Arthropod Ectoparasites of Two Rodent Species Occurring in Varied Elevations on Tanzania’s Second Highest Mountain
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
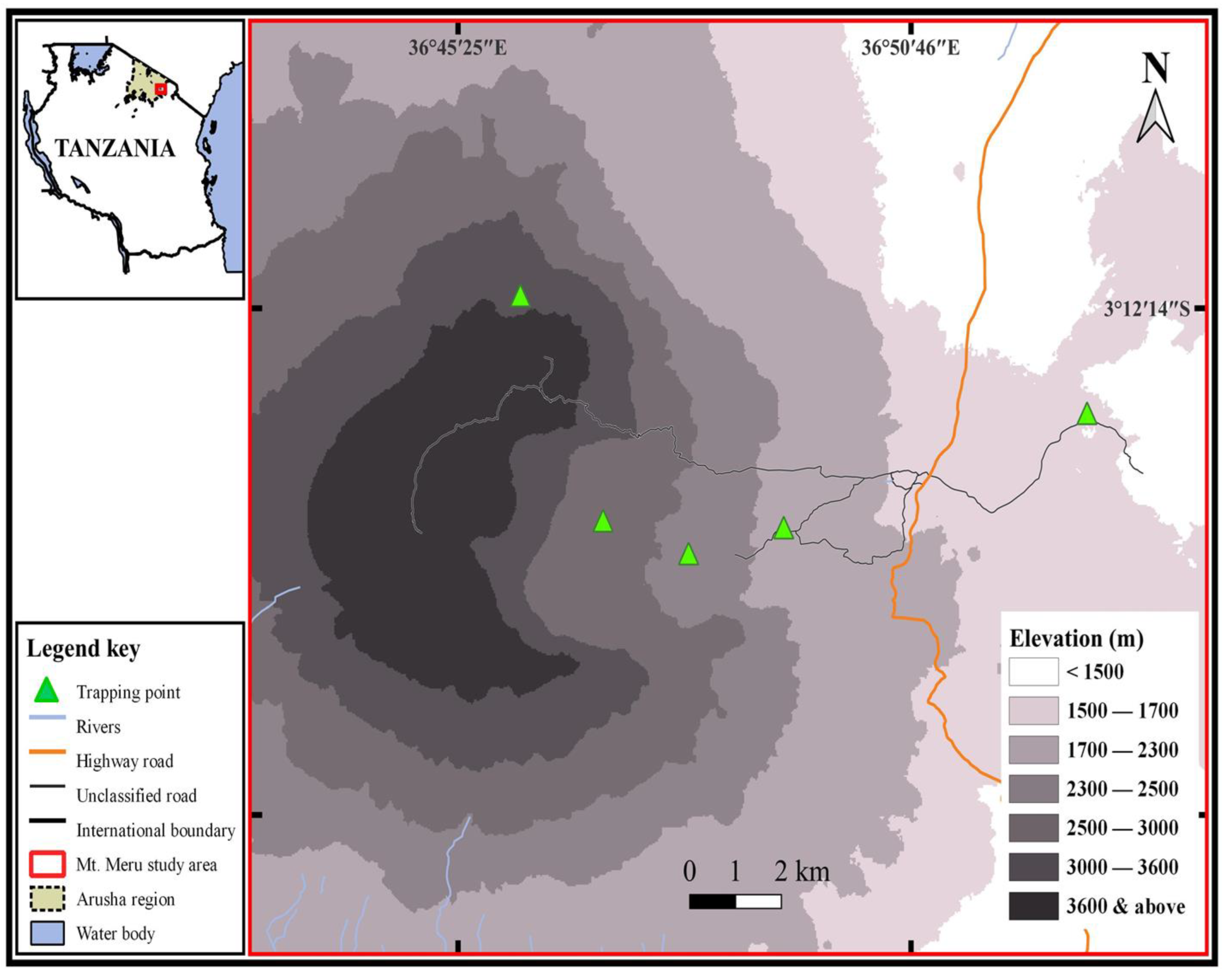
2.1. Study Area and Trapping Sites
- (1)
- Elevation 1500 m (3°13′20.766′′ S, 36°52′50.076′′ E)—This trapping site was located at the base of Mount Meru, and vegetation cover ranges from grassland, thicket, and bushland to woodland. Caesalpinia decapetala, Croton macrostachyus, Jacaranda mimosifolia, Ocimum gratissimum, Solanum incanum, Aerva lanata, Lantana trifoliata, and tussock grasses are among the notable plant species. Patches of Acacia trees are also prevalent.
- (2)
- Elevation 2000 m (3°14′33.102″ S, 36°49′15.528″ E)—This site was situated in a lower montane forest, with a dense canopy of trees of various species, including Diospyros abyssinica, Olea hochstetteri, Rhamnus prinoides, and Ficus thonningii.
- (3)
- Elevation 2500 m (3° 14′ 32.892′′ S, 36° 47′ 25.644′′ E)—This site was in the upper montane forest dominated by Juniperus procera and Podocarpus gracilior. Herbaceous plants, various lianas, and shrubs formed a thick understory, and the trees were often covered with epiphytes.
- (4)
- Elevation 2950 m (3°13′28.724′′ S, 36°47′7.782″ E) —This site was characterized as a transitional zone between habitats of higher montane forest and ericaceous heath.
- (5)
- Elevation 3500 m (3°13′6.192′′ S, 36°46′24.042′′ E)—This highest trapping site was located in the ericaceous heathland habitats dominated by Erica spp.
