Phylogenetic Diversity of the Red Swamp Crayfish Procambarus clarkii and Its Dispersal Pattern in Northern and Central Italy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
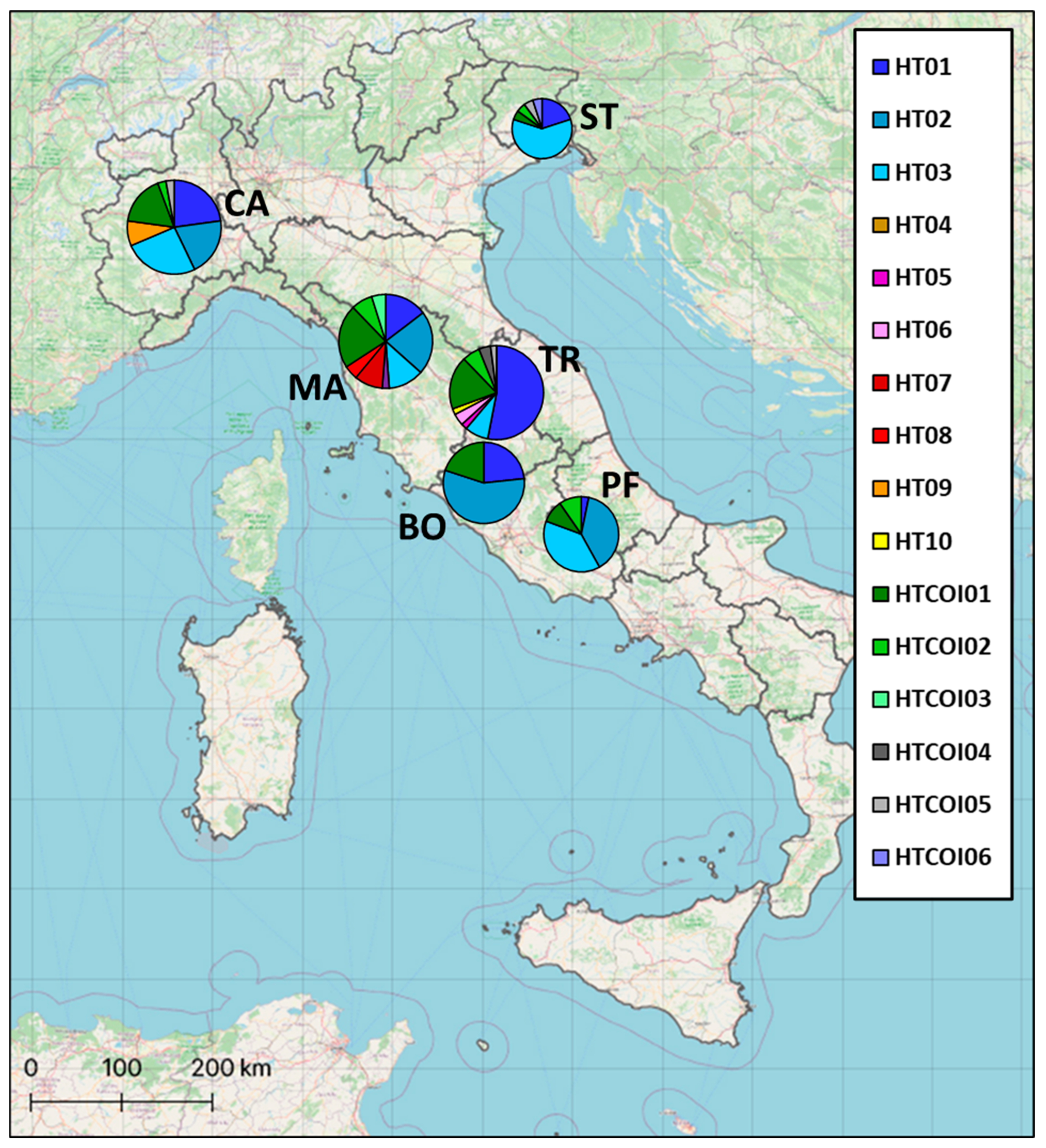
2.1. Sampling and Study Area
2.2. Genetic Analyses
3. Results and Discussion
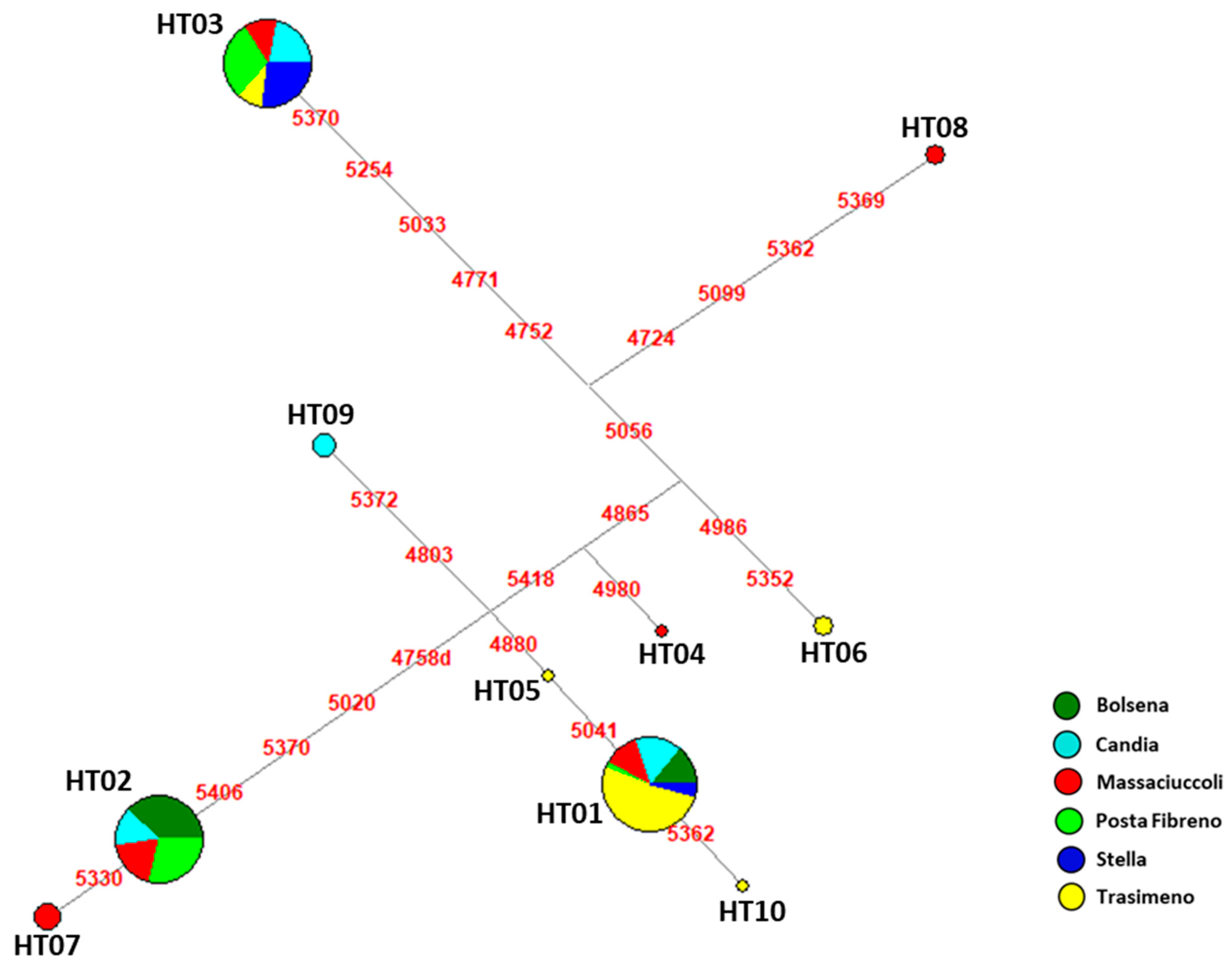
3.1. Genetic Analysis of Mitochondrial DNA Control Region
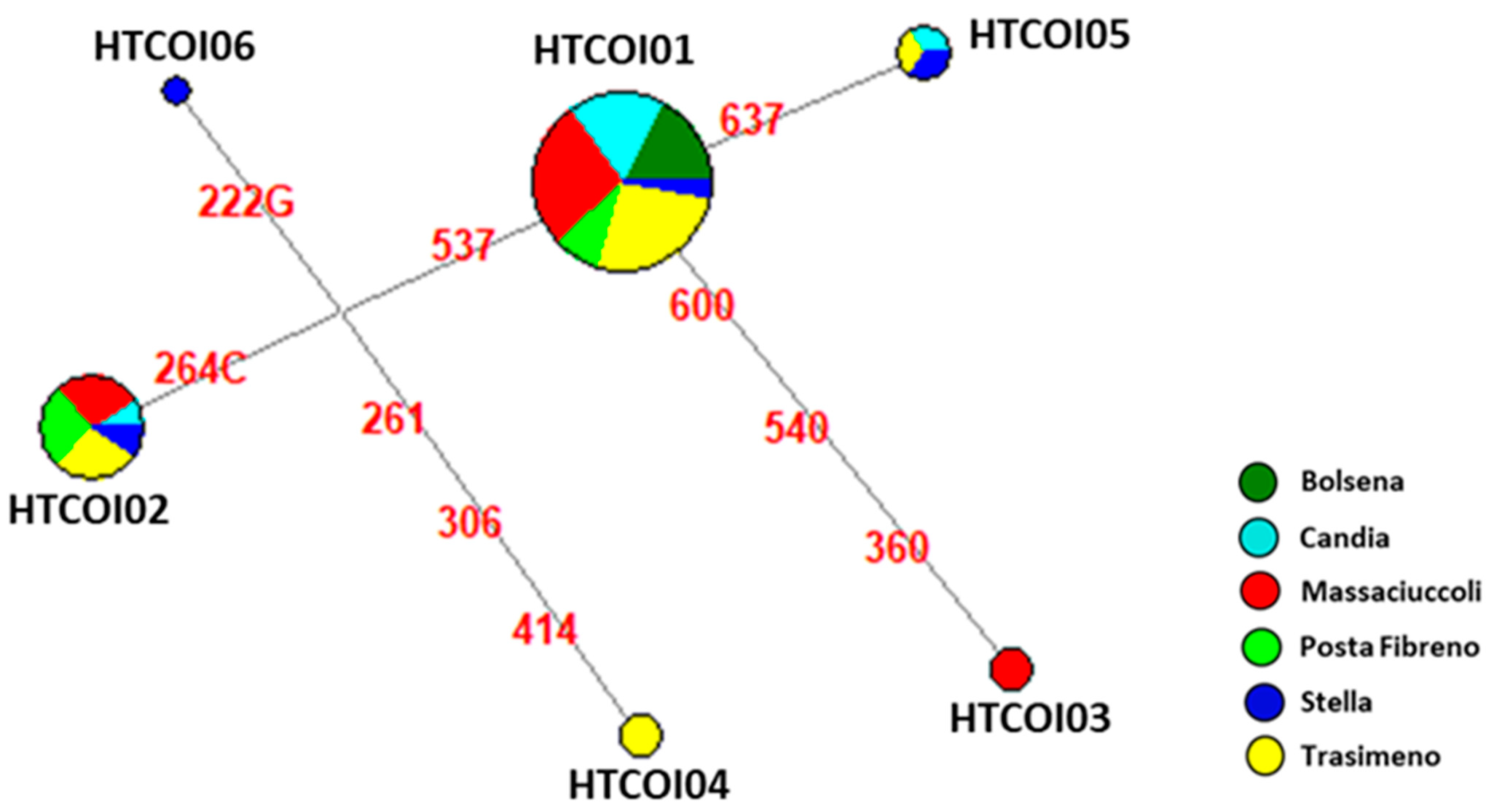
3.2. Genetic Analysis of Mitochondrial Cytochrome Oxidase Subunit I
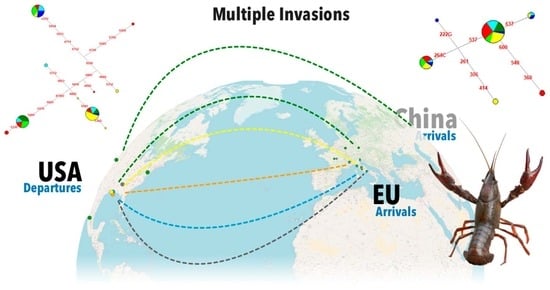
3.3. Worldwide Genetic Variability and Phylogeny
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blackburn, T.M.; Essl, F.; Evans, T.; Hulme, P.E.; Jeschke, J.M.; Kühn, I.; Kumschick, S.; Marková, Z.; Mrugała, A.; Nentwig, W.; et al. A Unified Classification of Alien Species Based on the Magnitude of Their Environmental Impacts. PLoS Biol. 2014, 12, e1001850. [Google Scholar] [CrossRef] [PubMed]
- Vilà, M.; Basnou, C.; Pyšek, P.; Josefsson, M.; Genovesi, P.; Gollasch, S.; Nentwig, W.; Olenin, S.; Roques, A.; Roy, D.; et al. How Well Do We Understand the Impacts of Alien Species on Ecosystem Services? A Pan-European, Cross-Taxa Assessment. Front. Ecol. Environ. 2010, 8, 135–144. [Google Scholar] [CrossRef]
- Hobbs, H.H. Crayfish Distribution, Adaptive Radiation and Evolution. In Freshwater Crayfish: Biology, Management and Exploitation; Holdich, D.M., Lowery, R.S., Eds.; Croom Helm Ltd.: London, UK, 1988; pp. 52–82. [Google Scholar]
- Clark, W.H.; Wrotenon, J.W. First Record of the Crayfish, Procambarus clarkii, from Idaho, U.S.A. (Decapoda, Cambaridae). Crustaceana 1978, 35, 317–319. [Google Scholar]
