Role of Goats in the Epidemiology of Coxiella burnetii
Abstract
Simple Summary
Abstract
1. Introduction
2. Coxiella burnetii: The Microorganism and Its Pathogenesis
3. Infection and Clinical Outcomes in Goats
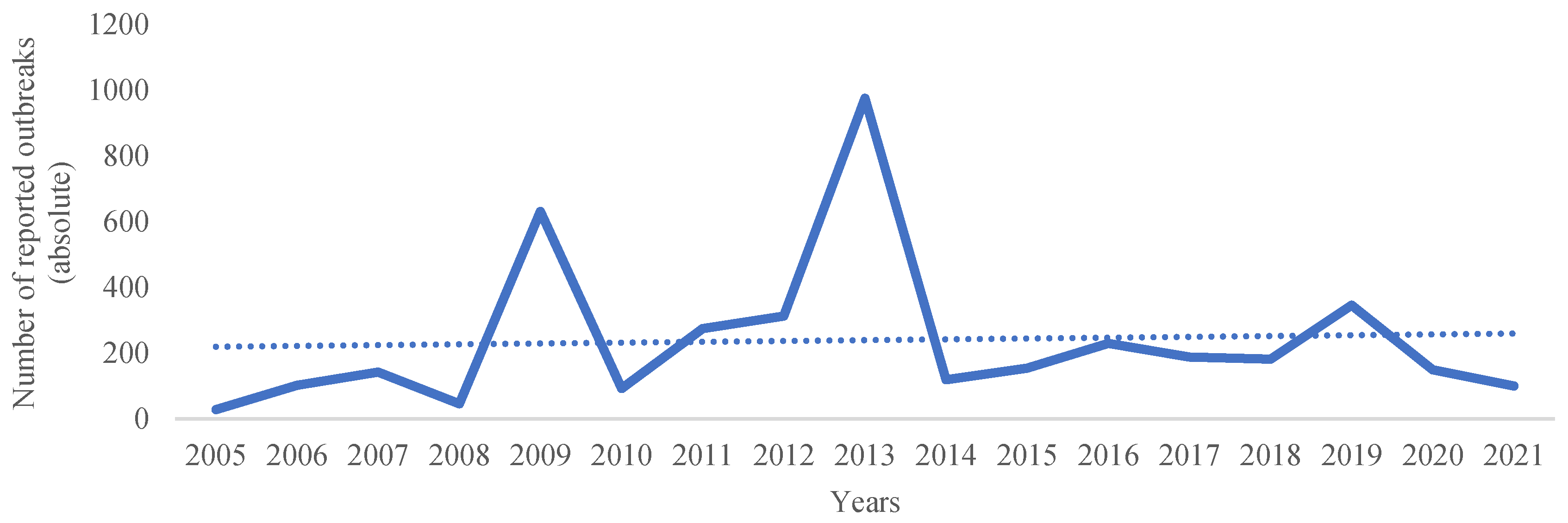
4. Epidemiological Highlights
| Country (Area) | Study Period | Type of Sample | Sampling Method | Number of Samples | Test | Cut-Off Value | Prevalence (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| Albania | 1995–1997 | Serum | - | 443 | ELISA | 0.4 | 8.8 | [136] |
| Bangladesh | 2009–2010 | Serum | Convenience | 529 | ELISA | 0.4 | 0.8 | [137] |
| Brazil | 2014–2015 | Serum | Convenience | 312 | ELISA | 0.4 | 55.1 | [138] |
| Canada | 2010–2012 | Serum | Multi-stage random | 2195 | ELISA | 0.4 | 32.5 | [123] |
| Ethiopia | - | Serum | Multi-stage random | 293 | ELISA | 0.4 | 35.5 | [139] |
| Great Britain | 2008 | Serum | Random stratified | 522 | ELISA | 0.4 | 0.8 | [124] |
| Greece | 2014–2015 | Serum | Convenience | 800 | ELISA | 0.4 | 14.4 | [140] |
| India | - | Serum | Convenience | 53 | ELISA | 0.4 | 5.7 | [141] |
| Iran | - | Serum | Multi-stage random | 241 | ELISA | 0.4 | 22.4 | [142] |
| Ireland (Republic of) | 2005–2007 | Serum | Random | 590 | ELISA | 0.4 | 0.3 | [125] |
| Italy | 2012 | Serum | Multi-stage random | 3185 | ELISA | 0.4 | 25.7 | [126] |
| Ivory Coast | 2012–2014 | Serum | Cluster | 622 | ELISA | 0.4 | 12.4 | [143] |
| Kenya | 2013 | Serum | Random | 280 | ELISA | 0.4 | 18.2 | [144] |
| Lebanon | 2014 | Serum | Random | 384 | ELISA | 0.4 | 17.2 | [127] |
| Reunion Island | 2011–2012 | Serum | Random | 134 | ELISA | 0.4 | 13.4 | [135] |
| Portugal | 2011 | Serum | Random | - | ELISA | 0.4 | 10.4 | [129] |
| Spain | 2007–2008 | Serum | Random | 115 | ELISA | 0.40 | 8.7 | [130] |
| Spain | 2015–2018 | Serum | Random | 135 | ELISA | 0.4 | 24.4 | [145] |
| Switzerland | 2011 | Serum | Random stratified | 321 | ELISA | 0.4 | 3.4 | [132] |
| The Gambia | 2012 | Serum | Multi-stage random | 484 | ELISA | 0.4 | 24.2 | [112] |
| The Netherlands | 2008 | Serum | Random | 3134 | ELISA | 0.4 | 7.8 | [133] |
| USA | 2012–2014 | Serum | Random | 608 | ELISA | 0.4 | 3.8 | [115] |
| Vietnam | 2016–2017 | Serum | Random | 1458 | ELISA | 0.4 | 4.1 | [146] |
5. Molecular Epidemiology: An Added Value
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Derrick, E.H. “Q” fever, a new fever entity: Clinical features, diagnosis and laboratory investigation. Med. J. Aust. 1937, 2, 281–299. [Google Scholar] [CrossRef]
- Davis, G.E.; Cox, H.R.; Parker, R.R.; Dyer, R.E. A Filter-Passing Infectious Agent Isolated from Ticks. Pub. Health Rep. 1938, 53, 2259–2282. [Google Scholar] [CrossRef]
- Bengtson, I.A. Immunological relationships between the rickettsiae of Australian and American “Q” fever. Pub. Health Rep. 1941, 56, 272–281. [Google Scholar] [CrossRef]
- Kaplan, M.M.; Bertagna, P. The geographical distribution of Q fever. Bull. World Health Organ. 1955, 13, 829–860. [Google Scholar] [PubMed]
- Caminopetros, J.P. La Q-fever en Grece: Le lait source de l’infection pour l’homme et les animaux. Ann. Parasite Paris 1948, 23, 107–118. [Google Scholar] [CrossRef]
- Fox-Lewis, A.; Isteed, K.; Austin, P.; Thompson-Faiva, H.; Wolfgang, J.; Ussher, J.E. A case of imported Q fever in New Zealand. NZMJ 2019, 132, 92–94. [Google Scholar]
- van den Brom, R.; van Engelen, E.; Roest, H.I.; van der Hoek, W.; Vellema, P. Coxiella burnetii infections in sheep or goats: An opinionated review. Vet. Microbiol. 2015, 181, 119–129. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (WOAH). WAHIS Interface. Available online: https://wahis.woah.org/#/home (accessed on 15 September 2022).