2.2. Rodent Trapping
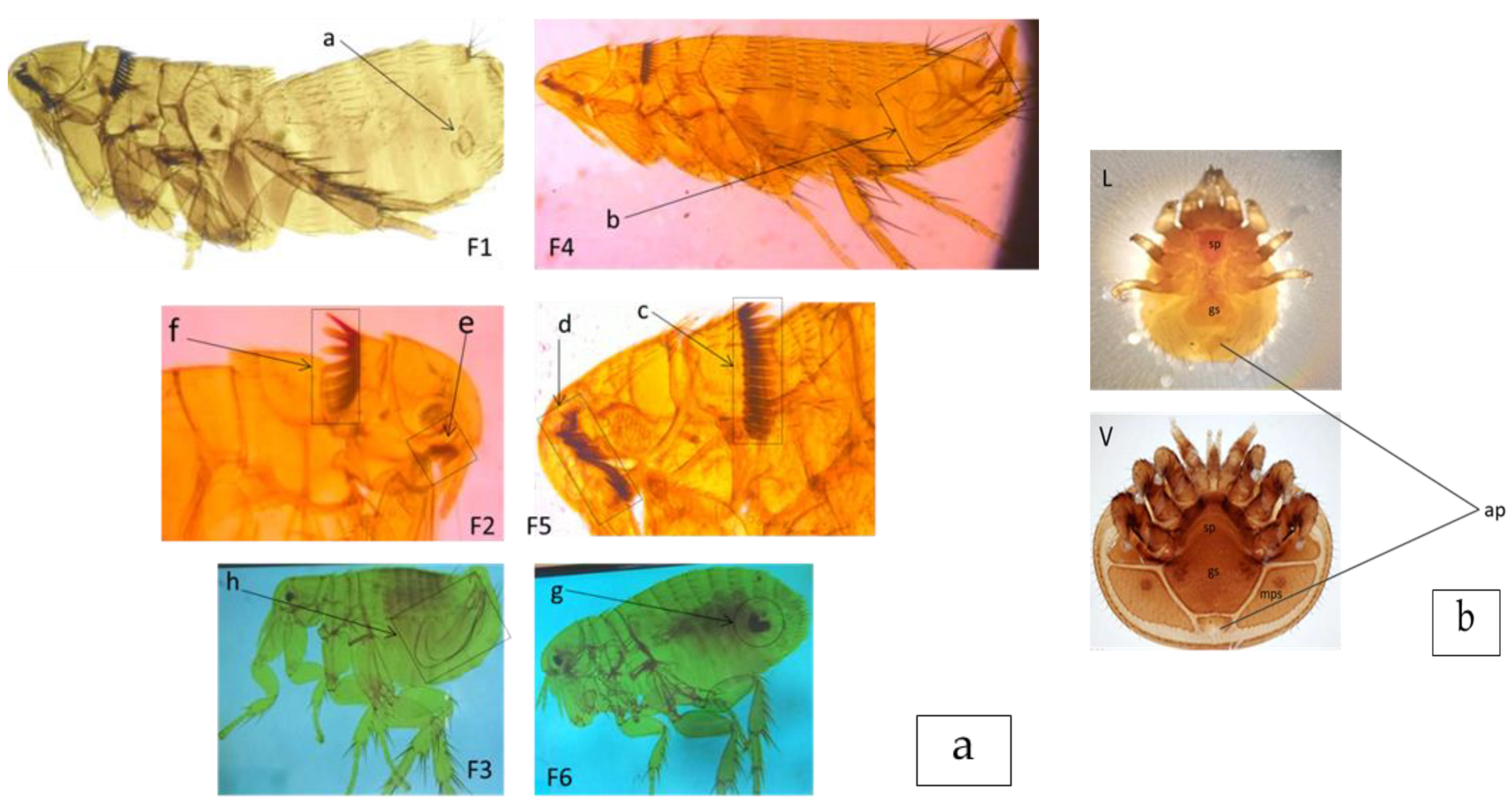
2.3. Ectoparasite Collection and Identification
2.4. Molecular Identification of Fleas and Mites
2.5. Data Analysis
3. Results
3.1. Morphological and Molecular Identification Results of Fleas and Mites
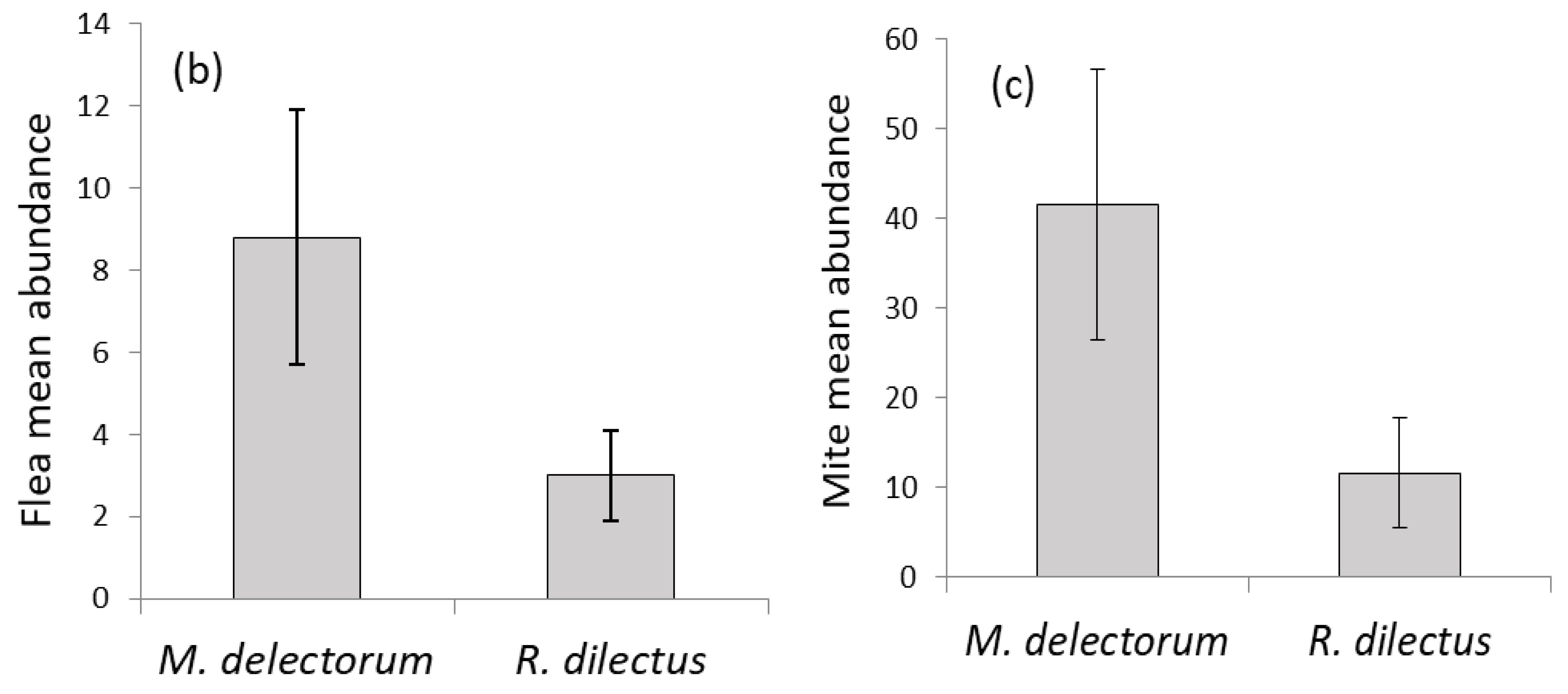
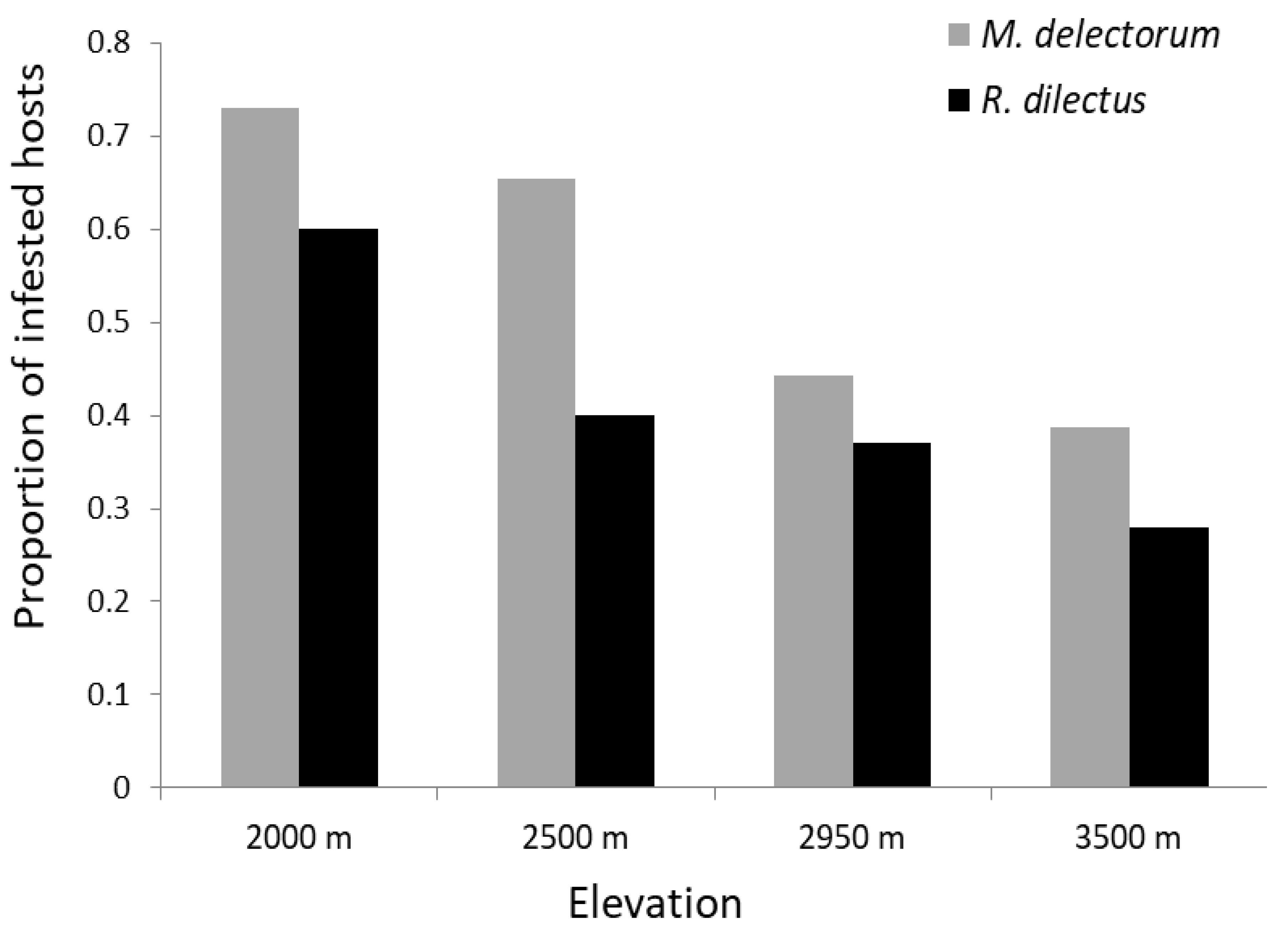
3.2. Quantitative Descriptors of Ectoparasite Infestation
3.3. Assessment of Parameters Influencing Ectoparasite Occurrence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krasnov, B.R.; Shenbrot, G.I.; Khokhlova, I.S.; Poulin, R. Geographical Variation in the “Bottom-Up” Control of Diversity: Fleas and Their Small Mammalian Hosts. Glob. Ecol. Biogeogr. 2007, 16, 179–186. [Google Scholar] [CrossRef]
- Wall, R.L.; Shearer, D. Veterinary Ectoparasites: Biology, Pathology & Control, 2nd ed.; Blackwell Science: London, UK, 2001; 275p. [Google Scholar]
- Anderson, D.L.; Trueman, J.W. Varroa jacobsoni(Acari: Varroidae) is more than one species. Exp. Appl. Acarol. 2000, 24, 165–189. [Google Scholar] [CrossRef] [PubMed]
- Giliba, R.A.; Mpinga, I.H.; Ndimuligo, S.A.; Mpanda, M.M. Changing climate patterns risk the spread of Varroa destructor infestation of African honeybees in Tanzania. Ecol. Process. 2020, 9, 1–11. [Google Scholar] [CrossRef]
- Flores, J.M.; Gámiz, V.; Jiménez-Marín, Á.; Flores-Cortés, A.; Gil-Lebrero, S.; Garrido, J.J.; Hernando, M.D. Impact of Varroa Destructor and Associated Pathologies on the Colony Collapse Disorder Affecting Honeybees. Res. Vet. Sci. 2021, 135, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Gisder, S.; Genersch, E. Direct Evidence for Infection of Varroa Destructor Mites with the Bee-Pathogenic Deformed Wing Virus Variant B—but Not Variant a—Via Fluorescence-in Situ-Hybridization Analysis. J. Virol. 2020, 95, e01786-20. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Quintana, M.; Espinosa-Montaño, L.G.; Prieto-Merlos, D.; Koleoglu, G.; Petukhova, T.; Correa-Benítez, A.; Guzman-Novoa, E. Impact of Varroa Destructor and Deformed Wing Virus on Emergence, Cellular Immunity, Wing Integrity and Survivorship of Africanized Honeybees in Mexico. J. Invertebr. Pathol. 2019, 164, 43–48. [Google Scholar] [CrossRef]
- Eads, D.A.; Biggins, D.E.; Gage, K.L. Ecology and Management of Plague in Diverse Communities of Rodents and Fleas. Vector Borne Zoonotic Dis. 2020, 20, 888–896. [Google Scholar] [CrossRef]
- Eisen, R.J.; Borchert, J.N.; Mpanga, J.T.; Atiku, L.A.; MacMillan, K.; Boegler, K.A.; Montenieri, J.A.; Monaghan, A.; Gage, K.L. Flea Diversity as an Element for Persistence of Plague Bacteria in an East African Plague Focus. PLoS ONE 2012, 7, e35598. [Google Scholar] [CrossRef]
- Moore, S.M.; Monaghan, A.; Borchert, J.N.; Atiku, L.A.; Boegler, K.A.; Montenieri, J.; MacMillan, K.; Gage, K.L.; Eisen, R.J. Seasonal Fluctuations of Small Mammal and Flea Communities in a Ugandan Plague Focus: Evidence to Implicate Arvicanthis Niloticus and Crocidura Spp. As Key Hosts in Yersinia Pestis Transmission. Parasites Vectors 2015, 8, 11. [Google Scholar] [CrossRef]
