Infections of Tumor Prostheses: An Updated Review on Risk Factors, Microbiology, Diagnosis, and Treatment Strategies
Simple Summary
Abstract
1. Introduction
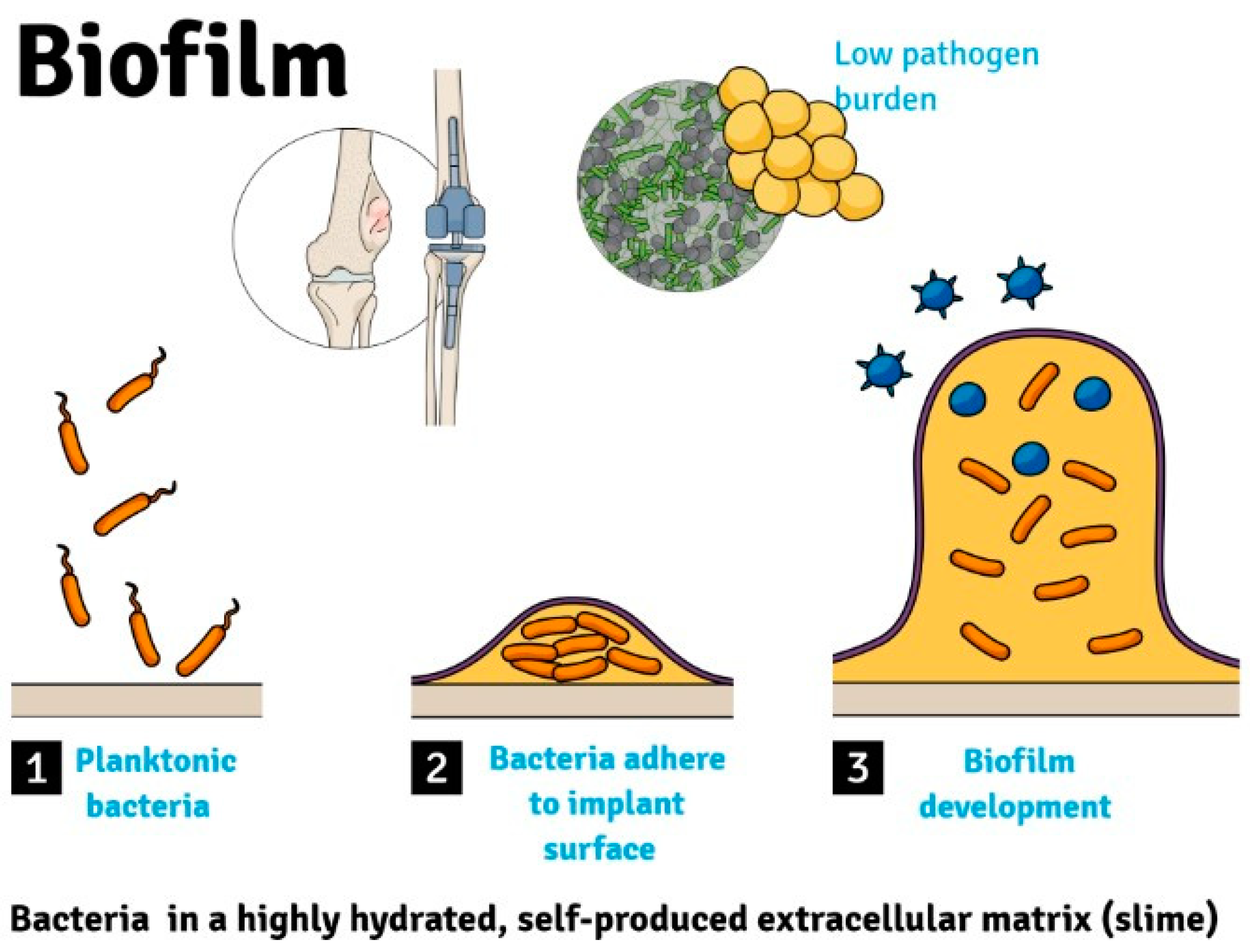
2. Pathophysiology
3. Epidemiology
4. Classification
5. Diagnosis
6. Risk Factors
7. Duration of Surgery
8. Antibiotic Prophylaxis
9. Chemotherapy and Radiation Therapy
10. Neutrophil Count
11. Surgical Drains
12. Cemented vs. Cementless Fixation
13. Surgical Treatment
13.1. Irrigation and Debridement
13.2. One-Stage Revision Surgery
13.3. Two-Stage Revision Surgery
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turcotte, R. Endoprosthetic replacements for bone tumors: Review of the most recent literature. Curr. Opin. Orthop. 2007, 18, 572–578. [Google Scholar] [CrossRef]
- DiCaprio, M.R.; Friedlaender, G.E. Malignant bone tumors: Limb sparing versus amputation. J. Am. Acad. Orthop. Surg. 2003, 11, 25–37. [Google Scholar] [CrossRef]
- Jeys, L.M.; Kulkarni, A.; Grimer, R.J.; Carter, S.R.; Tillman, R.M.; Abudu, A. Endoprosthetic reconstruction for the treatment of musculoskeletal tumors of the appendicular skeleton and pelvis. J. Bone Jt. Surg. Am. 2008, 90, 1265–1271. [Google Scholar] [CrossRef]
- Ahlmann, E.R.; Menendez, L.R.; Kermani, C.; Gotha, H. Survivorship and clinical outcome of modular endoprosthetic reconstruction for neoplastic disease of the lower limb. J. Bone Jt. Surg. Br. 2006, 88, 790–795. [Google Scholar] [CrossRef]
- Gitelis, S.; Yergler, J.D.; Sawlani, N.; Schiff, A.; Shott, S. Short and long term failure of the modular oncology knee prosthesis. Orthopedics 2008, 31, 362. [Google Scholar] [CrossRef]
- Shehadeh, A.; Noveau, J.; Malawer, M.; Henshaw, R. Late complications and survival of endoprosthetic reconstruction after resection of bone tumors. Clin. Orthop. Relat. Res. 2010, 468, 2885–2895. [Google Scholar] [CrossRef]
- Jeys, L.M.; Grimer, R.J.; Carter, S.R.; Tillman, R.M. Periprosthetic infection in patients treated for an orthopaedic oncological condition. J. Bone Jt. Surg. Am. 2005, 87, 842–849. [Google Scholar] [CrossRef]
- Grimer, R.J.; Belthur, M.; Chandrasekar, C.; Carter, S.R.; Tillman, R.M. Two-stage revision for infected endoprostheses used in tumor surgery. Clin. Orthop. Relat. Res. 2002, 395, 193–203. [Google Scholar] [CrossRef]
- Jeys, L.M.; Grimer, R.J.; Carter, S.R.; Tillman, R.M.; Abudu, A. Post operative infection and increased survival in osteosarcoma patients: Are they associated? Ann. Surg. Oncol. 2007, 14, 2887–2895. [Google Scholar] [CrossRef]
- Hardes, J.; von Eiff, C.; Streitbuerger, A.; Balke, M.; Budny, T.; Henrichs, M.P.; Hauschild, G.; Ahrens, H. Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. J. Surg. Oncol. 2010, 101, 389–395. [Google Scholar] [CrossRef]
- Moran, E.; Byren, I.; Atkins, B.L. The diagnosis and management of prosthetic joint infections. J. Antimicrob. Chemother. 2010, 65 (Suppl. S3), iii45–iii54. [Google Scholar] [CrossRef]
- Berbari, E.F.; Hanssen, A.D.; Duffy, M.C.; Steckelberg, J.M.; Ilstrup, D.M.; Harmsen, W.S.; Osmon, D.R. Risk factors for prosthetic joint infection: Case-control study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1998, 27, 1247–1254. [Google Scholar] [CrossRef]
- Morii, T.; Yabe, H.; Morioka, H.; Beppu, Y.; Chuman, H.; Kawai, A.; Takeda, K.; Kikuta, K.; Hosaka, S.; Yazawa, Y.; et al. Postoperative deep infection in tumor endoprosthesis reconstruction around the knee. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2010, 15, 331–339. [Google Scholar] [CrossRef]
- Nobile, M.; Navone, P.; Domeniconi, G.; Della Valle, A.; Daolio, P.A.; Buccino, N.A.; Auxilia, F. Surgical site infections in oncologic orthopaedic prosthetics surgery. Ann. Ig. Med. Prev. E Comunita 2015, 27, 711–717. [Google Scholar] [CrossRef]
- Haijie, L.; Dasen, L.; Tao, J.; Yi, Y.; Xiaodong, T.; Wei, G. Implant Survival and Complication Profiles of Endoprostheses for Treating Tumor Around the Knee in Adults: A Systematic Review of the Literature Over the Past 30 Years. J. Arthroplast. 2018, 33, 1275–1287.e3. [Google Scholar] [CrossRef]
- Kapoor, S.K.; Thiyam, R. Management of infection following reconstruction in bone tumors. J. Clin. Orthop. Trauma 2015, 6, 244. [Google Scholar] [CrossRef]