- de Valk, H. Q fever: New insights, still many queries. Eurosurveillance 2012, 17, 20062. [Google Scholar] [CrossRef] [PubMed]
- van Loenhout, J.A.F.; Paget, W.J.; Vercoulen, J.H.; Wijkmans, C.J.; Hautvast, J.L.A.; van der Velden, K. Assessing the long-term health impact of Q-fever in the Netherlands: A prospective cohort study started in 2007 on the largest documented Q-fever outbreak to date. BMC Infect. Dis. 2012, 12, 280. [Google Scholar] [CrossRef]
- Sidi-Boumedine, K.; Rousset, E.; Henning, K.; Ziller, M.; Niemczuck, K.; Roest, H.I.J.; Thiéry, R. Development of harmonised schemes for the monitoring and reporting of Q-fever in animals in the European Union. EFSA Support. Publ. 2010, 7, 48. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Panel on Animal Health and Welfare (AHAW); Scientific Opinion on Q Fever. EFSA J. 2010, 8, 1595. [Google Scholar]
- European Centre for Disease Prevention and Control. Risk Assessment on Q Fever—Technical Report; ECDC: Stockholm, Sweden, 2010; p. 40. [Google Scholar]
- Georgiev, M.; Afonso, A.; Neubauer, H.; Needham, H.; Thiéry, R.; Rodolakis, A.; Roest, J.; Stärk, K.D.; Stegeman, J.A.; Vellema, P.; et al. Q fever in humans and farm animals in four European countries, 1982 to 2010. Eurosurveillance 2013, 18, 20407. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Khan, A.U.; El-Adawy, H.; Mertens-Scholz, K.; Khan, I.; Neubauer, H.; Ho, Y.S. Research Trends and Hotspots of Q Fever Research: A Bibliometric Analysis 1990–2019. BioMed Res. Int. 2022, 2022, 9324471. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Alonso, R.; Basterretxea, M.; Barandika, J.F.; Hurtado, A.; Idiazabal, J.; Jado, I.; Beraza, X.; Montes, M.; Liendo, P.; García-Pérez, A.L. A Q Fever Outbreak with a High Rate of Abortions at a Dairy Goat Farm: Coxiella burnetii Shedding, Environmental Contamination, and Viability. Appl. Environ. Microbiol. 2018, 84, e01650-18. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.J.; Soares Magalhães, R.J. Airborne geographical dispersal of Q fever from livestock holdings to human communities: A systematic review and critical appraisal of evidence. BMC Infect. Dis. 2018, 18, 218. [Google Scholar] [CrossRef]
- Vellema, P.; Santman-Berends, I.; Dijkstra, F.; van Engelen, E.; Aalberts, M.; Ter Bogt-Kappert, C.; van den Brom, R. Dairy Sheep Played a Minor Role in the 2005-2010 Human Q Fever Outbreak in The Netherlands Compared to Dairy Goats. Pathogens 2021, 10, 1579. [Google Scholar] [CrossRef]
- Byeon, H.S.; Nattan, S.; Kim, J.H.; Han, S.T.; Chae, M.H.; Han, M.N.; Ahn, B.; Kim, Y.D.; Kim, H.S.; Jeong, H.W. Shedding and extensive and prolonged environmental contamination of goat farms of Q fever patients by Coxiella burnetii. Vet. Med. Sci. 2022, 8, 1264–1270. [Google Scholar] [CrossRef]
- Bond, K.A.; Vincent, G.; Wilks, C.R.; Franklin, L.; Sutton, B.; Stenos, J.; Cowan, R.; Lim, K.; Athan, E.; Harris, O.; et al. One Health approach to controlling a Q fever outbreak on an Australian goat farm. Epidemiol. Infect. 2016, 144, 1129–1141. [Google Scholar] [CrossRef]
- Panaiotov, S.; Ciccozzi, M.; Brankova, N.; Levterova, V.; Mitova-Tiholova, M.; Amicosante, M.; Rezza, G.; Kantardjiev, T. An outbreak of Q fever in Bulgaria. Ann. Dell’istituto Super. Sanità 2009, 45, 83–86. [Google Scholar]
- Genova-Kalou, P.; Vladimirova, N.; Stoitsova, S.; Krumova, S.; Kurchatova, A.; Kantardjiev, T. Q fever in Bulgaria: Laboratory and epidemiological findings on human cases and outbreaks, 2011 to 2017. Eurosurveillance 2019, 24, 1900119. [Google Scholar] [CrossRef]
- Huang, M.; Ma, J.; Jiao, J.; Li, C.; Chen, L.; Zhu, Z.; Ruan, F.; Xing, L.; Zheng, X.; Fu, M.; et al. The epidemic of Q fever in 2018 to 2019 in Zhuhai city of China determined by metagenomic next-generation sequencing. PLoS Negl. Trop. Dis. 2021, 15, e0009520. [Google Scholar] [CrossRef]
- Fishbein, D.B.; Raoult, D. A cluster of Coxiella burnetii infections associated with exposure to vaccinated goats and their unpasteurized dairy products. Am. J. Trop. Med. Hyg. 1992, 47, 35–40. [Google Scholar] [CrossRef]
- King, L.A.; Goirand, L.; Tissot-Dupont, H.; Giunta, B.; Giraud, C.; Colardelle, C.; Duquesne, V.; Rousset, E.; Aubert, M.; Thiéry, R.; et al. Outbreak of Q fever, Florac, Southern France, Spring 2007. Vector Borne Zoonotic Dis. 2011, 11, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Hatchette, T.F.; Hudson, R.C.; Schlech, W.F.; Campbell, N.A.; Hatchette, J.E.; Ratnam, S.; Raoult, D.; Donovan, C.; Marrie, T.J. Goat-Associated Q Fever: A New Disease in Newfoundland. Emerg. Infect. Dis. 2001, 7, 413–419. [Google Scholar] [CrossRef]
- Kovácová, E.; Kazár, J.; Simková, A. Clinical and serological analysis of a Q fever outbreak in western Slovakia with four-year follow-up. Eur. J. Clin. Microbiol. Infect. Dis. 1998, 17, 867–869. [Google Scholar] [PubMed]
- Gunther, M.J.; Heller, J.; Hayes, L.; Hernandez-Jover, M. Dairy goat producers’ understanding, knowledge and attitudes towards biosecurity and Q-fever in Australia. Prev. Vet. Med. 2019, 170, 104742. [Google Scholar] [CrossRef] [PubMed]
- van der Giessen, J.; Vlaanderen, F.; Kortbeek, T.; Swaan, C.; van den Kerkhof, H.; Broens, E.; Rijks, J.; Koene, M.; De Rosa, M.; Uiterwijk, M.; et al. Signalling and responding to zoonotic threats using a One Health approach: A decade of the Zoonoses Structure in the Netherlands, 2011 to 2021. Eurosurveillance 2022, 27, 2200039. [Google Scholar] [CrossRef]
- Jorm, L.R.; Lightfoot, N.F.; Morgan, K.L. An epidemiological study of an outbreak of Q fever in a secondary school. Epidemiol. Infect. 1990, 104, 467–477. [Google Scholar] [CrossRef]
- Clark, W.H.; Lennette, E.H.; Romer, M.S. Q fever in California. IX. An outbreak aboard a ship transporting goats. Am. J. Hyg. 1951, 54, 35–43. [Google Scholar]
- Bjork, A.; Marsden-Haug, N.; Nett, R.J.; Kersh, G.J.; Nicholson, W.; Gibson, D.; Szymanski, T.; Emery, M.; Kohrs, P.; Woodhall, D.; et al. First reported multistate human Q fever outbreak in the United States, 2011. Vector Borne Zoonotic Dis. 2014, 14, 111–117. [Google Scholar] [CrossRef]
- Preston, W. Bergey’s Manual of Determinative Bacteriology, 6th ed.; Bergey, D.H., Breed, R.S., Hitchens, A.P., Murray, E.G.D., Eds.; Williams & Wilkins: Baltimore, MD, USA, 1948. [Google Scholar]
