 63–73. [Google Scholar] [CrossRef]
- Britten, R.A.; Davis, L.K.; Johnson, A.M.; Keeney, S.; Siegel, A.; Sanford, L.D.; Singletary, S.J.; Lonart, G. Low (20 cGy) doses of 1 GeV/u 56Fe--particle radiation lead to a persistent reduction in the spatial learning ability of rats. Radiat. Res. 2012, 177, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Wyrobek, A.J.; Britten, R.A. Individual variations in dose response for spatial memory learning among outbred wistar rats exposed from 5 to 20 cGy of 56Fe particles. Environ. Mol. Mutagen. 2016, 57, 331–340. [Google Scholar] [CrossRef]
- Britten, R.A.; Jewell, J.S.; Duncan, V.D.; Davis, L.K.; Hadley, M.M.; Wyrobek, A.J. Spatial Memory Performance of Socially Mature Wistar Rats is Impaired after Exposure to Low (5 cGy) Doses of 1 GeV/n (48)Ti Particles. Radiat. Res. 2017, 187, 60–65. [Google Scholar] [CrossRef]
- Poulose, S.M.; Rabin, B.M.; Bielinski, D.F.; Kelly, M.E.; Miller, M.G.; Thanthaeng, N.; Shukitt-Hale, B. Neurochemical differences in learning and memory paradigms among rats supplemented with anthocyanin-rich blueberry diets and exposed to acute doses of 56Fe particles. Life Sci. Space Res. Amst. 2017, 12, 16–23. [Google Scholar] [CrossRef]
- Impey, S.; Jopson, T.; Pelz, C.; Tafessu, A.; Fareh, F.; Zuloaga, D.; Marzulla, T.; Riparip, L.K.; Stewart, B.; Rosi, S.; et al. Short- and long-term effects of 56Fe irradiation on cognition and hippocampal DNA methylation and gene expression. BMC Genom. 2016, 17, 825. [Google Scholar] [CrossRef]
- Rabin, B.; Shukitt-Hale, B.; Carrihill-Knoll, K. Effects of age on the disruption of cognitive performance by exposure to space radiation. J. Behav. Brain Sci. 2014, 4, 297–307. [Google Scholar] [CrossRef]
- Villasana, L.E.; Rosenthal, R.A.; Doctrow, S.R.; Pfankuch, T.; Zuloaga, D.G.; Garfinkel, A.M.; Raber, J. Effects of alpha-lipoic acid on associative and spatial memory of sham-irradiated and 56Fe-irradiated C57BL/6J male mice. Pharmacol. Biochem. Behav. 2013, 103, 487–493. [Google Scholar] [CrossRef]
- Dix, S.L.; Aggleton, J.P. Extending the spontaneous preference test of recognition: Evidence of object-location and object-context recognition. Behav. Brain Res. 1999, 99, 191–200. [Google Scholar] [CrossRef]
- Barker, G.R.; Bird, F.; Alexander, V.; Warburton, E.C. Recognition memory for objects, place, and temporal order: A disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 2948–2957. [Google Scholar] [CrossRef]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef]
- Albasser, M.M.; Chapman, R.J.; Amin, E.; Iordanova, M.D.; Vann, S.D.; Aggleton, J.P. New behavioral protocols to extend our knowledge of rodent object recognition memory. Learn Mem. 2010, 17, 407–419. [Google Scholar] [CrossRef]
- Yow, H.Y.; Ahmad, N.; Azmi, N.; Bakry, M.M. The effect of curcumin on anxiety and recognition memory in kainate model of epileptic rats. Indian J. Pharm. Sci. 2017, 79, 267–276. [Google Scholar] [CrossRef]
- Roman, E.; Karlsson, O. Increased anxiety-like behavior but no cognitive impairments in adult rats exposed to constant light conditions during perinatal development. Ups J. Med. Sci. 2013, 118, 222–227. [Google Scholar] [CrossRef]
- Luparini, M.R.; Del Vecchio, A.; Barillari, G.; Magnani, M.; Prosdocimi, M. Cognitive impairment in old rats: A comparison of object displacement, object recognition and water maze. Aging (Milano) 2000, 12, 264–273. [Google Scholar] [CrossRef]
- Sandi, C. Stress and cognition. Wiley Interdiscip. Rev. Cogn. Sci. 2013, 4, 245–261. [Google Scholar] [CrossRef]