- Haikukutu, L.; Lyaku, J.R.; Lyimo, C.; Kasanga, C.J.; Kandusi, S.E.; Rahelinirina, S.; Rasoamalala, F.; Rajerison, M.; Makundi, R. Plague in Tanzania: First Report of Sylvatic Plague in Morogoro Region, Persistence in Mbulu Focus, and Ongoing Quiescence in Lushoto and Iringa Foci. IJID Reg. 2022, 4, 105–110. [Google Scholar] [CrossRef]
- Zanet, S.; Miglio, G.; Ferrari, C.; Bassano, B.; Ferroglio, E.; von Hardenberg, A. Higher Risk of Gastrointestinal Parasite Infection at Lower Elevation Suggests Possible Constraints in the Distributional Niche of Alpine Marmots. PLoS ONE 2017, 12, e0182477. [Google Scholar] [CrossRef] [PubMed]
- Ebert, D.; Lipsitch, M.; Mangin, K.L. The Effect of Parasites on Host Population Density and Extinction: Experimental Epidemiology with Daphnia and Six Microparasites. Am. Nat. 2000, 156, 459–477. [Google Scholar] [CrossRef]
- Newey, S.; Dahl, F.; Willebrand, T.; Thirgood, S. Unstable Dynamics and Population Limitation in Mountain Hares. Biol. Rev. 2007, 82, 527–549. [Google Scholar] [CrossRef] [PubMed]
- Freed, L.A.; Medeiros, M.C.; Bodner, G.R. Explosive increase in ectoparasites in Hawaiian forest birds. J. Parasitol. 2008, 94, 1009–1021. [Google Scholar] [CrossRef]
- Poulin, R. Are there general laws in parasite ecology? Parasitology 2007, 134, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, H.; Bennett, N.C.; Ueckermann, E.A.; Lutermann, H. The Role of Host Traits, Season and Group Size on Parasite Burdens in a Cooperative Mammal. PLoS ONE 2011, 6, e27003. [Google Scholar] [CrossRef] [PubMed]
- Fichet-Calvet, E.; Wang, J.; Jomâa, I.; Ben Ismail, R.; Ashford, R.W. Patterns of the Tapeworm Raillietina Trapezoides Infection in the Fat Sand Rat Psammomys Obesus in Tunisia: Season, Climatic Conditions, Host Age and Crowding Effects. Parasitology 2003, 126, 481–492. [Google Scholar] [CrossRef]
- Soliman, S.; Marzouk, A.S.; Main, A.J.; Montasser, A.A. Effect of Sex, Size, and Age of Commensal Rat Hosts on the Infestation Parameters of Their Ectoparasites in a Rural Area of Egypt. J. Parasitol. Res. 2001, 87, 1308. [Google Scholar] [CrossRef]
- Postawa, T.; Szubert-Kruszyńska, A. Is Parasite Load Dependent on Host Aggregation Size?The Case of the Greater Mouse-Eared Bat Myotis Myotis(Mammalia: Chiroptera) and Its Parasitic Mite Spinturnix Myoti(Acari: Gamasida). Parasitol. Res. 2014, 113, 1803–1811. [Google Scholar] [CrossRef]
- Krasnov, A.; Skugor, S.; Todorcevic, M.; Glover, K.A.; Nilsen, F. Gene Expression in Atlantic Salmon Skin in Response to Infection with the Parasitic Copepod Lepeophtheirus Salmonis, Cortisol Implant, and Their Combination. BMC Genom. 2012, 13, 130. [Google Scholar] [CrossRef]
- Zhang, L.; Parsons, S.; Daszak, P.; Wei, L.; Zhu, G.; Zhang, S. Variation in the Abundance of Ectoparasitic Mites of Flat-Headed Bats. J. Mammal. 2010, 91, 136–143. [Google Scholar] [CrossRef]
- Gomez, D.; Sommaro, L.; Steinmann, A.; Chiappero, M.; Priotto, J. Movement Distances of Two Species of Sympatric Rodents in Linear Habitats of Central Argentine Agro-Ecosystems. Mamm. Biol. 2011, 76, 58–63. [Google Scholar] [CrossRef]
- Froeschke, G.; Harf, R.; Sommer, S.; Matthee, S. Effects of Precipitation on Parasite Burden along a Natural Climatic Gradient in Southern Africa—Implications for Possible Shifts in Infestation Patterns due to Global Changes. Oikos 2010, 119, 1029–1039. [Google Scholar] [CrossRef]
