Evaluation of Candidates for Systemic Analgesia and General Anesthesia in the Emerging Model Cephalopod, Euprymna berryi
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
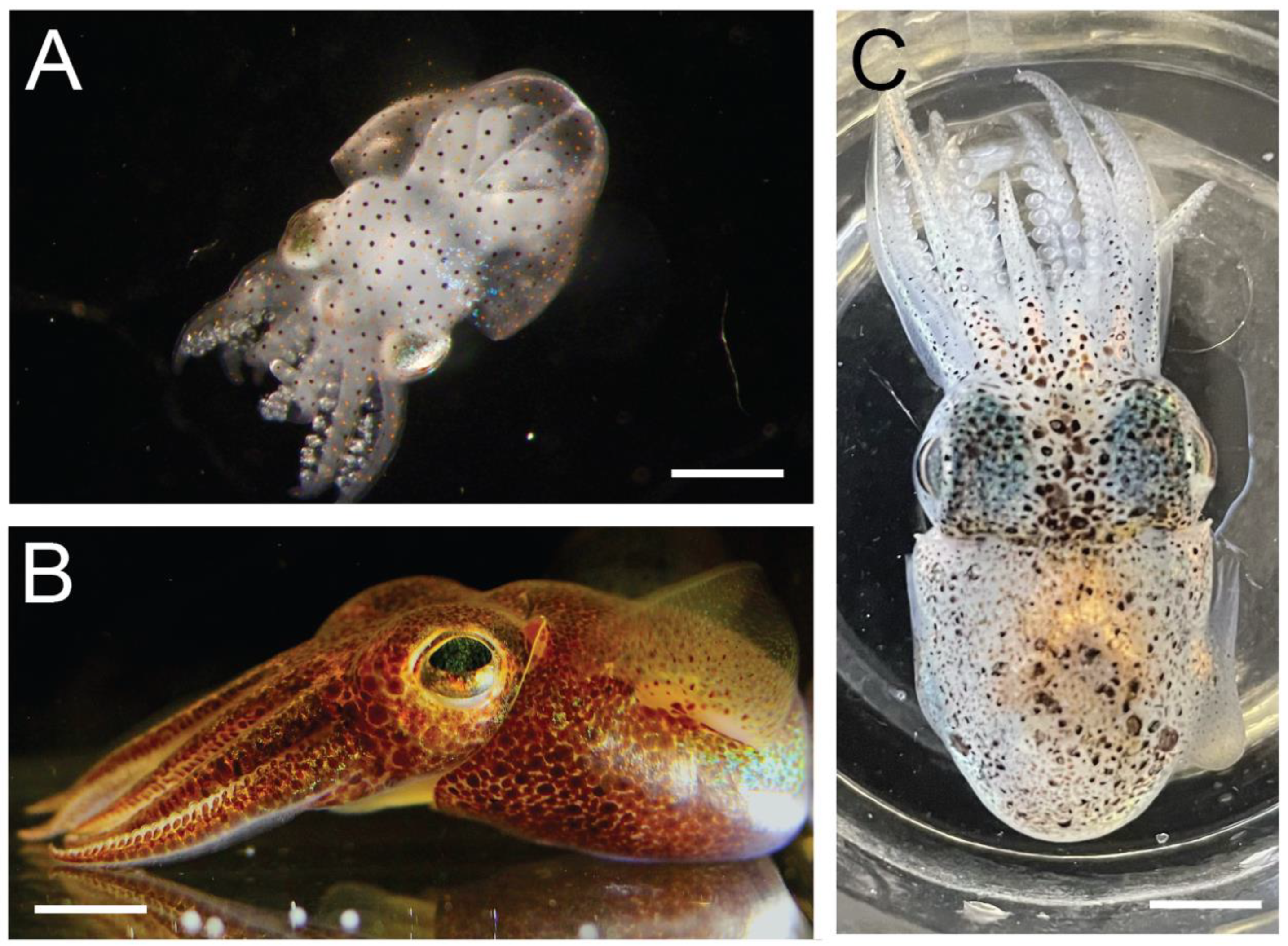
2.1. Animals
2.2. Analgesic Drugs and Dosages
2.3. Intramuscular Injections of Analgesic Drugs
2.4. Behavioral Assays of Analgesia Effects
2.5. Electrophysiological Measures of Analgesia
2.6. Responses to Painful Fin Pinch
2.7. Sequence Alignment Methods
2.8. General Anesthesia Trials
2.9. Data Analysis and Statistical Procedures
3. Results
3.1. Nociceptive Threshold Testing
3.2. Peripheral Nerve Excitability
3.3. Behavioral Response to Painful Sensory Input
3.4. Sequence Alignments
3.5. General Anesthesia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Brien, C.E.; Roumbedakis, K.; Winkelmann, I.E. The Current State of Cephalopod Science and Perspectives on the Most Critical Challenges Ahead from Three Early-Career Researchers. Front. Physiol. 2018, 9, 700. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, G.; Affuso, A.; Basil, J.; Cole, A.; de Girolamo, P.; D’Angelo, L.; Dickel, L.; Gestal, C.; Grasso, F.; Kuba, M.; et al. Guidelines for the Care and Welfare of Cephalopods in Research –A Consensus Based on an Initiative by CephRes, FELASA and the Boyd Group. Lab. Anim. 2015, 49, 1–90. [Google Scholar] [CrossRef] [PubMed]
- Birch, J.; Burn, C.; Schnell, A.; Browning, H.; Crump, A. Review of the Evidence of Sentience in Cephalopod Molluscs and Decapod Crustaceans; The London School of Economics and Political Science: London, UK, 2021. [Google Scholar]
- Canadian Council on Animal Care. Canadian Council on Animal Care CCAC Policy Statement; Canadian Council on Animal Care: Ottawa, ON, Canada, 1996. [Google Scholar]
- Moulton, S.; Holmes Norton, E.; McGovern, J.P.; Malinowski, T.; Huffman, J.; DelBene, S.K. Humane Care Handling Standards for Cephalopods: Letter to Xavier Becerra, Secretary, HHS and Lawrence Tabak, Act. Director. NIH: Bethesda, MD, USA, 2022. [Google Scholar]
- Jacquet, J.; Franks, B.; Pungor, J.; Mather, J.; Godfrey-Smith, P.; Marino, L.; Barord, G.; Safina, C.; Browning, H.; Veit, W.; et al. Petition to Include Cephalopods as “Animals” Deserving of Humane Treatment under the Public Health Service Policy on Humane Care and Use of Laboratory Animals. Harvard Law School Animal Law and Policy Clinic: Cambridge, MA, USA, 2020. [Google Scholar]
- Birch, J. Animal Sentience and the Precautionary Principle. Anim. Sentience 2017, 2, 1. [Google Scholar] [CrossRef]
- Alupay, J.S.; Hadjisolomou, S.P.; Crook, R.J. Arm Injury Produces Long-Term Behavioral and Neural Hypersensitivity in Octopus. Neurosci. Lett. 2014, 558, 137–142. [Google Scholar] [CrossRef]