- Shors, T.J. Acute stress rapidly and persistently enhances memory formation in the male rat. Neurobiol. Learn. Mem. 2001, 75, 10–29. [Google Scholar] [CrossRef]
- Edwards, E.J.; Edwards, M.S.; Lyvers, M. Cognitive trait anxiety, situational stress, and mental effort predict shifting efficiency: Implications for attentional control theory. Emotion 2015, 15, 350–359. [Google Scholar] [CrossRef]
- Sorg, B.; Whitney, P. The effect of trait anxiety and situational stress on working memory capacity. J. Res. Personal. 1992, 26, 235–241. [Google Scholar] [CrossRef]
- Duncko, R.; Cornwell, B.; Cui, L.; Merikangas, K.R.; Grillon, C. Acute exposure to stress improves performance in trace eyeblink conditioning and spatial learning tasks in healthy men. Learn. Mem. 2007, 14, 329–335. [Google Scholar] [CrossRef]
- Muir, J.L. Attention and stimulus processing in the rat. Brain Res. Cogn. Brain Res. 1996, 3, 215–225. [Google Scholar] [CrossRef]
- Light, K.R.; Grossman, H.; Kolata, S.; Wass, C.; Matzel, L.D. General learning ability regulates exploration through its influence on rate of habituation. Behav. Brain Res. 2011, 223, 297–309. [Google Scholar] [CrossRef]
- Belov, O.V.; Batmunkh, M.; Incerti, S.; Lkhagva, O. Radiation damage to neuronal cells: Simulating the energy deposition and water radiolysis in a small neural network. Phys. Med. PM Int. J. Devoted Appl. Phys. Med. Biol. Off. J. Ital. Assoc. Biomed. Phys. 2016, 32, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.R.; Everv, J.V. Quantum tunnelling in DNA. Chem. Phys. Lett. 1971, 8, 94–99. [Google Scholar] [CrossRef]
- Belov, O.V.; Krasavin, E.A.; Lyashko, M.S.; Batmunkh, M.; Sweilam, N.H. A quantitative model of the major pathways for radiation-induced DNA double-strand break repair. J. Theor. Biol. 2015, 366, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Grosshans, D.R.; Duman, J.G.; Gaber, M.W.; Sawakuchi, G. Particle Radiation Induced Neurotoxicity in the Central Nervous System. Int. J. Part 2018, 5, 74–83. [Google Scholar] [CrossRef]
- Batmunkh, M.; Belov, O.V.; Bayarchimeg, L.; Lhagva, O.; Sweilam, N.H. Estimation of the spatial energy deposition in CA1 pyramidal neurons under exposure to 12C and 56Fe ion beams. J. Radiat. Res. Appl. Sci. 2015, 8, 498–507. [Google Scholar] [CrossRef]
- Poulose, S.M.; Bielinski, D.F.; Carrihill-Knoll, K.; Rabin, B.M.; Shukitt-Hale, B. Exposure to 16O-particle radiation causes aging-like decrements in rats through increased oxidative stress, inflammation and loss of autophagy. Radiat. Res. 2011, 176, 761–769. [Google Scholar] [CrossRef]
- Anokhina, I.P.; Anokhin, P.K.; Kokhan, V.S. Combined Irradiation by Gamma Rays and Carbon Nuclei Increases the C/EBP-beta LIP Isoform Content in the Pituitary Gland of Rats. Dokl. Biol. Sci. Proc. Acad. Sci. USSR Biol. Sci. Sect./Transl. Russ. 2019, 488, 133–135. [Google Scholar] [CrossRef]
- Kiefer, J. Mutagenic effects of heavy charged particles. J. Radiat. Res. 2002, 43, S21–S25. [Google Scholar] [CrossRef]
- Greene-Schloesser, D.; Robbins, M.E.; Peiffer, A.M.; Shaw, E.G.; Wheeler, K.T.; Chan, M.D. Radiation-induced brain injury: A review. Front. Oncol. 2012, 2, 73. [Google Scholar] [CrossRef]
- Dickstein, D.L.; Talty, R.; Bresnahan, E.; Varghese, M.; Perry, B.; Janssen, W.G.M.; Sowa, A.; Giedzinski, E.; Apodaca, L.; Baulch, J.; et al. Alterations in synaptic density and myelination in response to exposure to high-energy charged particles. J. Comp. Neurol. 2018, 526, 2845–2855. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, D.M.; Chien, L.-C.; Cucinotta, F.A.; Pluth, J.M. Comparison of signaling profiles in the low dose range following low and high LET radiation. Life Sci. Space Res. 2020, 25, 28–41. [Google Scholar] [CrossRef]