- Capanna, R.; Morris, H.G.; Campanacci, D.; Del Ben, M.; Campanacci, M. Modular uncemented prosthetic reconstruction after resection of tumours of the distal femur. J. Bone Jt. Surg. Br. 1994, 76, 178–186. [Google Scholar] [CrossRef]
- Racano, A.; Pazionis, T.; Farrokhyar, F.; Deheshi, B.; Ghert, M. High infection rate outcomes in long-bone tumor surgery with endoprosthetic reconstruction in adults: A systematic review. Clin. Orthop. Relat. Res. 2013, 471, 2017–2027. [Google Scholar] [CrossRef]
- Henderson, E.R.; Groundland, J.S.; Pala, E.; Dennis, J.A.; Wooten, R.; Cheong, D.; Windhager, R.; Kotz, R.I.; Mercuri, M.; Funovics, P.T.; et al. Failure mode classification for tumor endoprostheses: Retrospective review of five institutions and a literature review. J. Bone Jt. Surg. Am. 2011, 93, 418–429. [Google Scholar] [CrossRef]
- Neel, M.D.; Wilkins, R.M.; Rao, B.N.; Kelly, C.M. Early multicenter experience with a noninvasive expandable prosthesis. Clin. Orthop. Relat. Res. 2003, 415, 72–81. [Google Scholar] [CrossRef]
- Palestro, C.J.; Torres, M.A. Radionuclide imaging in orthopedic infections. Semin. Nucl. Med. 1997, 27, 334–345. [Google Scholar] [CrossRef]
- Mulamba, L.; Ferrant, A.; Leners, N.; de Nayer, P.; Rombouts, J.J.; Vincent, A. Indium-111 leucocyte scanning in the evaluation of painful hip arthroplasty. Acta Orthop. Scand. 1983, 54, 695–697. [Google Scholar] [CrossRef]
- Love, C.; Marwin, S.E.; Tomas, M.B.; Krauss, E.S.; Tronco, G.G.; Bhargava, K.K.; Nichols, K.J.; Palestro, C.J. Diagnosing infection in the failed joint replacement: A comparison of coincidence detection 18F-FDG and 111In-labeled leukocyte/99mTc-sulfur colloid marrow imaging. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2004, 45, 1864–1871. [Google Scholar]
- Palestro, C.J.; Love, C.; Tronco, G.G.; Tomas, M.B.; Rini, J.N. Combined labeled leukocyte and technetium 99m sulfur colloid bone marrow imaging for diagnosing musculoskeletal infection. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2006, 26, 859–870. [Google Scholar] [CrossRef]
- Love, C.; Tomas, M.B.; Marwin, S.E.; Pugliese, P.V.; Palestro, C.J. Role of nuclear medicine in diagnosis of the infected joint replacement. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2001, 21, 1229–1238. [Google Scholar] [CrossRef]
- Achermann, Y.; Vogt, M.; Leunig, M.; Wüst, J.; Trampuz, A. Improved diagnosis of periprosthetic joint infection by multiplex PCR of sonication fluid from removed implants. J. Clin. Microbiol. 2010, 48, 1208–1214. [Google Scholar] [CrossRef]
- Bergin, P.F.; Doppelt, J.D.; Hamilton, W.G.; Mirick, G.E.; Jones, A.E.; Sritulanondha, S.; Helm, J.M.; Tuan, R.S. Detection of periprosthetic infections with use of ribosomal RNA-based polymerase chain reaction. J. Bone Jt. Surg. Am. 2010, 92, 654–663. [Google Scholar] [CrossRef]
- Marín, M.; Garcia-Lechuz, J.M.; Alonso, P.; Villanueva, M.; Alcalá, L.; Gimeno, M.; Cercenado, E.; Sánchez-Somolinos, M.; Radice, C.; Bouza, E. Role of universal 16S rRNA gene PCR and sequencing in diagnosis of prosthetic joint infection. J. Clin. Microbiol. 2012, 50, 583–589. [Google Scholar] [CrossRef]
- Morgenstern, C.; Cabric, S.; Perka, C.; Trampuz, A.; Renz, N. Synovial fluid multiplex PCR is superior to culture for detection of low-virulent pathogens causing periprosthetic joint infection. Diagn. Microbiol. Infect. Dis. 2018, 90, 115–119. [Google Scholar] [CrossRef]
- Higgins, E.; Suh, G.A.; Tande, A.J. Enhancing Diagnostics in Orthopedic Infections. J. Clin. Microbiol. 2022, 60, e0219621. [Google Scholar] [CrossRef]
- Esteban, J.; Gómez-Barrena, E. An update about molecular biology techniques to detect orthopaedic implant-related infections. EFORT Open Rev. 2021, 6, 93–100. [Google Scholar] [CrossRef]
- Bourbour, S.; Emaneini, M.; Jabalameli, M.; Mortazavi, S.M.J.; Tahmasebi, M.N.; Taghizadeh, A.; Sharafatvaziri, A.; Beigverdi, R.; Jabalameli, F. Efficacy of 16S rRNA variable regions high-resolution melt analysis for bacterial pathogens identification in periprosthetic joint infections. BMC Microbiol. 2021, 21, 112. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.S.; Parvizi, J. Diagnosis and Treatment of Culture-Negative Periprosthetic Joint Infection. J. Arthroplast. 2022, 37, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Šuster, K.; Podgornik, A.; Cör, A. An alternative molecular approach for rapid and specific detection of clinically relevant bacteria causing prosthetic joint infections with bacteriophage K. New Microbiol. 2020, 43, 107–114. [Google Scholar] [PubMed]