- Drancourt, M.; Roux, V.; Dang, L.V.; Tran-Hung, L.; Castex, D.; Chenal-Francisque, V.; Ogata, H.; Fournier, P.-E.; Crubézy, E.; Raoult, D. Genotyping, Orientalis-like Yersinia pestis, and Plague Pandemics. Emerg. Infect. Dis. 2004, 10, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, R.; Paulsen, I.T.; Eisen, J.A.; Read, T.D.; Nelson, K.E.; Nelson, W.C.; Ward, N.L.; Tettelin, H.; Davidsen, T.M.; Beanan, M.J.; et al. Complete genome sequence of the Q-fever pathogen Coxiella burnetii. Proc. Natl. Acad. Sci. USA 2003, 100, 5455–5460. [Google Scholar] [CrossRef] [PubMed]
- Sidi-Boumedine, K.; Adam, G.; Angen, O.; Aspan, A.; Bossers, A.; Roest, H.J.; Prigent, M.; Thiéry, R.; Rousset, E. Whole genome PCR scanning (WGPS) of Coxiella burnetii strains from ruminants. Microbes Infect. 2015, 17, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Sidi-Boumedine, K.; Duquesne, V.; Prigent, M.; Yang, E.; Joulié, A.; Thiéry, R.; Rousset, E. Impact of IS1111 insertion on the MLVA genotyping of Coxiella burnetii. Microbes Infect. 2015, 17, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Eldin, C.; Mélenotte, C.; Mediannikov, O.; Ghigo, E.; Million, M.; Edouard, S.; Mege, J.L.; Maurin, M.; Raoult, D. From Q Fever to Coxiella burnetii Infection: A Paradigm Change. Clin. Microbiol. Rev. 2017, 30, 115–190. [Google Scholar] [CrossRef]
- Mege, J.L.; Maurin, M.; Capo, C.; Raoult, D. Coxiella burnetii: The “query” fever bacterium—Model of immune subversion by a strictly intracellular microorganism. FEMS Microbiol. Rev. 1997, 19, 209–217. [Google Scholar]
- Beare, P.A.; Samuel, J.E.; Howe, D.; Virtaneva, K.; Porcella, S.F.; Heinzen, R.A. Genetic Diversity of the Q Fever Agent, Coxiella burnetii, Assessed by Microarray-Based Whole-Genome Comparisons. J. Bacteriol. 2006, 188, 2309–2324. [Google Scholar] [CrossRef] [PubMed]
- Denison, A.M.; Massung, R.F.; Thompson, H.E. Analysis of the O-antigen biosynthesis regions of phase II Isolates of Coxiella burnetii. FEMS Microbiol. Lettters 2007, 267, 102–107. [Google Scholar] [CrossRef]
- Hoover, T.A.; Culp, D.W.; Vodkin, M.H.; Williams, J.C.; Thompson, H.A. Chromosomal DNA Deletions Explain Phenotypic Characteristics of Two Antigenic Variants, Phase II and RSA 514 (Crazy), of the Coxiella burnetii Nine Mile Strain. Infect. Immun. 2002, 70, 6726–6733. [Google Scholar] [CrossRef]
- Kuley, R.; Smith, H.E.; Frangoulidis, D.; Smits, M.A.; Roest, H.I.J.; Bossers, A. Cell-Free Propagation of Coxiella burnetii Does Not Affect Its Relative Virulence. PLoS ONE 2015, 10, e0121661. [Google Scholar] [CrossRef]
- Coleman, S.A.; Fischer, E.R.; Howe, D.; Mead, D.J.; Heinzen, R.A. Temporal Analysis of Coxiella burnetii Morphological Differentiation. J. Bacteriol. 2004, 186, 7344–7352. [Google Scholar] [CrossRef]
- McCaul, T.F.; Williams, J.C. Developmental Cycle of Coxiella burnetii: Structure and Morphogenesis of Vegetative and Sporogenic Differentiations. J. Bacteriol. 1981, 147, 1063–1076. [Google Scholar] [CrossRef] [PubMed]
- Heinzen, R.A.; Hackstadt, T.; Samuel, J.E. Developmental biology of Coxiella burnetii. Trends Microbiol. 1999, 7, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, R.; Hendrix, L.R.; Samuel, J.E. Differential Expression of Translational Elements by Life Cycle Variants of Coxiella burnetii. Infect. Immun. 1999, 67, 6026–6033. [Google Scholar] [CrossRef] [PubMed]
- Sandoz, K.M.; Popham, D.L.; Beare, P.A.; Sturdevant, D.E.; Hansen, B.; Nair, V.; Heinzen, R.A. Transcriptional Profiling of Coxiella burnetii Reveals Extensive Cell Wall Remodeling in the Small Cell Variant Developmental Form. PLoS ONE 2016, 11, e0149957. [Google Scholar] [CrossRef] [PubMed]
- Shannon, J.G.; Heinzen, R.A. Adaptive immunity to the obligate intracellular pathogen Coxiella burnetii. Immunol. Res. 2009, 43, 138–148. [Google Scholar] [CrossRef]
- Amara, A.B.; Ghigo, E.; Le Priol, Y.; Lépolard, C.; Salcedo, S.P.; Lemichez, E.; Bretelle, F.; Capo, C.; Mege, J.L. Coxiella burnetii, the agent of Q fever, replicates within trophoblasts and induces a unique transcriptional response. PLoS ONE 2010, 5, e15315. [Google Scholar]
- Capo, C.; Moynault, A.; Collette, Y.; Olive, D.; Brown, E.J.; Raoult, D.; Mege, J.L. Coxiella burnetii avoids macrophage phagocytosis by interfering with spatial distribution of complement receptor 3. J. Immunol. 2003, 170, 4217–4225. [Google Scholar] [CrossRef]
- Dupuy, A.G.; Caron, E. Integrin-dependent phagocytosis: Spreading from microadhesion to new concepts. J. Cell Sci. 2008, 121, 1773–1783. [Google Scholar] [CrossRef]
- Meconi, S.; Jacomo, V.; Boquet, P.; Raoult, D.; Mege, J.L.; Capo, C. Coxiella burnetii Induces Reorganization of the Actin Cytoskeleton in Human Monocytes. Infect. Immun. 1998, 66, 5527–5533. [Google Scholar] [CrossRef] [PubMed]
- Meconi, S.; Capo, C.; Remacle-Bonnet, M.; Pommier, G.; Raoult, D.; Mege, J.L. Activation of protein tyrosine kinases by Coxiella burnetii: Role in actin cytoskeleton reorganization and bacterial phagocytosis. Infect. Immun. 2001, 69, 2520–2526. [Google Scholar] [CrossRef] [PubMed]
- Honstettre, A.; Ghigo, E.; Moynault, A.; Capo, C.; Toman, R.; Akira, S.; Takeuchi, O.; Lepidi, H.; Raoult, D.; Mege, J.L. Lipopolysaccharide from Coxiella burnetii is involved in bacterial phagocytosis, filamentous actin reorganization, and inflammatory responses through Toll-like receptor 4. J. Immunol. 2004, 172, 3695–3703. [Google Scholar] [CrossRef] [PubMed]
- van Schaik, E.J.; Chen, C.; Mertens, K.; Weber, M.M.; Samuel, J.E. Molecular pathogenesis of the obligate intracellular bacterium Coxiella burnetii. Nat. Rev. Microbiol. 2013, 11, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Heinzen, R.A.; Hackstadt, T. A developmental stage-specific histone H1 homolog of Coxiella burnetii. J. Bacteriol. 1996, 78, 5049–5052. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kinchen, J.M.; Ravichandran, K.S. Phagosome maturation: Going through the acid test. Nat. Rev. Mol. Cell Biol. 2008, 9, 781–795. [Google Scholar] [CrossRef]
- Howe, D.; Shannon, J.G.; Winfree, S.; Dorward, D.W.; Heinzen, R.A. Coxiella burnetii phase I and II variants replicate with similar kinetics in degradative phagolysosome-like compartments of human macrophages. Infect. Immun. 2010, 78, 3465–3474. [Google Scholar] [CrossRef]
- Berón, W.; Gutierrez, M.G.; Rabinovitch, M.; Colombo, M.I. Coxiella burnetii Localizes in a Rab7-Labeled Compartment with Autophagic Characteristics. Infect. Immun. 2002, 70, 5816–5821. [Google Scholar] [CrossRef]
- Schulze-Luehrmann, J.; Eckart, R.A.; Ölke, M.; Saftig, P.; Liebler-Tenorio, E.; Lührmann, A. LAMP proteins account for the maturation delay during the establishment of the Coxiella burnetii-containing vacuole. Cell. Microbiol. 2016, 18, 181–194. [Google Scholar] [CrossRef]
