Induced Models of Osteoarthritis in Animal Models: A Systematic Review
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Selection
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Search
2.5. Data Collection Process
2.6. Data Items
2.7. Risk of Bias
3. Results
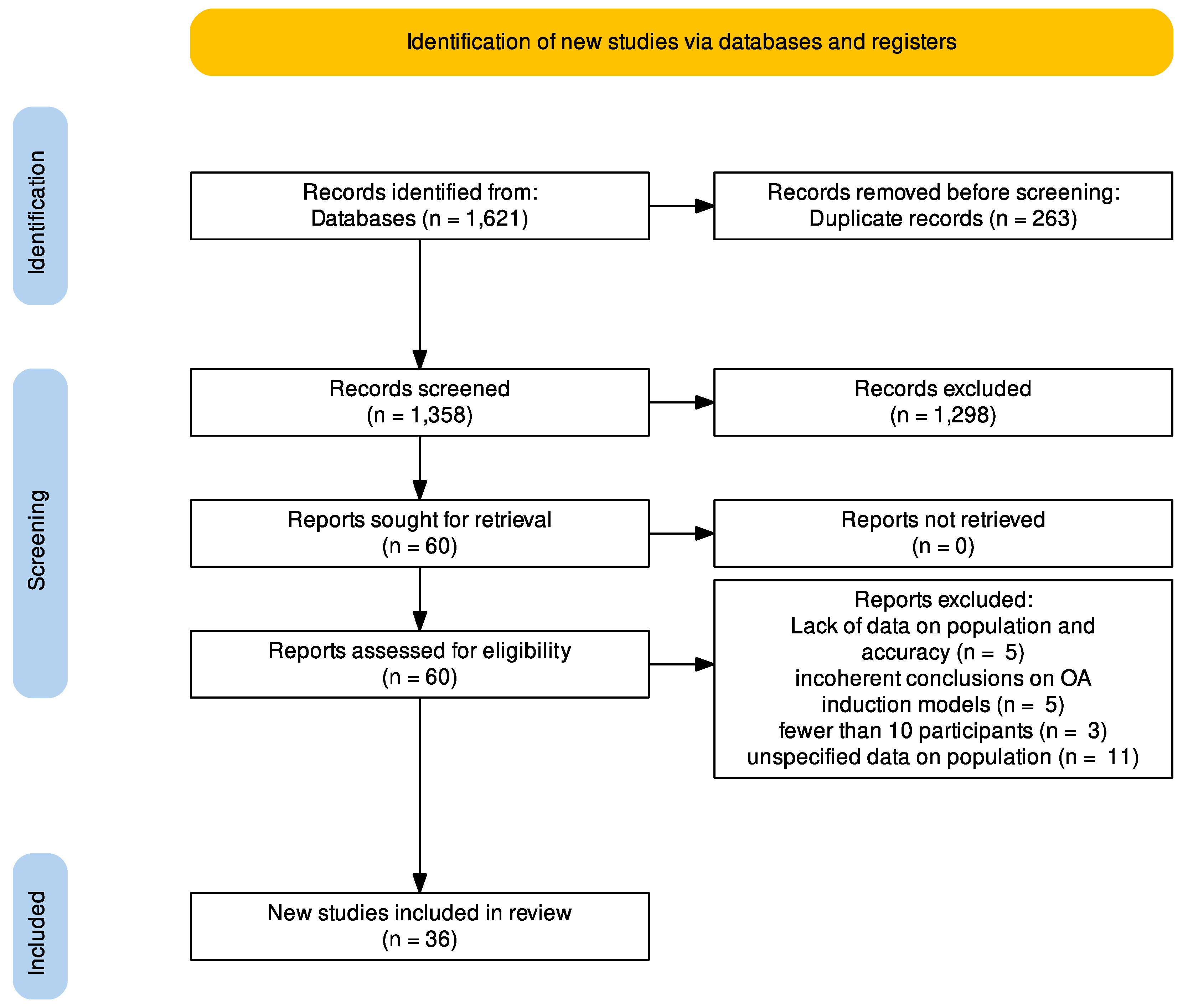
3.1. Study Selection
3.2. Study Characteristics
3.3. Quality of Evidence
3.4. Diagnostic Procedure
3.5. Animal Models
3.6. Type of Induction
3.7. Type of Joint Involved
4. Discussion
4.1. Mechanical Models
4.2. Surgically Induced Models
4.3. Chemical Models
4.4. Animal Models Characteristics
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korostynski, M.; Malek, N.; Piechota, M.; Starowicz, K. Cell-type-specific gene expression patterns in the knee cartilage in an osteoarthritis rat model. Funct. Integr. Genom. 2018, 18, 79–87. [Google Scholar] [CrossRef]
- Longo, U.G.; Loppini, M.; Fumo, C.; Rizzello, G.; Khan, W.S.; Maffulli, N.; Denaro, V. Osteoarthritis: New Insights in Animal Models. Open Orthop. J. 2012, 6, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Arunakul, M.; Tochigi, Y.; Goetz, J.E.; Diestelmeier, B.W.; Heiner, A.D.; Rudert, J.; Fredericks, D.C.; Brown, T.D.; McKinley, T.O. Replication of chronic abnormal cartilage loading by medial meniscus destabilization for modeling osteoarthritis in the rabbit knee in vivo. J. Orthop. Res. 2013, 31, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Sakitani, N.; Iwasawa, H.; Kohara, Y.; Takano, S.; Wakimoto, Y.; Kuroki, H.; Moriyama, H. Thinning of articular cartilage after joint unloading or immobilization. An experimental investigation of the pathogenesis in mice. Osteoarthr. Cartil. 2017, 25, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; McGuinness, L.A. PRISMA2020: R Package and ShinyApp for Producing PRISMA 2020 Compliant Flow Diagrams, Version 0.0.2; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022. [Google Scholar]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (MINORS): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.V.D.; Czeczko, N.G.; Malafaia, O.; Ribas Filho, J.M.; Garcia, J.B.S.; Miguel, M.T.; Zini, C.; Massignan, A.G. Osteoarthritis model induced by intra-articular monosodium iodoacetate in rats knee. Acta Cir. Bras. 2016, 31, 765–773. [Google Scholar] [CrossRef]
- Miyamoto, S.; Nakamura, J.; Ohtori, S.; Orita, S.; Omae, T.; Nakajima, T.; Suzuki, T.; Takahashi, K. Intra-articular injection of mono-iodoacetate induces osteoarthritis of the hip in rats. BMC Musculoskelet Disord. 2016, 17, 132. [Google Scholar] [CrossRef]
- Shuang, F.; Hou, S.-X.; Zhu, J.-L.; Liu, Y.; Zhou, Y.; Zhang, C.-L.; Tang, J.-G. Establishment of a rat model of lumbar facet joint osteoarthritis using intraarticular injection of urinary plasminogen activator. Sci. Rep. 2015, 5, 9828. [Google Scholar] [CrossRef]
- Shuang, F.; Zhu, J.; Song, K.; Hou, S.; Liu, Y.; Zhang, C.; Tang, J. Establishment of a Rat Model of Adjuvant-Induced Osteoarthritis of the Lumbar Facet Joint. Cell Biochem. Biophys. 2014, 70, 1545–1551. [Google Scholar] [CrossRef]
- Adães, S.; Mendonça, M.; Santos, T.N.; Castro-Lopes, J.M.; Ferreira-Gomes, J.; Neto, F.L. Intra-articular injection of collagenase in the knee of rats as an alternative model to study nociception associated with osteoarthritis. Thromb. Haemost. 2014, 16, R10. [Google Scholar] [CrossRef]
- Ferreira-Gomes, J.; Adães, S.; Sousa, R.M.; Mendonça, M.; Castro-Lopes, J.M. Dose-Dependent Expression of Neuronal Injury Markers during Experimental Osteoarthritis Induced by Monoiodoacetate in the Rat. Mol. Pain 2012, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Mohan, G.; Perilli, E.; Kuliwaba, J.S.; Humphries, J.M.; Parkinson, I.H.; Fazzalari, N.L. Application of in vivo micro-computed tomography in the temporal characterisation of subchondral bone architecture in a rat model of low-dose monosodium iodoacetate-induced osteoarthritis. Thromb. Haemost. 2011, 13, R210. [Google Scholar] [CrossRef] [PubMed]