- Crook, R.J.; Lewis, T.; Hanlon, R.T.; Walters, E.T. Peripheral Injury Induces Long-Term Sensitization of Defensive Responses to Visual and Tactile Stimuli in the Squid Loligo Pealeii, Lesueur 1821. J. Exp. Biol. 2011, 214, 3173–3185. [Google Scholar] [CrossRef] [Green Version]
- Howard, R.B.; Lopes, L.N.; Lardie, C.R.; Perez, P.P.; Crook, R.J. Early-Life Injury Produces Lifelong Neural Hyperexcitability, Cognitive Deficit and Altered Defensive Behaviour in the Squid Euprymna Scolopes. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20190281. [Google Scholar] [CrossRef] [Green Version]
- Crook, R.J. Somatotopic Organization of Mechanosensory Afferents in the Stellate Ganglion of the Squid, Euprymna. bioRxiv 2022, 10, 513268. [Google Scholar] [CrossRef]
- Crook, R.J.; Hanlon, R.T.; Walters, E.T. Squid Have Nociceptors That Display Widespread Long-Term Sensitization and Spontaneous Activity after Bodily Injury. J. Neurosci. 2013, 33, 10021–10026. [Google Scholar] [CrossRef] [Green Version]
- Crook, R.J. Behavioral and Neurophysiological Evidence Suggests Affective Pain Experience in Octopus. iScience 2021, 24, 102229. [Google Scholar] [CrossRef]
- Holst, M.A.; Howard, R.B.; Crook, R.J. Cephalopods. In Universities Federation for Animal Welfare Handbook on the Care and Management of Laboratory Animals (UFAW Handbook); Wiley: London, UK, 2022. [Google Scholar]
- Gavriouchkina, D.; Tan, Y.; Ziadi-Künzli, F.; Hasegawa, Y.; Piovani, L.; Zhang, L.; Sugimoto, C.; Luscombe, N.; Marlétaz, F.; Rokhsar, D.S. A Single-Cell Atlas of Bobtail Squid Visual and Nervous System Highlights Molecular Principles of Convergent Evolution. bioRxiv 2022. [Google Scholar] [CrossRef]
- Bazarini, S.N.; Crook, R.J. Environmental Estrogen Exposure Disrupts Sensory Processing and Nociceptive Plasticity in the Cephalopod Euprymna Scolopes. J. Exp. Biol. 2020, 223, jeb218008. [Google Scholar] [CrossRef] [PubMed]
- Seehafer, K.; Brophy, S.; Tom, S.R.; Crook, R.J. Ontogenetic and Experience-Dependent Changes in Defensive Behavior in Captive-Bred Hawaiian Bobtail Squid, Euprymna Scolopes. Front. Physiol. 2018, 9, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zepeda, E.A.; Veline, R.J.; Crook, R.J. Rapid Associative Learning and Stable Long-Term Memory in the Squid Euprymna Scolopes. Biol. Bull. 2017, 232, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Drerup, C.; Sykes, A.v.; Cooke, G.M. Behavioural Aspects of the Spotty Bobtail Squid Euprymna Parva (Cephalopoda: Sepiolidae). J. Exp. Mar. Biol. Ecol. 2020, 530–531, 151442. [Google Scholar] [CrossRef]
- Nyholm, S.v.; McFall-Ngai, M. The Winnowing: Establishing the Squid–Vibrio Symbiosis. Nat. Rev. Microbiol. 2004, 2, 632–642. [Google Scholar] [CrossRef]
- Lee, P.N.; McFall-Ngai, M.J.; Callaerts, P.; de Couet, H.G. The Hawaiian Bobtail Squid (Euprymna Scolopes): A Model to Study the Molecular Basis of Eukaryote-Prokaryote Mutualism and the Development and Evolution of Morphological Novelties in Cephalopods. Cold Spring Harb. Protoc. 2009, 2009, pdb-emo135. [Google Scholar] [CrossRef]