- Scalici, M.; Gherardi, F. Structure and Dynamics of an Invasive Population of the Red Swamp Crayfish (Procambarus clarkii) in a Mediterranean Wetland. Hydrobiologia 2007, 583, 309–319. [Google Scholar] [CrossRef]
- Piersanti, S.; Pallottini, M.; Salerno, G.; Goretti, E.; Elia, A.C.; Dörr, A.J.M.; Rebora, M. Resistance to Dehydration and Positive Hygrotaxis in the Invasive Red Swamp Crayfish Procambarus clarkii. Knowl. Manag. Aquat. Ecosyst. 2018, 419, 36. [Google Scholar] [CrossRef]
- Dörr, A.J.M.; Scalici, M.; Caldaroni, B.; Magara, G.; Scoparo, M.; Goretti, E.; Elia, A.C. Salinity Tolerance of the Invasive Red Swamp Crayfish Procambarus clarkii (Girard, 1852). Hydrobiologia 2020, 847, 2065–2081. [Google Scholar] [CrossRef]
- Tavoloni, T.; Stecconi, T.; Galarini, R.; Bacchiocchi, S.; Dörr, A.J.M.; Elia, A.C.; Giannotti, M.; Siracusa, M.; Stramenga, A.; Piersanti, A. BFRs (PBDEs and HBCDs) in Freshwater Species from Lake Trasimeno (Italy): The Singular Case of HBCDs in Red Swamp Crayfish. Sci. Total Environ. 2021, 758, 143585. [Google Scholar] [CrossRef]
- Selvaggi, R.; Pallottini, M.; Caldaroni, B.; Dörr, A.J.M.; Magara, G.; Gravina, P.; Grispoldi, L.; Cenci-Goga, B.; Goretti, E.; La Porta, G.; et al. Sex and Seasonal Differences in Metal Accumulation of Selected Tissues in Red Swamp Crayfish from Lake Trasimeno (Umbria, Italy). Environ. Sci. Pollut. Res. 2022, 30, 6234–6244. [Google Scholar] [CrossRef]
- Dörr, A.J.M.; Elia, A.C.; Rodolfi, M.; Garzoli, L.; Picco, A.M.; D’Amen, M.; Scalici, M. A Model of Co-Occurrence: Segregation and Aggregation Patterns in the Mycoflora of the Crayfish Procambarus clarkii in Lake Trasimeno (Central Italy). J. Limnol. 2012, 71, 14. [Google Scholar] [CrossRef]
- Dörr, A.J.M.; Rodolfi, M.; Scalici, M.; Elia, A.C.; Garzoli, L.; Picco, A.M. Phoma glomerata, a Potential New Threat to Italian Inland Waters. J. Nat. Conserv. 2011, 19, 370–373. [Google Scholar] [CrossRef]