- Ramme, A.J.; Lendhey, M.S.; Strauss, E.J.; Kennedy, O.D. A Biomechanical Study of Two Distinct Methods of Anterior Cruciate Ligament Rupture, and a Novel Surgical Reconstruction Technique, in a Small Animal Model of Posttraumatic Osteoarthritis. J. Knee Surg. 2017, 31, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.F.; Duan, X.; Quirk, J.D.; Holguin, N.; Schmidt, E.J.; Chinzei, N.; Silva, M.J.; Sandell, L.J. Post-Traumatic Osteoarthritis in Mice Following Mechanical Injury to the Synovial Joint. Sci. Rep. 2017, 7, 45223. [Google Scholar] [CrossRef]
- McCulloch, R.S.; Ashwell, M.S.; Maltecca, C.; O’Nan, A.T.; Mente, P.L. Progression of Gene Expression Changes following a Mechanical Injury to Articular Cartilage as a Model of Early Stage Osteoarthritis. Arthritis 2014, 2014, 371426. [Google Scholar] [CrossRef]
- Qin, J.; Chow, S.-H.; Guo, A.; Wong, W.-N.; Leung, K.-S.; Cheung, W.-H. Low magnitude high frequency vibration accelerated cartilage degeneration but improved epiphyseal bone formation in anterior cruciate ligament transect induced osteoarthritis rat model. Osteoarthr. Cartil. 2014, 22, 1061–1067. [Google Scholar] [CrossRef][Green Version]
- Horisberger, M.; Fortuna, R.; Valderrabano, V.; Herzog, W. Long-term repetitive mechanical loading of the knee joint by in vivo muscle stimulation accelerates cartilage degeneration and increases chondrocyte death in a rabbit model. Clin. Biomech. 2013, 28, 536–543. [Google Scholar] [CrossRef]
- Roemhildt, M.; Beynnon, B.; Gauthier, A.; Gardner-Morse, M.; Ertem, F.; Badger, G. Chronic in vivo load alteration induces degenerative changes in the rat tibiofemoral joint. Osteoarthr. Cartil. 2013, 21, 346–357. [Google Scholar] [CrossRef]
- O’Brien, E.J.; Beveridge, J.E.; Huebner, K.D.; Heard, B.J.; Tapper, J.E.; Shrive, N.G.; Frank, C.B. Osteoarthritis develops in the operated joint of an ovine model following ACL reconstruction with immediate anatomic reattachment of the native ACL. J. Orthop. Res. 2012, 31, 35–43. [Google Scholar] [CrossRef]
- Moodie, J.; Stok, K.; Müller, R.; Vincent, T.; Shefelbine, S. Multimodal imaging demonstrates concomitant changes in bone and cartilage after destabilisation of the medial meniscus and increased joint laxity. Osteoarthr. Cartil. 2011, 19, 163–170. [Google Scholar] [CrossRef]
- Vaseenon, T.; Tochigi, Y.; Heiner, A.D.; Goetz, J.; Baer, T.E.; Fredericks, D.C.; Martin, J.; Rudert, M.J.; Hillis, S.; Brown, T.D.; et al. Organ-level histological and biomechanical responses from localized osteoarticular injury in the rabbit knee. J. Orthop. Res. 2010, 29, 340–346. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Christiansen, B.; Anderson, M.; Lee, C.; Williams, J.; Yik, J.; Haudenschild, D. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthr. Cartil. 2012, 20, 773–782. [Google Scholar] [CrossRef]
- Killian, M.L.; Isaac, D.I.; Haut, R.C.; Déjardin, L.M.; Leetun, D.; Donahue, T.L.H. Traumatic anterior cruciate ligament tear and its implications on meniscal degradation: A preliminary novel lapine osteoarthritis model. J. Surg. Res. 2010, 164, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.C.; Dragomir, C.; Plumb, D.A.; Goldring, S.R.; Wright, T.M.; Goldring, M.B.; van der Meulen, M.C.H. In Vivo Cyclic Compression Causes Cartilage Degeneration and Subchondral Bone Changes in Mouse Tibiae. Arthritis Rheum. 2013, 65, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.C.; Dragomir, C.L.; Plumb, D.A.; Hsia, A.W.; Adebayo, O.O.; Goldring, S.R.; Wright, T.M.; Goldring, M.B.; van der Meulen, M.C. Progressive cell-mediated changes in articular cartilage and bone in mice are initiated by a single session of controlled cyclic compressive loading. J. Orthop. Res. 2016, 34, 1941–1949. [Google Scholar] [CrossRef]