- Wang, C.; Huang, Z.; Li, W.; Fang, X.; Zhang, W. Can metagenomic next-generation sequencing identify the pathogens responsible for culture-negative prosthetic joint infection? BMC Infect. Dis. 2020, 20, 253. [Google Scholar] [CrossRef]
- Torchia, M.T.; Austin, D.C.; Kunkel, S.T.; Dwyer, K.W.; Moschetti, W.E. Next-Generation Sequencing vs Culture-Based Methods for Diagnosing Periprosthetic Joint Infection After Total Knee Arthroplasty: A Cost-Effectiveness Analysis. J. Arthroplast. 2019, 34, 1333–1341. [Google Scholar] [CrossRef]
- Huang, Z.; Li, W.; Lee, G.-C.; Fang, X.; Xing, L.; Yang, B.; Lin, J.; Zhang, W. Metagenomic next-generation sequencing of synovial fluid demonstrates high accuracy in prosthetic joint infection diagnostics: MNGS for diagnosing PJI. Bone Jt. Res. 2020, 9, 440–449. [Google Scholar] [CrossRef]
- Wang, C.X.; Huang, Z.; Fang, X.; Li, W.; Yang, B.; Zhang, W. Comparison of broad-range polymerase chain reaction and metagenomic next-generation sequencing for the diagnosis of prosthetic joint infection. Int. J. Infect. Dis. 2020, 95, 8–12. [Google Scholar] [CrossRef]
- Rothenberg, A.C.; Wilson, A.E.; Hayes, J.P.; O’Malley, M.J.; Klatt, B.A. Sonication of Arthroplasty Implants Improves Accuracy of Periprosthetic Joint Infection Cultures. Clin. Orthop. Relat. Res. 2017, 475, 1827–1836. [Google Scholar] [CrossRef]
- Trampuz, A.; Piper, K.E.; Jacobson, M.J.; Hanssen, A.D.; Unni, K.K.; Osmon, D.R.; Mandrekar, J.N.; Cockerill, F.R.; Steckelberg, J.M.; Greenleaf, J.F.; et al. Sonication of removed hip and knee prostheses for diagnosis of infection. N. Engl. J. Med. 2007, 357, 654–663. [Google Scholar] [CrossRef]
- Portillo, M.E.; Salvadó, M.; Alier, A.; Martínez, S.; Sorli, L.; Horcajada, J.P.; Puig, L. Advantages of sonication fluid culture for the diagnosis of prosthetic joint infection. J. Infect. 2014, 69, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Puig-Verdié, L.; Alentorn-Geli, E.; González-Cuevas, A.; Sorlí, L.; Salvadó, M.; Alier, A.; Pelfort, X.; Portillo, M.E.; Horcajada, J.P. Implant sonication increases the diagnostic accuracy of infection in patients with delayed, but not early, orthopaedic implant failure. Bone Jt. J. 2013, 95-B, 244–249. [Google Scholar] [CrossRef]
- Sambri, A.; Maso, A.; Storni, E.; Megaloikonomos, P.D.; Igoumenou, V.G.; Errani, C.; Mavrogenis, A.F.; Bianchi, G. Sonication Improves the Diagnosis of Megaprosthetic Infections. Orthopedics 2019, 42, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Pugh, L.R.; Clarkson, P.W.; Phillips, A.E.; Biau, D.J.; Masri, B.A. Tumor endoprosthesis revision rates increase with peri-operative chemotherapy but are reduced with the use of cemented implant fixation. J. Arthroplast. 2014, 29, 1418–1422. [Google Scholar] [CrossRef]
- Brigman, B.E.; Hornicek, F.J.; Gebhardt, M.C.; Mankin, H.J. Allografts about the Knee in Young Patients with High-Grade Sarcoma. Clin. Orthop. Relat. Res. 2004, 421, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Hardes, J.; Gebert, C.; Schwappach, A.; Ahrens, H.; Streitburger, A.; Winkelmann, W.; Gosheger, G. Characteristics and outcome of infections associated with tumor endoprostheses. Arch. Orthop. Trauma Surg. 2006, 126, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Funovics, P.T.; Hipfl, C.; Hofstaetter, J.G.; Puchner, S.; Kotz, R.I.; Dominkus, M. Management of septic complications following modular endoprosthetic reconstruction of the proximal femur. Int. Orthop. 2011, 35, 1437–1444. [Google Scholar] [CrossRef]
- What Are the Significant Risk Factors for Surgical Site Infection/Periprosthetic Joint Infection (SSI/PJI) of an Oncologic Endoprosthesis Following Resection of a Malignant Bone Tumor? Available online: https://icmphilly.com/questions/what-are-the-significant-risk-factors-for-surgical-site-infection-periprosthetic-joint-infection-ssi-pji-of-an-oncologic-endoprosthesis-following-resection-of-a-malignant-bone-tumor/ (accessed on 20 December 2022).