- Peyer, S.M.; Pankey, M.S.; Oakley, T.H.; McFall-Ngai, M.J. Eye-Specification Genes in the Bacterial Light Organ of the Bobtail Squid Euprymna Scolopes, and Their Expression in Response to Symbiont Cues. Mech. Dev. 2014, 131, 111–126. [Google Scholar] [CrossRef]
- McFall-Ngai, M.; Heath-Heckman, E.A.C.C.; Gillette, A.A.; Peyer, S.M.; Harvie, E.A. The Secret Languages of Coevolved Symbioses: Insights from the Euprymna Scolopes-Vibrio Fischeri Symbiosis. Semin. Immunol. 2012, 24, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Nyholm, S.v.; Mcfall-Ngai, M.J. Sampling the Light-Organ Microenvironment of Euprymna Scolopes: Description of a Population of Host Cells in Association with the Bacterial Symbiont Vibrio Fischeri. Biol. Bull. 1998, 195, 89–97. [Google Scholar] [CrossRef]
- Hanlon, R.T.; Claes, M.F.; Ashcraft, S.E.; Dunlap, P.v. Laboratory Culture of the Sepiolid Squid Euprymna Scolopes: A Model System for Bacteria-Animal Symbiosis. Biol. Bull. 1997, 192, 364–374. [Google Scholar] [CrossRef]
- Jolly, J.; Hasegawa, Y.; Sugimoto, C.; Zhang, L.; Kawaura, R.; Sanchez, G.; Gavriouchkina, D.; Marlétaz, F.; Rokhsar, D. Lifecycle, Culture, and Maintenance of the Emerging Cephalopod Models Euprymna Berryi and Euprymna Morsei. Front. Mar. Sci. 2022, 9, 2453. [Google Scholar] [CrossRef]
- Butler-Struben, H.M.; Brophy, S.M.; Johnson, N.A.; Crook, R.J. In Vivo Recording of Neural and Behavioral Correlates of Anesthesia Induction, Reversal, and Euthanasia in Cephalopod Molluscs. Front. Physiol. 2018, 9, 109. [Google Scholar] [CrossRef] [Green Version]
- Abbo, L.A.; Himebaugh, N.E.; DeMelo, L.M.; Hanlon, R.T.; Crook, R.J. Anesthetic Efficacy of Magnesium Chloride and Ethyl Alcohol in Temperate Octopus and Cuttlefish Species. J. Am. Assoc. Lab. Anim. Sci. 2021, 60, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Sprecher, M.; Sprecher, S.G.; Spadavecchia, C. A Pilot Investigation of the Efficacy and Safety of Magnesium Chloride and Ethanol as Anesthetics in Loligo Vulgaris Embryos. Front. Physiol. 2022, 13, 1880. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.; Sneddon, L.; Spencer, J.; Chiao, C.C. Impact of Lidocaine on Pain-Related Grooming in Cuttlefish. Biology 2022, 11, 1560. [Google Scholar] [CrossRef] [PubMed]
- Cooke, G.M.; Anderson, D.B.; Begout, M.-L.; Dennison, N.; Osorio, D.; Tonkins, B.; Kristiansen, T.; Fiorito, G.; Galligioni, V.; Ponte, G.; et al. Prospective Severity Classification of Scientific Procedures in Cephalopods: Report of a COST FA1301 Working Group Survey. Lab. Anim. 2019, 53, 541–563. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.J.; James, L.E.; Bertelsen, M.F.; Wang, T. Analgesia for Non-Mammalian Vertebrates. Curr. Opin. Physiol. 2019, 11, 75–84. [Google Scholar] [CrossRef]
