Salmonella Phage CKT1 Effectively Controls the Vertical Transmission of Salmonella Pullorum in Adult Broiler Breeders
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Phage Isolation and Purification
2.3. Transmission Electron Microscopy
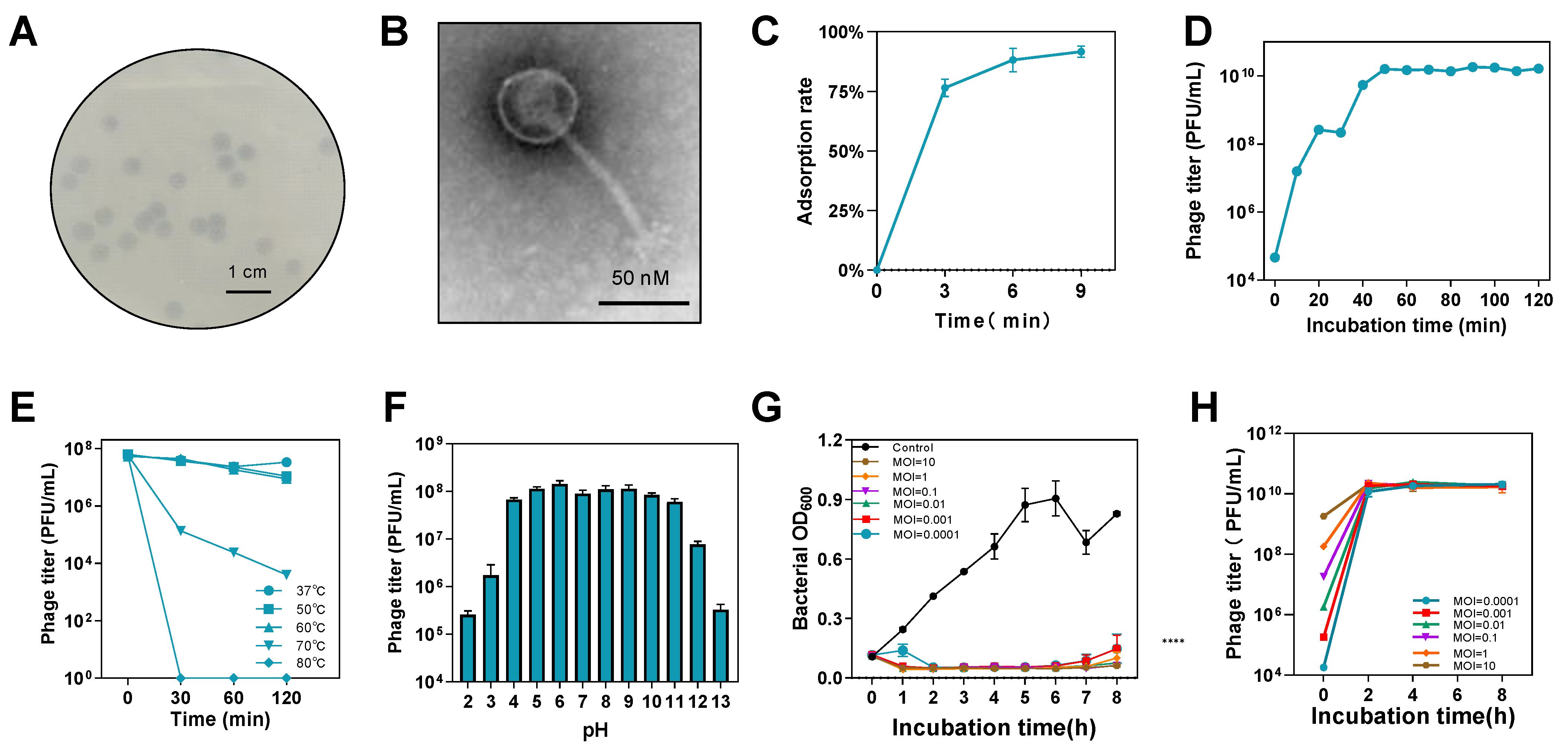
2.4. Phage Characterization Assay
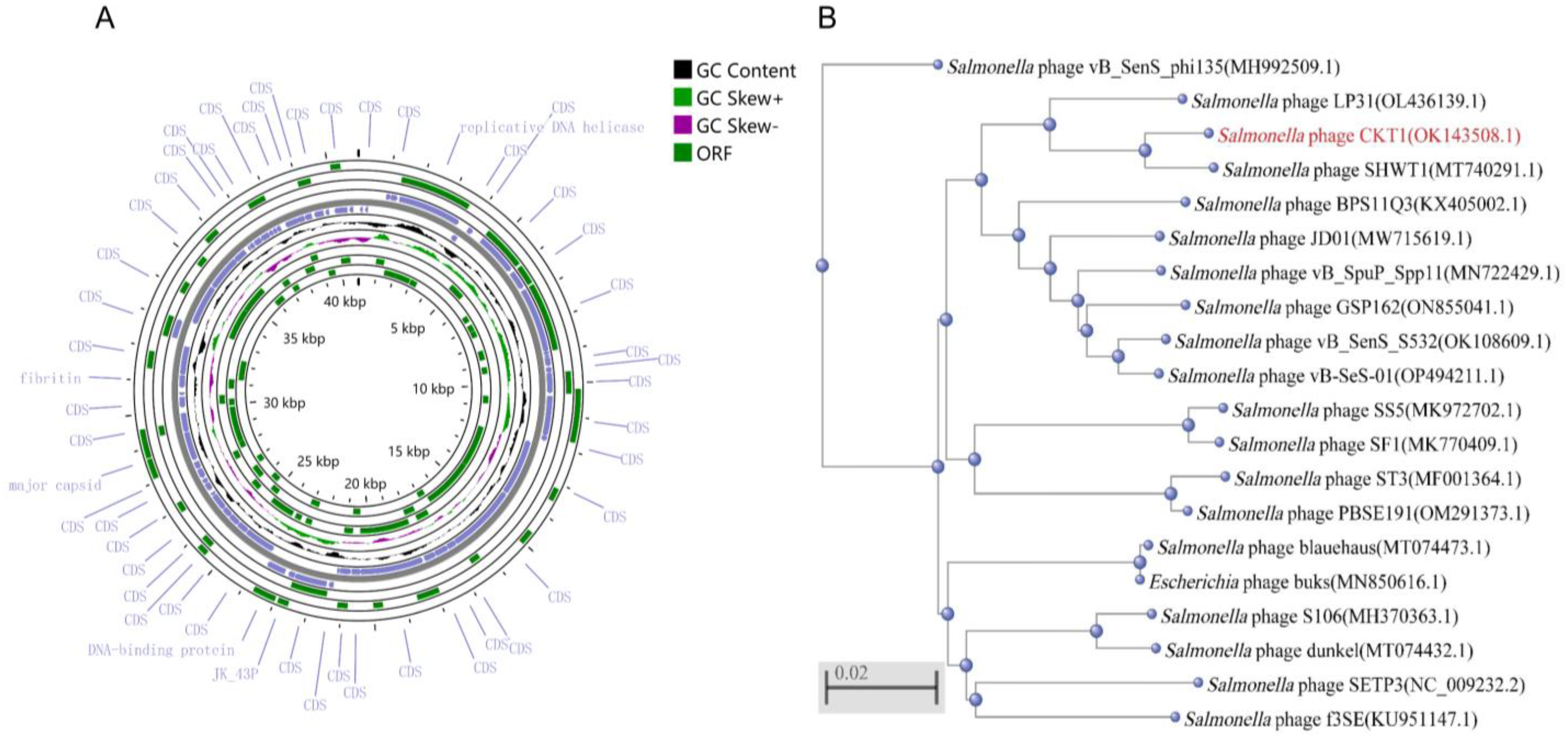
2.5. Phage Genomic Analysis
2.6. Phage Treatment in Salmonella-Infected Adult Chickens
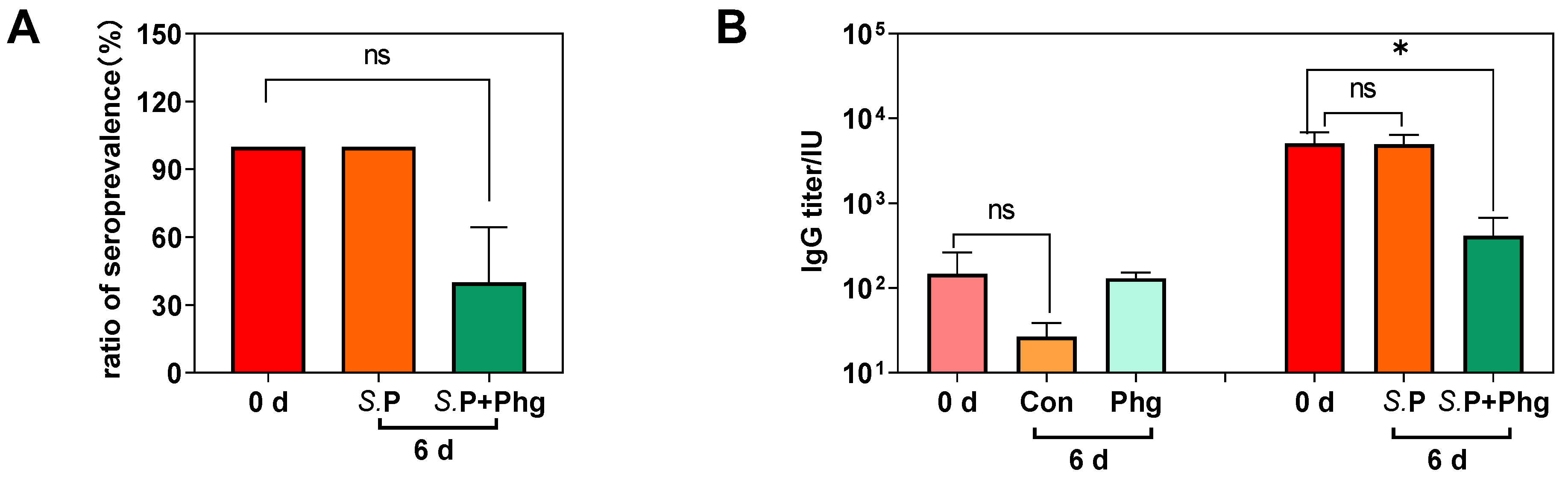
2.7. Serum Assays
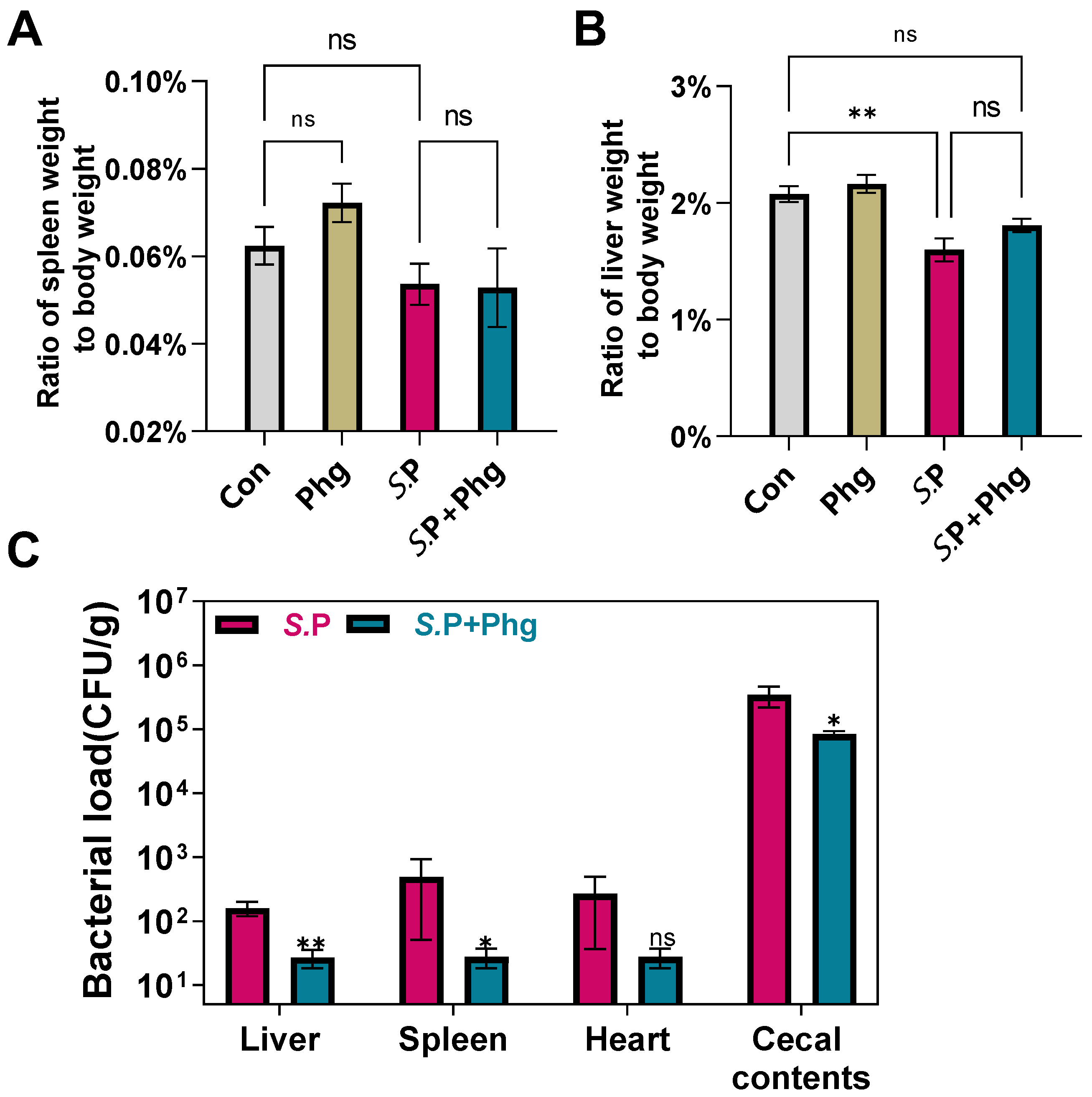
2.8. Detection of Bacterial Load in Various Tissues, Eggshell and Liquid Whole Egg
2.9. Detection of Bacterial Load in Cecal Contents
2.10. Detection of Bacterial Load in the Air
2.11. Statistical Analysis
3. Results
3.1. Characteristics of Phage CKT1
3.2. Genomic Analysis of Phage CKT1
3.3. Effects of Phage Treatment on the Salmonella-Specific Antibody in Chickens
3.4. Effects of Phage Treatment on the Bacterial Load in Various Tissues
3.5. Effects of Phage CKT1 on Bacterial Load in Reproductive System, Eggs, and Breeding Environment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Bacterial Species | Strain | Source | Phage CKT1 Lyiss Ability |
|---|---|---|---|
| Salmonella Pullorum | CVCC526 | CVCC | ++ |
| Salmonella Pullorum | SPG8 | Dead chick embryo | ++ |
| Salmonella Pullorum | ES4 | Dead chick embryo | ++ |
| Salmonella Pullorum | ES5 | Dead chick embryo | ++ |
| Salmonella Pullorum | ES6 | Dead chick embryo | ++ |
| Salmonella Pullorum | ES7 | Dead chick embryo | ++ |
| Salmonella Pullorum | ES11 | Dead chick embryo | ++ |
| Salmonella Pullorum | ES13 | Dead chick embryo | ++ |
| Salmonella Pullorum | ES14 | Dead chick embryo | ++ |
| Salmonella Pullorum | ES15 | Dead chick embryo | ++ |
| Salmonella Pullorum | ES16 | Dead chick embryo | ++ |
| Salmonella Pullorum | ES139 | Dead chick embryo | ++ |