- De Kort, H.; Mergeay, J.; Jacquemyn, H.; Honnay, O. Transatlantic Invasion Routes and Adaptive Potential in North American Populations of the Invasive Glossy Buckthorn, Frangula Alnus. Ann. Bot. 2016, 118, 1089–1099. [Google Scholar] [CrossRef]
- Paulson, E.L.; Martin, A.P. Discerning Invasion History in an Ephemerally Connected System: Landscape Genetics of Procambarus clarkii in Ash Meadows, Nevada. Biol. Invasions 2014, 16, 1719–1734. [Google Scholar] [CrossRef]
- Hänfling, B.; Edwards, F.; Gherardi, F. Invasive Alien Crustacea: Dispersal, Establishment, Impact and Control. BioControl 2011, 56, 573–595. [Google Scholar] [CrossRef]
- Lavergne, S.; Molofsky, J. Increased Genetic Variation and Evolutionary Potential Drive the Success of an Invasive Grass. Proc. Natl. Acad. Sci. USA 2007, 104, 3883–3888. [Google Scholar] [CrossRef]
- Acevedo-Limón, L.; Oficialdegui, F.J.; Sánchez, M.I.; Clavero, M. Historical, Human, and Environmental Drivers of Genetic Diversity in the Red Swamp Crayfish (Procambarus clarkii) Invading the Iberian Peninsula. Freshw. Biol. 2020, 65, 1460–1474. [Google Scholar] [CrossRef]
- Ladoukakis, E.D.; Zouros, E. Evolution and Inheritance of Animal Mitochondrial DNA: Rules and Exceptions. J. Biol. Res. Thessalon. 2017, 24, 2. [Google Scholar] [CrossRef]
- Raupach, M.J.; Radulovici, A.E. Looking Back on a Decade of Barcoding Crustaceans. ZooKeys 2015, 539, 53–81. [Google Scholar] [CrossRef]
- Spielmann, G.; Diedrich, J.; Haszprunar, G.; Busch, U.; Huber, I. Comparison of Three DNA Marker Regions for Identification of Food Relevant Crustaceans of the Order Decapoda. Eur. Food Res. Technol. 2019, 245, 987–995. [Google Scholar] [CrossRef]