- Sneddon, L.U. Evolution of Nociception and Pain: Evidence from Fish Models. Philos. Trans. R. Soc. B 2019, 374, 20190290. [Google Scholar] [CrossRef] [PubMed]
- Frederic, C.; Creighton, C.M.; Stevens, D.E. Updated Review of Fish Analgesia. JAALAS 2018, 57, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Martins, T.; Valentim, A.; Pereira, N.; Antunes, L.M. Anaesthetics and Analgesics Used in Adult Fish for Research: A Review. Lab. Anim. 2018, 53, 325–341. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: A Multiple Sequence Alignment Method with Reduced Time and Space Complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Priyam, A.; Woodcroft, B.J.; Rai, V.; Moghul, I.; Munagala, A.; Ter, F.; Chowdhary, H.; Pieniak, I.; Maynard, L.J.; Gibbins, M.A.; et al. Sequenceserver: A Modern Graphical User Interface for Custom BLAST Databases. Mol. Biol. Evol. 2019, 36, 2922–2924. [Google Scholar] [CrossRef]
- Belcaid, M.; Casaburi, G.; McAnulty, S.J.; Schmidbaur, H.; Suria, A.M.; Moriano-Gutierrez, S.; Sabrina Pankey, M.; Oakley, T.H.; Kremer, N.; Koch, E.J.; et al. Symbiotic Organs Shaped by Distinct Modes of Genome Evolution in Cephalopods. Proc. Natl. Acad. Sci. USA 2019, 116, 3030–3035. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ye, F.; Zhang, T.; Lv, S.; Zhou, L.; Du, D.; Lin, H.; Guo, F.; Luo, C.; Zhu, S. Structural Basis of Ketamine Action on Human NMDA Receptors. Nature 2021, 596, 301–305. [Google Scholar] [CrossRef]
- Orlando, B.J.; Lucido, M.J.; Malkowski, M.G. The Structure of Ibuprofen Bound to Cyclooxygenase-2. J. Struct. Biol. 2015, 189, 62. [Google Scholar] [CrossRef] [Green Version]
- Qu, L.; Zhou, Q.; Xu, Y.; Guo, Y.; Chen, X.; Yao, D.; Han, G.W.; Liu, Z.J.; Stevens, R.C.; Zhong, G.; et al. Structural Basis of the Diversity of Adrenergic Receptors. Cell Rep. 2019, 29, 2929–2935.e4. [Google Scholar] [CrossRef] [Green Version]
- Villiger, J.W.; Taylor, K.M. Buprenorphine: Characteristics of Binding Sites in the Rat Central Nervous System. Life Sci. 1981, 29, 2699–2708. [Google Scholar] [CrossRef]
- Wang, F.; Shen, W.; Cai, Y.; Zhang, X.; Du, H.; Lai, M.; Liu, H.; Kohli, E.; Zhou, W. Buprenorphine Reduces Methamphetamine Intake and Drug Seeking Behavior via Activating Nociceptin/Orphanin FQ Peptide Receptor in Rats. Front. Psychiatry 2022, 13, 983595. [Google Scholar] [CrossRef] [PubMed]
- Bloms-Funke, P.; Gillen, C.; Schuettler, A.J.; Wnendt, S. Agonistic Effects of the Opioid Buprenorphine on the Nociceptin/OFQ Receptor. Peptides 2000, 21, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Cerreta, A.J.; Masterson, C.A.; Lewbart, G.A.; Dise, D.R.; Papich, M.G. Pharmacokinetics of Ketorolac in Wild Eastern Box Turtles (Terrapene Carolina Carolina) after Single Intramuscular Administration. J. Vet. Pharmacol. Ther. 2019, 42, 154–159. [Google Scholar] [CrossRef]
