The Contribution of the Sheep and the Goat Model to the Study of Ovarian Ageing
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Collection
2.2. Paraffin Embedding
2.3. Haematoxylin and Eosin Staining
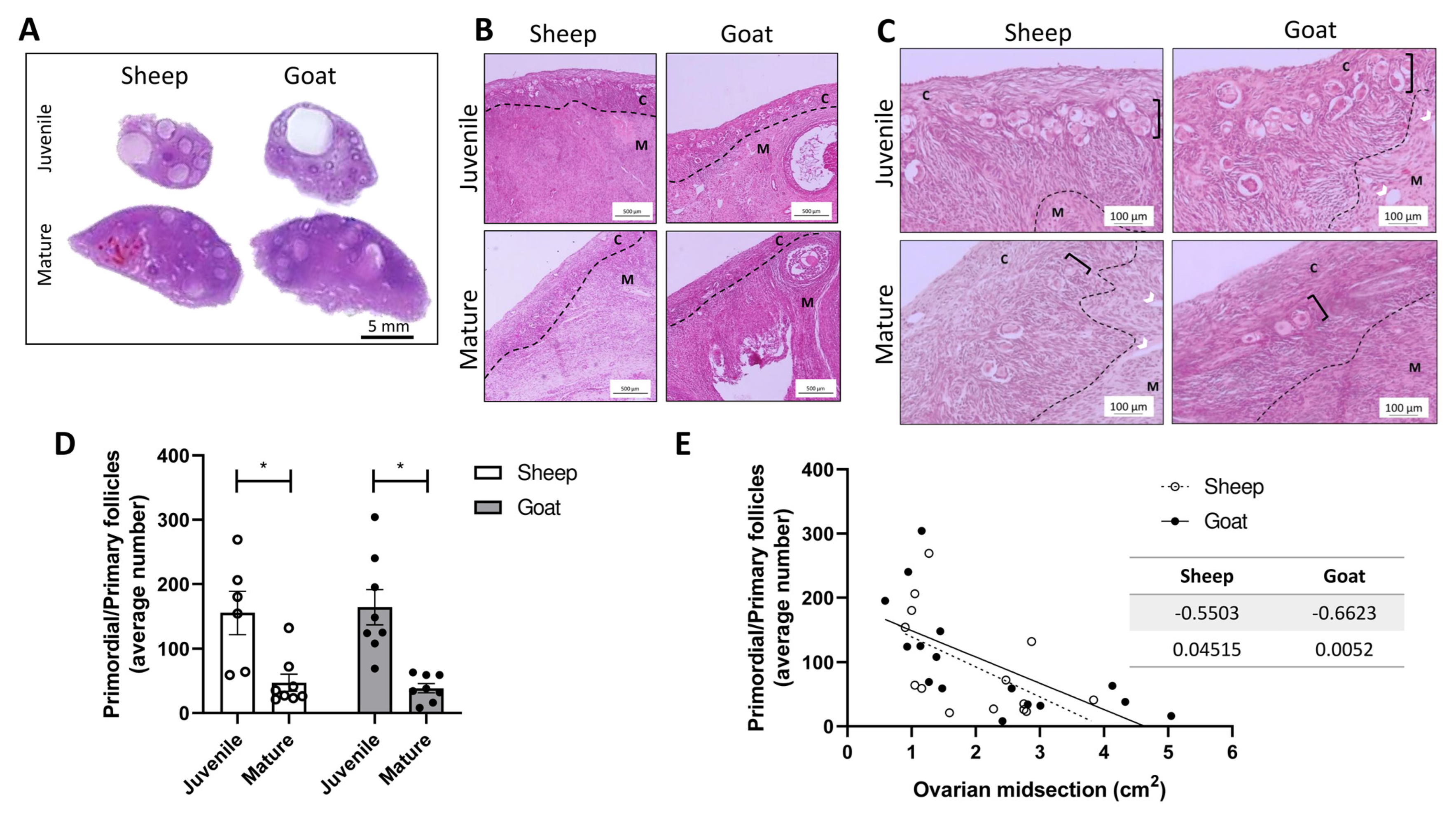
2.4. Follicle Counting at Ovarian Midsection
2.5. Determination of Ovarian Midsection Area
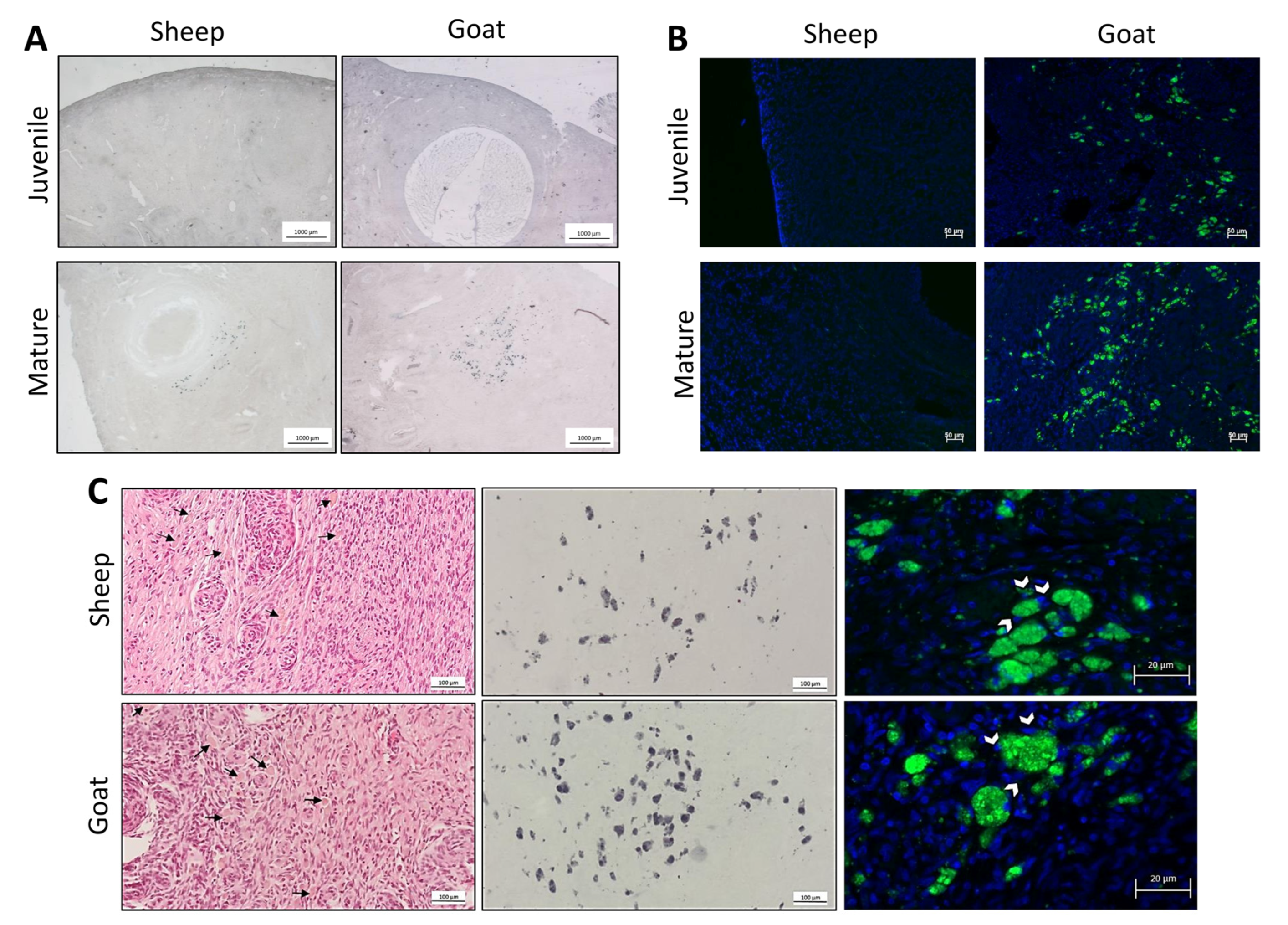
2.6. Sudan Black B Staining
2.7. Autofluorescence Detection
2.8. Statistical Analysis
3. Results
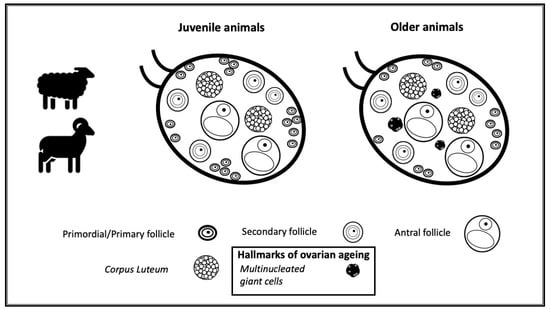
3.1. Morphological Analysis of Goats and Sheep Ovarian Tissue
3.2. Identification of the Population of Cells Considered Hallmarks of Ovarian Ageing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mills, M.; Rindfuss, R.R.; McDonald, P.; te Velde, E.; Force, E.R.A.S.T. Why do people postpone parenthood? Reasons and social policy incentives. Hum. Reprod. Update 2011, 17, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Shirasuna, K.; Iwata, H. Effect of aging on the female reproductive function. Contracept. Reprod. Med. 2017, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.R.; Knowlton, N.S.; Thyer, A.C.; Charleston, J.S.; Soules, M.R.; Klein, N.A. A new model of reproductive aging: The decline in ovarian non-growing follicle number from birth to menopause. Hum. Reprod. 2008, 23, 699–708. [Google Scholar] [CrossRef] [PubMed]
- te Velde, E.R.; Pearson, P.L. The variability of female reproductive ageing. Hum. Reprod. Update 2002, 8, 141–154. [Google Scholar] [CrossRef]
- Wallace, W.H.; Kelsey, T.W. Human ovarian reserve from conception to the menopause. PLoS ONE 2010, 5, e8772. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Song, Y.; Wang, L.; Ai, J.; Li, K. Aging conundrum: A perspective for ovarian aging. Front. Endocrinol. 2022, 13, 952471. [Google Scholar] [CrossRef]
- Lim, J.; Luderer, U. Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Biol. Reprod. 2011, 84, 775–782. [Google Scholar] [CrossRef]
- Matos, L.; Stevenson, D.; Gomes, F.; Silva-Carvalho, J.L.; Almeida, H. Superoxide dismutase expression in human cumulus oophorus cells. Mol. Hum. Reprod. 2009, 15, 411–419. [Google Scholar] [CrossRef]
- Timóteo-Ferreira, F.; Mendes, S.; Rocha, N.A.; Matos, L.; Rodrigues, A.R.; Almeida, H.; Silva, E. Apocynin Dietary Supplementation Delays Mouse Ovarian Ageing. Oxidative Med. Cell Longev. 2019, 2019, 5316984. [Google Scholar] [CrossRef]