- Lewis, J.; Hembree, W.; Furman, B.; Tippets, L.; Cattel, D.; Huebner, J.; Little, D.; DeFrate, L.; Kraus, V.; Guilak, F.; et al. Acute joint pathology and synovial inflammation is associated with increased intra-articular fracture severity in the mouse knee. Osteoarthr. Cartil. 2011, 19, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.E.; Main, R.P.; Xu, Q.; Walsh, D.J.; Schaffler, M.B.; Wright, T.M.; van der Meulen, M.C.H. Cancellous bone adaptation to tibial compression is not sex dependent in growing mice. J. Appl. Physiol. 2010, 109, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, K.A.; Chu, B.T.; Anderson, M.J.; Haudenschild, D.R.; Christiansen, B.A. Comparison of loading rate-dependent injury modes in a murine model of post-traumatic osteoarthritis. J. Orthop. Res. 2014, 32, 79–88. [Google Scholar] [CrossRef]
- Onur, T.S.; Wu, R.; Chu, S.; Chang, W.; Kim, H.T.; Dang, A.B. Joint instability and cartilage compression in a mouse model of posttraumatic osteoarthritis. J. Orthop. Res. 2014, 32, 318–323. [Google Scholar] [CrossRef]
- Poulet, B.; Hamilton, R.W.; Shefelbine, S.; Pitsillides, A.A. Characterizing a novel and adjustable noninvasive murine joint loading model. Arthritis Rheum. 2011, 63, 137–147. [Google Scholar] [CrossRef]
- Poulet, B.; Westerhof, T.; Hamilton, R.; Shefelbine, S.; Pitsillides, A. Spontaneous osteoarthritis in Str/ort mice is unlikely due to greater vulnerability to mechanical trauma. Osteoarthr. Cartil. 2013, 21, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Poulet, B.; de Souza, R.; Kent, A.; Saxon, L.; Barker, O.; Wilson, A.; Chang, Y.-M.; Cake, M.; Pitsillides, A. Intermittent applied mechanical loading induces subchondral bone thickening that may be intensified locally by contiguous articular cartilage lesions. Osteoarthr. Cartil. 2015, 23, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Holguin, N.; Silva, M.J.; Fu, M.; Liao, W.; Sandell, L.J. Early response of mouse joint tissue to noninvasive knee injury suggests treatment targets. Arthritis Rheumatol. 2014, 66, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- McNulty, M.; Loeser, R.; Davey, C.; Callahan, M.; Ferguson, C.; Carlson, C. Histopathology of naturally occurring and surgically induced osteoarthritis in mice. Osteoarthr. Cartil. 2012, 20, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.D.; A Mata, B.; Gabr, M.; Huebner, J.L.; Adams, S.B.; Kraus, V.B.; O Schmitt, D.; A Setton, L. Kinematic and dynamic gait compensations resulting from knee instability in a rat model of osteoarthritis. Thromb. Haemost. 2012, 14, R78. [Google Scholar] [CrossRef]
- Fischenich, K.; Pauly, H.; Button, K.; Fajardo, R.; DeCamp, C.; Haut, R.; Donahue, T.H. A study of acute and chronic tissue changes in surgical and traumatically-induced experimental models of knee joint injury using magnetic resonance imaging and micro-computed tomography. Osteoarthr. Cartil. 2016, 25, 561–569. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maerz, T.; Newton, M.D.; Kurdziel, M.D.; Altman, P.; Anderson, K.; Matthew, H.; Baker, K.C. Articular cartilage degeneration following anterior cruciate ligament injury: A comparison of surgical transection and noninvasive rupture as preclinical models of post-traumatic osteoarthritis. Osteoarthr. Cartil. 2016, 24, 1918–1927. [Google Scholar] [CrossRef]