- Hellweg, C.E.; Chishti, A.A.; Diegeler, S.; Spitta, L.F.; Henschenmacher, B.; Baumstark-Khan, C. Molecular Signaling in Response to Charged Particle Exposures and its Importance in Particle Therapy. Int. J. Part 2018, 5, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Machida, M.; Lonart, G.; Britten, R.A. Low (60 cGy) doses of 56Fe HZE-particle radiation lead to a persistent reduction in the glutamatergic readily releasable pool in rat hippocampal synaptosomes. Radiat. Res. 2010, 174, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Belov, O.V.; Belokopytova, K.V.; Kudrin, V.S.; Molokanov, A.G.; Shtemberg, A.S.; Bazyan, A.S. Neurochemical insights into the radiation protection of astronauts: Distinction between low- and moderate-LET radiation components. Phys. Med. PM Int. J. Devoted Appl. Phys. Med. Biol. Off. J. Ital. Assoc. Biomed. Phys. 2019, 57, 7–16. [Google Scholar] [CrossRef]
- Tubiana, M.; Feinendegen, L.E.; Yang, C.; Kaminski, J.M. The linear no-threshold relationship is inconsistent with radiation biologic and experimental data. Radiology 2009, 251, 13–22. [Google Scholar] [CrossRef]
- Jakob, B.; Dubiak-Szepietowska, M.; Janiel, E.; Schmidt, A.; Durante, M.; Taucher-Scholz, G. Differential Repair Protein Recruitment at Sites of Clustered and Isolated DNA Double-Strand Breaks Produced by High-Energy Heavy Ions. Sci. Rep. 2020, 10, 1443. [Google Scholar] [CrossRef]
- Kempf, S.J.; Janik, D.; Barjaktarovic, Z.; Braga-Tanaka, I., 3rd; Tanaka, S.; Neff, F.; Saran, A.; Larsen, M.R.; Tapio, S. Chronic low-dose-rate ionising radiation affects the hippocampal phosphoproteome in the ApoE-/- Alzheimer’s mouse model. Oncotarget 2016, 7, 71817–71832. [Google Scholar] [CrossRef]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Capilla-Gonzalez, V.; Guerrero-Cazares, H.; Bonsu, J.M.; Gonzalez-Perez, O.; Achanta, P.; Wong, J.; Garcia-Verdugo, J.M.; Quinones-Hinojosa, A. The subventricular zone is able to respond to a demyelinating lesion after localized radiation. Stem Cells 2014, 32, 59–69. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Z.; Weinstein, P.R.; Fike, J.R.; Liu, J. Environmental enrichment enhances neurogenesis and improves functional outcome after cranial irradiation. Eur. J. Neurosci. 2007, 25, 38–46. [Google Scholar] [CrossRef]
- Naumenko, V.S.; Kulikov, A.V.; Kondaurova, E.M.; Tsybko, A.S.; Kulikova, E.A.; Krasnov, I.B.; Shenkman, B.S.; Sychev, V.N.; Bazhenova, E.Y.; Sinyakova, N.A.; et al. Effect of actual long-term spaceflight on BDNF, TrkB, p75, BAX and BCL-XL genes expression in mouse brain regions. Neuroscience 2015, 284, 730–736. [Google Scholar] [CrossRef]
- Weber Boutros, S.; Unni, V.K.; Raber, J. An Adaptive Role for DNA Double-Strand Breaks in Hippocampus-Dependent Learning and Memory. Int. J. Mol. Sci. 2022, 23, 8352. [Google Scholar] [CrossRef]
- Konopka, A.; Atkin, J.D. The Role of DNA Damage in Neural Plasticity in Physiology and Neurodegeneration. Front. Cell. Neurosci. 2022, 16, 836885. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.M.; DeCicco-Skinner, K.L.; Roma, P.G.; Hienz, R.D. Individual differences in attentional deficits and dopaminergic protein levels following exposure to proton radiation. Radiat. Res. 2014, 181, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.W.; Nishiyama, N.C.; Pecaut, M.J.; Campbell-Beachler, M.; Gifford, P.; Haynes, K.E.; Becronis, C.; Gridley, D.S. Simulated Microgravity and Low-Dose/Low-Dose-Rate Radiation Induces Oxidative Damage in the Mouse Brain. Radiat. Res. 2016, 185, 647–657. [Google Scholar] [CrossRef]