- Schares, G.; Ziller, M.; Herrmann, D.C.; Globokar, M.V.; Pantchev, N.; Conraths, F.J. Seasonality in the Proportions of Domestic Cats Shedding Toxoplasma Gondii or Hammondia Hammondi Oocysts Is Associated with Climatic Factors. Int. J. Parasitol. 2016, 46, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Vicente, J.A.; Gutiérrez-Cabrera, A.E.; Flores-Villegas, A.L.; Lowenberger, C.; Benelli, G.; Salazar-Schettino, P.M.; Córdoba-Aguilar, A. What Makes an Effective Chagas Disease Vector? Factors Underlying Trypanosoma Cruzi-Triatomine Interactions. Acta Trop. 2018, 183, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Akbar, H.; Pinçon, C.; Aliouat-Denis, C.-M.; Derouiche, S.; Taylor, M.-L.; Pottier, M.; Carreto-Binaghi, L.-H.; González-González, A.E.; Courpon, A.; Barriel, V.; et al. Characterizing Pneumocystis in the Lungs of Bats: Understanding Pneumocystis Evolution and the Spread of Pneumocystis Organisms in Mammal Populations. Appl. Environ. Microbiol. 2012, 78, 8122–8136. [Google Scholar] [CrossRef] [PubMed]
- Kramm, M.M., III; Gutierrez, M.R.; Luepke, T.D.; Soria, C.; Lopez, R.R.; Cooper, S.M.; Davis, D.S.; Parker, I.D. Trypanosoma Cruziin free-ranging mammalian populations in south Texas, USA. J. Wildl. Dis. 2017, 53, 788–794. [Google Scholar] [CrossRef]
- Merino, S.; Møller, A.P. Host-parasite interactions and climate change. In Effects of Climate Change on Birds; Møller, A.P., Fiedler, W., Berthold, P., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 213–226. [Google Scholar]
- Moller, A.P.; Merino, S.; Soler, J.J.; Antonov, A.; Badás, E.P.; Calero-Torralbo, M.A.; de Lope, F.; Eeva, T.; Figuerola, J.; FlenstedJensen, E.; et al. Assessing the effects of climate on host-parasite interactions: A comparative study of European birds and their parasites. PLoS ONE 2013, 8, e82886. [Google Scholar] [CrossRef]
- Wood, C.L.; Vanhove, M.P.M. Is the World Wormier than It Used to Be? We’ll Never Know without Natural History Collections. J. Anim. Ecol. 2022, 92, 250–262. [Google Scholar] [CrossRef]
- Kirk, D.; O’Connor, M.I.; Mordecai, E.A. Scaling Effects of Temperature on Parasitism from Individuals to Populations. J. Anim. Ecol. 2022, 91, 2087–2102. [Google Scholar] [CrossRef]
- Rizzoli, A.; Tagliapietra, V.; Cagnacci, F.; Marini, G.; Arnoldi, D.; Rosso, F.; Rosà, R. Parasites and Wildlife in a Changing World: The Vector-Host- Pathogen Interaction as a Learning Case. Int. J. Parasitol. 2019, 9, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Pounds, J.A.; Bustamante, M.R.; Coloma, L.A.; Consuegra, J.A.; Fogden, M.P.L.; Foster, P.N.; La Marca, E.; Masters, K.L.; Merino-Viteri, A.; Puschendorf, R.; et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 2006, 439, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Vetaas, O.R. Mountain Biodiversity and Elevational Gradients. Front. Biogeogr. 2021, 13, e54146. [Google Scholar] [CrossRef]
- Heaney, L.R. Small Mammal Diversity along Elevational Gradients in the Philippines: An Assessment of Patterns and Hypotheses. Glob. Ecol. Biogeogr. 2001, 10, 15–39. [Google Scholar] [CrossRef]