- Mathews, K.A.; Paley, D.M.; Foster, R.A.; Valliant, A.E.; Young, S.S. A Comparison of Ketorolac with Flunixin, Butorphanol, and Oxymorphone in Controlling Postoperative Pain in Dogs. Can. Vet. J. 1996, 37, 557–567. [Google Scholar] [PubMed]
- Chiu, T.H.; Chen, M.J.; Yang, Y.R.; Yang, J.J.; Tang, F.I. Action of Dexmedetomidine on Rat Locus Coeruleus Neurones: Intracellular Recording in Vitro. Eur. J. Pharmacol. 1995, 285, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.; Riaz, S.; Hasan, S.; Iqbal, F.; Rice, T.; Syed, N. Mechanisms of Anesthetic Action and Neurotoxicity: Lessons from Molluscs. Front. Physiol. 2018, 8, 1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwijnenberg, R.; Muir, W. Evaluation of the Potential for Interaction between a Metaflumizone-Amitraz Combination and Dexmedetomidine Hydrochloride in Dogs-PubMed. Vet. Ther. 2009, 10, 40–45. [Google Scholar] [PubMed]
- Holst, M.M.; Hauver, C.M.; Stein, R.S.; Milano, B.L.; Levine, L.H.; Zink, A.G.; Watters, J.v.; Crook, R.J. Behavioral Changes in Senescent Giant Pacific Octopus (Enteroctopus Dofleini) Are Associated with Peripheral Neural Degeneration and Loss of Epithelial Tissue. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2022, 271, 111263. [Google Scholar] [CrossRef]
- Blednov, Y.; Stoffel, M.; Chang, S.; Harris, R.A. Potassium Channels as Targets for Ethanol: Studies of G-Protein-Coupled Inwardly Rectifying Potassium Channel 2 (GIRK2) Null Mutant Mice. ASPET J. Pharmacol. Exp. Ther. 2001, 298, 521–530. [Google Scholar]
- Bodhinathan, K.; Slesinger, P.A. Molecular Mechanism Underlying Ethanol Activation of G-Protein-Gated Inwardly Rectifying Potassium Channels. Proc. Natl. Acad. Sci. USA 2013, 110, 18309–18314. [Google Scholar] [CrossRef] [Green Version]
- Brodie, M.S.; Scholz, A.; Weiger, T.M.; Dopico, A.M. Ethanol Interactions with Calcium-Dependent Potassium Channels. Alcohol. Clin. Exp. Res. 2007, 31, 1625–1632. [Google Scholar] [CrossRef]
- Söderpalm, B.; Lidö, H.; Ericson, M. The Glycine Receptor—A Functionally Important Primary Brain Target of Ethanol. Alcohol. Clin. Exp. Res. 2017, 41, 1816–1830. [Google Scholar] [CrossRef] [PubMed]
- Perkins, D.I.; Trudell, J.R.; Crawford, D.K.; Alkana, R.L.; Davies, D.L. Molecular Targets and Mechanisms for Ethanol Action in Glycine Receptors. Pharmacol. Ther. 2010, 127, 53–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winlow, W.; Polese, G.; Moghadam, H.F.; Ahmed, I.A.; di Cosmo, A. Sense and Insensibility-An Appraisal of the Effects of Clinical Anesthetics on Gastropod and Cephalopod Molluscs as a Step to Improved Welfare of Cephalopods. Front. Physiol. 2018, 9, 1147. [Google Scholar] [CrossRef] [PubMed]





| Experiment Phase | Treatments | Count | Severity Classification |
|---|---|---|---|
| Analgesia: Nociceptive threshold testing | Control | 51 | Mild/Moderate |
| Dexdomitor | 6 | ||
| Ketorolac | 8 | ||
| Buprenex (low dose) | 10 | ||