| Salmonella Pullorum | ES141 | Dead chick embryo | ++ |
| Salmonella Pullorum | ES381 | Dead chick embryo | ++ |
| Salmonella Pullorum | ES384 | Dead chick embryo | ++ |
| Salmonella Pullorum | ES483 | Dead chick embryo | ++ |
| Salmonella Typhimurium | ST699 | Dead chick embryo | + |
| Salmonella Typhimurium | ST722 | Dead chick embryo | + |
| Salmonella Typhimurium | ST149 | Dead chick embryo | + |
| Salmonella Typhimurium | ST22 | Dead chick embryo | + |
| Salmonella Typhimurium | 149 | Dead chick embryo | + |
| Salmonella Typhimurium | 18Y15 | Dead chick embryo | + |
| Salmonella Enteritidis | F118 | Dead chick embryo | ++ |
| Salmonella Enteritidis | C9 | Dead chick embryo | ++ |
| Salmonella London | 151 | Dead chick embryo | − |
| Salmonella Kentucky | 147 | Dead chick embryo | − |
| Salmonella Kentucky | 18Y24 | Dead chick embryo | − |
| Salmonella Kentucky | 18Y23 | Dead chick embryo | − |
| Klebsiella pneumoniae | JAF2 | Chicken feces | − |
| Klebsiella pneumoniae | JA4 | Chicken feces | − |
| Escherichia coli | JA3-1 | Chicken feces | − |
| Escherichia coli | JAF1 | Chicken feces | − |
| Proteus mirabilis | FC2-2 | Chicken feces | − |
| Proteus mirabilis | T102-2 | Chicken feces | − |
References
- Wigley, P.; Berchieri, A., Jr.; Page, K.L.; Smith, A.L.; Barrow, P.A. Salmonella enterica serovar Pullorum persists in splenic macrophages and in the reproductive tract during persistent, disease-free carriage in chickens. Infect. Immun. 2001, 69, 7873–7879. [Google Scholar] [CrossRef] [PubMed]
- Wray, C.; Sojka, W.J. Reviews of the progress of dairy science: Bovine salmonellosis. J. Dairy Res. 1977, 44, 383–425. [Google Scholar] [CrossRef] [PubMed]