- Satkunananthan, P.; Anderson, M.; De Jesus, N.; Haudenschild, D.; Ripplinger, C.; Christiansen, B. In vivo fluorescence reflectance imaging of protease activity in a mouse model of post-traumatic osteoarthritis. Osteoarthr. Cartil. 2014, 22, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, P.A.; Murawski, C.D.; Hunt, K.J.; Andrews, C.L.; Longo, U.G.; McCollum, G.; Simpson, H.; Sofka, C.M.; Yoshimura, I.; Karlsson, J. Post-treatment Follow-up, Imaging, and Outcome Scores: Proceedings of the International Consensus Meeting on Cartilage Repair of the Ankle. Foot Ankle. Int. 2018, 39 (Suppl. 1), 68S–73S. [Google Scholar] [CrossRef]
- Longo, U.; Candela, V.; Berton, A.; De Salvatore, S.; Fioravanti, S.; Giannone, L.; Marchetti, A.; De Marinis, M.; Denaro, V. Biosensors for Detection of Biochemical Markers Relevant to Osteoarthritis. Biosensors 2021, 11, 31. [Google Scholar] [CrossRef]
- Lockwood, S.; Lopes, D.; McMahon, S.; Dickenson, A. Characterisation of peripheral and central components of the rat monoiodoacetate model of Osteoarthritis. Osteoarthr. Cartil. 2019, 27, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, B.; Guilak, F.; Lockwood, K.; Olson, S.; Pitsillides, A.; Sandell, L.; Silva, M.; van der Meulen, M.; Haudenschild, D. Non-invasive mouse models of post-traumatic osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- Poulet, B. Non-invasive Loading Model of Murine Osteoarthritis. Curr. Rheumatol. Rep. 2016, 18, 40. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Turkiewicz, A.; Petersson, I.; Björk, J.; Hawker, G.; Dahlberg, L.; Lohmander, L.; Englund, M. Current and future impact of osteoarthritis on health care: A population-based study with projections to year 2032. Osteoarthr. Cartil. 2014, 22, 1826–1832. [Google Scholar] [CrossRef]
- Lorenz, J.; Grässel, S. Experimental Osteoarthritis Models in Mice. Mouse Genet. Methods Protoc. 2014, 1194, 401–419. [Google Scholar]
- Foxa, G.E.; Liu, Y.; Turner, L.M.; Robling, A.G.; Yang, T.; Williams, B.O. Generation and Characterization of Mouse Models for Skeletal Disease. Methods Mol. Biol. 2021, 2221, 165–191. [Google Scholar] [PubMed]
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal models of osteoarthritis: Classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 2016, 11, 19. [Google Scholar] [CrossRef]
- Pitcher, T.; Sousa-Valente, J.; Malcangio, M. The Monoiodoacetate Model of Osteoarthritis Pain in the Mouse. J. Vis. Exp. 2016, 111, e53746. [Google Scholar]
- Sulaiman, S.Z.S.; Tan, W.M.; Radzi, R.; Shafie, I.N.F.; Ajat, M.; Mansor, R.; Mohamed, S.; Rahmad, N.; Ng, A.M.H.; Lau, S.F. Synovial fluid proteome profile of surgical versus chemical induced osteoarthritis in rabbits. Peerj 2022, 10, e12897. [Google Scholar] [CrossRef]
- McCoy, A.M. Animal Models of Osteoarthritis: Comparisons and Key Considerations. Vet. Pathol. 2015, 52, 803–818. [Google Scholar] [CrossRef]
- Serra, C.I.; Soler, C. Animal Models of Osteoarthritis in Small Mammals. Veter- Clin. North Am. Exot. Anim. Pr. 2019, 22, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.-F.; Xue, Y.; Zhang, J.-P.; Zhang, Z.-Q.; Li, W.-Y.; Cao, Y.-L.; Xu, J.-G. Similarities and differences between rat and mouse chondrocyte gene expression induced by IL-1β. J. Orthop. Surg. Res. 2022, 17, 70. [Google Scholar] [CrossRef] [PubMed]
- Longo, U.G.; Loppini, M.; Romeo, G.; Maffulli, N.; Denaro, V. Histological scoring systems for tissue-engineered, ex vivo and degenerative meniscus. Knee Surgery Sports Traumatol. Arthrosc. 2012, 21, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Longo, U.; Forriol, F.; Candela, V.; Tecce, S.; De Salvatore, S.; Altonaga, J.; Wallace, A.; Denaro, V. Arthroscopic Tenotomy of the Long Head of the Biceps Tendon and Section of the Anterior Joint Capsule Produce Moderate Osteoarthritic Changes in an Experimental Sheep Model. Int. J. Environ. Res. Public Heal. 2021, 18, 7471. [Google Scholar] [CrossRef] [PubMed]
