Cortisol Rapidly Facilitates Glucocorticoid Receptor Translocation to the Plasma Membrane in Primary Trout Hepatocytes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Antibodies
2.3. Animals
2.4. Hepatocyte Isolation and Cell Suspension
2.5. Immunofluorescent Labelling
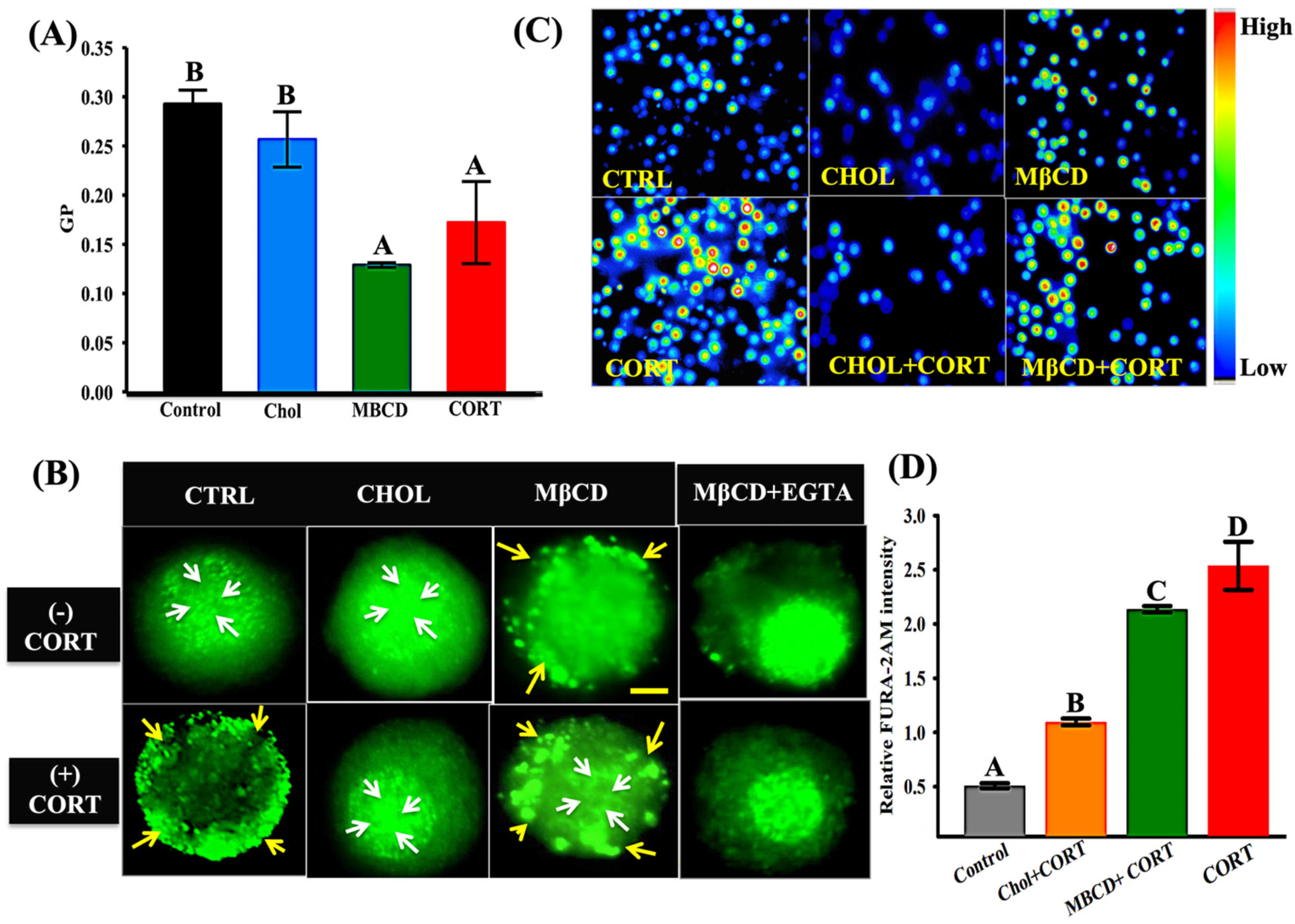
2.6. Cytosolic Ca2+ Measurements
2.7. Laurdan Generalized Polarization Imaging and Quantification
2.8. Data Acquisition and Analysis
3. Results
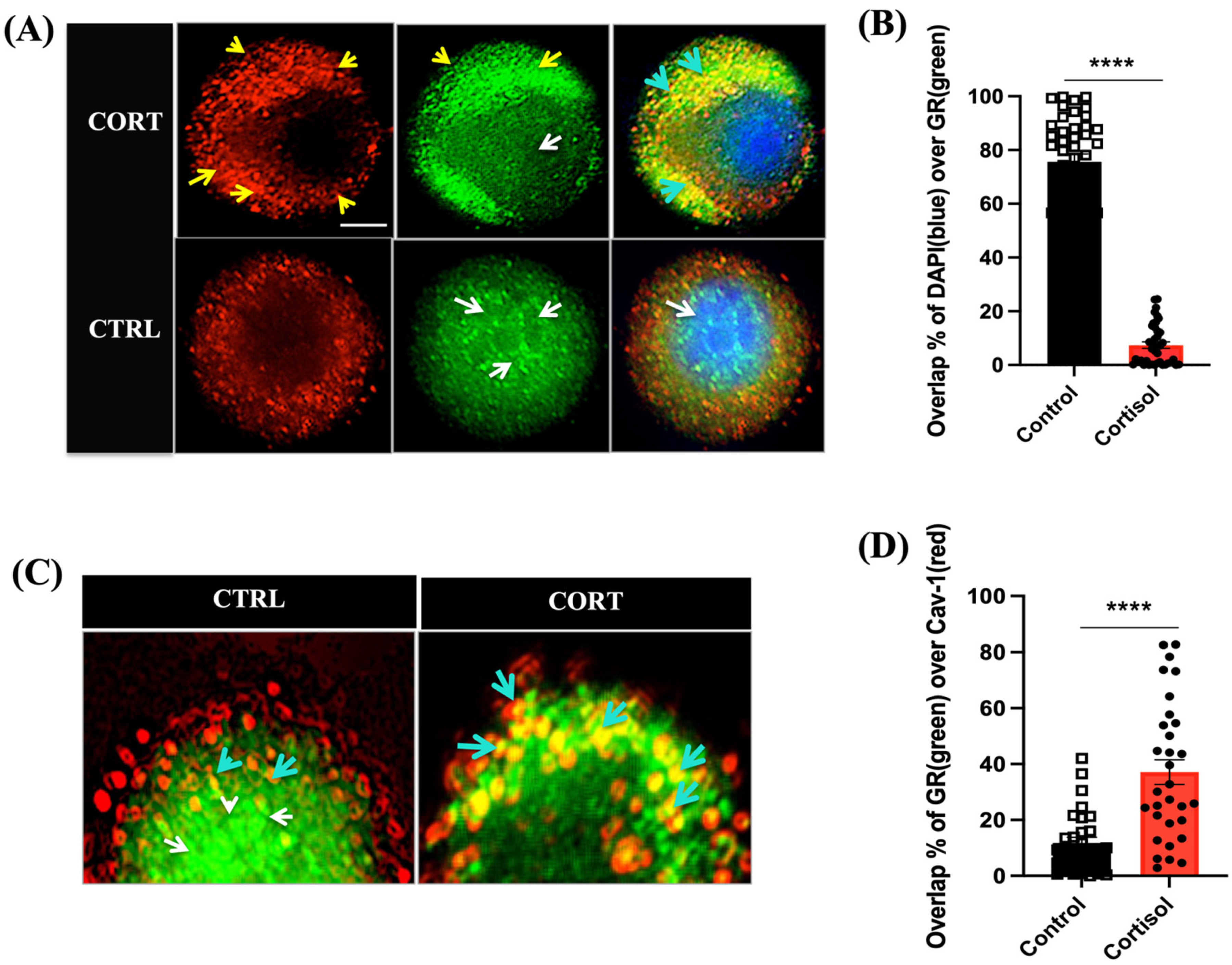
3.1. Cortisol Rapidly Translocate GR to the Plasma Membrane in Trout Hepatocytes
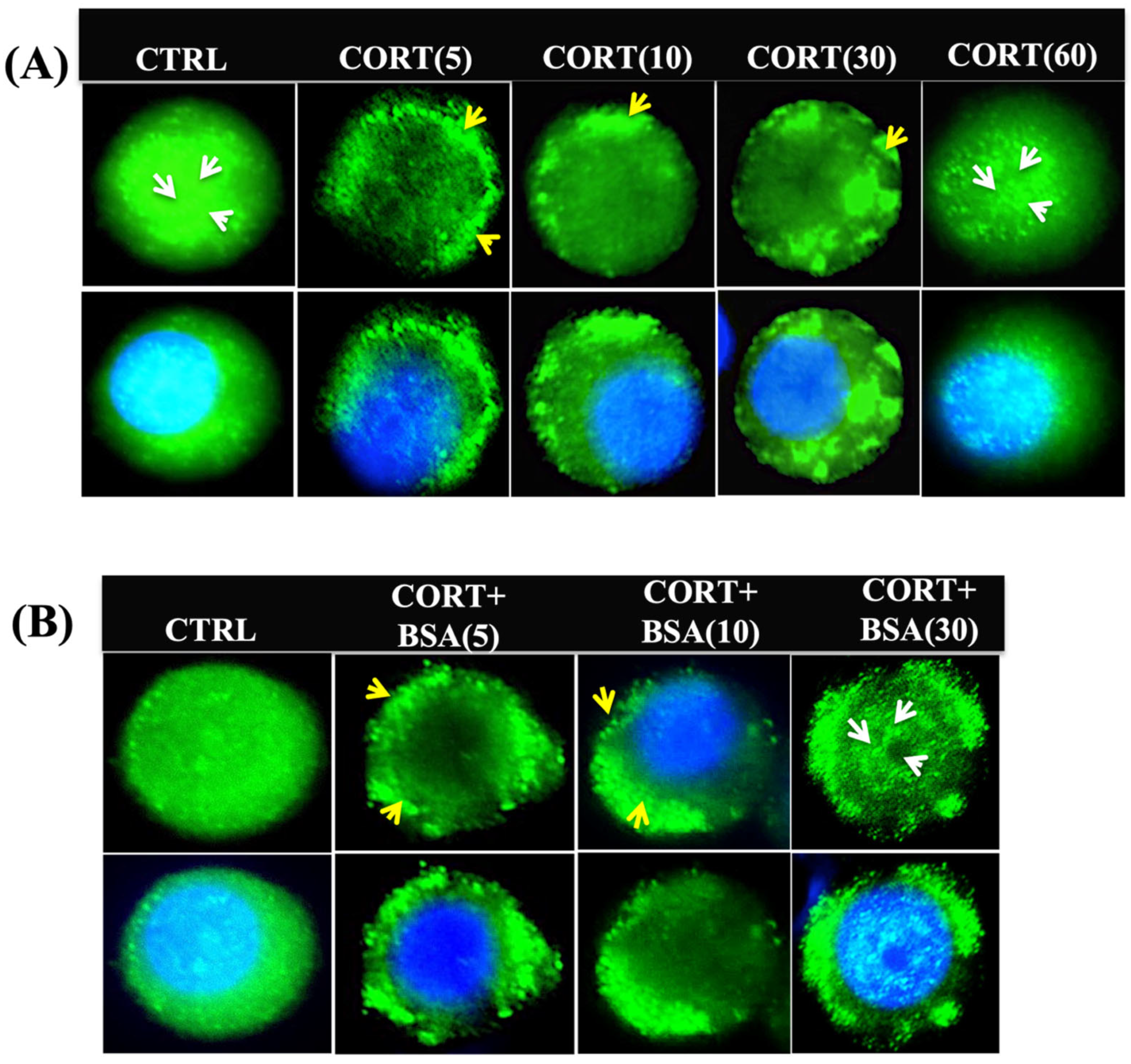
3.2. Cortisol Mediates Temporal Changes in GR Distribution in Trout Hepatocytes
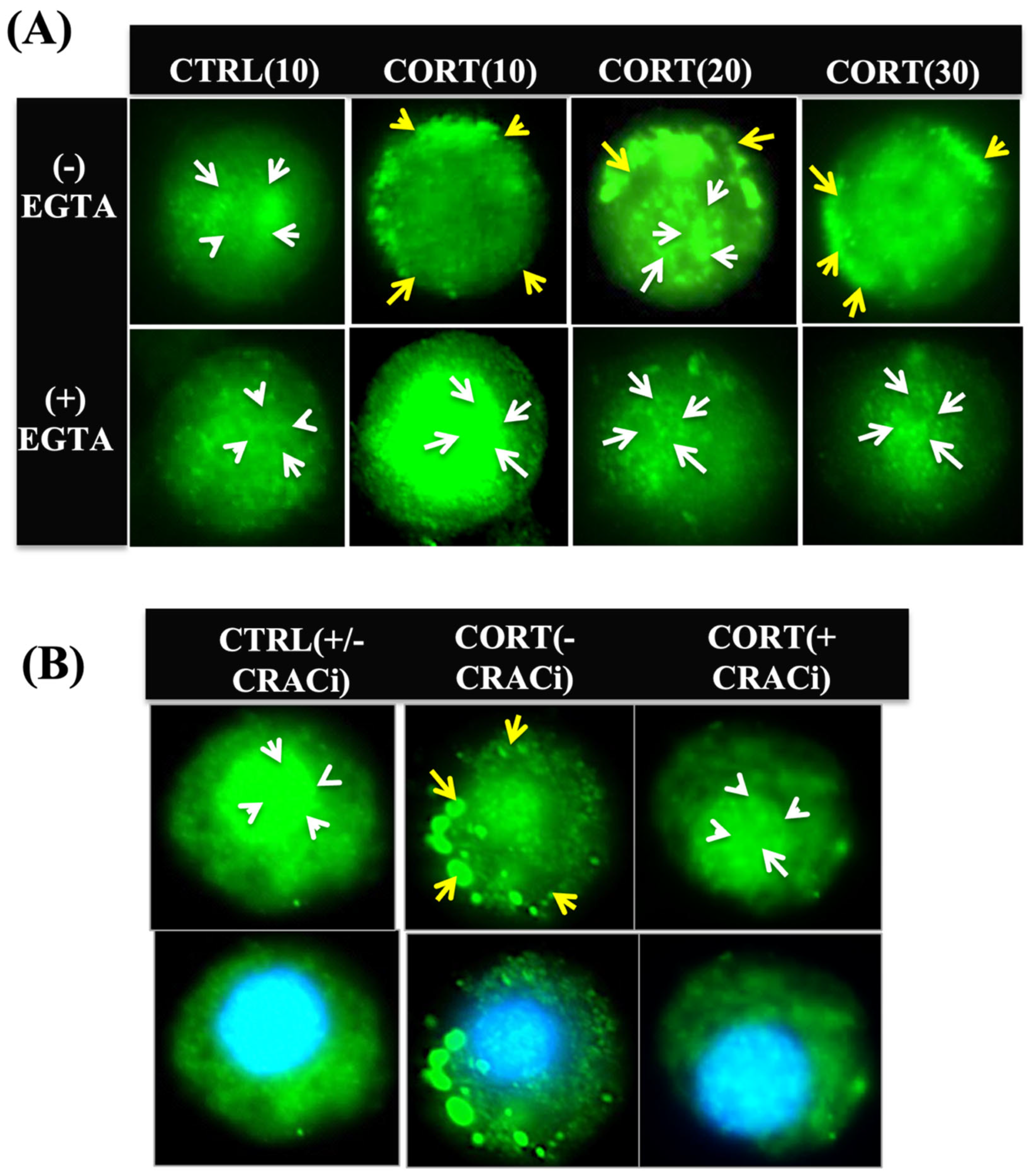
3.3. Cortisol-Mediated Membrane’s GR Localization Is Calcium-Dependent
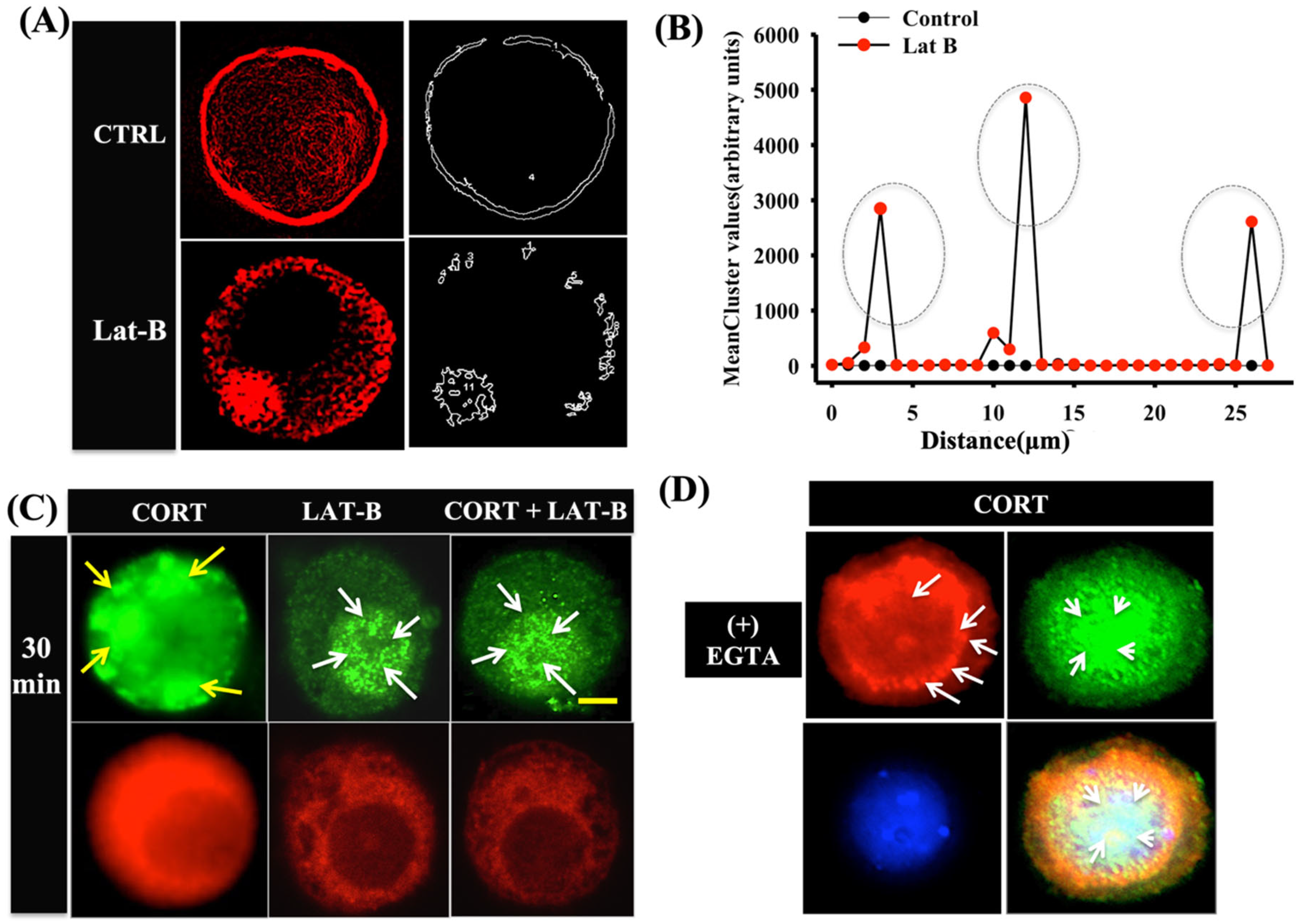
3.4. Cortisol-Mediated GR Translocation Involves F-Actin Framework
3.5. Cortisol-Mediated GR Translocation Is Modulated by Plasma Membrane Modifications
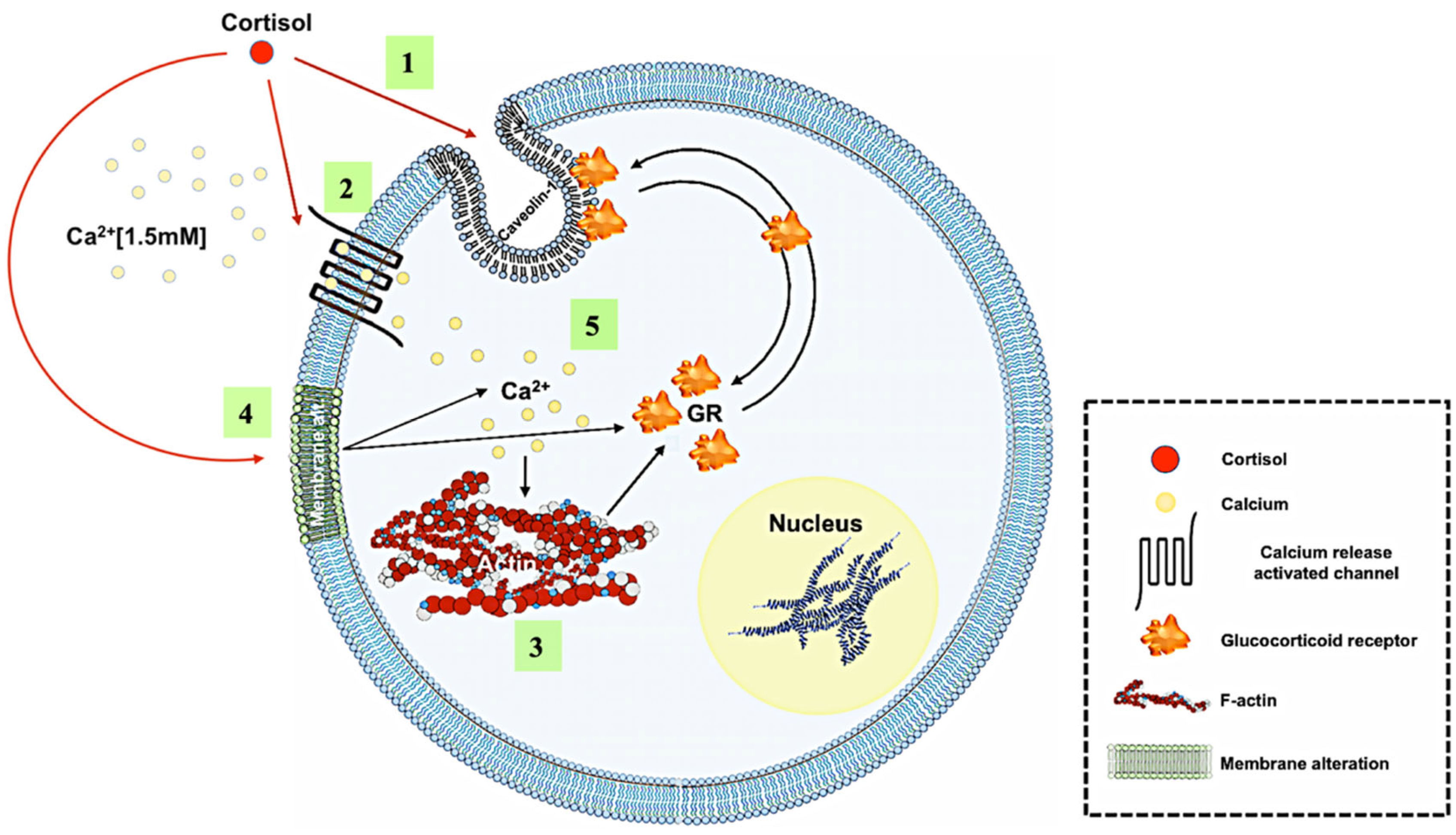
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mommsen, T.; Vijayan, M.; Moon, T. Cortisol in teleosts: Dynamics, mechanisms of action, and metabolic regulation. Rev. Fish Biol. Fish. 1999, 9, 211–268. [Google Scholar] [CrossRef]
- Nicolaides, N.C.; Kyratzi, E.; Lamprokostopoulou, A.; Chrousos, G.P.; Charmandari, E. Stress, the stress system and the role of glucocorticoids. Neuroimmunomodulation 2015, 22, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Faught, E.; Vijayan, M.M. The mineralocorticoid receptor is essential for stress axis regulation in zebrafish larvae. Sci. Rep. 2018, 8, 18081. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, W.M., III; Honeycutt, J.L.; Deck, C.A.; Borski, R.J. Nongenomic glucocorticoid effects and their mechanisms of action in vertebrates. Int. Rev. Cell Mol. Biol. 2019, 346, 51. [Google Scholar]
- Ayrout, M.; Simon, V.; Bernard, V.; Binart, N.; Cohen-Tannoudji, J.; Lombès, M.; Chauvin, S. A novel non genomic glucocorticoid signaling mediated by a membrane palmitoylated glucocorticoid receptor cross talks with GnRH in gonadotrope cells. Sci. Rep. 2017, 7, 1537. [Google Scholar] [CrossRef] [PubMed]