- Titus, S.; Li, F.; Stobezki, R.; Akula, K.; Unsal, E.; Jeong, K.; Dickler, M.; Robson, M.; Moy, F.; Goswami, S.; et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci. Transl. Med. 2013, 5, 172ra121. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Zeng, M.; Yuan, J.; Liu, M.; Yin, Y.; Wu, X.; Keefe, D.L.; Liu, L. Increased DNA damage and repair deficiency in granulosa cells are associated with ovarian aging in rhesus monkey. J. Assist. Reprod. Genet. 2015, 32, 1069–1078. [Google Scholar] [CrossRef]
- Tatone, C.; Heizenrieder, T.; Di Emidio, G.; Treffon, P.; Amicarelli, F.; Seidel, T.; Eichenlaub-Ritter, U. Evidence that carbonyl stress by methylglyoxal exposure induces DNA damage and spindle aberrations, affects mitochondrial integrity in mammalian oocytes and contributes to oocyte ageing. Hum. Reprod. 2011, 26, 1843–1859. [Google Scholar] [CrossRef] [PubMed]
- Tatone, C.; Carbone, M.C.; Falone, S.; Aimola, P.; Giardinelli, A.; Caserta, D.; Marci, R.; Pandolfi, A.; Ragnelli, A.M.; Amicarelli, F. Age-dependent changes in the expression of superoxide dismutases and catalase are associated with ultrastructural modifications in human granulosa cells. Mol. Hum. Reprod. 2006, 12, 655–660. [Google Scholar] [CrossRef] [PubMed]
- de Bruin, J.P.; Dorland, M.; Spek, E.R.; Posthuma, G.; van Haaften, M.; Looman, C.W.; te Velde, E.R. Age-related changes in the ultrastructure of the resting follicle pool in human ovaries. Biol. Reprod. 2004, 70, 419–424. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, R.; Hernández, J.; Martín-Vasallo, P.; Puopolo, M.; Palumbo, A.; Ávila, J. Expression Levels of the Oxidative Stress Response Gene ALDH3A2 in Granulosa-Lutein Cells Are Related to Female Age and Infertility Diagnosis. Reprod. Sci. 2016, 23, 604–609. [Google Scholar] [CrossRef]
- Ribeiro, A.; Freitas, C.; Matos, L.; Gouveia, A.; Gomes, F.; Silva Carvalho, J.L.; Almeida, H. Age-related expression of TGF beta family receptors in human cumulus oophorus cells. J. Assist. Reprod. Genet. 2017, 34, 1121–1129. [Google Scholar] [CrossRef]
- Wang, L.; Tang, J.; Tan, F.; Song, H.; Zhou, J.; Li, F. Oxidative stress in oocyte aging and female reproduction. J. Cell Physiol. 2021, 236, 7966–7983. [Google Scholar] [CrossRef]