| Author | Level of Evidence | Year | Animal | Sex | N of Samples | Induction | Type of Induction | Joint Involved | Endpoint |
|---|---|---|---|---|---|---|---|---|---|
| Adães et al. [11] | LOE 4 | 2014 | Wistar rats | M | 72 (n = 24) | C | Two IAI of collagenase | Knee | 1, 2, 3, 4, 5, 6 wk post-inj. |
| Allen, Kyle D et al. [36] | LOE 4 | 2012 | Lewis rats | U | 16 (n = 3) | S | MCL was transected | Knee | 9-24-28 d |
| Arunakul et al. [3] | LOE 4 | 2013 | NZW rabbits | M | 26 (n = 5) | S | MMD | Knee | 8 wk |
| Christiansen et al. [23] | LOE 4 | 2012 | C57BL/6N mice | M | 48 | M | Single overload cycle of tibial compression | Knee | 1, 3, 7, 14, 28, or 56 d |
| Etienne J O O’Brien et al. [20] | LOE 4 | 2012 | SC sheep | F | 29 (n = 5, n = 7) | S | Transection of ACL with consequent reconstruction | Knee | 0-4-20 wk |
| Farooq Rai et al. [15] | LOE 4 | 2017 | LGXSM-6 and LGXSM-33 mice | M | 88 (n = 44) | M | Axial tibial compression | Knee | 5, 9, 14, 28, 56 d |
| Fischenich et al. [37] | LOE 4 | 2016 | FG rabbits | M and F | 33 | S | ACLT, ACLF and mACLT | Knee | 4, 8, 12 wk |
| Geetha Mohan et al. [13] | LOE 4 | 2011 | Wistar rats | M | 12 | C | Injection of MIA | Knee | 0-2-6-10 wk |
| Horisberger et al. [18] | LOE 4 | 2013 | NZW rabbits | M | 24 (n = 8) | M | Joint loading by muscle stimulation | Knee | NS |
| Joana Ferreira-Gomes et al. [12] | LOE 4 | 2012 | Wistar rats | M | 54 (n = 5) | C | Injection of MIA | Knee | 3-7-14-21-31 d after MIA injection |
| Killian et al. [24] | LOE 4 | 2010 | FG rabbits | U | 11 | M | Tibiofemoral impaction resulting in ACL rupture or surgical ACL transection | Knee | 12 wk for traumatically torn and after 12 wk in ACL transected animals |
| Ko et al. [25] | LOE 4 | 2013 | C57BL/6 mice | M | 63 | M | Cyclic compression | Knee | 1, 2 and 6 wk |
| Ko et al. [26] | LOE 4 | 2016 | C57Bl/6 mice | M | 21 | M | VCL | Knee | 0, 1 and 2 wk |
| Korostynski et al. [1] | LOE 4 | 2018 | Wistar rats | M | 32 (n = 8) | C | IAI of MIA | Knee | 2, 14, 28 d |
| Lewis et al. [27] | LOE 4 | 2011 | C57BL/6 mice | M | 50 (n = 25) | M | Low and high energy fractures after loading | Knee | 0, 1, 3, 5 and 7 d post-fracture |
| Lynch et al. [28] | LOE 4 | 2010 | C57BL/6 mice | M and F | n = 14/sex | M | Dynamic compression of the left tibia | Knee | 2 wk of load evaluation |
| Lockwood et al. [29] | LOE 4 | 2013 | C57BL/6N mice | M | 80 | M | Tibial compressive overload | Knee | 0 d, 10 d, 12 wk or 16 wk |
| Maerz et al. [38] | LOE 4 | 2016 | Lewis rats | F | 36 (n = 6) | S | ACL rupture or ACL transection | Knee | 4 or 10 wk |
| McCulloch et al. [16] | LOE 4 | 2014 | Pig | U | 36 | M | Shear loading model | Knee | 3, 7, 14 d |
| McNulty et al. [35] | LOE 4 | 2012 | C57BL/6 mice | M | 11 | S | DDM surgery, Sham surgery | Knee | 2 months |
| Miyamoto et al. [8] | LOE 4 | 2016 | SD rats | M | 60 (n = 30) | C | IAI of MIA | Hip | 7, 14, 28, 42, 56 d |
| M.L. Roemhildt et al. [19] | LOE 4 | 2012 | SD rats | M | 25 (n = 5) | M | VLD implement | Knee | 0-6-20 wk |
| Moodie, J.P. et al. [21] | LOE 4 | 2011 | C57Bl6 mice | M | 38 | S | DMM surgery | Knee | 0-4-8 wk |
| Morais SV et al. [7] | LOE 4 | 2016 | Wistar rats | M | 48 (n = 24) | C | IAI of MIA | Knee | 1, 7, 14, 21, 28 d |
| Nomura et al. [4] | LOE 4 | 2017 | C57BL/6J mice | M | 24 (12 × 2; n = 4) | M | Hindlimb unloading | Knee | 2, 4, 8 wk |