- Ghigo, E.; Capo, C.; Tung, C.H.; Raoult, D.; Gorvel, J.P.; Mege, J.L. Coxiella burnetii survival in THP-1 monocytes involves the impairment of phagosome maturation: IFN-g mediates its restoration and bacterial killing. J. Immunol. 2002, 169, 4488–4495. [Google Scholar] [CrossRef] [PubMed]
- Howe, D.; Melnicáková, J.; Barák, I.; Heinzen, R.A. Maturation of the Coxiella burnetii parasitophorous vacuole requires bacterial protein synthesis but not replication. Cell. Microbiol. 2003, 5, 469–480. [Google Scholar] [CrossRef]
- Flannagan, R.S.; Jaumouillé, V.; Grinstein, S. The cell biology of phagocytosis. Ann. Rev. Pathol. 2012, 7, 61–98. [Google Scholar] [CrossRef] [PubMed]
- Barry, A.; Mege, J.; Ghigo, E. Hijacked phagosomes and leukocyte activation: An intimate relationship. J. Leukoc. Biol. 2011, 89, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Voth, D.E.; Heinzen, R.A. Lounging in a lysosome: The intracellular lifestyle of Coxiella burnetii. Cell. Microbiol. 2007, 9, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Pareja, M.E.M.; Bongiovanni, A.; Lafont, F.; Colombo, M.I. Alterations of the Coxiella burnetii Replicative Vacuole Membrane Integrity and Interplay with the Autophagy Pathway. Front. Cell. Infect. Microbiol. 2017, 7, 112. [Google Scholar] [CrossRef]
- Lührmann, A.; Roy, C.R. Coxiella burnetii inhibits activation of host cell apoptosis through a mechanism that involves preventing cytochrome c release from mitochondria. Infect. Immun. 2007, 75, 5282–5289. [Google Scholar] [CrossRef]
- Voth, D.E.; Howe, D.; Heinzen, R.A. Coxiella burnetii inhibits apoptosis in human THP-1 cells and monkey primary alveolar macrophages. Infect. Immun. 2007, 75, 4263–4271. [Google Scholar] [CrossRef] [PubMed]
- Voth, D.E.; Heinzen, R.A. Coxiella Type IV Secretion and Cellular Microbiology. Curr. Opin. Microbiol. 2009, 12, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Vázques, C.L.; Colombo, M.I. Coxiella burnetii modulates Beclin 1 and Bcl-2, preventing host cell apoptosis to generate a persistent bacterial infection. Cell. Death Differ. 2010, 17, 421–438. [Google Scholar] [CrossRef]
- Howe, D.; Mallavia, L.P. Coxiella burnetii Exhibits Morphological Change and Delays Phagolysosomal Fusion after Internalization by J774A. 1 Cells. Infect. Immun. 2000, 68, 3815–3821. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Nicas, M.; Hubbard, A.E.; Reingold, A.L. The Infectious Dose of Coxiella burnetii (Q Fever). Appl. Biosaf. 2006, 11, 32–41. [Google Scholar] [CrossRef]
- Brooke, R.J.; Mutters, N.T.; Péter, O.; Kretzschmar, M.E.E.; Teunis, P.F.M. Exposure to low doses of Coxiella burnetii caused high illness attack rates: Insights from combining human challenge and outbreak data. Epidemics 2015, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Brooke, R.J.; Kretzschmar, M.E.; Mutters, N.T.; Teunis, P.F. Human dose response relation for airborne exposure to Coxiella burnetii. BMC Infect. Dis. 2013, 13, 488. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.G.; MacDonald, L.J.; Hussain, S.K.; Sharma, U.M.; Kurten, R.C.; Voth, D.E. Virulent Coxiella burnetii Pathotypes Productively Infect Primary Human Alveolar Macrophages. Cell. Microbiol. 2013, 15, 1012–1025. [Google Scholar] [CrossRef] [PubMed]
- Marriott, H.M.; Dockrell, D.H. The role of the macrophage in lung disease mediated by bacteria. Exp. Lung Res. 2007, 33, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Louveau, C.; Lepidi, H.; Ricci, F.; Baylac, P.; Davoust, B.; Raoult, D. Q fever pneumonia: Virulence of C. burnetii pathovars in a murine model of aerosol infection. Infect. Immun. 2005, 73, 2469–2477. [Google Scholar] [CrossRef] [PubMed]
- Roest, H.I.J.; van Gelderen, B.; Dinkla, A.; Frangoulidis, D.; van Zijderveld, F.; Rebel, J.; van Keulen, L. Q fever in pregnant goats: Pathogenesis and excretion of C. burnetii. PLoS ONE 2012, 7, e48949. [Google Scholar] [CrossRef] [PubMed]
- Ammerdorffer, A.; Roest, H.I.; Dinkla, A.; Post, J.; Schoffelen, T.; van Deuren, M.; Sprong, T.; Rebel, J.M. The effect of C. burnetii infection on the cytokine response of PBMCs from pregnant goats. PLoS ONE 2014, 9, e109283. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.; Souriauy, A.; Buendía, A.J.; Arricau-Bouvery, N.; Matínez, C.M.; Salinas, J.; Rodolakis, A.; Navarro, J.A. Experimental Coxiella burnetii Infection in Pregnant Goats: A Histopathological and Immunohistochemical Study. J. Comp. Path. 2006, 135, 108–115. [Google Scholar] [CrossRef]
- Roest, H.I.J.; Dinkla, A.; Koets, A.P.; Post, J.; van Keulen, L. Experimental Coxiella burnetii infection in non-pregnant goats and the effect of breeding. Vet. Res. 2020, 51, 74. [Google Scholar] [CrossRef]
- Benoit, M.; Barbarat, B.; Bernard, A.; Olive, D.; Mege, J.L. Coxiella burnetii, the agent of Q fever, stimulates an atypical M2 activation program in human macrophages. Eur. J. Immunol. 2008, 38, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Ghigo, E.; Capo, C.; Raoult, D.; Mege, J.L. Interleukin-10 stimulates Coxiella burnetii replication in human monocytes through tumor necrosis factor down-modulation: Role in microbicidal defect of Q fever. Infect. Immun. 2001, 69, 2345–2352. [Google Scholar] [CrossRef] [PubMed]
- Ghigo, E.; Imbert, G.; Capo, C.; Raoult, D.; Mege, J.L. Interleukin-4 induces Coxiella burnetii replication in human monocytes but not in macrophages. Ann. New York Acad. Sci. 2003, 990, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Russell-Lodrigue, K.E.; Andoh, M.; Zhang, Y.; Hendrix, L.R.; Samuel, J.E. Mechanisms of vaccine-induced protective immunity against Coxiella burnetii infection in BALB/c mice. J. Immunol. 2007, 179, 8372–8380. [Google Scholar] [CrossRef] [PubMed]
- Roest, H.I.J.; Bossers, A.; Rebel, J.M.J. Q Fever Diagnosis and Control in Domestic Ruminants. Dev. Biol. 2013, 135, 183–189. [Google Scholar]
- Teunis, P.F.M.; Schimmer, B.; Notermans, D.W.; Leenders, A.C.A.P.; Wever, P.C.; Kretzschmar, M.E.E.; Schneeberger, P.M. Time-course of antibody responses against Coxiella burnetii following acute Q fever. Epidemiol. Infect. 2013, 141, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Langley, J.M.; Marrie, T.J.; Leblanc, J.C.; Almudevar, A.; Resch, L.; Raoult, D. Coxiella burnetii seropositivity in parturient women is associated with adverse pregnancy outcomes. Am. J. Obstet. Gynecol. 2003, 189, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Carcopino, X.; Raoult, D.; Bretelle, F.; On Boubli, L.; Stein, A. Managing Q Fever during Pregnancy: The Benefits of Long-Term Cotrimoxazole Therapy. Clin. Infect. Dis. 2007, 45, 548–555. [Google Scholar] [CrossRef]