- House, D.; Bishop, A.; Parry, C.; Dougan, G.; Wain, J. Typhoid fever: Pathogenesis and disease. Curr. Opin. Infect. Dis. 2001, 14, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Shivaprasad, H.L.; Timoney, J.F.; Morales, S.; Lucio, B.; Baker, R.C. Pathogenesis of Salmonella enteritidis infection in laying chickens. I. Studies on egg transmission, clinical signs, fecal shedding, and serologic responses. Avian Dis. 1990, 34, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Barrow, P.A.; Lovell, M.A. Experimental infection of egg-laying hens with Salmonella enteritidis phage type 4. Avian Pathol. 1991, 20, 335–348. [Google Scholar] [CrossRef]
- Eriksson, H.; Soderlund, R.; Ernholm, L.; Melin, L.; Jansson, D.S. Diagnostics, epidemiological observations and genomic subtyping in an outbreak of pullorum disease in non-commercial chickens. Vet. Microbiol. 2018, 217, 47–52. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, J.; Xu, M.; Zhu, C.; Yu, Y.; Liu, X.; Kelly, P.; Xu, B.; Wang, C. Prevalence and fimbrial genotype distribution of poultry Salmonella isolates in China (2006 to 2012). Appl. Environ. Microbiol. 2014, 80, 687–693. [Google Scholar] [CrossRef]
- Guo, X.; Wang, H.; Cheng, Y.; Zhang, W.; Luo, Q.; Wen, G.; Wang, G.; Shao, H.; Zhang, T. Quinolone resistance phenotype and genetic characterization of Salmonella enterica serovar Pullorum isolates in China, during 2011 to 2016. BMC Microbiol. 2018, 18, 225. [Google Scholar] [CrossRef]
- Henriques, A.; Sereno, R.; Almeida, A. Reducing Salmonella horizontal transmission during egg incubation by phage therapy. Foodborne Pathog. Dis. 2013, 10, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.S.; Jeong, J.; Lee, J.; Kim, S.; Min, W.; Myung, H. Therapeutic effects of bacteriophages against Salmonella gallinarum infection in chickens. J. Microbiol. Biotechnol. 2013, 23, 1478–1483. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Y.; Li, W.; Zhu, W.; Wang, J.; Wang, X. Application of a Novel Lytic Podoviridae Phage Pu20 for Biological Control of Drug-Resistant Salmonella in Liquid Eggs. Pathogens 2021, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak, Z.; Majewska, J.; Milczarek, M.; Owczarek, B.; Dabrowska, K. Circulation of Fluorescently Labelled Phage in a Murine Model. Viruses 2021, 13, 279. [Google Scholar] [CrossRef] [PubMed]
- Abedon, S.T.; Katsaounis, T.I. Basic Phage Mathematics. Methods Mol. Biol. 2018, 1681, 3–30. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhang, A.; Gu, J.; Zhao, R.; Pan, X.; Dai, Y.; Yin, L.; Zhang, Q.; Hu, X.; Wang, H.; et al. Evaluating Salmonella pullorum dissemination and shedding patterns and antibody production in infected chickens. BMC Vet. Res. 2022, 18, 240. [Google Scholar] [CrossRef]
- Kropinski, A.M.; Mazzocco, A.; Waddell, T.E.; Lingohr, E.; Johnson, R.P. Enumeration of bacteriophages by double agar overlay plaque assay. Methods Mol. Biol. 2009, 501, 69–76. [Google Scholar] [CrossRef]
- Shang, Y.; Sun, Q.; Chen, H.; Wu, Q.; Chen, M.; Yang, S.; Du, M.; Zha, F.; Ye, Q.; Zhang, J. Isolation and Characterization of a Novel Salmonella Phage vB_SalP_TR2. Front. Microbiol. 2021, 12, 664810. [Google Scholar] [CrossRef]
- Jeon, G.; Ahn, J. Evaluation of phage adsorption to Salmonella Typhimurium exposed to different levels of pH and antibiotic. Microb. Pathog. 2021, 150, 104726. [Google Scholar] [CrossRef]
- Danis-Wlodarczyk, K.; Olszak, T.; Arabski, M.; Wasik, S.; Majkowska-Skrobek, G.; Augustyniak, D.; Gula, G.; Briers, Y.; Jang, H.B.; Vandenheuvel, D.; et al. Characterization of the Newly Isolated Lytic Bacteriophages KTN6 and KT28 and Their Efficacy against Pseudomonas aeruginosa Biofilm. PLoS ONE 2015, 10, e0127603. [Google Scholar] [CrossRef]