| Onur et al. [30] | LOE 4 | 2013 | FVB strain mice | U | 21 | M | Axial compression | Knee | 1, 8 wk |
| Poulet et al. [31] | LOE 4 | 2011 | CBA mice | U | 35 | M | Cyclic loading compression | Knee | 2, 3 and 5 wk |
| Poulet et al. [32] | LOE 4 | 2013 | Str/ort mice | M | n = 3, n = 6, n = 8 | M | Compression with servo-hydraulic materials | Knee | 2 wk |
| Poulet et al. [33] | LOE 4 | 2015 | CBA mice | M | n = 8, n = 8, n = 5 | M | Compression with servo-hydraulic materials | Knee | 10, 13 wk |
| Qin et al. [17] | LOE 4 | 2014 | SD rats | F | 88 | S | ACLT | Knee | 6, 12, 18 wk |
| Ramme et al. [14] | LOE 4 | 2017 | SD rats | F | 24 | M | ACL biomechanical rupture and surgical transection | Knee | U |
| Satkunananthan et al. [39] | LOE 4 | 2014 | C57BL/6 mice | M and F | 54 | M | ACL rupture induced by tibial compression | Knee | 1-7 d |
| Shuang et al. [10] | LOE 4 | 2014 | SD rats | M | 60 | C | IAI of complete Freund’s adjuvant [9] | Spine | 3, 7, 14, 21 and 28 d |
| Shuang et al. [9] | LOE 4 | 2015 | SD rats | M | n = 88 | C | IAI of uPA [9] | Spine | 3, 7, 14, 28, 42, 56 d |
| Vaseenon, Tanawa et al. [22] | LOE 4 | 2011 | NZW rabbits | U | 40 | S | Sham surgery | Knee | 0-8-16 wk |
| Wu et al. [34] | LOE 4 | 2014 | C57BL/6 mice | M | n = 54 | M | Compressive joint loading | Knee | 5, 9, 14 d |
| Author | OA Score | Conclusions |
|---|---|---|
| Adães et al. [11] | Knee-bend and catwalk | It induces significant nociceptive alterations associated with OA changes |
| Allen, Kyle D et al. [36] | OARSI score present | First description of functional losses after medial meniscus injury in the rat with reported changes in gait dynamics |
| Arunakul et al. [3] | Mankin score | Results support the MMD technique as being an effective surgical insult modality to replicate injurious cumulative abnormal cartilage loading |
| Christiansen et al. [23] | OARSI scale | This model is a significant improvement over other mouse models of PTOA, since it induces an injury that is translatable to humans, easy to implement and highly reproducible. |
| Etienne J O O’Brien et al. [20] | Hellio Le Graverand protocol | Shows how a reconstruction model is not really helpful |
| Farooq Rai et al. [15] | High mechanical loading instigated whole knee-joint changes leading to PTOA | |
| Fischenich et al. [37] | Dependency of the model on the location, type and progression of damage over time | |
| Geetha Mohan et al. [13] | OARSI score = 17 | Low-dose MIA-induced OA rat model mimics the human disease condition and clearly demonstrates disease progression in the tibial subchondral bone in a timely manner |
| Horisberger et al. [18] | Mankin score | Muscular loading of physiological magnitude but excessive intensity caused chondrocyte deaths and onset of early OA in rabbit knees |
| Joana Ferreira-Gomes et al. [12] | Knee-bend and catwalk | Concentrated more on the neuronal aspect rather than the causes of OA, suggests that axonal injury and a regeneration response may be happening in this model of OA |
| Killian et al. [24] | This study has implications for the future use of lapine models for osteoarthritis, as it incorporates traumatic loading as a more realistic progression of osteoarthritis compared with surgically transected models | |
| Ko et al. [25] | OARSI scale | Noninvasive loading model, permits dissection of temporal and topographic changes in cartilage and bone |
| Ko et al. [26] | Osteomeasure histomorphometry system, OsteoMetrics, Decatur, GA | Demonstrate that a single session of noninvasive loading leads to the development of OA |
| Korostynski et al. [1] | The progression of cartilage damage is driven by the complex but precise regulation of gene patterns | |