- Parihar, V.K.; Allen, B.D.; Tran, K.K.; Chmielewski, N.N.; Craver, B.M.; Martirosian, V.; Morganti, J.M.; Rosi, S.; Vlkolinsky, R.; Acharya, M.M.; et al. Targeted overexpression of mitochondrial catalase prevents radiation-induced cognitive dysfunction. Antioxid. Redox Signal. 2015, 22, 78–91. [Google Scholar] [CrossRef]
- Belov, O.V.; Belokopytova, K.V.; Bazyan, A.S.; Kudrin, V.S.; Narkevich, V.B.; Ivanov, A.A.; Severiukhin, Y.S.; Timoshenko, G.N.; Krasavin, E.A. Exposure to 12C particles alters the normal dynamics of brain monoamine metabolism and behaviour in rats. Phys. Med. PM Int. J. Devoted Appl. Phys. Med. Biol. Off. J. Ital. Assoc. Biomed. Phys. 2016, 32, 1088–1094. [Google Scholar] [CrossRef]
- Kokhan, V.S. Some aspects of the effect of combined irradiation by gamma-rays and carbon nuclei (12C) on the serotonergic system in rat brain. J. Biomed. 2020, 16, 68–72. [Google Scholar] [CrossRef]
- Phelps, E.A.; LeDoux, J.E. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron 2005, 48, 175–187. [Google Scholar] [CrossRef]
- De la Torre, G.G. Cognitive neuroscience in space. Life 2014, 4, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Temple, M.D.; Kosik, K.S.; Steward, O. Spatial learning and memory is preserved in rats after early development in a microgravity environment. Neurobiol. Learn. Mem. 2002, 78, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Wollseiffen, P.; Klein, T.; Vogt, T.; Abeln, V.; Struder, H.K.; Stuckenschneider, T.; Sanders, M.; Claassen, J.; Askew, C.D.; Carnahan, H.; et al. Neurocognitive performance is enhanced during short periods of microgravity-Part 2. Physiol. Behav. 2019, 207, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.E.; Reschke, M.F.; Arrott, A.P.; Homick, J.L.; Lichtenberg, B.K. Otolith tilt-translation reinterpretation following prolonged weightlessness: Implications for preflight training. Aviat. Space Environ. Med. 1985, 56, 601–606. [Google Scholar]
- Miyachi, Y.; Yamada, T. Head-portion exposure to low-level X-rays reduces isolation-induced aggression of mouse, and involvement of the olfactory carnosine in modulation of the radiation effects. Behav. Brain Res. 1996, 81, 135–140. [Google Scholar] [CrossRef]
- Zhukova, N.A.; Ziablitskii, V.M.; Mikhal’skaia, T.; Tlepshukov, I.K. An experimental study of the early changes in the body after simultaneous radiation exposure at a low dose and stress. Radiatsionnaia Biol. Radioecol./Ross. Akad. Nauk 1996, 36, 371–375. [Google Scholar]
- Shtemberg, A.S. The combined effect of various forms of motor deprivation and gamma radiation on the higher nervous activity in rats. Aerosp. Environ. Med. 1997, 31, 38–43. [Google Scholar]
- Raber, J.; Holden, S.; Sudhakar, R.; Hall, R.; Glaeser, B.; Lenarczyk, M.; Rockwell, K.; Nawarawong, N.; Sterrett, J.; Perez, R.; et al. Effects of 5-Ion Beam Irradiation and Hindlimb Unloading on Metabolic Pathways in Plasma and Brain of Behaviorally Tested WAG/Rij Rats. Front. Physiol. 2021, 12, 746509. [Google Scholar] [CrossRef] [PubMed]
- Bellone, J.A.; Gifford, P.S.; Nishiyama, N.C.; Hartman, R.E.; Mao, X.W. Long-term effects of simulated microgravity and/or chronic exposure to low-dose gamma radiation on behavior and blood-brain barrier integrity. NPJ Microgravity 2016, 2, 16019. [Google Scholar] [CrossRef]