- Besemer, J.; Lomsadze, A.; Borodovsky, M. GeneMarkS: A self-training method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions. Nucleic Acids Res. 2001, 29, 2607–2618. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- McNair, K.; Bailey, B.A.; Edwards, R.A. PHACTS, a computational approach to classifying the lifestyle of phages. Bioinformatics 2012, 28, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, J.; Yu, J.; Yao, Z.; Sun, L.; Shen, Y.; Jin, Q. VFDB: A reference database for bacterial virulence factors. Nucleic Acids Res. 2005, 33, D325–D328. [Google Scholar] [CrossRef]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef] [PubMed]
- Wigley, P.; Hulme, S.D.; Powers, C.; Beal, R.K.; Berchieri, A., Jr.; Smith, A.; Barrow, P. Infection of the reproductive tract and eggs with Salmonella enterica serovar pullorum in the chicken is associated with suppression of cellular immunity at sexual maturity. Infect. Immun. 2005, 73, 2986–2990. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Guo, L.; Luan, Y.; Zhao, W.; Cui, L.; Hao, G.; Sun, S. Research Note: Effect of a live Salmonella Enteritidis vaccine against Salmonella Pullorum infection in breeder chickens. Poult. Sci. 2022, 102, 102308. [Google Scholar] [CrossRef] [PubMed]
- Tie, K.; Yuan, Y.; Yan, S.; Yu, X.; Zhang, Q.; Xu, H.; Zhang, Y.; Gu, J.; Sun, C.; Lei, L.; et al. Isolation and identification of Salmonella pullorum bacteriophage YSP2 and its use as a therapy for chicken diarrhea. Virus Genes 2018, 54, 446–456. [Google Scholar] [CrossRef]
- Al-Ajeeli, M.N.; Taylor, T.M.; Alvarado, C.Z.; Coufal, C.D. Comparison of eggshell surface sanitization technologies and impacts on consumer acceptability. Poult. Sci. 2016, 95, 1191–1197. [Google Scholar] [CrossRef]
- Nabil, N.M.; Tawakol, M.M.; Hassan, H.M. Assessing the impact of bacteriophages in the treatment of Salmonella in broiler chickens. Infect. Ecol. Epidemiol. 2018, 8, 1539056. [Google Scholar] [CrossRef]