- Das, C.; Thraya, M.; Vijayan, M.M. Nongenomic cortisol signaling in fish. Gen. Comp. Endocrinol. 2018, 265, 121–127. [Google Scholar] [CrossRef]
- Johnstone, W.M., III; Mills, K.A.; Alyea, R.A.; Thomas, P.; Borski, R.J. Characterization of membrane receptor binding activity for cortisol in the liver and kidney of the euryhaline teleost, Mozambique tilapia (Oreochromis mossambicus). Gen. Comp. Endocrinol. 2013, 192, 107–114. [Google Scholar] [CrossRef]
- Pedram, A.; Razandi, M.; Sainson, R.C.A.; Kim, J.K.; Hughes, C.C.; Levin, E.R. A conserved mechanism for steroid receptor translocation to the plasma membrane. J. Biol. Chem. 2007, 282, 22278–22288. [Google Scholar] [CrossRef]
- Adlanmerini, M.; Solinhac, R.; Abot, A.; Fabre, A.; Raymond-Letron, I.; Guihot, A.-L.; Boudou, F.; Sautier, L.; Vessieres, E.; Kim, S.H.; et al. Mutation of the palmitoylation site of estrogen receptor alpha in vivo reveals tissue-specific roles for membrane versus nuclear actions. Proc. Natl. Acad. Sci. USA. 2014, 111, E283–E290. [Google Scholar] [CrossRef]
- Kim, H.P.; Lee, J.Y.; Jeong, J.K.; Bae, S.W.; Lee, H.K.; Jo, I. Nongenomic stimulation of nitric oxide release by estrogen is mediated by estrogen receptor α localized in caveolae. Biochem. Biophys. Res. Commun. 1999, 263, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Razandi, M.; Oh, P.; Pedram, A.; Schnitzer, J.; Levin, E.R. ERs associate with and regulate the production of caveolin: Implications for signaling and cellular actions. Mol. Endocrinol. 2002, 16, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.L.; Schneider, M.C.; Zheng, Y.; Zhang, X.; Richie, J.P. Caveolin-1 interacts with androgen receptor A positive modulator of androgen receptor mediated transactivation. J. Biol. Chem. 2001, 276, 13442–13451. [Google Scholar] [CrossRef] [PubMed]
- Podar, K.; Tai, Y.-T.; Cole, C.E.; Hideshima, T.; Sattler, M.; Hamblin, A.; Mitsiades, N.; Schlossman, R.L.; Davies, F.E.; Morgan, G.J. Essential role of caveolae in interleukin-6-and insulin-like growth factor I-triggered Akt-1-mediated survival of multiple myeloma cells. J. Biol. Chem. 2003, 278, 5794–5801. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Butz, S.; Ying, Y.; Anderson, R.G.W. Tyrosine kinase receptors concentrated in caveolae-like domains from neuronal plasma membrane. J. Biol. Chem. 1997, 272, 3554–3559. [Google Scholar] [CrossRef]