- Brown, J.H. Mammals on Mountainsides: Elevational Patterns of Diversity. Glob. Ecol. Biogeogr. 2001, 10, 101–109. [Google Scholar] [CrossRef]
- McCain, C.M. The Mid-Domain Effect Applied to Elevational Gradients: Species Richness of Small Mammals in Costa Rica. J. Biogeogr. 2003, 31, 19–31. [Google Scholar] [CrossRef]
- Gebrezgiher, G.B.; Makundi, R.H.; Meheretu, Y.; Mulungu, L.S.; Katakweba, A.A.S. A Decade-Long Change in the Elevational Distribution of Non-Volant Small Mammals on Mount Meru, Tanzania. Diversity 2022, 14, 454. [Google Scholar] [CrossRef]
- Thomas, S.M.; Soka, G.E.; Mulungu, L.S. Influence of Vegetation Structure, Seasonality, and Soil Properties on Rodent Diversity and Community Assemblages in West Mount Kilimanjaro, Tanzania. Ecol. Evol. 2022, 12, e9211. [Google Scholar] [CrossRef]
- Stanley, W.T.; Kihaule, P.M. Elevational Distribution and Ecology of Small Mammals on Tanzania’s Second Highest Mountain. PLoS ONE 2016, 11, e0162009. [Google Scholar] [CrossRef]
- Krasnov, B.R.; Khokhlova, I.; Shenbrot, G. The effect of host density on ectoparasite distribution: An example of a rodent parasitized by fleas. Ecology 2002, 83, 164–175. [Google Scholar] [CrossRef]
- Morand, S.; Poulin, R. Density, Body Mass and Parasite Species Richness of Terrestrial Mammals. Evol. Ecol. 1998, 12, 717–727. [Google Scholar] [CrossRef]
- Meléndez, L.; Laiolo, P.; Mironov, S.; García, M.; Magaña, O.; Jovani, R. Climate-driven variation in the intensity of a host-symbiont animal interaction along a broad elevation gradient. PLoS ONE 2014, 9, e101942. [Google Scholar] [CrossRef]
- Baláž, I.; Ševčík, M.; Tulis, F.; Zigová, M.; Dudich, A. Diversity, Distribution and Changes in Communities of Fleas on Small Mammals along the Elevational Gradient from the Pannonian Plain to the Carpathian Mountains. Parasitology 2020, 148, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Castiglia, R.; Solano, E.; Makundi, R.H.; Hulselmans, J.; Verheyen, E.; Colangelo, P. Rapid Chromosomal Evolution in the Mesic Four-Striped Grass RatRhabdomys Dilectus(Rodentia, Muridae) Revealed by MtDNA Phylogeographic Analysis. J. Zool. Syst. Evol. Res. 2011, 50, 165–172. [Google Scholar] [CrossRef]
- Nicolas, V.; Mikula, O.; Lavrenchenko, L.A.; Šumbera, R.; Bartáková, V.; Bryjová, A.; Meheretu, Y.; Verheyen, E.; Missoup, A.D.; Lemmon, A.R.; et al. Phylogenomics of African Radiation of Praomyini(Muridae: Murinae) Rodents: First Fully Resolved Phylogeny, Evolutionary History and Delimitation of Extant Genera. Mol. Phylogenet. Evol. 2021, 163, 107263. [Google Scholar] [CrossRef]
- Cassola, F. “Praomys delectorum”. IUCN Red List of Threatened Species. 2016. [Google Scholar] [CrossRef]
- Makundi, R.H.; Massawe, A.W.; Borremans, B.; Laudisoit, A.; Katakweba, A. We Are Connected: Flea–Host Association Networks in the Plague Outbreak Focus in the Rift Valley, Northern Tanzania. Wildl. Res. 2015, 42, 196. [Google Scholar] [CrossRef]
- Animal-To-Human Diseases on the Rise in Africa, Warns UN Health Agency. Available online: https://news.un.org/en/story/2022/07/1122522 (accessed on 11 June 2022).