- Briley, S.M.; Jasti, S.; McCracken, J.M.; Hornick, J.E.; Fegley, B.; Pritchard, M.T.; Duncan, F.E. Reproductive age-associated fibrosis in the stroma of the mammalian ovary. Reproduction 2016, 152, 245–260. [Google Scholar] [CrossRef]
- Ansere, V.A.; Ali-Mondal, S.; Sathiaseelan, R.; Garcia, D.N.; Isola, J.V.V.; Henseb, J.D.; Saccon, T.D.; Ocañas, S.R.; Tooley, K.B.; Stout, M.B.; et al. Cellular hallmarks of aging emerge in the ovary prior to primordial follicle depletion. Mech. Ageing Dev. 2021, 194, 111425. [Google Scholar] [CrossRef]
- Vollenhoven, B.; Hunt, S. Ovarian ageing and the impact on female fertility. F1000Research 2018, 7, 1. [Google Scholar] [CrossRef]
- Foley, K.G.; Pritchard, M.T.; Duncan, F.E. Macrophage-derived multinucleated giant cells: Hallmarks of the aging ovary. Reproduction 2021, 161, V5–V9. [Google Scholar] [CrossRef]
- Alvites, R.D.; Branquinho, M.V.; Sousa, A.C.; Lopes, B.; Sousa, P.; Mendonça, C.; Atayde, L.M.; Maurício, A.C. Small Ruminants and Its Use in Regenerative Medicine: Recent Works and Future Perspectives. Biology 2021, 10, 249. [Google Scholar] [CrossRef]
- Feldman, A.T.; Wolfe, D. Tissue processing and hematoxylin and eosin staining. Methods Mol. Biol. 2014, 1180, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Salmonowicz, H.; Passos, J.F. Detecting senescence: A new method for an old pigment. Aging Cell 2017, 16, 1439. [Google Scholar] [CrossRef] [PubMed]
- Di Guardo, G. Lipofuscin, lipofuscin-like pigments and autofluorescence. Eur. J. Histochem. 2015, 59, 2485. [Google Scholar] [CrossRef] [PubMed]
- Comizzoli, P.; Ottinger, M.A. Understanding Reproductive Aging in Wildlife to Improve Animal Conservation and Human Reproductive Health. Front. Cell Dev. Biol. 2021, 9, 680471. [Google Scholar] [CrossRef] [PubMed]
- Sugianto, N.A.; Newman, C.; Macdonald, D.W.; Buesching, C.D. Reproductive and Somatic Senescence in the European Badger (Meles meles): Evidence from Lifetime Sex-Steroid Profiles. Zoology 2020, 141, 125803. [Google Scholar] [CrossRef] [PubMed]
- Mossa, F.; Jimenez-Krassel, F.; Scheetz, D.; Weber-Nielsen, M.; Evans, A.C.O.; Ireland, J.J. Anti-Mullerian Hormone (AMH) and fertility management in agricultural species. Reproduction 2017, 154, R1–R11. [Google Scholar] [CrossRef]