- Lennette, E.H.; Holmes, M.A.; Abinanti, F.R. Remove from marked Records, Q. Fever Studies. XIV. Observations on the Pathogenesis of the Experimental Infection induced in Sheep by the Intravenous Route. Am. J. Hyg. 1952, 55, 254–267. [Google Scholar]
- Martinov, S.P.; Neikov, P.; Popov, G.V. Experimental Q fever in sheep. Eur. J. Epidemiol. 1989, 5, 428–431. [Google Scholar] [CrossRef]
- Berri, M.; Rousset, E.; Hechard, C.; Champion, J.L.; Dufour, P.; Russo, P.; Rodolaskis, A. Progression of Q Fever and Coxiella burnetii shedding in milk after an outbreak of enzootic abortion in a goat herd. Vet. Rec. 2005, 156, 548–549. [Google Scholar] [CrossRef]
- Berri, M.; Rousset, E.; Champion, J.L.; Russo, P.; Rodolaskis, A. Goats may experience reproductive failures and shed Coxiella burnetii at two successive parturitions after a Q fever infection. Res. Vet. Sci. 2007, 83, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Van den Brom, R.; Vellema, P. Q fever outbreaks in small ruminants and people in the Netherlands. Small Rum. Res. 2009, 86, 74–79. [Google Scholar] [CrossRef]
- Arricau-Bouvery, N.; Rodolakis, A. Is Q fever an emerging or re-emerging Zoonosis? Vet. Res. 2005, 36, 327–349. [Google Scholar] [CrossRef]
- Eibach, R.; Bothe, F.; Runge, M.; Fischer, S.F.; Philipp, W.; Ganter, M. Q fever: Baseline monitoring of a sheep and a goat flock associated with human infections. Epidemiol. Infect. 2012, 140, 1939–1949. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roest, H.I.J.; Bossers, A.; van Zijderveld, F.R.; Rebel, J.M.L. Clinical microbiology of Coxiella burnetii and relevant aspects for the diagnosis and control of the zoonotic disease Q fever. Vet. Q. 2014, 33, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Tissot-Dupont, H.; Amadei, M.A.; Nezri, M.; Raoult, D. Wind in November, Q fever in December. Emerg. Infect. Dis. 2004, 10, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, M.M.T.; Borlée, F.; Smit, L.A.M.; de Bruin, A.; Janse, I.; Heederik, D.J.J.; Wouters, I.M. Detection of Coxiella burnetii in Ambient Air after a Large Q Fever Outbreak. PLoS ONE 2016, 11, e0151281. [Google Scholar] [CrossRef]
- Hermans, T.; Jeurissen, L.; Hackert, V.; Hoebe, C. Land-applied goat manure as a source of human Q-fever in the Netherlands, 2006–2010. PLoS ONE 2014, 9, e96607. [Google Scholar] [CrossRef] [PubMed]
- Hawker, J.I.; Ayres, J.G.; Blair, I.; Evans, M.R.; Smith, D.L.; Smith, E.G.; Burge, P.S.; Carpenter, M.J.; Caul, E.O.; Coupland, B.; et al. A large outbreak of Q fever in the West Midlands: Windborne spread into a metropolitan area? Commun. Dis. Public Health 1998, 1, 180–187. [Google Scholar]
- Schimmer, B.; Schegget, R.; Wegdam, M.; Züchner, L.; de Bruin, A.; Schneeberger, P.M.; Veenstra, T.; Vellema, P.; van der Hoek, W. The use of a geographic information system to identify a dairy goat farm as the most likely source of an urban Q-fever outbreak. BMC Infect. Dis. 2010, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Nusinovici, S.; Frössling, J.; Widgren, S.; Beaudeau, F.; Lindberg, A. Q fever infection in dairy cattle herds: Increased risk with high wind speed and low precipitation. Epidemiol. Infect. 2015, 143, 3316–3326. [Google Scholar] [CrossRef] [PubMed]
- Rodolakis, A.; Berri, M.; Héchard, C.; Caudron, C.; Souriau, A.; Bodier, C.C.; Blanchard, B.; Camuset, P.; Devillechaise, P.; Natorp, J.C.; et al. Comparison of Coxiella burnetii shedding in milk of dairy bovine, caprine and ovine herds. J. Dairy Sci. 2007, 90, 5352–5360. [Google Scholar] [CrossRef] [PubMed]
- Boarbi, S.; Mori, M.; Rousset, E.; Sidi-Boumedine, K.; Van Esbroeck, M.; Fretin, D. Prevalence and molecular typing of Coxiella burnetii in bulk tank milk in Belgian dairy goats, 2009–2013. Vet. Microbiol. 2014, 170, 117–124. [Google Scholar] [CrossRef]
- Rahimi, E.; Ameri, M.; Karim, G.; Doosti, A. Prevalence of Coxiella burnetii in bulk milk samples from dairy bovine, ovine, caprine, and camel herds in Iran as determined by polymerase chain reaction. Foodborne Pathog. Dis. 2011, 8, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Basanisi, M.G.; La Bella, G.; Nobili, G.; Raele, D.A.; Cafiero, M.A.; Coppola, R.; Damato, A.M.; Fraccalvieri, R.; Sottili, R.; La Salandra, G. Detection of Coxiella burnetii DNA in sheep and goat milk and dairy products by droplet digital PCR in south Italy. Int. J. Food Microbiol. 2022, 366, 109583. [Google Scholar] [CrossRef] [PubMed]
- Jodełko, A.; Szymańska-Czerwińska, M.; Rola, J.G.; Niemczuk, K. Molecular detection of Coxiella burnetii in small ruminants and genotyping of specimens collected from goats in Poland. BMC Vet. Res. 2021, 17, 341. [Google Scholar] [CrossRef]
- Anastácio, S.; Carolino, N.; Sidi-Boumedine, K.; da Silva, G.J. Q fever dairy herd status determination based on serological and molecular analysis of bulk tank milk. Transbound. Emerg. Dis. 2016, 63, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Fretz, R.; Schaeren, W.; Tanner, M.; Baumgartner, A. Screening of various foodstuffs for occurrence of Coxiella burnetii in Switzerland. Int. J. Food Microbiol. 2007, 116, 414–418. [Google Scholar] [CrossRef]
- Klaasen, M.; Roest, H.J.; van der Hoek, W.; Goossens, B.; Secka, A.; Stegeman, A. Coxiella burnetii seroprevalence in small ruminants in The Gambia. PLoS ONE 2014, 9, e85424. [Google Scholar] [CrossRef] [PubMed]
- van den Brom, R.; van Engelen, E.; Luttikholt, S.; Moll, L.; van Maanen, K.; Vellema, P. Coxiella burnetii in bulk tank milk samples from dairy goat and sheep farms in The Netherlands. Vet. Rec. 2012, 170, 310. [Google Scholar] [CrossRef]
- Can, H.Y.; Elmali, E.; Karagöz, A. Detection of Coxiella burnetii in cows’, goats’, and ewes’ bulk milk samples using polymerase chain reaction (PCR). Mljekarstvo 2015, 65, 26–31. [Google Scholar] [CrossRef]