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-joint infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef]
- Gaur, A.H.; Liu, T.; Knapp, K.M.; Daw, N.C.; Rao, B.N.; Neel, M.D.; Rodriguez-Galindo, C.; Brand, D.; Adderson, E.E. Infections in children and young adults with bone malignancies undergoing limb-sparing surgery. Cancer 2005, 104, 602–610. [Google Scholar] [CrossRef]
- Meijer, S.T.; Paulino Pereira, N.R.; Nota, S.P.F.T.; Ferrone, M.L.; Schwab, J.H.; Lozano Calderón, S.A. Factors associated with infection after reconstructive shoulder surgery for proximal humerus tumors. J. Shoulder Elbow Surg. 2017, 26, 931–938. [Google Scholar] [CrossRef]
- Jeys, L.M.; Luscombe, J.S.; Grimer, R.J.; Abudu, A.; Tillman, R.M.; Carter, S.R. The risks and benefits of radiotherapy with massive endoprosthetic replacement. J. Bone Jt. Surg. Br. 2007, 89, 1352–1355. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.J.; Capanna, R.; Gherlinzoni, F.; Bacci, G.; Ferruzzi, A.; Casadei, R.; Ferraro, A.; Cazzola, A.; Campanacci, M. Influence of chemotherapy on perioperative complications in limb salvage surgery for bone tumors. Cancer 1990, 65, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Dhanoa, A.; Ajit Singh, V.; Elbahri, H. Deep Infections after Endoprosthetic Replacement Operations in Orthopedic Oncology Patients. Surg. Infect. 2015, 16, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Mayilvahanan, N.; Paraskumar, M.; Sivaseelam, A.; Natarajan, S. Custom mega-prosthetic replacement for proximal humeral tumours. Int. Orthop. 2006, 30, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Bozic, K.J.; Ward, D.T.; Lau, E.C.; Chan, V.; Wetters, N.G.; Naziri, Q.; Odum, S.; Fehring, T.K.; Mont, M.A.; Gioe, T.J.; et al. Risk factors for periprosthetic joint infection following primary total hip arthroplasty: A case control study. J. Arthroplast. 2014, 29, 154–156. [Google Scholar] [CrossRef]
- Pugely, A.J.; Martin, C.T.; Gao, Y.; Schweizer, M.L.; Callaghan, J.J. The Incidence of and Risk Factors for 30-Day Surgical Site Infections Following Primary and Revision Total Joint Arthroplasty. J. Arthroplast. 2015, 30, 47–50. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, F.; Chen, W.; Liu, S.; Zhang, Q.; Zhang, Y. Risk factors for periprosthetic joint infection after total joint arthroplasty: A systematic review and meta-analysis. J. Hosp. Infect. 2015, 89, 82–89. [Google Scholar] [CrossRef]
- Namba, R.S.; Inacio, M.C.S.; Paxton, E.W. Risk factors associated with deep surgical site infections after primary total knee arthroplasty: An analysis of 56,216 knees. J. Bone Jt. Surg. Am. 2013, 95, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Chen, B.P.-H.; Soleas, I.M.; Ferko, N.C.; Cameron, C.G.; Hinoul, P. Prolonged Operative Duration Increases Risk of Surgical Site Infections: A Systematic Review. Surg. Infect. 2017, 18, 722–735. [Google Scholar] [CrossRef]
- Peersman, G.; Laskin, R.; Davis, J.; Peterson, M.G.E.; Richart, T. Prolonged operative time correlates with increased infection rate after total knee arthroplasty. HSS J. Musculoskelet. J. Hosp. Spec. Surg. 2006, 2, 70–72. [Google Scholar] [CrossRef]
- Pulido, L.; Ghanem, E.; Joshi, A.; Purtill, J.J.; Parvizi, J. Periprosthetic joint infection: The incidence, timing, and predisposing factors. Clin. Orthop. Relat. Res. 2008, 466, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- De Boer, A.S.; Geubbels, E.L.; Wille, J.; Mintjes-de Groot, A.J. Risk assessment for surgical site infections following total hip and total knee prostheses. J. Chemother. Florence Italy 2001, 13 (Suppl. S4), 42–47. [Google Scholar] [CrossRef] [PubMed]
- Wymenga, A.B.; van Horn, J.R.; Theeuwes, A.; Muytjens, H.L.; Slooff, T.J. Perioperative factors associated with septic arthritis after arthroplasty. Prospective multicenter study of 362 knee and 2651 hip operations. Acta Orthop. Scand. 1992, 63, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Previous Fracture Surgery Is a Major Risk Factor of Infection after Total Knee Arthroplasty—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/21541707/ (accessed on 31 October 2022).
- Crowe, B.; Payne, A.; Evangelista, P.J.; Stachel, A.; Phillips, M.S.; Slover, J.D.; Inneh, I.A.; Iorio, R.; Bosco, J.A. Risk Factors for Infection Following Total Knee Arthroplasty: A Series of 3836 Cases from One Institution. J. Arthroplast. 2015, 30, 2275–2278. [Google Scholar] [CrossRef]
- Naranje, S.; Lendway, L.; Mehle, S.; Gioe, T.J. Does Operative Time Affect Infection Rate in Primary Total Knee Arthroplasty? Clin. Orthop. Relat. Res. 2015, 473, 64–69. [Google Scholar] [CrossRef]
- AlBuhairan, B.; Hind, D.; Hutchinson, A. Antibiotic prophylaxis for wound infections in total joint arthroplasty: A systematic review. J. Bone Jt. Surg. Br. 2008, 90, 915–919. [Google Scholar] [CrossRef]
- Yates, A.J. American Association of Hip and Knee Surgeons Evidence-Based Medicine Committee Postoperative prophylactic antibiotics in total joint arthroplasty. Arthroplast. Today 2018, 4, 130–131. [Google Scholar] [CrossRef]
- What Is the Most Appropriate Perioperative Prophylactic Antibiotic (Agent, Route and Number of Doses) for Patients Undergoing Primary Total Joint Arthroplasty (TJA) to Reduce the Risk of Subsequent Surgical Site Infections/Periprosthetic Joint Infections (SSIs/PJIs)? [Internet]. ICM Philly. 2019. Available online: https://icmphilly.com/questions/what-is-the-most-appropriate-perioperative-prophylactic-antibiotic-agent-route-and-number-of-doses-for-patients-undergoing-primary-total-joint-arthroplasty-tja-to-reduce-the-risk-of-subsequent/ (accessed on 20 December 2022).