- Woods, L.; Perez-Garcia, V.; Kieckbusch, J.; Wang, X.; DeMayo, F.; Colucci, F.; Hemberger, M. Decidualisation and placentation defects are a major cause of age-related reproductive decline. Nat. Commun. 2017, 8, 352. [Google Scholar] [CrossRef]
- Jayasekara, S.; Sharma, R.P.; Drown, D.B. Immunotoxic potential of N-ethyl, N-nitrosourea (ENU) in CD1 mice. Clin. Exp. Immunol. 1989, 77, 294–298. [Google Scholar]
- Oktay, K.; Karlikaya, G.G.; Aydin, B.A. Ovarian cryopreservation and transplantation: Basic aspects. Mol. Cell Endocrinol. 2000, 169, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.T.; Campbell, B.; de Souza, C.; Telfer, E. Long-term ovarian function in sheep after ovariectomy and autotransplantation of cryopreserved cortical strips. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 113 (Suppl. S1), S55–S59. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo, J.A.; De Santiago-Miramontes, M.A.; Carrillo, E. Season of birth modifies puberty in female and male goats raised under subtropical conditions. Animal 2007, 1, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.M.; Valencak, T.G. A short life on the farm: Aging and longevity in agricultural, large-bodied mammals. Geroscience 2020, 42, 909–922. [Google Scholar] [CrossRef] [PubMed]
- American College of, O.; Gynecologists Committee on Gynecologic, P.; Practice, C. Female age-related fertility decline. Committee Opinion No. 589. Fertil. Steril. 2014, 101, 633–634. [Google Scholar] [CrossRef]
- Kelsey, T.W.; Dodwell, S.K.; Wilkinson, A.G.; Greve, T.; Andersen, C.Y.; Anderson, R.A.; Wallace, W.H. Ovarian volume throughout life: A validated normative model. PLoS ONE 2013, 8, e71465. [Google Scholar] [CrossRef]
- Pavlik, E.J.; DePriest, P.D.; Gallion, H.H.; Ueland, F.R.; Reedy, M.B.; Kryscio, R.J.; van Nagell, J.R., Jr. Ovarian volume related to age. Gynecol. Oncol. 2000, 77, 410–412. [Google Scholar] [CrossRef]
- Hall, J.E. Endocrinology of the Menopause. Endocrinol. Metab. Clin. 2015, 44, 485–496. [Google Scholar] [CrossRef]
- Park, C.J.; Oh, J.E.; Feng, J.; Cho, Y.M.; Qiao, H.; Ko, C. Lifetime changes of the oocyte pool: Contributing factors with a focus on ovulatory inflammation. Clin. Exp. Reprod. Med. 2022, 49, 16–25. [Google Scholar] [CrossRef]