- Bronstein, O.; Kroh, A.; Haring, E. Mind the Gap! The Mitochondrial Control Region and Its Power as a Phylogenetic Marker in Echinoids. BMC Evol. Biol. 2018, 18, 80. [Google Scholar] [CrossRef]
- Oficialdegui, F.J.; Clavero, M.; Sánchez, M.I.; Green, A.J.; Boyero, L.; Michot, T.C.; Klose, K.; Kawai, T.; Lejeusne, C. Unravelling the Global Invasion Routes of a Worldwide Invader, the Red Swamp Crayfish (Procambarus clarkii). Freshw. Biol. 2019, 64, 1382–1400. [Google Scholar] [CrossRef]
- Li, Y.; Guo, X.; Cao, X.; Deng, W.; Luo, W.; Wang, W. Population Genetic Structure and Post-Establishment Dispersal Patterns of the Red Swamp Crayfish Procambarus clarkii in China. PLoS ONE 2012, 7, e40652. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhou, L.; Li, X.; Lu, D. Population Genetic Structure of the Invasive Red Swamp Crayfish in China Revealed by ITS1 Variation. Biochem. Genet. 2013, 51, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Kang, J.; Chen, W.; He, S. DNA Barcoding for the Identification of Common Economic Aquatic Products in Central China and Its Application for the Supervision of the Market Trade. Food Control 2016, 61, 79–91. [Google Scholar] [CrossRef]
- Liu, Q.-N.; Chai, X.-Y.; Jiang, S.-H.; Zhou, C.-L.; Xuan, F.-J.; Tang, B.-P. Characterization of the Complete Mitochondrial Genome of the Red Crayfish, Procambarus clarkii (Decapoda: Cambaridae). Mitochondrial DNA Part A 2016, 27, 3458–3459. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Feng, M.; Tang, Y.; Yang, C.; Meng, X.; Nie, G. Species Diversity of Freshwater Shrimp in Henan Province, China, Based on Morphological Characters and COI Mitochondrial Gene. Ecol. Evol. 2021, 11, 10502–10514. [Google Scholar] [CrossRef]