- Wilkenfeld, S.R.; Lin, C.; Frigo, D.E. Communication between genomic and non-genomic signaling events coordinate steroid hormone actions. Steroids 2018, 133, 2–7. [Google Scholar] [CrossRef]
- Samarasinghe, R.A.; Di Maio, R.; Volonte, D.; Galbiati, F.; Lewis, M.; Romero, G.; DeFranco, D.B. Nongenomic glucocorticoid receptor action regulates gap junction intercellular communication and neural progenitor cell proliferation. Proc. Natl. Acad. Sci. USA 2011, 108, 16657–16662. [Google Scholar] [CrossRef]
- Dindia, L.; Murray, J.; Faught, E.; Davis, T.L.; Leonenko, Z.; Vijayan, M.M. Novel Nongenomic Signaling by Glucocorticoid May Involve Changes to Liver Membrane Order in Rainbow Trout. PLoS ONE 2012, 7, e46859. [Google Scholar] [CrossRef]
- Dindia, L.; Faught, L.E.; Leonenko, Z.; Thomas, R.; Vijayan, M.M. Rapid cortisol signaling in response to acute stress involves changes in plasma membrane order in rainbow trout liver. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1157–E1166. [Google Scholar] [CrossRef]
- Espinoza, M.B.; Aedo, J.E.; Zuloaga, R.; Valenzuela, C.; Molina, A.; Valdés, J.A. Cortisol Induces Reactive Oxygen Species Through a Membrane Glucocorticoid Receptor in Rainbow Trout Myotubes. J. Cell. Biochem. 2017, 118, 718–725. [Google Scholar] [CrossRef]
- Hirabayashi, T.; Kume, K.; Hirose, K.; Yokomizo, T.; Iino, M.; Itoh, H.; Shimizu, T. Critical duration of intracellular Ca2+ response required for continuous translocation and activation of cytosolic phospholipase A2. J. Biol. Chem. 1999, 274, 5163–5169. [Google Scholar] [CrossRef]
- Price, L.S.; Langeslag, M.; ten Klooster, J.P.; Hordijk, P.L.; Jalink, K.; Collard, J.G. Calcium signaling regulates translocation and activation of Rac. J. Biol. Chem. 2003, 278, 39413–39421. [Google Scholar] [CrossRef] [PubMed]
- Das, C.; Rout, M.K.; Wildering, W.C.; Vijayan, M.M. Cortisol modulates calcium release-activated calcium channel gating in fish hepatocytes. Sci. Rep. 2021, 11, 9621. [Google Scholar] [CrossRef] [PubMed]
- Prakriya, M.; Lewis, R.S. Store-Operated Calcium Channels. Physiol. Rev. 2015, 95, 1383–1436. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Romagnoli, A.; Agnoletto, C.; Bergamelli, L.; Sorrentino, G.; Brini, M.; Pozzan, T.; Meldolesi, J.; Pinton, P.; Rizzuto, R. Translocation of signalling proteins to the plasma membrane revealed by a new bioluminescent procedure. BMC Cell Biol. 2011, 12, 27. [Google Scholar] [CrossRef]