- Odebode, A.; Adekunle, A.; Stajich, J.; Adeonipekun, P. Airborne fungi spores distribution in various locations in Lagos, Nigeria. Environ. Monit. Assess. 2020, 192, 87. [Google Scholar] [CrossRef]
- Berrang, M.E.; Frank, J.F.; Buhr, R.J.; Bailey, J.S.; Cox, N.A. Eggshell membrane structure and penetration by Salmonella typhimurium. J. Food Prot. 1999, 62, 73–76. [Google Scholar] [CrossRef]
- Wang, H.; Slavik, M.F. Bacterial penetration into eggs washed with various chemicals and stored at different temperatures and times. J. Food Prot. 1998, 61, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Wigley, P.; Jones, M.A.; Barrow, P.A. Salmonella enterica serovar Pullorum requires the Salmonella pathogenicity island 2 type III secretion system for virulence and carriage in the chicken. Avian Pathol. 2002, 31, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liang, L.; Cui, K.; Li, P.; Hao, G.; Sun, S. Salmonella phage CKT1 significantly relieves the body weight loss of chicks by normalizing the abnormal intestinal microbiome caused by hypervirulent Salmonella Pullorum. Poult. Sci. 2022, 101, 101668. [Google Scholar] [CrossRef] [PubMed]
- Bodner, K.; Melkonian, A.L.; Covert, M.W. The Enemy of My Enemy: New Insights Regarding Bacteriophage-Mammalian Cell Interactions. Trends Microbiol. 2021, 29, 528–541. [Google Scholar] [CrossRef]
- Bichet, M.C.; Chin, W.H.; Richards, W.; Lin, Y.W.; Avellaneda-Franco, L.; Hernandez, C.A.; Oddo, A.; Chernyavskiy, O.; Hilsenstein, V.; Neild, A.; et al. Bacteriophage uptake by mammalian cell layers represents a potential sink that may impact phage therapy. iScience 2021, 24, 102287. [Google Scholar] [CrossRef]
- Nguyen, S.; Baker, K.; Padman, B.S.; Patwa, R.; Dunstan, R.A.; Weston, T.A.; Schlosser, K.; Bailey, B.; Lithgow, T.; Lazarou, M.; et al. Bacteriophage Transcytosis Provides a Mechanism To Cross Epithelial Cell Layers. mBio 2017, 8, e01874-17. [Google Scholar] [CrossRef]