| Lewis et al. [27] | Mankin score | This study demonstrates that articular fracture is associated with a loss of chondrocyte viability and increased levels of systemic biomarkers |
| Lynch et al. [28] | For all cancellous measures, the response to tibial compression did not differ between male and female mice | |
| Lockwood et al. [29] | OARSI score | These studies further characterize the non-invasive knee-injury mouse model |
| Maerz et al. [38] | Mankin score | AC degeneration is a time-, compartment- and injury-dependent cascade |
| McCulloch et al. [16] | The chondrocytes in the shear specimens are attempting repairs but are unable to mount a successful effort | |
| McNulty et al. [35] | Mankin score | Provided surgically induced models using a comprehensive histological grading scheme |
| Miyamoto et al. [8] | Mankin score | Intra-articular injection of MIA consistently causes progressive hip OA |
| M.L. Roemhildt et al. [19] | Shows VLD is a better replicate of how OA really develops | |
| Moodie et al. [21] | Graded but without scaling grade | Increase in AP laxity suggests that DMM surgery redistributes loading posteriorly on the medial plateau, resulting in bone and cartilage loss |
| Morais SV et al. [7] | OA induced by intra-articular MIA is a good model to be used in related research | |
| Nomura et al. [4] | Thinning of articular cartilage induced by mechanical unloading may be mediated by metabolic changes in chondrocytes | |
| Onur et al. [30] | Pritzker grading scale | Axial compression without joint instability does not appear to be sufficient for inducing PTOA |
| Poulet et al. [31] | This model offers opportunities to study the effects of various loading magnitudes and regimens on joint health and disease | |
| Poulet et al. [32] | Load application appears to accelerate OA | |
| Poulet et al. [33] | Concomitant AC-damage aggravates focal SCB-thickening induced by applied loading | |
| Qin et al. [17] | OARSI score | LMHFV accelerated cartilage degeneration and deterioration of OA and promoted bone formation in affected distal femur epiphysis |
| Ramme et al. [14] | ACL reconstruction and joint assessment will be useful in future joint-injury/PTOA studies in small-animal models | |
| Satkunananthan et al. [39] | OARSI scale | Describes the dynamic protease profile following traumatic knee injury and establishes FRI as a useful analysis method |
| Shuang et al. [10] | OARSI score | Injection of complete Freund’s adjuvant caused local synovitis in early stage, and the inflammation was similar with osteoarthritis |
| Shuang et al. [9] | OARSI score | This animal model is convenient and shows good resemblance of human facet joint OA pathology |
| Vaseenon, Tanawa et al. [22] | Mankin score | The accompanying localized incongruity involved the onset of biomechanical abnormality consistent with causing cartilage degeneration |
| Wu et al. [34] | Established a murine model of knee-joint trauma with different degrees of overloading in vivo |
| Author | Clearly Stated Aim | Inclusion of Consecutive Patients | Prospective Data Collection | Checkpoints Appropriate to Study Aim | Unbiased Assessment of Study Endpoint | Follow-Up Period | Appropriate to <5% Lost to Follow-Up | Prospective Calculation of Study Size | Total Score |
|---|---|---|---|---|---|---|---|---|---|
| Adães, Mendonça et al., 2014 [11] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| Allen, Mata et al., 2012 [36] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| Arunakul, Tochigi et al., 2013 [3] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| Christiansen et al., 2012 [23] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| Fischenich, Pauly et al., 2017 [37] | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 12 |