- Quan, A. Origins of the Invasive Red Swamp Crayfish (Procambarus clarkii) in the Santa Monica Mountains. Aquat. Invasions 2014, 9, 211–219. [Google Scholar] [CrossRef]
- Braband, A.; Kawai, T.; Scholtz, G. The Phylogenetic Position of the East Asian Freshwater Crayfish Cambaroides within the Northern Hemisphere Astacoidea (Crustacea, Decapoda, Astacida) Based on Molecular Data. J. Zool. Syst. Evol. Res. 2006, 44, 17–24. [Google Scholar] [CrossRef]
- Filipová, L.; Grandjean, F.; Chucholl, C.; Soes, D.M.; Petrusek, A. Identification of Exotic North American Crayfish in Europe by DNA Barcoding. Knowl. Manag. Aquat. Ecosyst. 2011, 401, 11. [Google Scholar] [CrossRef]
- Ackefors, H. The Positive Effects of Established Crayfish Introductions in Europe. In Crayfish in Europe as Alien Species; Routledge: Rotterdam, The Netherlands, 1999; pp. 49–61. ISBN 978-1-315-14046-9. [Google Scholar]
- Almerão, M.P.; Delaunay, C.; Coignet, A.; Peiró, D.F.; Pinet, F.; Souty-Grosset, C. Genetic Diversity of the Invasive Crayfish Procambarus clarkii in France. Limnologica 2018, 69, 135–141. [Google Scholar] [CrossRef]
- Mestre, A.; Butlin, R.K.; Kelso, W.E.; Romaire, R.; Bonvillain, C.P.; Monrós, J.S.; Mesquita-Joanes, F. Contrasting Patterns of Genetic Diversity and Spatial Structure in an Invasive Symbiont-Host Association. Biol. Invasions 2016, 18, 3175–3191. [Google Scholar] [CrossRef]
- Delmastro, G. Sull’acclimatazione Del Gambero Della Louisiana Procambarus clarkii (Girard, 1852) Nelle Acque Dolci Italiane (Crustacea Decapoda Cambaridae). Pianura—Supplemento di Provincia Nuova 1992, 4, 5–10. [Google Scholar]