- Maleko, D.D.; Mbassa, G.N.; Maanga, W.F.; Sisya, E.S. Impacts of wildlife-livestock interactions in and around Arusha National Park, Tanzania. Curr. Res. J. Biol. Sci. 2012, 4, 471–476. [Google Scholar]
- Bussmann, R.W. Vegetation zonation and nomenclature of African Mountains—An overview. Lyonia 2006, 11, 41–66. [Google Scholar]
- Happold, D.C.D.; Kingdon, J. Mammals of Africa. Volume III: Rodents, Hares and Rabbits; David, C.D., Ed.; Happold: Bath, UK; Bloomsbury: London, UK, 2013. [Google Scholar]
- Hoffmann, A.; Decher, J.; Rovero, F.; Schaer, J.; Voigt, C.; Wibbelt, G. Field methods and techniques for monitoring mammals. J. Dedic. Capacit. Build. Taxon. Collect. Manag. 2010, 8, 482–529. [Google Scholar]
- Walker, A. The Arthropods of Humans and Domestic Animals: A Guide to Preliminary Identification; Chapman & Hall: London UK; New York, NY, USA, 1994; p. 231. [Google Scholar]
- Anne, M.Z.; Gary, A.C.; Susan, E.L.; Mason, V.R. Veterinary Clinical Parasitology, 9th ed.; Wiley Blackwell: Hoboken, NJ, USA, 2021. [Google Scholar]
- Mathison, B.A.; Pritt, B.S. Laboratory Identification of Arthropod Ectoparasites. Clin. Microbiol. 2014, 27, 48–67. [Google Scholar] [CrossRef] [PubMed]
- Yakub, A.; Amrito, B.; Abdullah, S.M. Morphological Identification and Prevalence of the Dog Flea Ctenocephalides Canis (Curtis, 1826) and the Cat Flea(Ctenocephalides Felis (Bouché, 1835) in Dhaka City, Bangladesh. Parasitology 2020, 54, 163–172. [Google Scholar] [CrossRef]
- Campbell, J.D.; Bennett, S.; Krueger, L.; Morgan, T.; Nguyen, K.; Penicks, A.; Sun, S.; Cummings, R.; Martinez, D.; Quinn, N. Flea ‘in around: A look at the identification, preservation, clearing, and mounting of Siphonaptera. Proc. Vertebr. Pest Conf. 2018, 28, 163–172. [Google Scholar]
- Zhu, Q.; Hastriter, M.W.; Whiting, M.F.; Dittmar, K. Fleas(Siphonaptera) Are Cretaceous, and Evolved with Theria. Mol. Phylogenet. Evol. 2015, 90, 129–139. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Geneious Software, version 2022.1.1.; Biomatters Inc.: Auckland, New Zealand.
- Kumar, S.; Stecher, G.; Tamura, K. MEGA11: Molecular Evolutionary Genetics Analysis Version 11.0 for Bigger Datasets. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Reiczigel, J.; Marozzi, M.; Fábián, I.; Rózsa, L. Biostatistics for parasitologists–a primer to quantitative parasitology. Trends Parasitol. 2019, 35, 277–281. [Google Scholar] [CrossRef]
- Stanko, M.; Miklisová, D.; Goüy de Bellocq, J.; Morand, S. Mammal Density and Patterns of Ectoparasite Species Richness and Abundance. Oecologia 2002, 131, 289–295. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A Protocol for Data Exploration to Avoid Common Statistical Problems. Methods Ecol. Evol. 2009, 1, 3–14. [Google Scholar] [CrossRef]
- Anderson, D.R.; Burnham, K.P. Avoiding Pitfalls When Using Information-Theoretic Methods. J. Wildl. Manag. 2002, 66, 912–918. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Methods in Ecology and Evolution. 2008. Available online: http://softlibre.unizar.es/manuales/aplicaciones/r/fullrefman.pdf (accessed on 23 June 2022).
- Varroa (Jacobsoni) Mite (326). Available online: https://apps.lucidcentral.org/ppp/text/web_full/entities/varroa_jacobsoni_mite_326.htm (accessed on 10 November 2022).