- Berríos-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017, 152, 784. [Google Scholar] [CrossRef]
- Prokuski, L. Prophylactic antibiotics in orthopaedic surgery. J. Am. Acad. Orthop. Surg. 2008, 16, 283–293. [Google Scholar] [CrossRef]
- Ghert, M.; Deheshi, B.; Holt, G.; Randall, R.L.; Ferguson, P.; Wunder, J.; Turcotte, R.; Werier, J.; Clarkson, P.; Damron, T.; et al. Prophylactic antibiotic regimens in tumour surgery (PARITY): Protocol for a multicentre randomised controlled study. BMJ Open 2012, 2, e002197. [Google Scholar] [CrossRef]
- Gahm, J.; Ljung Konstantinidou, A.; Lagergren, J.; Sandelin, K.; Glimåker, M.; Johansson, H.; Wickman, M.; de Boniface, J.; Frisell, J. Effectiveness of Single vs Multiple Doses of Prophylactic Intravenous Antibiotics in Implant-Based Breast Reconstruction: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2231583. [Google Scholar] [CrossRef] [PubMed]
- Gafter-Gvili, A.; Fraser, A.; Paul, M.; Vidal, L.; Lawrie, T.A.; van de Wetering, M.D.; Kremer, L.C.; Leibovici, L. Antibiotic prophylaxis for bacterial infections in afebrile neutropenic patients following chemotherapy. Cochrane Database Syst. Rev. 2012, 2012, CD004386. [Google Scholar] [CrossRef] [PubMed]
- Gafter-Gvili, A.; Paul, M.; Fraser, A.; Leibovici, L. Effect of quinolone prophylaxis in afebrile neutropenic patients on microbial resistance: Systematic review and meta-analysis. J. Antimicrob. Chemother. 2007, 59, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Cruciani, M.; Rampazzo, R.; Malena, M.; Lazzarini, L.; Todeschini, G.; Messori, A.; Concia, E. Prophylaxis with fluoroquinolones for bacterial infections in neutropenic patients: A meta-analysis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1996, 23, 795–805. [Google Scholar] [CrossRef]
- Morii, T.; Morioka, H.; Ueda, T.; Araki, N.; Hashimoto, N.; Kawai, A.; Mochizuki, K.; Ichimura, S. Deep infection in tumor endoprosthesis around the knee: A multi-institutional study by the Japanese musculoskeletal oncology group. BMC Musculoskelet. Disord. 2013, 14, 51. [Google Scholar] [CrossRef]
- Sevelda, F.; Schuh, R.; Hofstaetter, J.G.; Schinhan, M.; Windhager, R.; Funovics, P.T. Total Femur Replacement After Tumor Resection: Limb Salvage Usually Achieved But Complications and Failures are Common. Clin. Orthop. Relat. Res. 2015, 473, 2079–2087. [Google Scholar] [CrossRef]
- Ji, T.; Guo, W.; Yang, R.L.; Tang, X.D.; Wang, Y.F. Modular hemipelvic endoprosthesis reconstruction--experience in 100 patients with mid-term follow-up results. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2013, 39, 53–60. [Google Scholar] [CrossRef]
- Peel, T.; May, D.; Buising, K.; Thursky, K.; Slavin, M.; Choong, P. Infective complications following tumour endoprosthesis surgery for bone and soft tissue tumours. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2014, 40, 1087–1094. [Google Scholar] [CrossRef]
- Morris, C.D.; Sepkowitz, K.; Fonshell, C.; Margetson, N.; Eagan, J.; Miransky, J.; Boland, P.J.; Healey, J. Prospective identification of risk factors for wound infection after lower extremity oncologic surgery. Ann. Surg. Oncol. 2003, 10, 778–782. [Google Scholar] [CrossRef]
- Biau, D.; Faure, F.; Katsahian, S.; Jeanrot, C.; Tomeno, B.; Anract, P. Survival of total knee replacement with a megaprosthesis after bone tumor resection. J. Bone Jt. Surg. Am. 2006, 88, 1285–1293. [Google Scholar] [CrossRef]
- Miwa, S.; Shirai, T.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Tada, K.; Kajino, Y.; Inatani, H.; Higuchi, T.; Abe, K.; et al. Risk factors for postoperative deep infection in bone tumors. PLoS ONE 2017, 12, e0187438. [Google Scholar] [CrossRef]
- Demura, S.; Kawahara, N.; Murakami, H.; Nambu, K.; Kato, S.; Yoshioka, K.; Okayama, T.; Tomita, K. Surgical site infection in spinal metastasis: Risk factors and countermeasures. Spine 2009, 34, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Lentino, J.R. Prosthetic joint infections: Bane of orthopedists, challenge for infectious disease specialists. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2003, 36, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.M.; O’Sullivan, B.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Hammond, A.; Benk, V.; Kandel, R.; et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2005, 75, 48–53. [Google Scholar] [CrossRef]
- Cannon, C.P.; Ballo, M.T.; Zagars, G.K.; Mirza, A.N.; Lin, P.P.; Lewis, V.O.; Yasko, A.W.; Benjamin, R.S.; Pisters, P.W.T. Complications of combined modality treatment of primary lower extremity soft-tissue sarcomas. Cancer 2006, 107, 2455–2461. [Google Scholar] [CrossRef] [PubMed]
- Itshayek, E.; Yamada, J.; Bilsky, M.; Schmidt, M.; Shaffrey, C.; Gerszten, P.; Polly, D.; Gokaslan, Z.; Varga, P.P.; Fisher, C.G. Timing of surgery and radiotherapy in the management of metastatic spine disease: A systematic review. Int. J. Oncol. 2010, 36, 533–544. [Google Scholar]
- Montassier, E.; Gastinne, T.; Vangay, P.; Al-Ghalith, G.A.; Bruley des Varannes, S.; Massart, S.; Moreau, P.; Potel, G.; de La Cochetière, M.F.; Batard, E.; et al. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment. Pharmacol. Ther. 2015, 42, 515–528. [Google Scholar] [CrossRef]
- Hardes, J.; Henrichs, M.-P.; Gosheger, G.; Guder, W.; Nottrott, M.; Andreou, D.; Bormann, E.; Eveslage, M.; Hauschild, G.; Streitbürger, A. Tumour endoprosthesis replacement in the proximal tibia after intra-articular knee resection in patients with sarcoma and recurrent giant cell tumour. Int. Orthop. 2018, 42, 2475–2481. [Google Scholar] [CrossRef]