- Li, Y.; Guo, X.; Chen, L.; Bai, X.; Wei, X.; Zhou, X.; Huang, S.; Wang, W. Inferring Invasion History of Red Swamp Crayfish (Procambarus clarkii) in China from Mitochondrial Control Region and Nuclear Intron Sequences. Int. J. Mol. Sci. 2015, 16, 14623–14639. [Google Scholar] [CrossRef]
- Dörr, A.J.M.; Scoparo, M.; Cardinali, I.; La Porta, G.; Caldaroni, B.; Magara, G.; Pallottini, M.; Selvaggi, R.; Cenci-Goga, B.; Goretti, E.; et al. Population Ecology and Genetic Diversity of the Invasive Alien Species Procambarus clarkii in Lake Trasimeno (Italy). Biology 2021, 10, 1059. [Google Scholar] [CrossRef]
- Barbaresi, S.; Gherardi, F.; Mengoni, A.; Souty-Grosset, C. Genetics and Invasion Biology in Fresh Waters: A Pilot Study of Procambarus clarkii in Europe. In Biological Invaders in Inland Waters: Profiles, Distribution, and Threats; Gherardi, F., Ed.; Invading Nature—Springer Series in Invasion Ecology; Springer: Dordrecht, The Netherlands, 2007; Volume 2, pp. 381–400. ISBN 978-1-4020-6028-1. [Google Scholar]
- Orrù, F.; Deiana, A.M.; Cau, A. Conservazione Della Biodiversità e Specie Alloctone Invasive: Il Gambero Procambarus clarkii Nelle Acque Interne Della Sardegna. Studi Trentini Sci. Nat. 2009, 86, 162. [Google Scholar]
- Di Leo, C.; Faraone, F.P.; Lo Valvo, M. A New Record of the Redswamp Crayfish, Procambarus clarkii (Girard, 1852) (Crustacea Cambaridae), in Sicily, Italy. Biodivers. J. 2014, 5, 425–428. [Google Scholar]
- Cipriani, A. Il Lago Di Posta Fibreno (FR). Mem. Descr. Carta Geol. d’It. 2020, 106, 165–176. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA Primers for Amplification of Mitochondrial Cytochrome c Oxidase Subunit I from Diverse Metazoan Invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Kim, S.; Park, M.-H.; Jung, J.-H.; Ahn, D.-H.; Sultana, T.; Kim, S.; Park, J.-K.; Choi, H.-G.; Min, G.-S. The Mitochondrial Genomes of Cambaroides Similis and Procambarus clarkii (Decapoda: Astacidea: Cambaridae): The Phylogenetic Implications for Reptantia: Complete Mitochondrial Genomes of Northern Hemisphere Crayfish. Zool. Scr. 2012, 41, 281–292. [Google Scholar] [CrossRef]