- Woodroffe, R. Managing Disease Threats to Wild Mammals. Anim. Conserv. 1999, 2, 185–193. [Google Scholar] [CrossRef]
- Altizer, S.; Harvell, D.; Friedle, E. Rapid Evolutionary Dynamics and Disease Threats to Biodiversity. Trends Ecol. Evol. 2003, 18, 589–596. [Google Scholar] [CrossRef]
- Lyles, A.M.; Dobson, A.P. Infectious disease and intensive management: Population dynamics, threatened hosts and their parasites. Zoo Wildl. Med. 1993, 24, 315–326. [Google Scholar]
- McCallum, H.; Dobson, A. Detecting Disease and Parasite Threats to Endangered Species and Ecosystems. Trends Ecol. Evol. 1995, 10, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.B.; Jones, K.E.; Nunn, C.L.; Altizer, S. Infectious Diseases and Extinction Risk in Wild Mammals. Conserv. Biol. 2007, 21, 1269–1279. [Google Scholar] [CrossRef]
- Lareschi, M.; Krasnov, B.R. Determinants of Ectoparasite Assemblage Structure on Rodent Hosts from South American Marshlands: The Effect of Host Species, Locality and Season. Med. Vet. Entomol. 2010, 24, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Yonas, M.; Welegerima, K.; Laudisoit, A.; Bauer, H.; Gebrehiwot, K.; Deckers, S.; Katakweba, A.; Makundi, R.; Leirs, H. Preliminary Investigation on Rodent-Ectoparasite Associations in the Highlands of Tigray, Northern Ethiopia: Implications for Potential Zoonoses. Integr. Zool. 2011, 6, 366–374. [Google Scholar] [CrossRef]
- Krasnov, B.R.; Shenbrot, G.I.; Khokhlova, I.S.; Vinarski, M.; Korallo-Vinarskaya, N.; Poulin, R. Geographical Patterns of Abundance: Testing Expectations of the “Abundance Optimum” Model in Two Taxa of Ectoparasitic Arthropods. J. Biogeogr. 2008, 35, 2187–2194. [Google Scholar] [CrossRef]
- Cruz, L.D.; Fernandes, F.R.; Linhares, A.X. Similarities among Ectoparasite Fauna of Sigmodontine Rodents: Phylogenetic and Geographical Influences. Parasitology 2012, 139, 1749–1756. [Google Scholar] [CrossRef]
- Delfinado, M.D.; Baker, E.W. Varroidae, a new family of mites on honeybees (Mesostigmata: Acarina). J. Wash. Acad. Sci. 1974, 1, 4–10. [Google Scholar]
- Medzhitov, R.; Schneider, D.S.; Soares, M.P. Disease Tolerance as a Defense Strategy. Science 2012, 335, 936–941. [Google Scholar] [CrossRef]
- da Amaral, C.H.L.; Bergmann, F.B.; dos Santos, P.R.S.; Silveira, T.; Krüger, R.F. How Do Seasonality and Host Traits Influence the Distribution Patterns of Parasites on Juveniles and Adults of Columba Livia? Acta Trop. 2017, 176, 305–310. [Google Scholar] [CrossRef]
- Shilereyo, M.; Magige, F.; Ranke, P.S.; Ogutu, J.O.; Røskaft, E. Ectoparasite Load of Small Mammals in the Serengeti Ecosystem: Effects of Land Use, Season, Host Species, Age, Sex and Breeding Status. Parasitol. Res. 2022, 121, 823–838. [Google Scholar] [CrossRef]
- Matthee, S.; McGEOCH, M.A.; Krasnov, B.R. Parasite-specifc variation and the extent of male-biased parasitism; an example with a South African rodent and ectoparasitic arthropods. Parasitology 2010, 137, 651–660. [Google Scholar] [CrossRef]
- Comas, M. Body Condition, Sex and Elevation in Relation to Mite Parasitism in a High Mountain Gecko. J. Zool. 2020, 310, 298–305. [Google Scholar] [CrossRef]
- Scantlebury, M.; Maher McWilliams, M.; Marks, N.J.; Dick, J.T.A.; Edgar, H.; Lutermann, H. Effects of Life-History Traits on Parasite Load in Grey Squirrels. J. Zool. 2010, 282, 246–255. [Google Scholar] [CrossRef]
- Slob, A.K.; van der Werff Ten Bosch, J.J. Sex Differences in Body Growth in the Rat. Physiol. Behav. 1975, 14, 353–361. [Google Scholar] [CrossRef]
- Kowalski, K.; Bogdziewicz, M.; Eichert, U.; Rychlik, L. Sex Differences in Flea Infections among Rodent Hosts: Is There a Male Bias? Parasitol. Res. 2014, 114, 337–341. [Google Scholar] [CrossRef]
- Young, H.S.; Dirzo, R.; McCauley, D.J.; Agwanda, B.; Cattaneo, L.; Dittmar, K.; Eckerlin, R.P.; Fleischer, R.C.; Helgen, L.E.; Hintz, A.; et al. Drivers of Intensity and Prevalence of Flea Parasitism on Small Mammals in East African Savanna Ecosystems. J. Parasitol. 2015, 101, 327. [Google Scholar] [CrossRef]
- Postawa, T.; Nagy, Z. Variation of Parasitism Patterns in Bats during Hibernation: The Effect of Host Species, Resources, Health Status, and Hibernation Period. Parasitol. Res. 2016, 115, 3767–3778. [Google Scholar] [CrossRef]
- Krasnov, B.R.; Vinarski, M.V.; Korallo-Vinarskaya, N.P.; Shenbrot, G.I.; Khokhlova, I.S. Species Associations in Arthropod Ectoparasite Infracommunities Are Spatially and Temporally Variable and Affected by Environmental Factors. Ecol. Entomol. 2021, 46, 1254–1265. [Google Scholar] [CrossRef]
- Singleton, G. Population Dynamics of Mus Musculus and Its Parasites in Mallee Wheatlands in Victoria during and after a Drought. Wildl. Res. 1985, 12, 437. [Google Scholar] [CrossRef]