| Mohan, Perilli et al., 2011 [13] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| Horisberger, Fortuna et al., 2013 [18] | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 2 | 8 |
| Ferreira-Gomes, Adães et al., 2012 [12] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| Killian et al., 2010 [24] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| Ko et al., 2013 [25] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| Ko et al., 2016 [26] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| Korostynski, Malek et al., 2018 [1] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| Lewis et al., 2011 [27] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| Lynch et al., 2010 [28] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| Lockwook et al., 2014 [29] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| Maerz et al., 2016 [38] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| McCulloch et al., 2014 [16] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| McNulty et al., 2012 [35] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| Miyamoto, Nakamura et al., 2016 [8] | 2 | 2 | 2 | 1 | 0 | 0 | 0 | 2 | 9 |
| Moodie et al., 2011 [21] | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 2 | 8 |
| Morais, Czeczko et al., 2016 [7] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| Nomura, Sakitani et al., 2017 [4] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| O’Brien et al., 2012 [20] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| Onur et al., 2013 [30] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| Poulet et al., 2011 [31] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| Poulet et al. 2013 [32] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| Poulet et al., 2015 [33] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| Qin, Chow et al., 2014 [17] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| Ramme, Lendhey et al., 2018 [14] | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 2 | 8 |
| Rai et al. 2017 [15] | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 2 | 10 |
| Roemhildt, Beynnon et al., 2013 [19] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| Satkunananthan et al., 2014 [39] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| Shuang, Hou et al., 2015 [9] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| Shuang, Zhu et al., 2014 [10] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| Vaseenon, Tochigi et al., 2011 [22] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
| Wu et al., 2014 [34] | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longo, U.G.; Papalia, R.; De Salvatore, S.; Picozzi, R.; Sarubbi, A.; Denaro, V. Induced Models of Osteoarthritis in Animal Models: A Systematic Review. Biology 2023, 12, 283. https://doi.org/10.3390/biology12020283
Longo UG, Papalia R, De Salvatore S, Picozzi R, Sarubbi A, Denaro V. Induced Models of Osteoarthritis in Animal Models: A Systematic Review. Biology. 2023; 12(2):283. https://doi.org/10.3390/biology12020283
Chicago/Turabian StyleLongo, Umile Giuseppe, Rocco Papalia, Sergio De Salvatore, Riccardo Picozzi, Antonio Sarubbi, and Vincenzo Denaro. 2023. "Induced Models of Osteoarthritis in Animal Models: A Systematic Review" Biology 12, no. 2: 283. https://doi.org/10.3390/biology12020283
APA StyleLongo, U. G., Papalia, R., De Salvatore, S., Picozzi, R., Sarubbi, A., & Denaro, V. (2023). Induced Models of Osteoarthritis in Animal Models: A Systematic Review. Biology, 12(2), 283. https://doi.org/10.3390/biology12020283