- Quintana, A.; Schwarz, E.C.; Schwindling, C.; Lipp, P.; Kaestner, L.; Hoth, M. Sustained activity of calcium release-activated calcium channels requires translocation of mitochondria to the plasma membrane. J. Biol. Chem. 2006, 281, 40302–40309. [Google Scholar] [CrossRef]
- Chang, W.-C.; Nelson, C.; Parekh, A.B.; Chang, W.-C.; Nelson, C.; Parekh, A.B. Ca2+ influx through CRAC channels activates cytosolic phospholipase A2, leukotriene C4 secretion, and expression of c-fos through ERK-dependent and-independent pathways in mast cells. FASEB J. 2006, 20, 2381–2383. [Google Scholar] [CrossRef]
- Hartzell, C.A.; Jankowska, K.I.; Burkhardt, J.K.; Lewis, R.S. Calcium influx through CRAC channels controls actin organization and dynamics at the immune synapse. eLife 2016, 5, e14850. [Google Scholar] [CrossRef]
- Van Deurs, B.; Roepstorff, K.; Hommelgaard, A.M.; Sandvig, K. Caveolae: Anchored, multifunctional platforms in the lipid ocean. Trends Cell Biol. 2003, 13, 92–100. [Google Scholar] [CrossRef]
- Sathiyaa, R.; Vijayan, M.M. Autoregulation of glucocorticoid receptor by cortisol in rainbow trout hepatocytes. Am. J. Physiol. Physiol. 2003, 284, C1508–C1515. [Google Scholar] [CrossRef]
- Luo, X.; Wang, D.; Zhu, X.; Wang, G.; You, Y.; Ning, Z.; Li, Y.; Jin, S.; Huang, Y.; Hu, Y.; et al. Autophagic degradation of caveolin-1 promotes liver sinusoidal endothelial cells defenestration. Cell Death Dis. 2018, 9, 576. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choi, J.; Yang, M.J.; Hong, S.P.; Lee, C.-K.; Kubota, Y.; Lim, D.-S.; Koh, G.Y. A MST1-FOXO1 cascade establishes endothelial tip cell polarity and facilitates sprouting angiogenesis. Nat. Commun. 2019, 10, 838. [Google Scholar] [CrossRef] [PubMed]
- Eylenstein, A.; Gehring, E.-M.; Heise, N.; Shumilina, E.; Schmidt, S.; Szteyn, K.; Munzer, P.; Nurbaeva, M.K.; Eichenmuller, M.; Tyan, L.; et al. Stimulation of Ca2+-channel Orai1/STIM1 by serum- and glucocorticoid-inducible kinase 1 (SGK1). FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2011, 25, 2012–2021. [Google Scholar] [CrossRef]