| Buprenex (high dose) | 7 | ||
| Acetominophen | 6 | ||
| Ketamine | 6 | ||
| n = 94 | |||
| Analgesia: Electrophysiology | Ketorolac | 8 | Non-recovery |
| Buprenex | 8 | ||
| Dexdomitor | 8 | ||
| n = 24 | |||
| Analgesia: Pain behavior | Control | 4 | Moderate/Severe |
| Buprenex | 4 | ||
| Dexdomitor | 4 | ||
| Ketorolac | 4 | ||
| n = 16 | |||
| General anesthesia | Hatchling | 16 | Mild |
| Juvenile/sub-adult | 6 | ||
| Senescent adult | 11 | ||
| n = 33 | |||
| Total in Mild/Moderate | 127 | ||
| Total in Moderate/Severe | 16 | ||
| Total Non-recovery | 24 | ||
| Total squid used in this study | n = 167 | ||
| Agent | Schedule | Classification | Route | Dose | Predicted Target |
|---|---|---|---|---|---|
| Acetaminophen | Over the Counter (OTC) | Pain Reliever & Antipyretic | Oral (PO) | 0.14 mg/kg | COX-2 prostaglandin pathway, other targets |
| Ketorolac | Prescription (Rx) | Non-Steroidal Anti-Inflammatory (NSAID) | Intramuscular (IM) | 3–6 mg/kg | COX-2 prostaglandin pathway |
| Dexmedetomidine | Prescription (Rx) | Sedative | Intramuscular (IM) | 0.005 mg/kg | Alpha-2 adrenoreceptor |
| Ketamine | Schedule Three (III) | Dissociative Anesthetic | Intramuscular (IM) | 10 mg/kg | NMDA receptor, likely other targets |
| Buprenorphine | Schedule Three (III) | Opioid | Intramuscular (IM) | Low: 0.015 mg/kg | Mu-opioid and kappa-opioid receptors, possible met-enkephalin receptors |
| High: 0.15 mg/kg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deutsch, S.; Parsons, R.; Shia, J.; Detmering, S.; Seng, C.; Ng, A.; Uribe, J.; Manahan, M.; Friedman, A.; Winters-Bostwick, G.; et al. Evaluation of Candidates for Systemic Analgesia and General Anesthesia in the Emerging Model Cephalopod, Euprymna berryi. Biology 2023, 12, 201. https://doi.org/10.3390/biology12020201
Deutsch S, Parsons R, Shia J, Detmering S, Seng C, Ng A, Uribe J, Manahan M, Friedman A, Winters-Bostwick G, et al. Evaluation of Candidates for Systemic Analgesia and General Anesthesia in the Emerging Model Cephalopod, Euprymna berryi. Biology. 2023; 12(2):201. https://doi.org/10.3390/biology12020201
Chicago/Turabian StyleDeutsch, Skyler, Rachel Parsons, Jonathan Shia, Sarah Detmering, Christopher Seng, Alyssa Ng, Jacqueline Uribe, Megan Manahan, Amanda Friedman, Gabrielle Winters-Bostwick, and et al. 2023. "Evaluation of Candidates for Systemic Analgesia and General Anesthesia in the Emerging Model Cephalopod, Euprymna berryi" Biology 12, no. 2: 201. https://doi.org/10.3390/biology12020201
APA StyleDeutsch, S., Parsons, R., Shia, J., Detmering, S., Seng, C., Ng, A., Uribe, J., Manahan, M., Friedman, A., Winters-Bostwick, G., & Crook, R. J. (2023). Evaluation of Candidates for Systemic Analgesia and General Anesthesia in the Emerging Model Cephalopod, Euprymna berryi. Biology, 12(2), 201. https://doi.org/10.3390/biology12020201