- Tao, C.; Yi, Z.; Zhang, Y.; Wang, Y.; Zhu, H.; Afayibo, D.J.A.; Li, T.; Tian, M.; Qi, J.; Ding, C.; et al. Characterization of a Broad-Host-Range Lytic Phage SHWT1 Against Multidrug-Resistant Salmonella and Evaluation of Its Therapeutic Efficacy in vitro and in vivo. Front. Vet. Sci. 2021, 8, 683853. [Google Scholar] [CrossRef]
- Gordillo Altamirano, F.L.; Barr, J.J. Phage Therapy in the Postantibiotic Era. Clin. Microbiol. Rev. 2019, 32, e00066-18. [Google Scholar] [CrossRef]
- Li, M.; Lin, H.; Jing, Y.; Wang, J. Broad-host-range Salmonella bacteriophage STP4-a and its potential application evaluation in poultry industry. Poult. Sci. 2020, 99, 3643–3654. [Google Scholar] [CrossRef]
- Santos, S.B.; Kropinski, A.M.; Ceyssens, P.J.; Ackermann, H.W.; Villegas, A.; Lavigne, R.; Krylov, V.N.; Carvalho, C.M.; Ferreira, E.C.; Azeredo, J. Genomic and proteomic characterization of the broad-host-range Salmonella phage PVP-SE1: Creation of a new phage genus. J. Virol. 2011, 85, 11265–11273. [Google Scholar] [CrossRef]
- Gorski, A.; Miedzybrodzki, R.; Borysowski, J.; Dabrowska, K.; Wierzbicki, P.; Ohams, M.; Korczak-Kowalska, G.; Olszowska-Zaremba, N.; Lusiak-Szelachowska, M.; Klak, M.; et al. Phage as a modulator of immune responses: Practical implications for phage therapy. Adv. Virus. Res. 2012, 83, 41–71. [Google Scholar] [CrossRef] [PubMed]
- Hodyra-Stefaniak, K.; Miernikiewicz, P.; Drapala, J.; Drab, M.; Jonczyk-Matysiak, E.; Lecion, D.; Kazmierczak, Z.; Beta, W.; Majewska, J.; Harhala, M.; et al. Mammalian Host-Versus-Phage immune response determines phage fate in vivo. Sci. Rep. 2015, 5, 14802. [Google Scholar] [CrossRef] [PubMed]