- Krasnov, B.R.; Shenbrot, G.I.; Medvedev, S.G.; Vatschenok, V.S.; Khokhlova, I.S. Host–Habitat Relations as an Important Determinant of Spatial Distribution of Flea Assemblages(Siphonaptera) on Rodents in the Negev Desert. Parasitology 1997, 114, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Lomolino, M.V. Elevation Gradients of Species-Density: Historical and Prospective Views. Glob. Ecol. Biogeogr. 2001, 10, 3–13. [Google Scholar] [CrossRef]
- Zamora-Vilchis, I.; Williams, S.E.; Johnson, C.N. Environmental Temperature Affects Prevalence of Blood Parasites of Birds on an Elevation Gradient: Implications for Disease in a Warming Climate. PLoS ONE 2012, 7, e39208. [Google Scholar] [CrossRef]
- Alvarez-Ruiz, L.; Megía-Palma, R.; Reguera, S.; Ruiz, S.; Zamora-Camacho, F.J.; Figuerola, J.; Moreno-Rueda, G. Opposed Elevational Variation in Prevalence and Intensity of Endoparasites and Their Vectors in a Lizard. Curr. Zool. 2018, 64, 197–204. [Google Scholar] [CrossRef]
- Nziza, J.; Tumushime, J.C.; Cranfield, M.; Ntwari, A.E.; Modrý, D.; Mudakikwa, A.; Gilardi, K.; Šlapeta, J. Fleas from Domestic Dogs and Rodents in Rwanda Carry Rickettsia Asembonensis and Bartonella Tribocorum. Med. Vet. Entomol. 2018, 33, 177–184. [Google Scholar] [CrossRef]
- Mullins, K.; Canal, E.; Ouch, P.; Prasetyo, D.; Tagoe, J.; Attram, N.; Yeboah, C.; Kumordjie, S.; Fox, A.; Letizia, A.G.; et al. Bartonella Species in Cambodia, Ghana, Laos, and Peru: Results from Vector and Serosurveys. Vector Borne Zoonotic Dis. 2023, 23, 9–17. [Google Scholar] [CrossRef]
- Diaz, J.H. Mite-Transmitted Dermatoses and Infectious Diseases in Returning Travelers. J. Travel Med. 2010, 17, 21–31. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Guo, X.-G.; Ding, F.; Lv, Y.; Yin, P.-W.; Song, W.-Y.; Zhao, C.-F.; Zhang, Z.-W.; Fan, R.; Peng, P.-Y.; et al. Infestation of Oriental House Rat (Rattus Tanezumi) with Chigger Mites Varies along Environmental Gradients across Five Provincial Regions of Southwest China. Int. J. Environ. Res. Public Health 2023, 20, 2203. [Google Scholar] [CrossRef]




| Host (Infested /Examined) | Ectoparasite Taxa | 1500 m N = 6 n = 3 | 2000 m N = 83 n = 57 | 2500 m N = 201 n = 130 | 2950 m N = 70 n = 29 | 3500 m N = 38 n = 15 | Total N = 398 n = 234 |
|---|---|---|---|---|---|---|---|
| Montemys delectorum (211/335) | Xenopsylla cheopis | 0 | 0 | 0 | 0 | 0 | 0 |
| Dinopsyllus ellobius | 0 | 22(0.28) | 20(0.21) | 2(0.05) | 0 | 44 | |
| Ctenophthalmus calceatus cabirus | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mite | 0 | 52(0.67) | 121(0.62) | 24(0.56) | 11(0.60) | 208 | |
| Total | 0 | 74(0.95) | 141(0.72) | 26(0.64) | 11(0.60) | 252 | |
| Rhabdomys dilectus (23/63) | Xenopsylla cheopis | 2(0.05) | 0 | 0 | 0 | 0 | 2 |
| Dinopsyllus ellobius | 0 | 0 | 2(0.40) | 5(0.19) | 0 | 7 | |
| Ctenophthalmus calceatus cabirus | 0 | 0 | 1(0.20) | 4(0.15) | 1(0.05) | 6 | |
| Mite | 3(0.75) | 0 | 0 | 37(1.37) | 18 (0.82) | 58 | |
| Total | 5(1.25) | 0 | 3(0.6) | 46(1.70) | 19(0.86) | 73 | |
| Overall ectoparasites (MA ± SE) | 0.16 ± 0.10 | 0.29 ± 0.17 | 0.19 ± 0.12 | 0.16 ± 0.08 | 0.12 ± 0.09 |
| Ectoparasite Taxa | %P | %D |
|---|---|---|
| Xenopsylla cheopis | 0.25 | 0.62 |
| Dinopsyllus ellobius | 7.11 | 15.69 |
| Ctenophthalmus calceatus cabirus | 0.76 | 1.85 |
| Over all fleas | 8.12 | 18.15 |
| Mites | 50.68 | 81.85 |
| Variables | Estimate | SE | 95% LCL | 95% UCL |
|---|---|---|---|---|
| Prevalence | ||||
| Host species (Md) | 1.22 | 0.30 | 0.32 | 5.21 |
| Host sex (Male) | 2.56 | 0.28 | 0.39 | 4.69 |
| Temperature (°C) | 0.50 | 0.12 | 0.28 | 3.44 |
| Abundance | ||||
| Host species (Md) | 0.42 | 0.14 | 0.04 | 8.06 |
| Host sex (Male) | 0.93 | 0.12 | 0.13 | 9.17 |
| Temperature (°C) | 2.10 | 0.99 | 1.16 | 4.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebrezgiher, G.B.; Makundi, R.H.; Katakweba, A.A.S.; Belmain, S.R.; Lyimo, C.M.; Meheretu, Y. Arthropod Ectoparasites of Two Rodent Species Occurring in Varied Elevations on Tanzania’s Second Highest Mountain. Biology 2023, 12, 394. https://doi.org/10.3390/biology12030394
Gebrezgiher GB, Makundi RH, Katakweba AAS, Belmain SR, Lyimo CM, Meheretu Y. Arthropod Ectoparasites of Two Rodent Species Occurring in Varied Elevations on Tanzania’s Second Highest Mountain. Biology. 2023; 12(3):394. https://doi.org/10.3390/biology12030394
Chicago/Turabian StyleGebrezgiher, Genet B., Rhodes H. Makundi, Abdul A. S. Katakweba, Steven R. Belmain, Charles M. Lyimo, and Yonas Meheretu. 2023. "Arthropod Ectoparasites of Two Rodent Species Occurring in Varied Elevations on Tanzania’s Second Highest Mountain" Biology 12, no. 3: 394. https://doi.org/10.3390/biology12030394
APA StyleGebrezgiher, G. B., Makundi, R. H., Katakweba, A. A. S., Belmain, S. R., Lyimo, C. M., & Meheretu, Y. (2023). Arthropod Ectoparasites of Two Rodent Species Occurring in Varied Elevations on Tanzania’s Second Highest Mountain. Biology, 12(3), 394. https://doi.org/10.3390/biology12030394