- Kokhan, V.S.; Kudrin, V.S.; Shtemberg, A.S. Serotonin and Noradrenaline Metabolism in the Brain of Rats under the Combined Action of Radiation and Hypogravity in a Ground-based Experiment. Neurochem. J. 2019, 36, 65–70. [Google Scholar] [CrossRef]
- Overbey, E.G.; Paul, A.M.; da Silveira, W.A.; Tahimic, C.G.T.; Reinsch, S.S.; Szewczyk, N.; Stanbouly, S.; Wang, C.; Galazka, J.M.; Mao, X.W. Mice Exposed to Combined Chronic Low-Dose Irradiation and Modeled Microgravity Develop Long-Term Neurological Sequelae. Int. J. Mol. Sci. 2019, 20, 4094. [Google Scholar] [CrossRef]
- Mizumatsu, S.; Monje, M.L.; Morhardt, D.R.; Rola, R.; Palmer, T.D.; Fike, J.R. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003, 63, 4021–4027. [Google Scholar] [PubMed]
- Rola, R.; Raber, J.; Rizk, A.; Otsuka, S.; VandenBerg, S.R.; Morhardt, D.R.; Fike, J.R. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp. Neurol. 2004, 188, 316–330. [Google Scholar] [CrossRef] [PubMed]
- Raber, J.; Rola, R.; LeFevour, A.; Morhardt, D.; Curley, J.; Mizumatsu, S.; VandenBerg, S.R.; Fike, J.R. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat. Res. 2004, 162, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Mineyeva, O.A.; Barykina, N.V.; Bezriadnov, D.V.; Latushkin, S.T.; Ryazanov, A.I.; Unezhev, V.N.; Shuvaev, S.A.; Usova, S.V.; Lazutkin, A.A. Suppressed neurogenesis without cognitive deficits: Effects of fast neutron irradiation in mice. Neuroreport 2019, 30, 538–543. [Google Scholar] [CrossRef]
- Hernandez-Rabaza, V.; Llorens-Martin, M.; Velazquez-Sanchez, C.; Ferragud, A.; Arcusa, A.; Gumus, H.G.; Gomez-Pinedo, U.; Perez-Villalba, A.; Rosello, J.; Trejo, J.L.; et al. Inhibition of adult hippocampal neurogenesis disrupts contextual learning but spares spatial working memory, long-term conditional rule retention and spatial reversal. Neuroscience 2009, 159, 59–68. [Google Scholar] [CrossRef]
- Jaholkowski, P.; Kiryk, A.; Jedynak, P.; Ben Abdallah, N.M.; Knapska, E.; Kowalczyk, A.; Piechal, A.; Blecharz-Klin, K.; Figiel, I.; Lioudyno, V.; et al. New hippocampal neurons are not obligatory for memory formation; cyclin D2 knockout mice with no adult brain neurogenesis show learning. Learn. Mem. 2009, 16, 439–451. [Google Scholar] [CrossRef]
- Garthe, A.; Kempermann, G. An old test for new neurons: Refining the Morris water maze to study the functional relevance of adult hippocampal neurogenesis. Front. Neurosci. 2013, 7, 63. [Google Scholar] [CrossRef]
- Greene-Schloesser, D.; Moore, E.; Robbins, M.E. Molecular pathways: Radiation-induced cognitive impairment. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 2294–2300. [Google Scholar] [CrossRef]
- Ramirez-Amaya, V.; Balderas, I.; Sandoval, J.; Escobar, M.L.; Bermudez-Rattoni, F. Spatial long-term memory is related to mossy fiber synaptogenesis. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 7340–7348. [Google Scholar] [CrossRef]
- Sweet, T.B.; Hurley, S.D.; Wu, M.D.; Olschowka, J.A.; Williams, J.P.; O’Banion, M.K. Neurogenic Effects of Low-Dose Whole-Body HZE (Fe) Ion and Gamma Irradiation. Radiat. Res. 2016, 186, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Zanni, G.; Deutsch, H.M.; Rivera, P.D.; Shih, H.Y.; LeBlanc, J.A.; Amaral, W.Z.; Lucero, M.J.; Redfield, R.L.; DeSalle, M.J.; Chen, B.P.C.; et al. Whole-Body 12C Irradiation Transiently Decreases Mouse Hippocampal Dentate Gyrus Proliferation and Immature Neuron Number, but Does Not Change New Neuron Survival Rate. Int. J. Mol. Sci. 2018, 19, 3078. [Google Scholar] [CrossRef]