- Bai, J.; Behera, M.; Bruner, D.W. The gut microbiome, symptoms, and targeted interventions in children with cancer: A systematic review. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2018, 26, 427–439. [Google Scholar] [CrossRef]
- Nycz, B.T.; Dominguez, S.R.; Friedman, D.; Hilden, J.M.; Ir, D.; Robertson, C.E.; Frank, D.N. Evaluation of bloodstream infections, Clostridium difficile infections, and gut microbiota in pediatric oncology patients. PLoS ONE 2018, 13, e0191232. [Google Scholar] [CrossRef]
- Galloway-Peña, J.R.; Smith, D.P.; Sahasrabhojane, P.; Ajami, N.J.; Wadsworth, W.D.; Daver, N.G.; Chemaly, R.F.; Marsh, L.; Ghantoji, S.S.; Pemmaraju, N.; et al. The role of the gastrointestinal microbiome in infectious complications during induction chemotherapy for acute myeloid leukemia. Cancer 2016, 122, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- Hakim, H.; Dallas, R.; Wolf, J.; Tang, L.; Schultz-Cherry, S.; Darling, V.; Johnson, C.; Karlsson, E.A.; Chang, T.-C.; Jeha, S.; et al. Gut Microbiome Composition Predicts Infection Risk During Chemotherapy in Children With Acute Lymphoblastic Leukemia. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2018, 67, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Montassier, E.; Al-Ghalith, G.A.; Ward, T.; Corvec, S.; Gastinne, T.; Potel, G.; Moreau, P.; De La Cochetiere, M.F.; Batard, E.; Knights, D. Erratum to: Pretreatment gut microbiome predicts chemotherapy-related bloodstream infection. Genome Med. 2016, 8, 61. [Google Scholar] [CrossRef]
- Teoh, F.; Pavelka, N. How Chemotherapy Increases the Risk of Systemic Candidiasis in Cancer Patients: Current Paradigm and Future Directions. Pathogens 2016, 5, 6. [Google Scholar] [CrossRef]
- Newburger, P.E.; Dale, D.C. Evaluation and management of patients with isolated neutropenia. Semin. Hematol. 2013, 50, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Denham, J.W.; Hauer-Jensen, M. The radiotherapeutic injury—A complex “wound”. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2002, 63, 129–145. [Google Scholar] [CrossRef]
- Griffin, A.M.; Dickie, C.I.; Catton, C.N.; Chung, P.W.M.; Ferguson, P.C.; Wunder, J.S.; O’Sullivan, B. The influence of time interval between preoperative radiation and surgical resection on the development of wound healing complications in extremity soft tissue sarcoma. Ann. Surg. Oncol. 2015, 22, 2824–2830. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Hozumi, T.; Yamakawa, K.; Goto, T.; Kondo, T. Risk factors for surgical site infection after posterior fixation surgery and intraoperative radiotherapy for spinal metastases. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2016, 25, 1034–1038. [Google Scholar] [CrossRef]
- Keam, J.; Bilsky, M.H.; Laufer, I.; Shi, W.; Zhang, Z.; Tam, M.; Zatcky, J.; Lovelock, D.M.; Yamada, Y. No association between excessive wound complications and preoperative high-dose, hypofractionated, image-guided radiation therapy for spine metastasis. J. Neurosurg. Spine 2014, 20, 411–420. [Google Scholar] [CrossRef]
- Bodey, G.P.; Buckley, M.; Sathe, Y.S.; Freireich, E.J. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann. Intern. Med. 1966, 64, 328–340. [Google Scholar] [CrossRef]
- Lima, S.S.S.; França, M.S.; Godoi, C.C.G.; Martinho, G.H.; de Jesus, L.A.; Romanelli, R.M.; Clemente, W.T. Neutropenic patients and their infectious complications at a University Hospital. Rev. Bras. Hematol. E Hemoter. 2013, 35, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Natour, R.H.A.; Ashley, S.W.; Tavakkolizadeh, A. 797 Outcomes of Abdominal Surgery in Neutropenic Patients. Gastroenterology 2010, 5 (Suppl. S1), S-860. [Google Scholar] [CrossRef]
- Gulack, B.C.; Englum, B.R.; Lo, D.D.; Nussbaum, D.P.; Keenan, J.E.; Scarborough, J.E.; Shapiro, M.L. Leukopenia is associated with worse but not prohibitive outcomes following emergent abdominal surgery. J. Trauma Acute Care Surg. 2015, 79, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Dogar, S.A.; Khan, M.A.M. Implantable port devices in paediatric oncology patients: A clinical experience from a tertiary care hospital. JPMA J. Pak. Med. Assoc. 2013, 63, 1248–1251. [Google Scholar] [PubMed]
- Lerman, D.M.; Blank, A.T.; Billig, J.I.; Karia, R.; Rapp, T.B. Identification of Risk Factors for Acute Surgical Site Infections in Musculoskeletal Tumor Patients Using CDC/NHSN Criteria. Bull. Hosp. Jt. Dis. 2013 2015, 73, 233–238. [Google Scholar] [PubMed]
- Rossi, B.; Zoccali, C. Surgical Site Infections in Treatment of Musculoskeletal Tumors: Experience from a Single Oncologic Orthopedic Institution. J. Orthop. Oncol. 2016, 2, 2. [Google Scholar] [CrossRef]
- Morii, T.; Mochizuki, K.; Tajima, T.; Ichimura, S.; Satomi, K. Surgical site infection in malignant soft tissue tumors. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2012, 17, 51–57. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Ong, K.L.; Lau, E.; Bozic, K.J.; Berry, D.; Parvizi, J. Prosthetic joint infection risk after TKA in the Medicare population. Clin. Orthop. Relat. Res. 2010, 468, 52–56. [Google Scholar] [CrossRef]
- Patel, V.P.; Walsh, M.; Sehgal, B.; Preston, C.; DeWal, H.; Di Cesare, P.E. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J. Bone Jt. Surg. Am. 2007, 89, 33–38. [Google Scholar] [CrossRef]
- Parker, M.J.; Roberts, C.P.; Hay, D. Closed suction drainage for hip and knee arthroplasty. A meta-analysis. J. Bone Jt. Surg. Am. 2004, 86, 1146–1152. [Google Scholar] [CrossRef]
- Reiffel, A.J.; Barie, P.S.; Spector, J.A. A multi-disciplinary review of the potential association between closed-suction drains and surgical site infection. Surg. Infect. 2013, 14, 244–269. [Google Scholar] [CrossRef] [PubMed]