- Diercks, B.-P.; Werner, R.; Weidemuller, P.; Czarniak, F.; Hernandez, L.; Lehmann, C.; Rosche, A.; Kruger, A.; Kaufmann, U.; Vaeth, M.; et al. ORAI1, STIM1/2, and RYR1 shape subsecond Ca(2+) microdomains upon T cell activation. Sci. Signal. 2018, 11, eaat0358. [Google Scholar] [CrossRef] [PubMed]
- Schrader, M.; Bahlmann, K.; Giese, G.; Hell, S.W. 4Pi-confocal imaging in fixed biological specimens. Biophys. J. 1998, 75, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Sathiyaa, R.; Campbell, T.; Vijayan, M.M. Cortisol modulates HSP90 mRNA expression in primary cultures of trout hepatocytes. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2001, 129, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Shiue, Y.-L.; Tailiu, J.-J.; Liou, J.-F.; Lu, H.-T.; Tai, C.; Shiau, J.-W.; Chen, L.-R. Establishment of the long-term in vitro culture system for chicken primordial germ cells. Reprod. Domest. Anim. 2009, 44, 55–61. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, Y.; Huang, G.; Ye, B.; Liu, B.; Wu, J.; Du, Y.; He, L.; Fan, Z. lnc-beta-Catm elicits EZH2-dependent beta-catenin stabilization and sustains liver CSC self-renewal. Nat. Struct. Mol. Biol. 2016, 23, 631–639. [Google Scholar] [CrossRef]
- Manders, E.M.M.; Verbeek, F.J.; Aten, J.A. Measurement of co-localization of objects in dual-colour confocal images. J. Microsc. 1993, 169, 375–382. [Google Scholar] [CrossRef]
- Bootman, M.D.; Rietdorf, K.; Collins, T.; Walker, S.; Sanderson, M. Ca2+-Sensitive Fluorescent Dyes and Intracellular Ca2+ Imaging. Cold Spring Harb. Protoc. 2013, 2013, 83–99. [Google Scholar] [CrossRef]
- Gaus, K.; Gratton, E.; Kable, E.P.W.; Jones, A.S.; Gelissen, I.; Kritharides, L.; Jessup, W. Visualizing lipid structure and raft domains in living cells with two-photon microscopy. Proc. Natl. Acad. Sci. USA 2003, 100, 15554–15559. [Google Scholar] [CrossRef]
- Parasassi, T.; De Stasio, G.; Ravagnan, G.; Rusch, R.M.; Gratton, E. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys. J. 1991, 60, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, S.; Kusunose, N.; Taniguchi, M.; Akamine, T.; Kanado, Y.; Ozono, Y.; Masuda, T.; Kohro, Y.; Matsunaga, N.; Tsuda, M. Glucocorticoid regulation of ATP release from spinal astrocytes underlies diurnal exacerbation of neuropathic mechanical allodynia. Nat. Commun. 2016, 7, 13102. [Google Scholar] [CrossRef] [PubMed]