- Sweet, T.B.; Panda, N.; Hein, A.M.; Das, S.L.; Hurley, S.D.; Olschowka, J.A.; Williams, J.P.; O’Banion, M.K. Central nervous system effects of whole-body proton irradiation. Radiat. Res. 2014, 182, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Acharya, M.M.; Patel, N.H.; Craver, B.M.; Tran, K.K.; Giedzinski, E.; Tseng, B.P.; Parihar, V.K.; Limoli, C.L. Consequences of low dose ionizing radiation exposure on the hippocampal microenvironment. PLoS ONE 2015, 10, e0128316. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Kim, S.; Jung, U.; Kim, I.; Kim, J.K.; Roh, C. Effect of acute and fractionated irradiation on hippocampal neurogenesis. Molecules 2012, 17, 9462–9468. [Google Scholar] [CrossRef]
- Shinohara, C.; Gobbel, G.T.; Lamborn, K.R.; Tada, E.; Fike, J.R. Apoptosis in the subependyma of young adult rats after single and fractionated doses of X-rays. Cancer Res. 1997, 57, 2694–2702. [Google Scholar]
- Balentova, S.; Adamkov, M. Molecular, Cellular and Functional Effects of Radiation-Induced Brain Injury: A Review. Int. J. Mol. Sci. 2015, 16, 27796–27815. [Google Scholar] [CrossRef]
- Tan, Y.F.; Rosenzweig, S.; Jaffray, D.; Wojtowicz, J.M. Depletion of new neurons by image guided irradiation. Front. Neurosci. 2011, 5, 59. [Google Scholar] [CrossRef]
- Tada, E.; Parent, J.M.; Lowenstein, D.H.; Fike, J.R. X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neuroscience 2000, 99, 33–41. [Google Scholar] [CrossRef]
- Shi, L.; Molina, D.P.; Robbins, M.E.; Wheeler, K.T.; Brunso-Bechtold, J.K. Hippocampal neuron number is unchanged 1 year after fractionated whole-brain irradiation at middle age. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 526–532. [Google Scholar] [CrossRef]
- Monje, M.L.; Toda, H.; Palmer, T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science 2003, 302, 1760–1765. [Google Scholar] [CrossRef]
- Lumniczky, K.; Szatmari, T.; Safrany, G. Ionizing Radiation-Induced Immune and Inflammatory Reactions in the Brain. Front. Immunol. 2017, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Rola, R.; Sarkissian, V.; Obenaus, A.; Nelson, G.A.; Otsuka, S.; Limoli, C.L.; Fike, J.R. High-LET radiation induces inflammation and persistent changes in markers of hippocampal neurogenesis. Radiat. Res. 2005, 164, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ma, Z.-W.; Ho, F.-M.; Sethi, G.; Tang, F.R. Dual Effects of miR-181b-2-3p/SOX21 Interaction on Microglia and Neural Stem Cells after Gamma Irradiation. Cells 2023, 12, 649. [Google Scholar] [CrossRef]
- Oh, S.B.; Park, H.R.; Jang, Y.J.; Choi, S.Y.; Son, T.G.; Lee, J. Baicalein attenuates impaired hippocampal neurogenesis and the neurocognitive deficits induced by gamma-ray radiation. Br. J. Pharmacol. 2013, 168, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Raber, J.; Fuentes Anaya, A.; Torres, E.R.S.; Lee, J.; Boutros, S.; Grygoryev, D.; Hammer, A.; Kasschau, K.D.; Sharpton, T.J.; Turker, M.S.; et al. Effects of Six Sequential Charged Particle Beams on Behavioral and Cognitive Performance in B6D2F1 Female and Male Mice. Front. Physiol. 2020, 11, 959. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.J.; Wray, S.; Sattler, R.; Zhang, C. Editorial: Mechanisms of Action in Neurodegenerative Proteinopathies. Front. Neurosci. 2022, 16, 968994. [Google Scholar] [CrossRef]