- Wu, R.; Van der Hoek, K.H.; Ryan, N.K.; Norman, R.J.; Robker, R.L. Macrophage contributions to ovarian function. Hum. Reprod. Update 2004, 10, 119–133. [Google Scholar] [CrossRef]
- Milde, R.; Ritter, J.; Tennent, G.A.; Loesch, A.; Martinez, F.O.; Gordon, S.; Pepys, M.B.; Verschoor, A.; Helming, L. Multinucleated Giant Cells Are Specialized for Complement-Mediated Phagocytosis and Large Target Destruction. Cell Rep. 2015, 13, 1937–1948. [Google Scholar] [CrossRef] [PubMed]
- Roszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef] [PubMed]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- de Souza, C.J.; Campbell, B.K.; Baird, D.T. Incipient ovarian failure associated with raised levels of follicle stimulating hormone and reduced levels of inhibin A in older sheep. Hum. Reprod. 1998, 13, 3016–3022. [Google Scholar] [CrossRef]
- Fransolet, M.; Labied, S.; Henry, L.; Masereel, M.C.; Rozet, E.; Kirschvink, N.; Nisolle, M.; Munaut, C. Strategies for using the sheep ovarian cortex as a model in reproductive medicine. PLoS ONE 2014, 9, e91073. [Google Scholar] [CrossRef]
- Paulini, F.; Silva, R.C.; Rolo, J.L.; Lucci, C.M. Ultrastructural changes in oocytes during folliculogenesis in domestic mammals. J. Ovarian Res. 2014, 7, 102. [Google Scholar] [CrossRef]
- Lucci, C.M.; Silva, R.V.; Carvalho, C.A.; Figueiredo, R.; Bao, N. Light microscopical and ultrastructural characterization of goat preantral follicles. Small Rumin. Res. 2001, 41, 61–69. [Google Scholar] [CrossRef]
- Fatet, A.; Pellicer-Rubio, M.T.; Leboeuf, B. Reproductive cycle of goats. Anim. Reprod. Sci. 2011, 124, 211–219. [Google Scholar] [CrossRef]
- Barberino, R.S.; Macedo, T.J.S.; Lins, T.; Menezes, V.G.; Silva, R.L.S.; Monte, A.P.O.; Palheta, R.C., Jr.; Smitz, J.E.J.; Matos, M.H.T. Immunolocalization of melatonin receptor type 1 in the sheep ovary and involvement of the PI3K/Akt/FOXO3a signaling pathway in the effects of melatonin on survival and in vitro activation of primordial follicles. Mol. Reprod. Dev. 2022, 89, 485–497. [Google Scholar] [CrossRef]
- Ford, E.A.; Beckett, E.L.; Roman, S.D.; McLaughlin, E.A.; Sutherland, J.M. Advances in human primordial follicle activation and premature ovarian insufficiency. Reproduction 2020, 159, R15–R29. [Google Scholar] [CrossRef]