- Sankar, B.; Ray, P.; Rai, J. Suction drain tip culture in orthopaedic surgery: A prospective study of 214 clean operations. Int. Orthop. 2004, 28, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Durai, R.; Mownah, A.; Ng, P.C.H. Use of drains in surgery: A review. J. Perioper. Pract. 2009, 19, 180–186. [Google Scholar] [CrossRef]
- Parker, M.J.; Livingstone, V.; Clifton, R.; McKee, A. Closed suction surgical wound drainage after orthopaedic surgery. Cochrane Database Syst. Rev. 2007, CD001825. [Google Scholar] [CrossRef] [PubMed]
- Strahovnik, A.; Fokter, S.K.; Kotnik, M. Comparison of drainage techniques on prolonged serous drainage after total hip arthroplasty. J. Arthroplast. 2010, 25, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Bratzler, D.W.; Houck, P.M.; Richards, C.; Steele, L.; Dellinger, E.P.; Fry, D.E.; Wright, C.; Ma, A.; Carr, K.; Red, L. Use of antimicrobial prophylaxis for major surgery: Baseline results from the National Surgical Infection Prevention Project. Arch. Surg. Chic. Ill 1960 2005, 140, 174–182. [Google Scholar] [CrossRef]
- Willett, K.M.; Simmons, C.D.; Bentley, G. The effect of suction drains after total hip replacement. J. Bone Jt. Surg. Br. 1988, 70, 607–610. [Google Scholar] [CrossRef]
- Jeys, L.M.; Grimer, R.J.; Carter, S.R.; Tillman, R.M. Risk of amputation following limb salvage surgery with endoprosthetic replacement, in a consecutive series of 1261 patients. Int. Orthop. 2003, 27, 160–163. [Google Scholar] [CrossRef]
- Zeegen, E.N.; Aponte-Tinao, L.A.; Hornicek, F.J.; Gebhardt, M.C.; Mankin, H.J. Survivorship analysis of 141 modular metallic endoprostheses at early followup. Clin. Orthop. Relat. Res. 2004, 420, 239–250. [Google Scholar] [CrossRef]
- Bickels, J.; Wittig, J.C.; Kollender, Y.; Henshaw, R.M.; Kellar-Graney, K.L.; Meller, I.; Malawer, M.M. Distal femur resection with endoprosthetic reconstruction: A long-term followup study. Clin. Orthop. Relat. Res. 2002, 400, 225–235. [Google Scholar] [CrossRef]
- Sharma, S.; Turcotte, R.E.; Isler, M.H.; Wong, C. Experience with cemented large segment endoprostheses for tumors. Clin. Orthop. Relat. Res. 2007, 459, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Mittermayer, F.; Krepler, P.; Dominkus, M.; Schwameis, E.; Sluga, M.; Heinzl, H.; Kotz, R. Long-term followup of uncemented tumor endoprostheses for the lower extremity. Clin. Orthop. Relat. Res. 2001, 388, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Unwin, P.S.; Cannon, S.R.; Grimer, R.J.; Kemp, H.B.; Sneath, R.S.; Walker, P.S. Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb. J. Bone Jt. Surg. Br. 1996, 78, 5–13. [Google Scholar] [CrossRef]
- Griffin, A.M.; Parsons, J.A.; Davis, A.M.; Bell, R.S.; Wunder, J.S. Uncemented tumor endoprostheses at the knee: Root causes of failure. Clin. Orthop. Relat. Res. 2005, 438, 71–79. [Google Scholar] [CrossRef]
- Morii, T.; Morioka, H.; Ueda, T.; Araki, N.; Hashimoto, N.; Kawai, A.; Takeuchi, K.; Anazawa, U.; Mochizuki, K.; Ichimura, S. Functional analysis of cases of tumor endoprostheses with deep infection around the knee: A multi institutional study by the Japanese Musculoskeletal Oncology Group (JMOG). J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2013, 18, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Hardes, J.; Ahrens, H.; Gosheger, G.; Nottrott, M.; Dieckmann, R.; Henrichs, M.-P.; Streitbürger, A. Management of complications in megaprostheses. Unfallchirurg 2014, 117, 607–613. [Google Scholar] [CrossRef]
- Pala, E.; Mavrogenis, A.F.; Angelini, A.; Henderson, E.R.; Douglas Letson, G.; Ruggieri, P. Cemented versus cementless endoprostheses for lower limb salvage surgery. J. BUON Off. J. Balk. Union Oncol. 2013, 18, 496–503. [Google Scholar]
- Li, X.; Moretti, V.M.; Ashana, A.O.; Lackman, R.D. Perioperative infection rate in patients with osteosarcomas treated with resection and prosthetic reconstruction. Clin. Orthop. Relat. Res. 2011, 469, 2889–2894. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Pala, E.; Angelini, A.; Calabro, T.; Romagnoli, C.; Romantini, M.; Drago, G.; Ruggieri, P. Infected Prostheses after Lower-Extremity Bone Tumor Resection: Clinical Outcomes of 100 Patients. Surg. Infect. 2015, 16, 267–275. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Papagelopoulos, P.J.; Coll-Mesa, L.; Pala, E.; Guerra, G.; Ruggieri, P. Infected tumor prostheses. Orthopedics 2011, 34, 991–998; quiz 999–1000. [Google Scholar] [CrossRef]
- Daniel Allison MD, M.B.A.; Eddie Huang, M.D.; Elke Ahlmann, M.D.; Scott Carney, M.D.; Ling Wang, P.-C.; Lawrence Menendez, M.D. Peri-Prosthetic Infection in the Orthopedic Tumor Patient. Reconstr. Rev. 2014, 4. [Google Scholar] [CrossRef]
- Holzer, G.; Windhager, R.; Kotz, R. One-stage revision surgery for infected megaprostheses. J. Bone Jt. Surg. Br. 1997, 79, 31–35. [Google Scholar] [CrossRef]
- Buchholz, H.W.; Elson, R.A.; Engelbrecht, E.; Lodenkämper, H.; Röttger, J.; Siegel, A. Management of deep infection of total hip replacement. J. Bone Jt. Surg. Br. 1981, 63-B, 342–353. [Google Scholar] [CrossRef]
- Stockley, I.; Mockford, B.J.; Hoad-Reddick, A.; Norman, P. The use of two-stage exchange arthroplasty with depot antibiotics in the absence of long-term antibiotic therapy in infected total hip replacement. J. Bone Jt. Surg. Br. 2008, 90, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Bejon, P.; Berendt, A.; Atkins, B.L.; Green, N.; Parry, H.; Masters, S.; McLardy-Smith, P.; Gundle, R.; Byren, I. Two-stage revision for prosthetic joint infection: Predictors of outcome and the role of reimplantation microbiology. J. Antimicrob. Chemother. 2010, 65, 569–575. [Google Scholar] [CrossRef]