- Bauer, A.E.; Hubbard, K.R.; Johnson, A.J.; Messick, J.B.; Weng, H.Y.; Pogranichniy, R.M. A cross sectional study evaluating the prevalence of Coxiella burnetii, potential risk factors for infection, and agreement between diagnostic methods in goats in Indiana. Prev. Vet. Med. 2016, 126, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Loftis, A.D.; Priestley, R.A.; Massung, R.F. Detection of Coxiella burnetii in Commercially Available Raw Milk from the United States. Foodborne Pathog. Dis. 2010, 7, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Gale, P.; Kelly, L.; Mearns, R.; Duggan, J.; Snary, E.L. Q fever through consumption of unpasteurised milk and milk products—A risk profile and exposure assessment. J. Appl. Microbiol. 2015, 118, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Eldin, C.; Angelakis, E.; Renvoisé, A.; Raoult, D. Coxiella burnetii DNA, but not viable bacteria, in dairy products in France. Am. J. Trop. Med. Hyg. 2013, 88, 765–769. [Google Scholar] [CrossRef]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards). Scientific Opinion on the public health risks related to the consumption of raw drinking milk. EFSA J. 2015, 13, 3940. [Google Scholar] [CrossRef]
- Enright, J.B.; Sadler, W.W.; Thomas, R.C. Pasteurization of milk containing the organism of Q Fever. Am. J. Public Health 1957, 47, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Wittwer, M.; Hammer, P.; Runge, M.; Valentin-Weigand, P.; Neubauer, H.; Henning, K.; Mertens-Scholz, K. Inactivation Kinetics of Coxiella burnetii During High-Temperature Short-Time Pasteurization of Milk. Front. Microbiol. 2022, 12, 753871. [Google Scholar] [CrossRef] [PubMed]
- Arricau-Bouvery, N.; Souriau, A.; Lechopier, P.; Rodolakis, A. Experimental Coxiella burnetii infection in pregnant goats: Excretion routes. Vet. Res. 2003, 34, 423–433. [Google Scholar] [CrossRef]
- Meadows, S.; Jones-Bitton, A.; McEwen, S.; Jansen, J.; Menzies, P. Coxiella burnetii seropositivity and associated risk factors in goats in Ontario, Canada. Prev. Vet. Med. 2015, 121, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Lambton, S.L.; Smith, R.P.; Gillard, K.; Horigan, M.; Farren, C.; Pritchard, G.C. Serological survey using ELISA to determine the prevalence of Coxiella burnetii infection (Q fever) in sheep and goats in Great Britain. Epidemiol. Infect. 2016, 144, 19–24. [Google Scholar] [CrossRef]
- Ryan, E.; Kirby, M.; Clegg, T.; Collins, D.M. Seroprevalence of Coxiella burnetii antibodies in sheep and goats in the Republic of Ireland. Vet. Rec. 2011, 169, 280. [Google Scholar] [CrossRef]
- Rizzo, F.; Vitale, N.; Ballardini, M.; Borromeo, V.; Luzzago, C.; Chiavacci, L.; Mandola, M.L. Q fever seroprevalence and risk factors in sheep and goats in northwest Italy. Prev. Vet. Med. 2016, 130, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Dabaja, M.F.; Greco, G.; Villari, S.; Vesco, G.; Bayan, A.; Bazzal, B.E.; Ibrahim, E.; Gargano, V.; Sciacca, C.; Lelli, R.; et al. Occurrence and risk factors of Coxiella burnetii in domestic ruminants in Lebanon. Comp. Immunol. Microbiol. Infect. Dis. 2019, 64, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Kampen, A.H.; Hopp, P.; Groneng, G.M.; Melkild, I.; Urdahl, A.M.; Karlsson, A.C.; Tharaldsen, J. No indication of Coxiella burnetii infection in Norwegian farmed ruminants. BMC Vet. Res. 2012, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Anastacio, S.; Tavares, N.; Carolino, N.; Sidi-Boumedine, K.; da Silva, G.J. Serological evidence of exposure to Coxiella burnetii in sheep and goats in central Portugal. Vet. Microbiol. 2013, 167, 500–505. [Google Scholar] [CrossRef]
- Ruiz-Fons, F.; Astobiza, I.; Barandika, J.F.; Hurtado, A.; Atxaerandio, R.; Juste, R.A.; Garcia-Perez, A.L. Seroepidemiological study of Q fever in domestic ruminants in semi-extensive grazing systems. BMC Vet. Res. 2010, 6, 3. [Google Scholar] [CrossRef]
- Ohlson, A.; Malmsten, J.; Frössling, J.; Bölske, G.; Aspán, A.; Dalin, A.M.; Lindberg, A. Surveys on Coxiella burnetii infections in Swedish cattle, sheep, goats and moose. Acta Vet. Scand. 2014, 56, 39. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Magouras, I.; Hunninghaus, J.; Scherrer, S.; Wittenbrink, M.M.; Hamburger, A.; Stärk, K.D.; Schüpbach-Regula, G. Coxiella burnetii Infections in Small Ruminants and Humans in Switzerland. Transbound. Emerg. Dis. 2017, 64, 204–212. [Google Scholar] [CrossRef] [PubMed]
- van den Brom, R.; Moll, L.; van Schaik, G.; Vellema, P. Demography of Q fever seroprevalence in sheep and goats in The Netherlands in 2008. Prev. Vet. Med. 2013, 109, 76–82. [Google Scholar] [CrossRef]
- Schimmer, B.; Luttikholt, S.; Hautvast, J.L.A.; Graat, E.A.M.; Vellema, P.; van Duynhoven, Y.T.H.P. Seroprevalence and risk factors of Q fever in goats on commercial dairy goat farms in the Netherlands, 2009-2010. BMC Vet. Res. 2011, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, E.; Esnault, O.; Beral, M.; Naze, F.; Michault, A. Emergence of Coxiella burnetii in Ruminants on Reunion Island? Prevalence and Risk Factors. PLoS Neglect. Trop. Dis. 2014, 8, e3055. [Google Scholar] [CrossRef] [PubMed]
- Cekani, M.; Papa, A.; Kota, M.; Velo, E.; Berxholi, K. Report of a serological study of Coxiella burnetii in domestic animals in Albania. Vet. J. 2008, 175, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.; Rahman, M.S.; Khan, S.U.; Mikolon, A.; Osmani, M.G.; Gurley, E.S.; Shanta, I.S.; Paul, S.K.; Macfarlane-Berry, L.; Islam, A.; et al. Serological Evidence of Coxiella burnetii Infection in Cattle and Goats in Bangladesh. Ecohealth 2015, 12, 354–358. [Google Scholar] [CrossRef]
- de Oliveira, J.M.B.; Rozental, T.; de Lemos, E.R.S.; Forneas, D.; Ortega-Mora, L.M.; Porto, W.J.N.; da Fonseca Oliveira, A.A.; Mota, R.A. Coxiella burnetii in dairy goats with a history of reproductive disorders in Brazil. Acta Trop. 2018, 183, 19–22. [Google Scholar] [CrossRef]
- Tesfaye, A.; Sahele, M.; Sori, T.; Guyassa, C.; Garoma, A. Seroprevalence and associated risk factors for chlamydiosis, coxiellosis and brucellosis in sheep and goats in Borana pastoral area, southern Ethiopia. BMC Vet. Res. 2020, 16, 145. [Google Scholar] [CrossRef] [PubMed]
- Filioussis, G.; Theodoridis, A.; Papadopoulos, D.; Gelasakis, A.I.; Vouraki, S.; Bramis, G.; Arsenos, G. Serological prevalence of Coxiella burnetii in dairy goats and ewes diagnosed with adverse pregnancy outcomes in Greece. Ann. Agric. Environ. Med. 2017, 24, 702–705. [Google Scholar] [CrossRef]
- Vaidya, V.M.; Malik, S.V.S.; Bhilegaonkar, K.N.; Rathore, R.S.; Kaur, S.; Barbuddhe, S.B. Prevalence of Q fever in domestic animals with reproductive disorders. Comp. Immunol. Microbiol. Infect. Dis. 2010, 33, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Ezatkhah, M.; Alimolaei, M.; Khalili, M.; Sharifi, H. Seroepidemiological study of Q fever in small ruminants from Southeast Iran. J. Infect. Public Health 2015, 8, 170–176. [Google Scholar] [CrossRef]