- Bouckaert, R.R. An Efficient Coalescent Epoch Model for Bayesian Phylogenetic Inference. Syst. Biol. 2022, 71, 1549–1560. [Google Scholar] [CrossRef]
- Barbaresi, S.; Fani, R.; Gherardi, F.; Mengoni, A.; Souty-Grosset, C. Genetic Variability in European Populations of an Invasive American Crayfish: Preliminary Results. Biol. Invasions 2003, 5, 269–274. [Google Scholar] [CrossRef]
- Torres, E.; Álvarez, F. Genetic Variation in Native and Introduced Populations of the Red Swamp Crayfish Procambarus clarkii (Girard, 1852) (Crustacea, Decapoda, Cambaridae) in Mexico and Costa Rica. Aquat. Invasions 2012, 7, 235–241. [Google Scholar] [CrossRef]
- Loureiro, T.G.; Anastácio, P.M.S.G.; Araujo, P.B.; Souty-Grosset, C.; Almerão, M.P. Red Swamp Crayfish: Biology, Ecology and Invasion—An Overview. Nauplius 2015, 23, 1–19. [Google Scholar] [CrossRef]
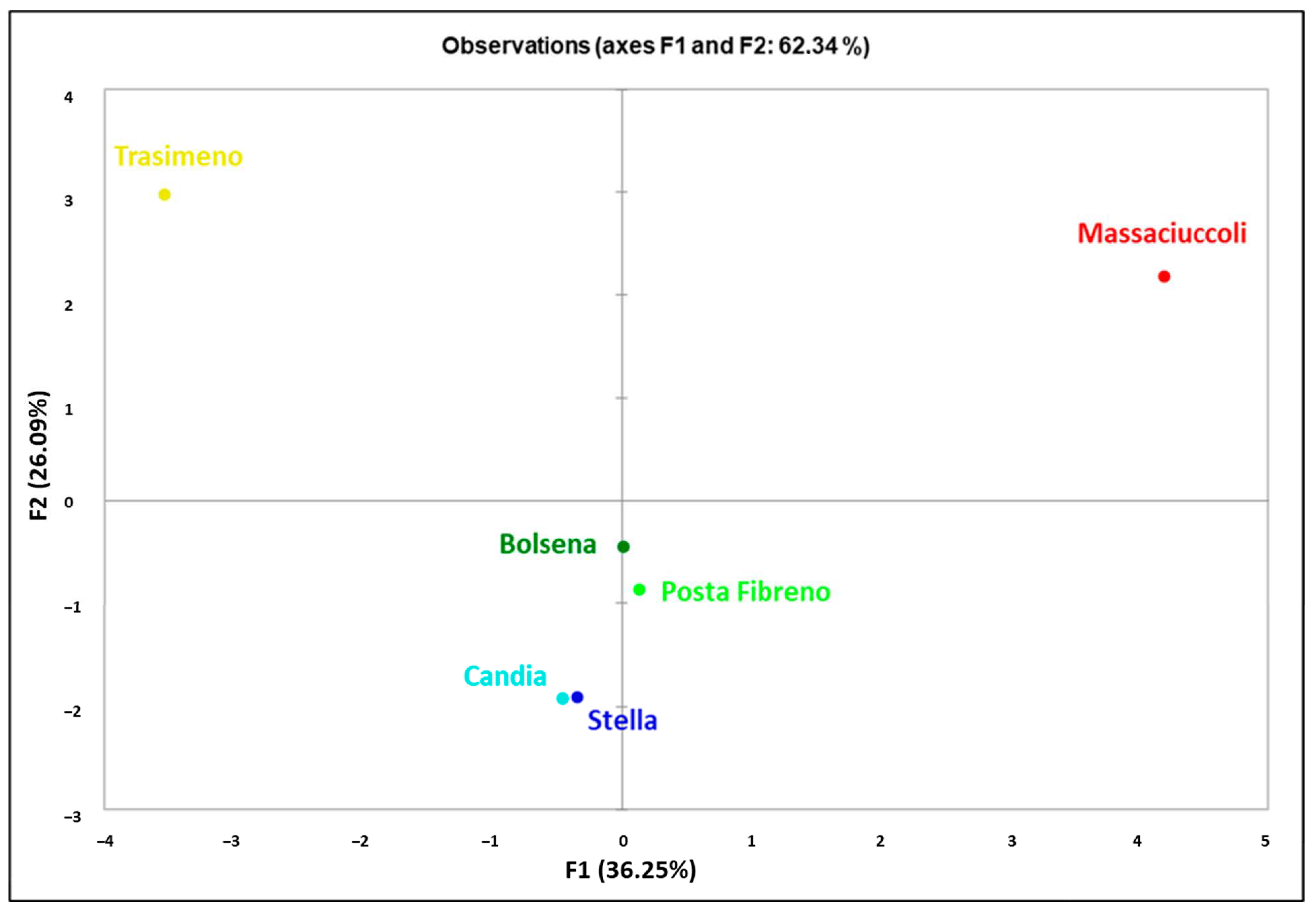
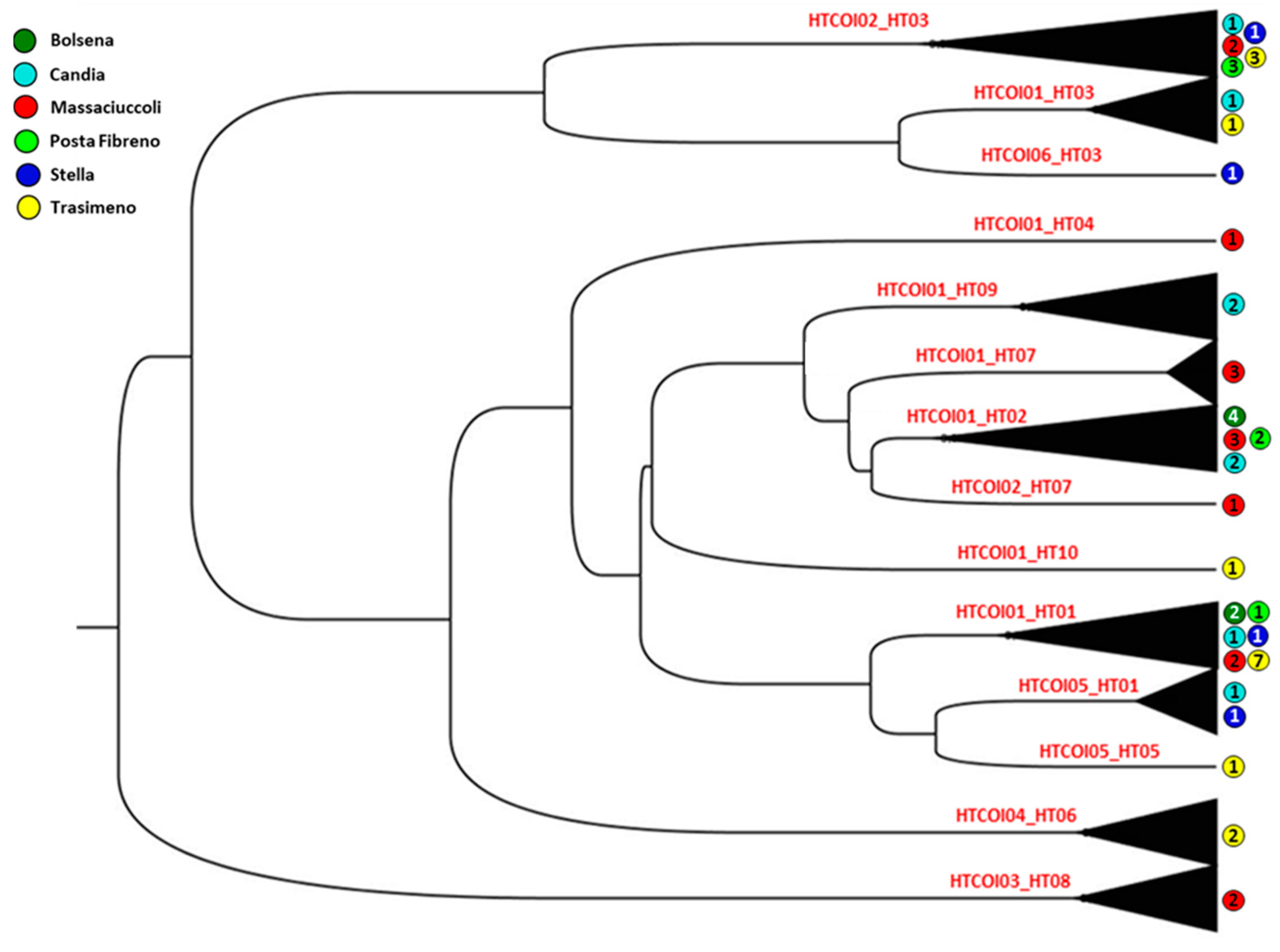
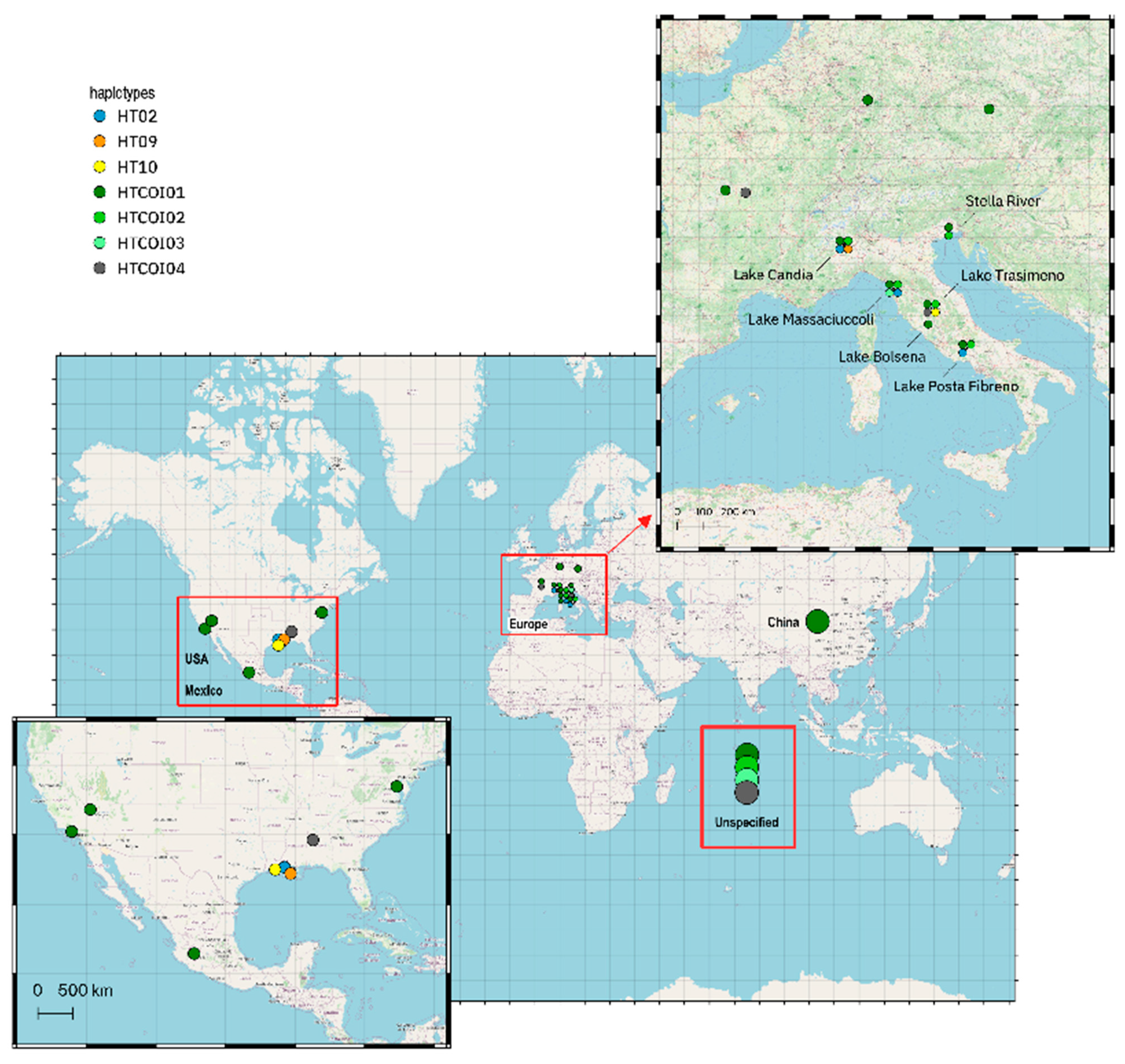
| Basin | Basin Code | N | π | Nh | Hd | S | k |
|---|---|---|---|---|---|---|---|
| Bolsena | BO | 24 | 0.003 | 2 | 0.431 | 5 | 2.156 |
| Candia | CA | 27 | 0.008 | 4 | 0.749 | 14 | 5.806 |
| Massaciuccoli | MA | 27 | 0.008 | 6 | 0.806 | 18 | 5.607 |
| Posta Fibreno | PF | 25 | 0.007 | 3 | 0.560 | 12 | 4.920 |
| Stella | ST | 16 | 0.006 | 2 | 0.400 | 10 | 4.000 |
| Trasimeno | TR | 34 | 0.004 | 5 | 0.408 | 13 | 2.806 |
| All samples | 153 | 0.008 | 10 | 0.726 | 22 | 5.455 |
| Basin | Basin Code | N | π | Nh | Hd | S | k |