- Roach, D.R.; Leung, C.Y.; Henry, M.; Morello, E.; Singh, D.; Di Santo, J.P.; Weitz, J.S.; Debarbieux, L. Synergy between the Host Immune System and Bacteriophage Is Essential for Successful Phage Therapy against an Acute Respiratory Pathogen. Cell Host Microbe 2017, 22, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Majewska, J.; Kazmierczak, Z.; Lahutta, K.; Lecion, D.; Szymczak, A.; Miernikiewicz, P.; Drapala, J.; Harhala, M.; Marek-Bukowiec, K.; Jedruchniewicz, N.; et al. Induction of Phage-Specific Antibodies by Two Therapeutic Staphylococcal Bacteriophages Administered per os. Front. Immunol. 2019, 10, 2607. [Google Scholar] [CrossRef] [PubMed]


| Sample Source from Groups | Group 1 | S.P Group | S.P + Phg Group |
|---|---|---|---|
| Eggshell | 6/9 | 9/11 | 3/10 |
| Liquid whole egg | 4/9 | 6/11 | 1/10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, K.; Li, P.; Huang, J.; Lin, F.; Li, R.; Cao, D.; Hao, G.; Sun, S. Salmonella Phage CKT1 Effectively Controls the Vertical Transmission of Salmonella Pullorum in Adult Broiler Breeders. Biology 2023, 12, 312. https://doi.org/10.3390/biology12020312
Cui K, Li P, Huang J, Lin F, Li R, Cao D, Hao G, Sun S. Salmonella Phage CKT1 Effectively Controls the Vertical Transmission of Salmonella Pullorum in Adult Broiler Breeders. Biology. 2023; 12(2):312. https://doi.org/10.3390/biology12020312
Chicago/Turabian StyleCui, Ketong, Peiyong Li, Jiaqi Huang, Fang Lin, Ruibo Li, Dingguo Cao, Guijuan Hao, and Shuhong Sun. 2023. "Salmonella Phage CKT1 Effectively Controls the Vertical Transmission of Salmonella Pullorum in Adult Broiler Breeders" Biology 12, no. 2: 312. https://doi.org/10.3390/biology12020312
APA StyleCui, K., Li, P., Huang, J., Lin, F., Li, R., Cao, D., Hao, G., & Sun, S. (2023). Salmonella Phage CKT1 Effectively Controls the Vertical Transmission of Salmonella Pullorum in Adult Broiler Breeders. Biology, 12(2), 312. https://doi.org/10.3390/biology12020312