- Kanouté, Y.B.; Gragnon, B.G.; Schindler, C.; Bonfoh, B.; Schelling, E. Epidemiology of brucellosis, Q Fever and Rift Valley Fever at the human and livestock interface in northern Côte d’Ivoire. Acta Trop. 2017, 165, 66–75. [Google Scholar] [CrossRef]
- Larson, P.S.; Espira, L.; Grabow, C.; Wang, C.A.; Muloi, D.; Browne, A.S.; Deem, S.L.; Fèvre, E.M.; Foufopoulos, J.; Hardin, R.; et al. The sero-epidemiology of Coxiella burnetii (Q fever) across livestock species and herding contexts in Laikipia County, Kenya. Zoonoses Public Health 2019, 66, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Espí, A.; Del Cerro, A.; Oleaga, Á.; Rodríguez-Pérez, M.; López, C.M.; Hurtado, A.; Rodríguez-Martínez, L.D.; Barandika, J.F.; García-Pérez, A.L. One Health Approach: An Overview of Q Fever in Livestock, Wildlife and Humans in Asturias (Northwestern Spain). Animals 2021, 11, 1395. [Google Scholar] [CrossRef] [PubMed]
- Burns, R.J.L.; Douangngeun, B.; Theppangna, W.; Khounsy, S.; Mukaka, M.; Selleck, P.W.; Hansson, E.; Wegner, M.D.; Windsor, P.A.; Blacksell, S.D. Serosurveillance of Coxiellosis (Q-fever) and Brucellosis in goats in selected provinces of Lao People’s Democratic Republic. PLoS Negl. Trop. Dis. 2018, 12, e0006411. [Google Scholar] [CrossRef] [PubMed]
- Schack, M.; Sachse, S.; Rödel, J.; Frangoulidis, D.; Pletz, M.W.; Rohde, G.U.; Straube, E.; Boden, K. Coxiella burnetii (Q fever) as a cause of community-acquired pneumonia during the warm season in Germany. Epidemiol. Infect. 2014, 142, 1905–1910. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Q fever. In ECDC. Annual Epidemiological Report for 2019; ECDC: Stockholm, Sweden, 2021. [Google Scholar]
- European Centre for Disease Prevention and Control. Annual Epidemiological Report 2011, Reporting on 2009 Surveillance Data and 2010 Epidemic Intelligence Data; ECDC: Stockholm, Sweden, 2011; p. 224. [Google Scholar]
- European Centre for Disease Prevention and Control. Annual Epidemiological Report 2014, Emerging and Vector-Borne Diseases; ECDC: Stockholm, Sweden, 2014; p. 52. [Google Scholar]
- Cutler, S.J.; Bouzid, M.; Cutler, R.R. Q fever. J. Infect. 2007, 54, 313–318. [Google Scholar] [CrossRef]
- Szymanska-Czerwinska, M.; Galinska, E.M.; Niemczuk, K.; Knap, J.P. Prevalence of Coxiella burnetii Infection in Humans Occupationally Exposed to Animals in Poland. Vector Borne Zoonotic Dis. 2015, 15, 261–267. [Google Scholar] [CrossRef]
- Valencia, M.C.S.; Rodriguez, C.O.; Puñet, O.G.; Giral, I.B. Q fever seroprevalence and associated risk factors among students from the Veterinary School of Zaragoza, Spain. Eur. J. Epidemiol. 2000, 16, 469–476. [Google Scholar] [CrossRef]
- Dorko, E.; Rimárová, K.; Kecerová, A.; Pilipčinec, E.; Dudríková, E.; Lovayová, V.; Petrovičová, J.; Boroš, E. Potential association between Coxiella burnetii seroprevalence and selected risk factors among veterinary students in Slovakia. Ann. Agric. Environ. Med. 2011, 18, 47–53. [Google Scholar]
- de Rooij, M.M.; Schimmer, B.; Versteeg, B.; Schneeberger, P.; Berends, B.R.; Heederik, D.; van der Hoek, W.; Wouters, I.M. Risk factors of Coxiella burnetii (Q fever) seropositivity in veterinary medicine students. PLoS ONE 2012, 7, e32108. [Google Scholar] [CrossRef][Green Version]
- Njeru, J.; Henning, K.; Pletz, M.W.; Heller, R.; Forstner, C.; Kariuki, S.; Fèvre, E.M.; Neubauer, H. Febrile patients admitted to remote hospitals in Northeastern Kenya: Seroprevalence, risk factors and a clinical prediction tool for Q-Fever. BMC Infect. Dis. 2016, 16, 244. [Google Scholar] [CrossRef]
- Schimmer, B.; Lenferink, A.; Schneeberger, P.; Aangenend, H.; Vellema, P.; Hautvast, J.; van Duynhoven, Y. Seroprevalence and Risk Factors for Coxiella burnetii (Q Fever) Seropositivity in Dairy Goat Farmers’ Households in The Netherlands, 2009–2010. PLoS ONE 2012, 7, e42364. [Google Scholar] [CrossRef]
- Glazunova, O.; Roux, V.; Freylikman, O.; Sekeyova, Z.; Fournous, G.; Tyczka, J.; Tokarevich, N.; Kovacova, E.; Marrie, T.J.; Raoult, D. Coxiella burnetii Genotyping. Emerg. Infect. Dis. 2005, 11, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Svraka, S.; Toman, R.; Skultety, L.; Slaba, K.; Homan, W.L. Establishment of a genotyping scheme for Coxiella burnetii. FEMS Microbiol. Lett. 2006, 254, 268–274. [Google Scholar] [CrossRef]
- Astobiza, I.; Tilburg, J.J.; Piñero, A.; Hurtado, A.; García-Pérez, A.L.; Nabuurs-Franssen, M.H.; Klaassen, C.H. Genotyping of Coxiella burnetii from domestic ruminants in northern Spain. BMC Vet. Res. 2012, 8, 241. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.S.; Tilburg, J.J.H.C.; Botelho, A.; Barahona, M.J.; Núncio, M.S.; Nabuurs-Franssen, M.H.; Klaassen, C.H.W. Genotypic diversity of clinical Coxiella burnetii isolates from Portugal based on MST and MLVA typing. Int. J. Med. Microbiol. 2012, 302, 253–256. [Google Scholar] [CrossRef]
- Ceglie, L.; Guerrini, E.; Rampazzo, E.; Barberio, A.; Tilburg, J.J.; Hagen, F.; Lucchese, L.; Zuliani, F.; Marangon, S.; Natale, A. Molecular characterization by MLVA of Coxiella burnetii strains infecting dairy cows and goats of north-eastern Italy. Microbes Infect. 2015, 17, 776–781. [Google Scholar] [CrossRef]
- Joulié, A.; Sidi-Boumedine, K.; Bailly, X.; Gasqui, P.; Barry, S.; Jaffrelo, L.; Poncet, C.; Abrial, D.; Yang, E.; Animal diagnostic laboratories consortium; et al. Molecular epidemiology of Coxiella burnetii in French livestock reveals the existence of three main genotype clusters and suggests species-specific associations as well as regional stability. Infect. Genet Evol. 2017, 48, 142–149. [Google Scholar] [CrossRef]
- Tilburg, J.J.H.C.; Rossen, J.W.; van Hannen, E.J.; Melchers, W.J.; Hermans, M.H.; van de Bovenkamp, J.; Roest, H.J.; de Bruin, A.; Nabuurs-Franssen, M.H.; Horrevorts, A.M.; et al. Genotypic diversity of Coxiella burnetii in the 2007–2010 Q fever outbreak episodes in The Netherlands. J. Clin. Microbiol. 2012, 50, 1076–1078. [Google Scholar] [CrossRef][Green Version]
- Sulyok, K.M.; Kreizinger, Z.; Hornstra, H.M.; Pearson, T.; Szigeti, A.; Dán, Á.; Balla, E.; Keim, P.S.; Gyuranecz, M. Genotyping of Coxiella burnetii from domestic ruminants and human in Hungary: Indication of various genotypes. BMC Vet. Res. 2014, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Rousset, E.; Sidi-Boumedine, K.; Kadra, B.; Kupcsullk, B. Chapter 3.1.17—Q fever. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 8th ed.; World Organization for Animal Health: Paris, France, 2018; Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-manual-online-access/ (accessed on 30 September 2022).