|---|---|---|---|---|---|---|---|
| Bolsena | BO | 6 | 0.000 | 1 | 0.000 | 0 | 0.000 |
| Candia | CA | 8 | 0.001 | 3 | 0.464 | 3 | 0.750 |
| Massaciuccoli | MA | 14 | 0.003 | 3 | 0.560 | 5 | 1.516 |
| Posta Fibreno | PF | 6 | 0.002 | 2 | 0.600 | 2 | 1.200 |
| Stella | ST | 4 | 0.004 | 4 | 1.000 | 4 | 2.167 |
| Trasimeno | TR | 15 | 0.003 | 4 | 0.619 | 6 | 1.695 |
| All samples | 53 | 0.002 | 6 | 0.549 | 10 | 1.322 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scoparo, M.; Cardinali, I.; La Porta, G.; Caldaroni, B.; Magara, G.; Dörr, A.J.M.; Elia, A.C.; Lancioni, H. Phylogenetic Diversity of the Red Swamp Crayfish Procambarus clarkii and Its Dispersal Pattern in Northern and Central Italy. Biology 2023, 12, 313. https://doi.org/10.3390/biology12020313
Scoparo M, Cardinali I, La Porta G, Caldaroni B, Magara G, Dörr AJM, Elia AC, Lancioni H. Phylogenetic Diversity of the Red Swamp Crayfish Procambarus clarkii and Its Dispersal Pattern in Northern and Central Italy. Biology. 2023; 12(2):313. https://doi.org/10.3390/biology12020313
Chicago/Turabian StyleScoparo, Melissa, Irene Cardinali, Gianandrea La Porta, Barbara Caldaroni, Gabriele Magara, Ambrosius Josef Martin Dörr, Antonia Concetta Elia, and Hovirag Lancioni. 2023. "Phylogenetic Diversity of the Red Swamp Crayfish Procambarus clarkii and Its Dispersal Pattern in Northern and Central Italy" Biology 12, no. 2: 313. https://doi.org/10.3390/biology12020313
APA StyleScoparo, M., Cardinali, I., La Porta, G., Caldaroni, B., Magara, G., Dörr, A. J. M., Elia, A. C., & Lancioni, H. (2023). Phylogenetic Diversity of the Red Swamp Crayfish Procambarus clarkii and Its Dispersal Pattern in Northern and Central Italy. Biology, 12(2), 313. https://doi.org/10.3390/biology12020313