- Turner, A.S. The sheep as a model for osteoporosis in humans. Vet. J. 2002, 163, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.I.; Richards, R.G.; Milz, S.; Schneider, E.; Pearce, S.G. Animal models for implant biomaterial research in bone: A review. Eur. Cell Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dias, I.R.; Camassa, J.A.; Bordelo, J.A.; Babo, P.S.; Viegas, C.A.; Dourado, N.; Reis, R.L.; Gomes, M.E. Preclinical and Translational Studies in Small Ruminants (Sheep and Goat) as Models for Osteoporosis Research. Curr. Osteoporos. Rep. 2018, 16, 182–197. [Google Scholar] [CrossRef] [PubMed]
- Camassa, J.A.; Diogo, C.C.; Sousa, C.P.; Azevedo, J.T.; Viegas, C.A.; Reis, R.L.; Dourado, N.; Dias, I.R. Bone turnover markers in sheep and goat: A review of the scientific literature. An. Acad. Bras. Cienc. 2017, 89, 231–245. [Google Scholar] [CrossRef] [PubMed]
| Sheep | Goat | |
|---|---|---|
| Juvenile | 8 | 7 |
| Mature | 8 | 8 |
| Sudan Black B | Autofluorescence | |||
|---|---|---|---|---|
| Sheep | Goat | Sheep | Goat | |
| Juvenile | 0% | 0% | 0% | 0% |
| Mature | 80% | 67% | 100% | 62% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montenegro, L.; Magalhães, P.; Guerreiro, A.C.; Brandão, C.; Pinto, A.; Almeida, H.; Martins-Bessa, A.; Silva, E. The Contribution of the Sheep and the Goat Model to the Study of Ovarian Ageing. Biology 2023, 12, 270. https://doi.org/10.3390/biology12020270
Montenegro L, Magalhães P, Guerreiro AC, Brandão C, Pinto A, Almeida H, Martins-Bessa A, Silva E. The Contribution of the Sheep and the Goat Model to the Study of Ovarian Ageing. Biology. 2023; 12(2):270. https://doi.org/10.3390/biology12020270
Chicago/Turabian StyleMontenegro, Luís, Paulo Magalhães, Adriana Costa Guerreiro, Catarina Brandão, Anabela Pinto, Henrique Almeida, Ana Martins-Bessa, and Elisabete Silva. 2023. "The Contribution of the Sheep and the Goat Model to the Study of Ovarian Ageing" Biology 12, no. 2: 270. https://doi.org/10.3390/biology12020270
APA StyleMontenegro, L., Magalhães, P., Guerreiro, A. C., Brandão, C., Pinto, A., Almeida, H., Martins-Bessa, A., & Silva, E. (2023). The Contribution of the Sheep and the Goat Model to the Study of Ovarian Ageing. Biology, 12(2), 270. https://doi.org/10.3390/biology12020270