- Morton, W.M.; Ayscough, K.R.; McLaughlin, P.J. Latrunculin alters the actin-monomer subunit interface to prevent polymerization. Nat. Cell Biol. 2000, 2, 376. [Google Scholar] [CrossRef] [PubMed]
- Patki, V.; Buxton, J.; Chawla, A.; Lifshitz, L.; Fogarty, K.; Carrington, W.; Tuft, R.; Corvera, S. Insulin action on GLUT4 traffic visualized in single 3T3-l1 adipocytes by using ultra-fast microscopy. Mol. Biol. Cell 2001, 12, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Christian, A.E.; Haynes, M.P.; Phillips, M.C.; Rothblat, G.H. Use of cyclodextrins for manipulating cellular cholesterol content. J. Lipid Res. 1997, 38, 2264–2272. [Google Scholar] [CrossRef] [PubMed]
- Harris, F.M.; Best, K.B.; Bell, J.D. Use of laurdan fluorescence intensity and polarization to distinguish between changes in membrane fluidity and phospholipid order. Biochim. Biophys. Acta (BBA)-Biomembr. 2002, 1565, 123–128. [Google Scholar] [CrossRef]
- Fra, A.M.; Mastroianni, N.; Mancini, M.; Pasqualetto, E.; Sitia, R. Human caveolin-1 and caveolin-2 are closely linked genes colocalized with WI-5336 in a region of 7q31 frequently deleted in tumors. Genomics 1999, 56, 355–356. [Google Scholar] [CrossRef]
- Faught, E.; Vijayan, M.M. Glucocorticoid and mineralocorticoid receptor activation modulates postnatal growth. J. Endocrinol. 2020, 244, 261–271. [Google Scholar] [CrossRef]
- Prunet, P.; Sturm, A.; Milla, S. Multiple corticosteroid receptors in fish: From old ideas to new concepts. Gen. Comp. Endocrinol. 2006, 147, 17–23. [Google Scholar] [CrossRef]
- Bury, N.R.; Sturm, A. Evolution of the corticosteroid receptor signalling pathway in fish. Gen. Comp. Endocrinol. 2007, 153, 47–56. [Google Scholar] [CrossRef]
- Jasmin, J.-F.; Yang, M.; Iacovitti, L.; Lisanti, M.P. Genetic ablation of caveolin-1 increases neural stem cell proliferation in the subventricular zone (SVZ) of the adult mouse brain. Cell Cycle 2009, 8, 3978–3983. [Google Scholar] [CrossRef] [PubMed]
- Gametchu, B. Glucocorticoid receptor-like antigen in lymphoma cell membranes: Correlation to cell lysis. Science 1987, 236, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Stojadinovic, O.; Sawaya, A.; Pastar, I.; Tomic-Canic, M. Glucocorticoid receptor localizes to adherens junctions at the plasma membrane of keratinocytes. PLoS ONE 2013, 8, e63453. [Google Scholar] [CrossRef] [PubMed]