- Database on Multi Spacers Typing of Coxiella burnetii. Available online: https://ifr48.timone.univ-mrs.fr/mst/coxiella_burnetii/ (accessed on 25 September 2022).
- Hemsley, C.M.; Essex-Lopresti, A.; Norville, I.H.; Titball, R.W. Correlating Genotyping Data of Coxiella burnetii with Genomic Groups. Pathogens 2021, 10, 604. [Google Scholar] [CrossRef]
- Pearson, T.; Hornstra, H.M.; Hilsabeck, R.; Gates, L.T.; Olivas, S.M.; Birdsell, D.M.; Hall, C.M.; German, S.; Cook, J.M.; Seymour, M.L.; et al. High prevalence and two dominant host-specific genotypes of Coxiella burnetii in U.S. milk. BMC Microbiol. 2014, 14, 41. [Google Scholar] [CrossRef] [PubMed]
| Country (Area) | Period | Reference |
|---|---|---|
| Australia | 2012–2014 | [20] |
| Bulgaria | 2004 | [21] |
| 2007–2011 | [22] | |
| China | 2018–2019 | [23] |
| France | - | [24] |
| 2007 | [25] | |
| Newfoundland | 1999 | [26] |
| Slovakia | 1993 | [27] |
| The Netherlands | 2007–2020 | [28,29] |
| United Kingdom | 1987 | [30] |
| USA | - | [31] |
| 2011 | [32] |
| Country (Area) | Study Period | Type of Sample | Number of Samples | Test | Prevalence (%) | Reference |
|---|---|---|---|---|---|---|
| Belgium | 2009–2013 | BTM a | 1924 | Real-time PCR | 12.1 | [106] |
| France | - | BTM a | 120 | PCR | 19.0 | [105] |
| Iran | 2008 | BTM a | 110 | Nested PCR | 4.5 | [107] |
| Italy | 2018–2020 | Milk | 68 | PCR | 25.0 | [108] |
| Cheese | 15 | PCR | 6.7 | |||
| Poland | - | BTM a | 35 | Real-time PCR | 54.3 | [109] |
| Portugal | 2009–2013 | BTM a | 12 | Real-time PCR | 0.0 | [110] |
| Switzerland | 2006 | Milk | 39 | Nested PCR | 0.0 | [111] |
| The Gambia | 2012 | Milk | 33 | PCR | 2.94 | [112] |
| The Netherlands | 2008 | BTM a | 292 | Real-time PCR | 32.9 | [113] |
| Turkey | - | Milk | 50 | PCR | 4.0 | [114] |
| USA | 2012 | Milk | 387 | Real-time PCR | 2.5 | [115] |
| Country (Area) | Study Period | Type of Sample | Sampling Method | Number | Test | Cut-Off Value | Prevalence (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| Canada | 2010–2012 | Serum | Multi-stage random | 76 | ELISA | 0.4 | 63.2 | [123] |
| Great Britain | 2008 | Serum | Random stratified | 145 | ELISA | 0.4 | 3.0 | [124] |
| Ireland (Republic of) | 2005–2007 | Serum | Random | 66 | ELISA | 0.4 | 1.5 | [125] |
| Italy | 2012 | Serum | Multi-stage random | 206 | ELISA | 0.4 | 19.5 | [126] |
| Lebanon | 2014 | Serum | Random | 128 | ELISA | 0.4 | 45.3 | [127] |
| Norway | 2009 | BTM a | Random | 348 | ELISA | 0.4 | 0 | [128] |
| Portugal | 2011 | Serum | Random | 52 | ELISA | 0.30 | 28.8 | [129] |
| Spain | 2007–2008 | Serum | Random | 11 | ELISA | 0.40 | 45.0 | [130] |
| Sweden | 2010 | BTM a | Random | 58 | ELISA | 0.4 | 1.7 | [131] |
| Switzerland | 2011 | Serum | Random stratified | 72 | ELISA | 0.4 | 11.1 | [132] |
| The Netherlands | 2008 | Serum | Random | 442 | ELISA | 0.4 | 17.9 | [133] |
| USA | 2012–2014 | Serum | Random | 89 | ELISA | 0.4 | 11.5 | [115] |
| MST | Species | Country | Reference |
|---|---|---|---|
| 8 | Goat | Spain | [160,167] |
| Sheep | Spain | ||
| Human | Portugal, France, and USA | ||
| 13 | Goat | Portugal, Spain | [160,167] |
| Sheep | Spain | ||
| Cattle | Spain | ||
| Human | Portugal | ||
| Ticks | France | ||
| 18 | Goat | Germany, Spain | [160,167] |
| Sheep | Germany | ||
| Cattle | Poland | ||
| Human | France, Greece, Italy, Poland, Slovakia, and Romania | ||
| 30 | Goat | Namibia | [167] |
| 32 | Goat | Austria | [167] |
| Human | France and Germany | ||
| 53 | Goat | France | [167] |
| 58 | Goat | Libano | [167] |
| 61 | Goat | Poland | [167] |
| Cattle | Iran | [167] | |
| 62 | Goat | Iran | [167] |
| Sheep | [167] | ||
| Cattle | [167] | ||
| 66 to 70 | Goat | Thailand | [167] |
| 74 | Goat | Brazil | [167] |
| Cattle | [167] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anastácio, S.; de Sousa, S.R.; Saavedra, M.J.; da Silva, G.J. Role of Goats in the Epidemiology of Coxiella burnetii. Biology 2022, 11, 1703. https://doi.org/10.3390/biology11121703
Anastácio S, de Sousa SR, Saavedra MJ, da Silva GJ. Role of Goats in the Epidemiology of Coxiella burnetii. Biology. 2022; 11(12):1703. https://doi.org/10.3390/biology11121703
Chicago/Turabian StyleAnastácio, Sofia, Sérgio Ramalho de Sousa, Maria José Saavedra, and Gabriela Jorge da Silva. 2022. "Role of Goats in the Epidemiology of Coxiella burnetii" Biology 11, no. 12: 1703. https://doi.org/10.3390/biology11121703
APA StyleAnastácio, S., de Sousa, S. R., Saavedra, M. J., & da Silva, G. J. (2022). Role of Goats in the Epidemiology of Coxiella burnetii. Biology, 11(12), 1703. https://doi.org/10.3390/biology11121703