- Wang, B.; Tanaka, K.; Ji, B.; Ono, M.; Fang, Y.; Ninomiya, Y.; Maruyama, K.; Izumi-Nakajima, N.; Begum, N.; Higuchi, M.; et al. Total body 100-mGy X-irradiation does not induce Alzheimer’s disease-like pathogenesis or memory impairment in mice. J. Radiat. Res. 2014, 55, 84–96. [Google Scholar] [CrossRef]
- Villemagne, V.L.; Dore, V.; Burnham, S.C.; Masters, C.L.; Rowe, C.C. Imaging tau and amyloid-beta proteinopathies in Alzheimer disease and other conditions. Nat. Rev. Neurol. 2018, 14, 225–236. [Google Scholar] [CrossRef]
- Cherry, J.D.; Liu, B.; Frost, J.L.; Lemere, C.A.; Williams, J.P.; Olschowka, J.A.; O’Banion, M.K. Galactic cosmic radiation leads to cognitive impairment and increased abeta plaque accumulation in a mouse model of Alzheimer’s disease. PLoS ONE 2012, 7, e53275. [Google Scholar] [CrossRef]
- Rudobeck, E.; Bellone, J.A.; Szucs, A.; Bonnick, K.; Mehrotra-Carter, S.; Badaut, J.; Nelson, G.A.; Hartman, R.E.; Vlkolinsky, R. Low-dose proton radiation effects in a transgenic mouse model of Alzheimer’s disease—Implications for space travel. PLoS ONE 2017, 12, e0186168. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Vats, A.; Taneja, V. Toxic species in amyloid disorders: Oligomers or mature fibrils. Ann. Indian Acad. Neurol. 2015, 18, 138–145. [Google Scholar] [CrossRef]
- Kokhan, V.S.; Afanasyeva, M.A.; Van’kin, G.I. alpha-Synuclein knockout mice have cognitive impairments. Behav. Brain Res. 2012, 231, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Tsuji, M. Protofibrils of Amyloid-beta are Important Targets of a Disease-Modifying Approach for Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 952. [Google Scholar] [CrossRef] [PubMed]
- van Dyck, C.H. Anti-Amyloid-beta Monoclonal Antibodies for Alzheimer’s Disease: Pitfalls and Promise. Biol. Psychiatry 2018, 83, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Cantu, D.; Walker, K.; Andresen, L.; Taylor-Weiner, A.; Hampton, D.; Tesco, G.; Dulla, C.G. Traumatic Brain Injury Increases Cortical Glutamate Network Activity by Compromising GABAergic Control. Cereb Cortex 2015, 25, 2306–2320. [Google Scholar] [CrossRef]
- Dams-O’Connor, K.; Guetta, G.; Hahn-Ketter, A.E.; Fedor, A. Traumatic brain injury as a risk factor for Alzheimer’s disease: Current knowledge and future directions. Neurodegener Dis. Manag. 2016, 6, 417–429. [Google Scholar] [CrossRef]
- Bevelacqua, J.J.; Mortazavi, S.M.J. Alzheimer’s Disease: Possible Mechanisms Behind Neurohormesis Induced by Exposure to Low Doses of Ionizing Radiation. J. Biomed. Phys. Eng. 2018, 8, 153–156. [Google Scholar] [CrossRef]
- Wilson, G.D.; Marples, B. A New Use for an Old Treatment: Radiation Therapy and Alzheimer’s Disease. Radiat. Res. 2016, 185, 443–448. [Google Scholar] [CrossRef]
- Sahebi, R.; Aghaei, M.; Halvaei, S.; Alizadeh, A. The role of microgravity in cancer: A dual-edge sword. Multidiscip. Cancer Investig. 2017, 1, 1–5. [Google Scholar] [CrossRef]
| Object | Dose and Composition (Age of Irradiation) | Condition of Irradiation | Anxious Behavior (Age of Testing) | Reference |
|---|---|---|---|---|
| Sprague-Dawley male rats | γ-rays, 661.7 keV, 0.5 or 1, or 2, or 4 Gy; 4He, 0.9 keV/µm, 1 or 5, or 10, or 50, or 100 mGy (~45–50 days, relying on [77]) | Acute | Increased except 4 Gy γ-rays (2.5–6.5 months) | [74] |
| C57BL/6 male mice | H+, 0.5 keV/µm, 0.5 or 1 Gy (6 months) | Acute | Increased (15 months) | [75] |
| Wistar male rats | H+, 0.4 keV/µm, 1.5 Gy combined with γ-rays, 3 Gy (3 months) | γ-rays fractionated × 6; nuclei—acute | Not changed (5 months) | [39] |
| Wistar male rats | 12C, 0.4 keV/µm, 0.14 Gy combined with γ-rays, 661.7 keV, 0.4 Gy (3 months) | Acute | Increased (3 or/and 11 months) | [9,54] |