- Bartholome, B.; Spies, C.M.; Gaber, T.; Schuchmann, S.; Berki, T.; Kunkel, D.; Bienert, M.; Radbruch, A.; Burmester, G.-R.; Lauster, R.; et al. Membrane glucocorticoid receptors (mGCR) are expressed in normal human peripheral blood mononuclear cells and up-regulated after in vitro stimulation and in patients with rheumatoid arthritis. FASEB J. 2004, 18, 70–80. [Google Scholar] [CrossRef]
- Jarman, K.E.; Moretti, P.A.B.; Zebol, J.R.; Pitson, S.M. Translocation of sphingosine kinase 1 to the plasma membrane is mediated by calcium-and integrin-binding protein 1. J. Biol. Chem. 2010, 285, 483–492. [Google Scholar] [CrossRef]
- Papakonstanti, E.A.; Kampa, M.; Castanas, E.; Stournaras, C. A Rapid, Nongenomic, Signaling Pathway Regulates the Actin Reorganization Induced by Activation of Membrane Testosterone Receptors. Mol. Endocrinol. 2003, 17, 870–881. [Google Scholar] [CrossRef]
- Tong, P.; Khayat, Z.A.; Huang, C.; Patel, N.; Ueyama, A.; Klip, A. Insulin-induced cortical actin remodeling promotes GLUT4 insertion at muscle cell membrane ruffles. J. Clin. Investig. 2001, 108, 371–381. [Google Scholar] [CrossRef]
- Hehnly, H.; Stamnes, M. Regulating cytoskeleton-based vesicle motility. FEBS Lett. 2007, 581, 2112–2118. [Google Scholar] [CrossRef]
- Sylow, L.; Nielsen, I.L.; Kleinert, M.; Møller, L.L.V.; Ploug, T.; Schjerling, P.; Bilan, P.J.; Klip, A.; Jensen, T.E.; Richter, E.A. Rac1 governs exercise-stimulated glucose uptake in skeletal muscle through regulation of GLUT4 translocation in mice. J. Physiol. 2016, 594, 4997–5008. [Google Scholar] [CrossRef]
- Galigniana, M.D.; Housley, P.R.; DeFranco, D.B.; Pratt, W.B. Inhibition of glucocorticoid receptor nucleocytoplasmic shuttling by okadaic acid requires intact cytoskeleton. J. Biol. Chem. 1999, 274, 16222–16227. [Google Scholar] [CrossRef]
- Davey, G.E.; Murmann, P.; Hoechli, M.; Tanaka, T.; Heizmann, C.W. Calcium-dependent translocation of S100A11 requires tubulin filaments. Biochim. Biophys. Acta-Mol. Cell Res. 2000, 1498, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, R.-M.; Trifaro, J.-M.; Carbajal, M.E.; Okawara, Y.; Vitale, M.L. Calcium-Dependent Actin Filament-Severing Protein Scinderin Levels and Localization in Bovine Testis, Epididymis, and Spermatozoa1. Biol. Reprod. 1999, 60, 1128–1136. [Google Scholar] [CrossRef]