- Flint, M.N.; Griffin, A.M.; Bell, R.S.; Wunder, J.S.; Ferguson, P.C. Two-stage revision of infected uncemented lower extremity tumor endoprostheses. J. Arthroplast. 2007, 22, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Mihalko, W.M.; Shields, J.S.; Ries, M.; Saleh, K.J. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J. Bone Jt. Surg. Am. 2007, 89, 871–882. [Google Scholar] [CrossRef]
- Ure, K.J.; Amstutz, H.C.; Nasser, S.; Schmalzried, T.P. Direct-exchange arthroplasty for the treatment of infection after total hip replacement. An average ten-year follow-up. J. Bone Jt. Surg. Am. 1998, 80, 961–968. [Google Scholar] [CrossRef]
- Younger, A.S.; Duncan, C.P.; Masri, B.A. Treatment of infection associated with segmental bone loss in the proximal part of the femur in two stages with use of an antibiotic-loaded interval prosthesis. J. Bone Jt. Surg. Am. 1998, 80, 60–69. [Google Scholar] [CrossRef]
- Donati, F.; Di Giacomo, G.; D’Adamio, S.; Ziranu, A.; Careri, S.; Rosa, M.A.; Maccauro, G. Silver-Coated Hip Megaprosthesis in Oncological Limb Savage Surgery. BioMed Res. Int. 2016, 2016, e9079041. [Google Scholar] [CrossRef]
- Wafa, H.; Grimer, R.J.; Reddy, K.; Jeys, L.; Abudu, A.; Carter, S.R.; Tillman, R.M. Retrospective evaluation of the incidence of early periprosthetic infection with silver-treated endoprostheses in high-risk patients: Case-control study. Bone Jt. J. 2015, 97-B, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Zajonz, D.; Zieme, A.; Prietzel, T.; Moche, M.; Tiepoldt, S.; Roth, A.; Josten, C.; von Salis-Soglio, G.F.; Heyde, C.-E.; Ghanem, M. Periprosthetic joint infections in modular endoprostheses of the lower extremities: A retrospective observational study in 101 patients. Patient Saf. Surg. 2016, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Romanò, C.L.; Tsantes, A.G.; Papadopoulos, D.V.; Tsuchiya, H.; Benzakour, T.; Benevenia, J.; Del Sel, H.; Drago, L.; Mavrogenis, A.F. Infectious disease specialists and teamwork strategies worldwide: The World Association against Infection in Orthopedics and Trauma (WAIOT) and SICOT continue to cooperate in fighting musculoskeletal infections. SICOT J. 2022, 8, E1. [Google Scholar] [CrossRef] [PubMed]
| Classification | Time Period |
|---|---|
| Class I (Early or acute) | Within 4 weeks following surgery |
| Class II | 4 weeks to 2 years following surgery |
| Class III (Late or chronic) | >2 years |
| Modalities | Studies | Findings | Comments |
|---|---|---|---|
| Imaging | X-rays | Bone loss and implant loosening | Low sensitivity and specificity |
| Bone scintigraphy Combined use of labeled leukocyte and Tc-99m sulfur colloid | High activity on labeled leukocyte. Low Activity on Tc 99m-sulfur colloid | Moderate sensitivity and specificity | |
| Ultrasound | Joint effusion or synovial hypertrophy | Operated-dependent, low specificity | |
| MRI | High signal intensity in T2 sequence | Usually not helpful due to artifacts | |
| Laboratory | Blood workup | Elevated ESR, CRP | Commonly elevated during the first 2 weeks |
| Elevated WBC count | Commonly elevated during the first 2 weeks | ||
| Joint aspiration | Cultures | Sensitivity 70–90%, specificity 67–91% | |
| Histological examination | - | ||
| Nucleic amplification techniques | PCR, NGS | ||
| Implant Sonication | Cultures | - | |
| Nucleic amplification techniques | PCR, NGS | ||
| Tissue biopsy | Cultures | Sensitivity 70–90%, specificity 67–91% | |
| Histological examination | - | ||
| Nucleic amplification techniques | PCR, NGS |
| Societies | Recommendations |
|---|---|
| International Consensus Meeting on Periprosthetic Infections [70] | One dose preoperatively and for the following 24 h postoperatively |
| Centers for Disease Control and Prevention [71] | One dose preoperatively without redosing |
| American Association of Hip and Knee Surgeons [69] | Postoperative antibiotics should be continued for 24 h |
| American Academy of Orthopaedic Surgeons [72] | Postoperative antibiotics should be continued for 24 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsantes, A.G.; Altsitzioglou, P.; Papadopoulos, D.V.; Lorenzo, D.; Romanò, C.L.; Benzakour, T.; Tsukamoto, S.; Errani, C.; Angelini, A.; Mavrogenis, A.F. Infections of Tumor Prostheses: An Updated Review on Risk Factors, Microbiology, Diagnosis, and Treatment Strategies. Biology 2023, 12, 314. https://doi.org/10.3390/biology12020314
Tsantes AG, Altsitzioglou P, Papadopoulos DV, Lorenzo D, Romanò CL, Benzakour T, Tsukamoto S, Errani C, Angelini A, Mavrogenis AF. Infections of Tumor Prostheses: An Updated Review on Risk Factors, Microbiology, Diagnosis, and Treatment Strategies. Biology. 2023; 12(2):314. https://doi.org/10.3390/biology12020314
Chicago/Turabian StyleTsantes, Andreas G., Pavlos Altsitzioglou, Dimitrios V. Papadopoulos, Drago Lorenzo, Carlo Luca Romanò, Thami Benzakour, Shinji Tsukamoto, Costantino Errani, Andrea Angelini, and Andreas F. Mavrogenis. 2023. "Infections of Tumor Prostheses: An Updated Review on Risk Factors, Microbiology, Diagnosis, and Treatment Strategies" Biology 12, no. 2: 314. https://doi.org/10.3390/biology12020314
APA StyleTsantes, A. G., Altsitzioglou, P., Papadopoulos, D. V., Lorenzo, D., Romanò, C. L., Benzakour, T., Tsukamoto, S., Errani, C., Angelini, A., & Mavrogenis, A. F. (2023). Infections of Tumor Prostheses: An Updated Review on Risk Factors, Microbiology, Diagnosis, and Treatment Strategies. Biology, 12(2), 314. https://doi.org/10.3390/biology12020314