| C57BL/6J male mice | 28Si, 67 keV/µm, 0.2 or 1 Gy (~70 days) | Acute | Increased only at 1 Gy (4 months) | [48] |
| Thy1-EGFP male mice | 48Ti, 126 keV/µm, 0.3 Gy (6 months) | Acute | Increased (~12 months) | [58] |
| Fischer 344 male rats | 48Ti, 134 keV/µm, 10 or 100 mGy (15 months); 16O, 14.2 keV/µm, 1 mGy; 4He, 0.9 keV/µm, 0.1 or 0.5, or 1 mGy (15 months) | Acute | Increased only when irradiated by 48Ti or 16O (15 months) | [76] |
| Fischer 344 male rats | 48Ti, 134 keV/µm, 10 or 100 mGy (2 or 11 months); 16O, 14.2 keV/µm, 1 mGy; 4He, 0.9 keV/µm, 0.1 or 0.5, or 1 mGy (2 or 11 months) | Acute | Not changed (2 or 11 months) | |
| Sprague-Dawley male rats | 56Fe, 147 keV/µm, 1.5 Gy (3 months) | Acute | Not changed (6 months) | [73] |
| C57BL/6J male mice | mixed 33 beams of nuclei (H+, 4He, 12C, 16O, 28Si, 48Ti, 56Fe) with different energy, 0.75 Gy totally (6 months) | Acute | Not changed (10.5 months) | [37] |
| C3H male mice and BALB/c female mice | 252Cf source of neutrons and γ-rays, 0.12 or 0.2, or 0.4 Gy (2 months) | Chronic, 400 days | Increased only at 0.4 Gy, C3H mice (20 and 23 month); not changed in other | [12] |
| Object | Dose and Composition | Behavior | Protein Aggregation | Reference |
|---|---|---|---|---|
| C57BL/6J Jms mice | 100 mGy X-ray, 200 keV | MWM not changed. | Aβ, tau, and phospho-tau plaque in hippocampus not changed. | [201] |
| APP/PS1 mice | 56Fe, 147 keV/μm, 0.1 (male and female) or 1 Gy (only female) | Impairment of contextual fear conditioning (only 1 Gy, male); recognition memory decreased. | Increased Aβ plaque. | [203] |
| APP/PS1 mice | H+, 0.57 keV/µm, 1 Gy, but not 0.1 or 0.5 Gy | MWM and Barnes maze not changed. | Increased Aβ plaque in the neocortex, but not hippocampus. | [204] |
| C57BL/6J Jms mice | 12C, 15 keV/µm, 50 or 100 mGy | MWM not changed. | APP, Aβ, tau, and phospho-tau plaque in hippocampal CA1 regions not changed. | [49] |
| APP/PS1 mice, C57BL/6J mice | 56Fe, 147 keV/µm, 0.1 or 0.5 Gy | Improved motor learning (0.5 Gy, female APP/PS1); reduced grip strength (female APP/PS1); spatial memory in the Y maze not changed. | Decreased Aβ plaque (APP/PS1); not changed (C57BL/6J). | [16] |
| Tau P301S mice 5xFAD mice | 12C, 10.3 keV/μm, 0.18 Gy combined with γ-rays, 0.24 Gy | Anxiolytic effect and stimulated orientation and exploratory behavior (Tau P301S); improved odor discrimination (5xFAD). | Not studied. | [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokhan, V.S.; Dobynde, M.I. The Effects of Galactic Cosmic Rays on the Central Nervous System: From Negative to Unexpectedly Positive Effects That Astronauts May Encounter. Biology 2023, 12, 400. https://doi.org/10.3390/biology12030400
Kokhan VS, Dobynde MI. The Effects of Galactic Cosmic Rays on the Central Nervous System: From Negative to Unexpectedly Positive Effects That Astronauts May Encounter. Biology. 2023; 12(3):400. https://doi.org/10.3390/biology12030400
Chicago/Turabian StyleKokhan, Viktor S., and Mikhail I. Dobynde. 2023. "The Effects of Galactic Cosmic Rays on the Central Nervous System: From Negative to Unexpectedly Positive Effects That Astronauts May Encounter" Biology 12, no. 3: 400. https://doi.org/10.3390/biology12030400
APA StyleKokhan, V. S., & Dobynde, M. I. (2023). The Effects of Galactic Cosmic Rays on the Central Nervous System: From Negative to Unexpectedly Positive Effects That Astronauts May Encounter. Biology, 12(3), 400. https://doi.org/10.3390/biology12030400