- Lundbaek, J.A.; Andersen, O.S.; Werge, T.; Nielsen, C. Cholesterol-induced protein sorting: An analysis of energetic feasibility. Biophys. J. 2003, 84, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Schekman, R. How sterols regulate protein sorting and traffic. Proc. Natl. Acad. Sci. USA 2007, 104, 6496–6497. [Google Scholar] [CrossRef] [PubMed]
- Coyan, F.C.; Loussouarn, G. Cholesterol regulation of ion channels. Channels 2013, 7, 415–416. [Google Scholar] [CrossRef] [PubMed]
- Zidovetzki, R.; Levitan, I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: Evidence, misconceptions and control strategies. Biochim. Biophys. Acta 2007, 1768, 1311–1324. [Google Scholar] [CrossRef] [PubMed]
- Derler, I.; Jardin, I.; Stathopulos, P.B.; Muik, M.; Fahrner, M.; Zayats, V.; Pandey, S.K.; Poteser, M.; Lackner, B.; Absolonova, M. Cholesterol modulates Orai1 channel function. Sci. Signal. 2016, 9, ra10. [Google Scholar] [CrossRef] [PubMed]
- Panettieri, R.A.; Schaafsma, D.; Amrani, Y.; Koziol-White, C.; Ostrom, R.; Tliba, O. Non-genomic Effects of Glucocorticoids: An Updated View. Trends Pharmacol. Sci. 2019, 40, 38–49. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, C.; Vijayan, M.M. Cortisol Rapidly Facilitates Glucocorticoid Receptor Translocation to the Plasma Membrane in Primary Trout Hepatocytes. Biology 2023, 12, 311. https://doi.org/10.3390/biology12020311
Das C, Vijayan MM. Cortisol Rapidly Facilitates Glucocorticoid Receptor Translocation to the Plasma Membrane in Primary Trout Hepatocytes. Biology. 2023; 12(2):311. https://doi.org/10.3390/biology12020311
Chicago/Turabian StyleDas, Chinmayee, and Mathilakath M. Vijayan. 2023. "Cortisol Rapidly Facilitates Glucocorticoid Receptor Translocation to the Plasma Membrane in Primary Trout Hepatocytes" Biology 12, no. 2: 311. https://doi.org/10.3390/biology12020311
APA StyleDas, C., & Vijayan, M. M. (2023). Cortisol Rapidly Facilitates Glucocorticoid Receptor Translocation to the Plasma Membrane in Primary Trout Hepatocytes. Biology, 12(2), 311. https://doi.org/10.3390/biology12020311




